Abstract
Background
Bacterial meningitis remains a significant cause of neonatal and childhood morbidity and mortality in many countries of the world, particularly in developing countries. In some instances, children recover but remain impaired as a result of neurological sequelae such as hearing loss, developmental delay and cognitive impairment.
Objectives
To assess the effectiveness and safety of adjunctive corticosteroids in reducing death and neurological sequelae in neonates with bacterial meningitis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 7), MEDLINE via PubMed (1966 to July 2015), African Index Medicus (up to January 2015), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (up to July 2015), EMBASE (up to July 2015) and the metaRegister of Controlled Trials (mRCT) for ongoing trials.
Selection criteria
All randomised controlled trials (RCTs) or quasi‐RCTs of adjunctive corticosteroids for treatment of neonates with bacterial meningitis.
Data collection and analysis
Two review authors independently assessed and extracted data on methods, participants, interventions and outcomes (all‐cause death until hospital discharge, presence of sensorineural deafness at one year and presence of neurological deficits or developmental delay at two years, adverse events). Risk ratio (RR), risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) were calculated when appropriate. We assessed quality using the Cochrane risk of bias assessment tool and the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system.
Main results
We found two trials with 132 participants that met our inclusion criteria. One of the included trials was a quasi‐randomised trial.
Adjunctive corticosteroids reduced the risk of death (typical RR 0.46, 95% confidence interval (CI) 0.24 to 0.88; typical RD ‐0.19, 95% CI ‐0.33 to ‐0.04; NNTB = 6; two studies, 132 participants, very low‐quality evidence) but did not have a significant effect on the number of infants with sensorineural deafness at two years (RR 1.80, 95% CI 0.18 to 18.21; RD 0.04, 95% CI ‐0.12 to 0.21; one study, 38 participants, low‐quality evidence). In one trial, dexamethasone reduced the likelihood of hearing loss at four to 10 weeks post discharge (RR 0.41, 95% CI 0.17 to 0.98; RD ‐0.25, 95% CI ‐0.48 to ‐0.01; one study, 59 participants, low‐quality evidence). Data reported on the other outcomes of interest were insufficient.
Authors' conclusions
Very low‐quality data from two randomised controlled trials suggest that some reduction in death and hearing loss may result from use of adjunctive steroids alongside standard antibiotic therapy for treatment of patients with neonatal meningitis. Benefit is not yet seen with regards to reduction in neurological sequelae. Researchers who wish to clarify these findings must conduct more robustly designed trials with greater numbers of participants, evaluating more relevant outcomes and providing adequate follow‐up.
Plain language summary
Use of corticosteroids for treatment of the newborn with bacterial meningitis
Review question: Does use of adjuvant corticosteroids in neonates with bacterial meningitis reduce the risk of death and the possibility of neurodevelopmental sequelae?
Background: Neonatal meningitis is a common cause of death and long‐term disability among children everywhere, particularly in developing countries. In this review, we investigated the benefits and safety of adjunctive corticosteroids in the treatment of neonatal meningitis.
Study characteristics: We identified two studies for inclusion.
Results: We found that giving steroids to babies affected with meningitis may reduce the number of children who would die or become deaf from the disease. However, most of this benefit was observed in only one trial. As of now, it appears as though steroids are not helpful with regard to preventing developmental delay. We are not able to make far reaching conclusions at this time, as the evidence that we found is limited and of low quality and could change if more results from larger and better designed studies become available.
