Abstract
Exposure to hypoxia is known to induce a reduction in core body temperature as a protective mechanism, which has been shown in both animals and humans. The purpose of this study was to test if acute exposure to normobaric hypoxia (NH) induces anapyrexia in adult humans in association with decreased peripheral oxygen saturation (). Ten healthy male subjects were seated in atmospheres of normobaric normoxia 21% (NN21), NH 17% (NH17), and 13% (NH13) O2 for 60 minutes in a counterbalanced manner. Rectal temperature (Tre) was continuously monitored together with the quantification of metabolic heat production (MHP) and body heat storage (S). Baseline physiological measurements showed no differences between the three conditions. was significantly decreased in NH17 and NH13 compared with NN21 (p≤0.001). Tre decreased following 60 minutes of resting in all conditions, but, independent of the conditions, showed no association between Tre and levels of hypoxic . There was also no significant difference in either MHP or S between conditions. The present results showed no evidence of hypoxia-induced anapyrexia in adult humans during 1 hour of resting after exposure to NH either at 13% or 17% .
Keywords: body temperature, heat storage, hypoxia, metabolic heat production
Introduction
Exposure to hypoxia evokes numerous physiological adjustments to compensate for a low partial pressure of oxygen () in the inspired air, leading to insufficient supply into the body compared with sea level. These compensatory responses include, for example, increases in alveolar ventilation, cardiac output, and sympathetic excitation (Steiner and Branco, 2002; Wilkins et al., 2006).
Hypoxia has also been known to induce a reduction in core body temperature (Tc), termed hypoxia-induced anapyrexia, which has been experimentally demonstrated in both animal and human studies (Gellhorn and Janus, 1936; Miller and Westheimer, 1966; Schubring, 1986; DiPasquale et al., 2015). This physiologic response is thought to be a protective mechanism of the body against low availability. Thus, to achieve homeostasis through, for example, a reduction in consumption (), a leftward shift of the oxyhemoglobin dissociation curve results in improved delivery to the body (Krogh, 1914; Wood, 1991; Steiner and Branco, 2002).
While the exact underlying physiologic and neuronal pathways governing anapyrexia remain unclear, it has been suggested that a downward resetting of the thermoregulatory set point triggered by peripheral and/or central chemoreflex activation may be a contributing factor (Wood, 1991; Steiner and Branco, 2002). There have also been a number of putative mediators thought to contribute to the development of hypoxia-induced anapyrexia discussed in review articles by Wood (1991) and Steiner and Branco (2002). More recent reports suggest other possible modulators of hypoxia-induced anapyrexia, including neurotransmitters such as hydrogen sulfide, endogenous opioids, nitric oxide, serotonin, adenosine, and dopamine (Scarpellini Cda et al., 2009; Branco et al., 2010; Kwiatkoski et al., 2012).
Despite ample evidence in animal studies, the presence of hypoxia-induced anapyrexia in adult humans is challenged by equivocal results of bidirectional responses in Tc (Cipriano and Goldman, 1975; Blatteis and Lutherer,1976; Coste et al., 2009; DiPasquale et al., 2015). Moreover, hypoxia-dependent reduction in Tc is much less clear in some studies due to confounding factors (e.g., acute mountain sickness, cold ambient temperature) and/or as an indirect finding rather than testing for hypoxia-induced anapyrexia (Coste et al., 2004, 2009). The recent study by DiPasquale et al. (2015) demonstrated significantly decreased rectal temperature (Tre) in adult humans during acute exposure to normobaric hypoxia (NH) in a thermoneutral ambient condition. In that study, the decrease in Tre was observed to be as much as 0.3C in less than an hour, with a clear dose-response relationship between peripheral saturation () and Tre, leading to a conclusion that is a strong predictor of hypoxia-induced anapyrexia. However, their experimental trials of normoxia and hypoxia were not isolated, but carried out on the same day, making it somewhat difficult to characterize Tre responses between conditions. Therefore, there is a possibility of the carryover effect on Tre response to a subsequent hypoxic condition and separate exposures would provide clearer results, although both continuous and separate exposures to hypoxia are considered validated methods (Scott et al., 2008). Furthermore, it is not clear from their results if the observed anapyrexia was due to hypometabolism (e.g., reduced metabolic heat production [MHP]), increased heat loss (e.g., greater heat exchange between the body and environment), or a combination of both.