Summary of findings
Summary of findings for the main comparison. Adjunctive steroid versus placebo for reducing death in neonatal bacterial meningitis.
| Adjunctive steroid versus placebo for reducing death in neonatal bacterial meningitis | ||||||
| Patient or population: neonates with clinical and microbiological features of bacterial meningitis Settings: neonatal care facilities in both developed and developing countries Intervention: adjunctive steroid versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Adjunctive steroid vs placebo | |||||
| All‐cause death until hospital discharge | Study population | RR 0.46 (0.24 to 0.88) | 132 (2 studies) | ⊕⊝⊝⊝ very lowa‐e |
Daoud 1999 was a quasi‐randomised trial. Mathur 2013 was an open trial The direction of effect was different in the 2 studies Concern has been expressed regarding imprecision, as both trials enrolled few infants |
|
| 354 per 1000 | 163 per 1000 (85 to 312) | |||||
| Medium‐risk population | ||||||
| 340 per 1000 | 156 per 1000 (82 to 299) | |||||
| Number of participants with developmental delay | Study population | RR 0.77 (0.32 to 1.87) | 38 (1 study) | ⊕⊕⊝⊝ lowa,d | Daoud 1999 was a quasi‐randomised trial | |
| 389 per 1000 | 300 per 1000 (124 to 727) | |||||
| Medium‐risk population | ||||||
| 389 per 1000 | 300 per 1000 (124 to 727) | |||||
| Hearing loss at 2 years of age | Study population | RR 1.8 (0.18 to 18.21) | 38 (1 study) | ⊕⊕⊝⊝ lowa,d |
Daoud 1999 was a quasi‐randomised trial Concern has been expressed regarding imprecision, as Daoud 1999 enrolled only 52 infants |
|
| 56 per 1000 | 101 per 1000 (10 to 1000) | |||||
| Medium‐risk population | ||||||
| 56 per 1000 | 101 per 1000 (10 to 1000) | |||||
| Hearing loss at 4 to 10 weeks after discharge | Study population | RR 0.41 (0.17 to 0.98) | 59 (1 study) | ⊕⊕⊝⊝ lowb,e,f |
Mathur 2013 was an open trial, and duration of follow‐up was rather short Concern has been expressed regarding imprecision, as Mathur 2013 enrolled only 80 infants |
|
| 417 per 1000 | 171 per 1000 (71 to 409) | |||||
| Medium‐risk population | ||||||
| 417 per 1000 | 171 per 1000 (71 to 409) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aDaoud 1999 was a quasi‐randomised trial.
bMathur 2013 was an open trial. cThe direction of effect was different in the two studies.
dConcern has been expressed regarding imprecision, as Daoud 1999 enrolled only 52 infants.
eConcern has been expressed regarding imprecision, as Mathur 2013 enrolled only 80 infants.
fIn Mathur 2013, the duration of follow‐up was rather short.
Background
Description of the condition
Epidemiology
Meningitis, an inflammatory condition of the leptomeninges (covering of the brain), is a serious and potentially fatal infectious condition of the central nervous system. Meningitis occurs more commonly in the neonatal period than at any other time in life (Delouvois 1991) because of the increased susceptibility of newborn infants to infection by virtue of their immature cellular and humoral immunity.
Improved antibiotic therapy and supportive care have led to a remarkable decline in the mortality associated with neonatal meningitis since the 1990s. Mortality in the developed world has dropped from close to 50% to about 10%, although morbidity among survivors remains high (15% to 60%) (Harvey 1999). Although underreporting remains a challenge in the developing world, mortality associated with neonatal meningitis is still as high as 40% to 60%, and morbidity figures vary greatly (Stoll 1997; Airede 2008).
A high proportion of survivors of neonatal meningitis develop chronic impairment with serious medical and psychosocial implications, such as cerebral palsy, mental retardation, seizure disorder, hemiplegia, deafness and blindness (Airede 2008).
Pathogenesis
Major pathogens associated with newborn meningitis include group B β‐haemolytic Streptococcus (GBS), Escherichia coli, Streptococcus pneumoniae, Listeria monocytogenes and other Gram‐negative bacilli. The pattern of bacteriological aetiology in neonatal meningitis differs in some developing parts of the world; E. coli still predominates in some settings (Laving 2003), Staphylococcus aureus predominates according to a Nigerian report (Airede 2008) and some articles have described predominance of GBS in Taiwan (Chang 2003).