Therefore, the present study tested the hypothesis that acute exposure to NH results in a reduction in Tc predicted by in a dose-response manner to different levels of hypoxia. Furthermore, the present study also quantified MHP and body heat storage (S) to clarify if hypometabolism or increased body heat loss, singly or in combination, contributes to hypoxia-dependent anapyrexia.
Materials and Methods
The Institutional Review Board at Kent State University approved this study and all subjects signed a consent form before participation. The current study was carried out in a repeated measures within-subjects design. The study included three experimental trials: normobaric normoxia (21% : NN21) and two levels of NH (17% : NH17 and 13% : NH13). Experimental trials were counterbalanced and separated by at least 7 days.
Subjects
Ten healthy nonsmoking men (23.4±3.0 years; 179.5± 5.2cm; 83.3±9.1kg; 10.7%±6.0% body fat) volunteered to participate in the current study. Subjects were excluded if they reported the presence or history of cardiovascular disease, metabolic disorder, respiratory disease, or were exposed to NH or an altitude above 2500m within 2 months before participation. Women were not included in this study due to established gender difference in thermoregulation (Wagner and Horvath, 1985).
Experimental procedures
Before participation, subjects underwent prescreening with a medical questionnaire and were introduced to the NH chamber and the study procedures. Subjects were also instructed to abstain from strenuous exercise, caffeine, and alcohol at least 24 hours before each experimental trial. On the day of the experimental trial, subjects reported to the Exercise Physiology Laboratory at Kent State University at the same time of the day, following a 3-hour self-reported fast intended to stabilize substrate utilization (Ruby and Robergs, 1994). Upon arrival at the laboratory, subjects were dressed in t-shirts and shorts and self-inserted a flexible rectal thermocouple 13 cm past the anal sphincter, after which study instrumentation occurred. Then, subjects sat on a chair quietly for ~30 minutes in a thermoneutral condition (25°C, 40% relative humidity) and baseline measurements of , , Tre, and skin temperature (Tsk) were obtained. Following baseline measurements, subjects walked ~3m into the hypoxic chamber (Colorado Altitude Training, Louisville, CO) and, after ~5 minutes of sensor instrumentation, sat upright on a chair quietly for 60 minutes where the atmospheric levels were maintained at 21%, 17%, or 13% according to the assigned trial of the day.
Instrumentation
Tre and Tsk were measured continuously using thermocouples (ITP082–25, Nikkiso; Therm Co., Ltd., Japan) connected to a data logger (Model N543, Nikkiso; Therm Co., Ltd.). Five skin thermocouples were affixed with a transparent permeable film on chest, forearm, thigh, triceps, and calf for the calculation of weighted mean skin temperature (Toner et al., 1985). Consequently, the thermal gradient (TG) between the body core and skin was calculated as follows: Tre-Tsk; and mean body temperature (Tb) was calculated as follows: 0.64 (Tre) +0.36 (Tsk) (Burton, 1935). was measured by indirect open-circuit spirometry (True Max 2400; Parvo Medics, Sandy, UT) and averaged at 1-minute intervals. was measured by digit pulse oximeter (Oxy-Go, Roslyn, NY) at 14–15th, 29–30th, 44–45th, and 59–60th minutes.
Measurement of thermogenesis was determined through calculation of MHP (Cena and Clark, 1981):
where RER is respiratory exchange ratio, is the caloric equivalent of a liter of oxygen when carbohydrates are oxidized (21.1kJ), and is the caloric equivalent of a liter of oxygen when fat is oxidized (19.6kJ). BSA is the body surface area (m2) estimated as ; where is the weight of body (kg) and is the height of body (m).
Body heat storage (S; kJ m−2) was calculated as follows:
where is the body mass (kg) and is the average heat capacity of the body (3.48kJ/kg/C).
Statistical analyses
Using a statistical software package (SPSS v.19.0), two-way (three conditions by five time points) repeated measures analysis of variance (ANOVA) was performed to ascertain the effect of hypoxia on dependent variables. When ANOVA indicated a significant interaction, post hoc pair-wise comparison with least significant differences was performed. Statistical significance was set at p<0.05 and all data are presented as mean±standard deviation (SD).
Results
Baseline measurements showed no differences in any dependent variables between conditions (NN21, NH17, and NH13).
There was a significant condition by time interaction for (F=18.924, p≤0.001) so that was significantly decreased immediately following exposure to hypoxia in NH17 (6% reduction) and NH13 (13% reduction), but not in NN21, and remained unchanged during 60 minutes of resting with a clear dose-response to the severity of hypoxia (p≤0.001).
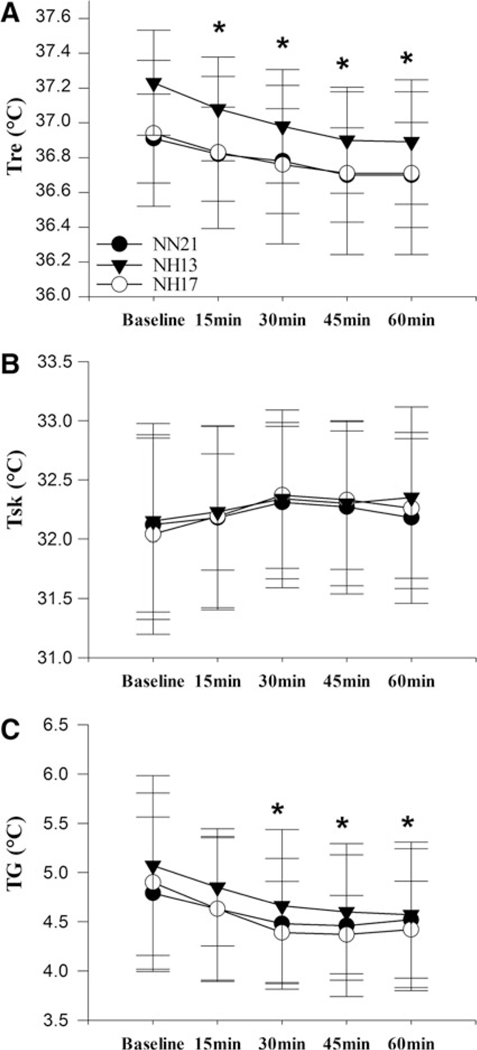
There was no significant interaction found for Tre (F=1.658, p=0.124), and Tre trended to decrease in all conditions by 0.2°C±0.1°C, 0.3°C±0.1°C, and 0.3°C±0.2°C following 60 minutes of resting compared with baseline in NN21, NH17, and NH13, respectively (Fig. 1A). Tsk was also unchanged following 60 minutes of resting compared with baseline in NN21, NH17, and NH13, respectively (Fig. 1B), and therefore no significant difference was observed in TG trending a reduction by 0.3°C±0.6°C, 0.5°C±0.9°C, and 0.5°C±0.7°C in NN21, NH17, and NH13, respectively (Fig. 1C).
FIG. 1. (A).
Rectal temperature and (B) mean skin temperature and (C) thermal gradient between Tre and Tsk response to 60 minutes of resting in NN21, NH17, and NH13 conditions; *p≤0.05 compared with baseline at given time point, mean±SD. No significant condition by time interaction between the conditions. NH, normobaric hypoxia; NN, normobaric normoxia; SD, standard deviation; Tre, rectal temperature; Tsk, skin temperature.
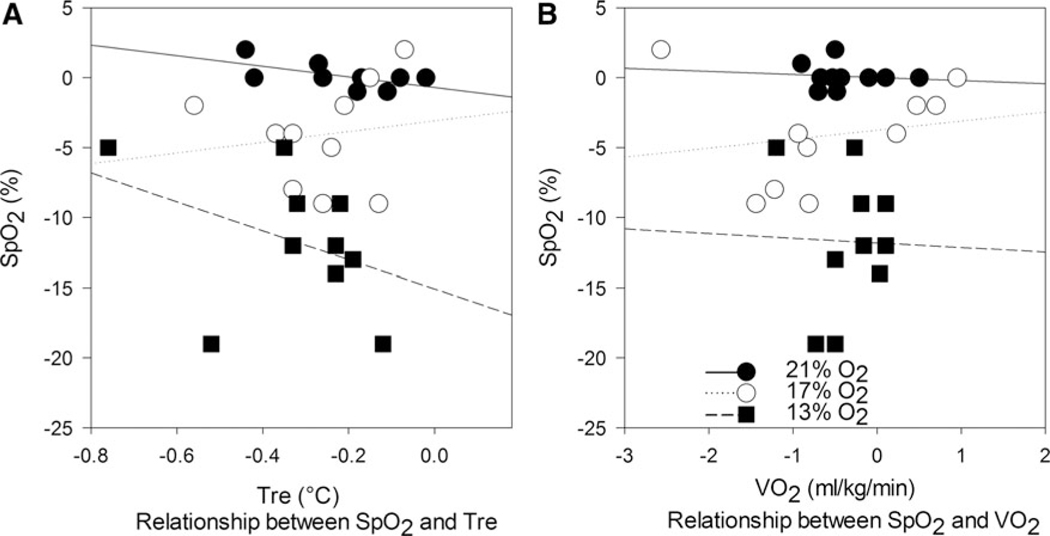
Consequently, no meaningful association was identified between and Tre; NN21 (R2 =0.352, p=0.071), NH17 (R2 =0.021, p=0.693), and NH13 (R2 =0.155, p=0.260) (Fig. 2A); or between and ; NN21 (R2 =0.011, p= 0.772), NH17 (R2 =0.036, p=0.598), and NH13 (R2 =0.001, p=0.938) (Fig. 2B).
FIG. 2.
(A) Relationship between changes in and Tre and (B) relationship between changes in and across three conditions. , peripheral oxygen saturation; , oxygen uptake.
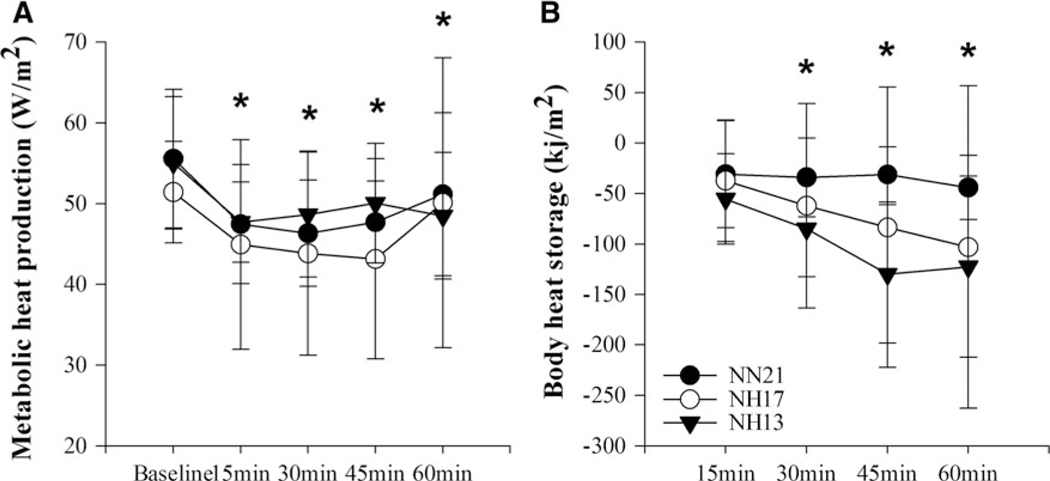
There was no significant interaction found for either MHP (F=0.531, p=0.829) or S (F=1.344, p=0.254) such that MHP was reduced by 4.4±11.0, 1.3±16.6, and 6.6±8.7W·m−2 (Fig. 3A) and S was reduced by 44.1±31.7, 103.1±159.8, and 122.6±89.9kJ · m−2 following 60 minutes of resting compared with baseline in NN21, NH17, and NH13, respectively (Fig. 3B). Therefore, no evidence of hypoxia-induced hypometabolism or increased heat loss leading to a significant reduction in Tre was observed.
FIG. 3.
(A) MHP and (B) body heat storage response to 60 minutes of resting in NN21, NH17, and NH13 conditions; *p≤0.05 compared with baseline at given time point, mean±SD. No significant condition by time interaction between the conditions. MHP, metabolic heat production.
Discussion
The present study was conducted to determine hypoxia-induced reduction in Tc in adult humans as well as to quantify thermoregulatory effector responses to acute exposure to different severities of NH. Contrary to previous findings (DiPasquale et al., 2015), the current results showed neither significant hypoxia-induced reduction in Tre nor meaningful association between and Tre during 1 hour of passive exposure to NH13 and NH17. Furthermore, there were no significant signs of hypometabolism or increased heat loss as a possible effector pathway for anapyrexia when determined by MHP and S, respectively.
The present findings support a previous study that showed reduction in Tre was more dependent on ambient temperature, but not significantly influenced by altitude in thermoneutral ambient temperatures (e.g., 21°C and 26.5°C), without a noted difference in heat production, heat loss, or core-skin gradient between sea level and altitudes of 2500 and 5000m (Cipriano and Goldman, 1975). Other studies, although not quite aimed at testing hypoxia-induced anapyrexia, were also in line with the present results, in that no difference in Tre between sea level and altitudes (e.g., 3330 and 4360m) in ambient temperature of 26C was noted (Blatteis and Lutherer, 1976) and Tre increased following 8 hours of exposure to a simulated altitude of 2438 and 3657m in adult humans (Coste et al., 2009).
On the contrary, a study by Robinson and Haymes (1990) observed a significant reduction in Tre during 90 minutes of breathing 12% O2 in neutral (25°C) and cold (8°C) conditions, together with reduced O2 uptake. This result suggests that anapyrexia might occur in adult humans and results, in part, from reduced metabolic rate, while a degree of reduction in Tre was not specified in their report. A more recent study by DiPasquale et al. (2015) reported that Tre was significantly reduced by a mean of 0.13°C and 0.25°C following only 30 minutes of exposure to 14% and 12% O2, respectively. These reported reductions in Tre during NH, apart from the previously mentioned shortcoming in their study design, are seemingly small changes and similar to Tre responses in the present results (0.2°C-0.3°C). Such a small reduction in Tre may be the body’s natural response to transitioning into a resting state. In fact, nonexercise activity thermogenesis has been reported to be lowered by ~9% during motionless seating than standing, likely due to a decreased muscle tone (Levine et al., 2000). In addition, it is plausible that vasomotor adjustments occurred during rest in the thermally comfortable environment so that elevated cutaneous vasodilation alters blood distribution between the core and skin, leading to a temporal decrease in Tre. This decrease might have been incorrectly interpreted as anapyrexia. Nonetheless, a responsible mechanism(s) leading to a consistent reduction in Tre during rest in both normoxia and hypoxia in this study is not clear.
Apart from the debate on the existence of thermoregulatory set point (Cabanac, 2006), we hypothesized that hypoxia-induced anapyrexia, through the downward resetting of the thermoregulatory set point (Wood, 1991; Steiner and Branco, 2002), would be more pertinent to a chronic long-term exposure to hypoxia. Thus, we speculated that either decreased thermogenesis or increased heat loss, singly or in combination, would be responsible for reduction in Tre during an acute exposure to hypoxia, which were also previously documented for human neonates and other mammals (Gellhorn and Janus, 1936; Miller and Westheimer, 1966; Schubring, 1986; Barros et al., 2001; Tattersall and Milsom, 2003; Scott et al., 2008; Mortola and Maskrey, 2011; Cadena and Tattersall, 2014). Contrary to our expectation, no significant differences in MHP or S were observed between the conditions regardless of Tre responses. This indicated that such observations in other mammals and human neonates may have limited translational value in adult humans. Nonetheless, it is important to emphasize that most reported cases of hypoxia-induced anapyrexia are shown in small animals, including human neonates, in a time-dependent manner (Schubring, 1986; Barros et al., 2001; Tattersall and Milsom, 2003; Scott et al., 2008; Mortola and Maskrey,2011; Cadena and Tattersall, 2014).This implies that the intensity of Tb reduction is likely dependent on the body size as well as on the duration of exposure, more specifically body surface area-to-mass ratio, which is a major factor in dry heat exchange. Therefore, a significant Tb reduction in adult humans (if hypoxia-induced alteration in metabolism and/or heat loss plays a main role) may be difficult to accomplish during a short period of hypoxic exposure due to a smaller surface area-to-mass ratio.
In conclusion, we did not observe hypoxia-induced anapyrexia in adult humans during 1 hour of resting exposure to NH either at 13% or 17% O2. Additionally, there were no significant changes in either resting thermogenesis or heat loss in NH compared with normoxia. Further studies are warranted to determine if the present results would differ under different conditions (e.g., temperature effect of hypoxemia brought about by extended duration [>60 minutes] of normobaria at various levels of hypoxia; effect of NH and hypobaric hypoxia on Tc, thermogenesis, and heat loss responses). Future studies are also suggested to examine the role of other previously proposed mediators (Steiner and Branco, 2002; Branco et al., 2010) for hypoxic anapyrexia in adult humans. Measurement of these putative mediators, along with the assessment on thermoregulatory responses in a longer duration of hypoxic exposure, may provide a better understanding of hypoxic anapyrexia in adult humans.
Acknowledgments
The study subjects who generously volunteered their time to participate in this study are much appreciated.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of National Institute for Occupational Safety and Health. Mention of commercial products does not constitute endorsement by the National Institute for Occupational Safety and Health.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- Barros RC, Zimmer ME, Branco LG, and Milsom WK. (2001). Hypoxic metabolic response of the golden-mantled ground squirrel. J Appl Physiol (1985) 91:603–612. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, and Lutherer LO. (1976). Effect of altitude exposure on thermoregulatory response of man to cold. J Appl Physiol 41:848±858. [DOI] [PubMed] [Google Scholar]
- Branco LG, Bicego KC, Carnio EC, and Pittman QJ. (2010). Gaseous neurotransmitters and their role in anapyrexia. Front Biosci (Elite Ed) 2:948–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AC. (1935). Human calorimetry II. The average temperature of the tissues of the body three figures. J Nutr 9: 261–280. [Google Scholar]
- Cabanac M. (2006). Adjustable set point: To honor Harold T. Hammel. J Appl Physiol (1985) 100:1338–1346. [DOI] [PubMed] [Google Scholar]
- Cadena V, and Tattersall GJ. (2014). Body temperature regulation during acclimation to cold and hypoxia in rats. J Therm Biol 46:56–64. [DOI] [PubMed] [Google Scholar]
- Cena K, and Clark JA. (1981). Bioengineering, Thermal Physiology and Comfort. New York, NY: Elsevier. [Google Scholar]
- Cipriano LF, and Goldman RF. (1975). Thermal responses of unclothed men exposed to both cold temperatures and high altitudes. J Appl Physiol 39:796–800. [DOI] [PubMed] [Google Scholar]
- Coste O, Beaumont M, Batejat D, Beers PV, and Touitou Y. (2004). Prolonged mild hypoxia modifies human circadian core body temperature and may be associated with sleep disturbances. Chronobiol Int 21:419–433. [DOI] [PubMed] [Google Scholar]
- Coste O, Van Beers P, and Touitou Y. (2009). Hypoxia-induced changes in recovery sleep, core body temperature, urinary 6-sulphatoxymelatonin and free cortisol after a simulated long-duration flight. J Sleep Res 18:454–465. [DOI] [PubMed] [Google Scholar]
- DiPasquale DM, Kolkhorst FW, and Buono MJ. (2015). Acute normobaric hypoxia reduces body temperature in humans. High Alt Med Biol 16:61–66. [DOI] [PubMed] [Google Scholar]
- Gellhorn E, and Janus A. (1936). The influence of partial pressure of O2 on body temperature. Am J Physiol 116:327–329. [Google Scholar]
- Krogh A. (1914). The quantitative relation between temperature and standard metabolism in animals. Intern Z Phys Chem Biol 1:491–508. [Google Scholar]
- Kwiatkoski M, Soriano RN, Francescato HD, Batalhao ME, Coimbra TM, Carnio EC, and Branco LG. (2012). Hydrogen sulfide as a cryogenic mediator of hypoxia-induced anapyrexia. Neuroscience 201:146–156. [DOI] [PubMed] [Google Scholar]
- Levine JA, Schleusner SJ, and Jensen MD. (2000). Energy expenditure of nonexercise activity. Am J Clin Nutr 72: 1451–1454. [DOI] [PubMed] [Google Scholar]
- Miller DL, and Westheimer FH. (1966). Interaction of gamma-phenylpropyl triphosphate with cations. J Am Chem Soc 88: 1514–1517. [DOI] [PubMed] [Google Scholar]
- Mortola JP, and Maskrey M. (2011). Metabolism, temperature, and ventilation. Compr Physiol 1:1679–1709. [DOI] [PubMed] [Google Scholar]
- Robinson KA, and Haymes EM. (1990). Metabolic effects of exposure to hypoxia plus cold at rest and during exercise in humans. J Appl Physiol (1985) 68:720–725. [DOI] [PubMed] [Google Scholar]
- Ruby BC, and Robergs RA. (1994). Gender differences in substrate utilisation during exercise. J Sports Med 17:393–410. [DOI] [PubMed] [Google Scholar]
- Scarpellini Cda S, Gargaglioni LH, Branco LG, and Bicego KC. (2009). Role of preoptic opioid receptors in the body temperature reduction during hypoxia. Brain Res 1286: 66–74. [DOI] [PubMed] [Google Scholar]
- Schubring C. (1986). Temperature regulation in healthy and resuscitated newborns immediately after birth. J Perinat Med 14:27–33. [DOI] [PubMed] [Google Scholar]
- Scott GR, Cadena V, Tattersall GJ, and Milsom WK. (2008). Body temperature depression and peripheral heat loss accompany the metabolic and ventilatory responses to hypoxia in low and high altitude birds. J Exp Biol 211:1326–1335. [DOI] [PubMed] [Google Scholar]
- Steiner AA, and Branco LG. (2002). Hypoxia-induced anapyrexia: implications and putative mediators. Annu Rev Physiol 64:263–288. [DOI] [PubMed] [Google Scholar]
- Tattersall GJ, and Milsom WK. (2003). Transient peripheral warming accompanies the hypoxic metabolic response in the golden-mantled ground squirrel. J Exp Biol 206:33–42. [DOI] [PubMed] [Google Scholar]
- Toner MM, Sawka MN, Holden WL, and Pandolf KB. (1985). Comparison of thermal responses between rest and leg exercise in water. J Appl Physiol (1985) 59:248–253. [DOI] [PubMed] [Google Scholar]
- Wagner JA, and Horvath SM. (1985). Cardiovascular reactions to cold exposures differ with age and gender. J Appl Physiol (1985) 58:187–192. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Schrage WG, Liu Z, Hancock KC, and Joyner MJ. (2006). Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol (1985) 101: 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SC. (1991). Interactions between hypoxia and hypothermia. Annu Rev Physiol 53:71–85. [DOI] [PubMed] [Google Scholar]