The mortality and morbidity of neonatal meningitis are related to inflammatory damage to neural tissues. Components of bacterial cell membrane in the meninges provoke the release of cytokines such as interleukins (IL‐1β), tumour necrosis factor (TNF)‐γ and platelet aggregation factor (PAF). These agents in turn offset an inflammatory cascade that ultimately results in cerebral oedema, elevated intracranial pressure, reduced cerebral perfusion, cerebritis, neuritis and vasculitis (Mustafa 1990). The end result of these pathological features consists of ischaemia, infarction and atrophy of neural tissues.
Clinical features
Clinical features of bacterial meningitis, which are usually subtle and non‐specific in the early phase of the illness, include abnormal temperature (hypothermia or fever), poor cry, poor skin colour, poor feeding, irritability, lethargy and respiratory distress, and can be easily overlooked. Late in the illness, classical features of raised intracranial pressure such as full and tense fontanelles, setting‐sun eye appearance, retrocollis, opisthotonus (when the neck of the infant is twisted backwards) and seizures can occur. If meningitis is not recognised and treated appropriately, complications such as the syndrome of inappropriate antidiuretic hormone secretion (SIADH), disseminated intravascular coagulation and hydrocephalus can develop.
Diagnosis
The diagnosis of bacterial meningitis is usually confirmed following bacteriological analysis (microscopy and culture) of cerebrospinal fluid (CSF). When bacteriological culture of CSF is difficult or impossible, the diagnosis can be made from specific microscopic and biochemical abnormalities of the CSF. Cell count greater than 32/mm3, protein level greater than 150 mg/dL and glucose level less than 1 mmol/L or less than 50% of simultaneously determined random blood glucose are suggestive of bacterial meningitis. Serological methods such as polymerase chain reaction (PCR) may detect the antigens of bacterial organisms in the CSF.
Description of the intervention
Corticosteroids have metabolic and regulatory effects in humans. Regulatory functions include anti‐inflammatory and immunosuppressive roles. Specifically, corticosteroids decrease influx and activity of leucocytes during acute inflammation, decrease activities of cytokine‐secreting T cells, decrease production and activities of cytokines and decrease production of immunoglobulin G (IgG) and complement components in the blood.
In addition to endogenously produced corticosteroids such as hydrocortisone and corticosterone, many synthetic corticosteroids such as dexamethasone and betamethasone are available. In serious infections such as meningitis, corticosteroids are commonly administered through the intravenous route. Research has led to suggestions that administration of dexamethasone before or with first doses of antibiotics may be more beneficial than administration of dexamethasone after antibiotic therapy is instituted (Odio 1991).
Adverse effects of corticosteroid therapy include glucose intolerance, systemic hypertension, benign intracranial hypertension, poor response to infection and injury, easy bruising, cataracts and gastrointestinal bleeding (Rang 2003). Many of these adverse effects are uncommon following corticosteroid use of short duration (McIntyre 1997).
How the intervention might work
Bacterial meningitis in the newborn infant is characterised by high risk of mortality and serious neurological sequelae among most survivors. Most sequelae are believed to occur as a result of damage to neural tissues during the acute inflammatory process that characterises bacterial meningitis.
Corticosteroids when administered as adjunctive treatment in bacterial meningitis may help attenuate the acute inflammatory process, while antibiotics clear the pathogenic microorganisms. This process has the potential to improve clinical outcomes in the short term and over the long term.
Why it is important to do this review
Although adjuvant corticosteroids have traditionally been used in the treatment of children of postneonatal age and adults with meningitis, randomised and non‐randomised studies have provided conflicting reports concerning the effectiveness of adjuvant dexamethasone in improving survival and reducing neurological deficits, including hearing loss, among children with meningitis, particularly those in low‐income countries (Brouwer 2010).
Controlled trials among US children eight weeks to 12 years of age with meningitis have shown that audiological and neurological outcomes were similar among dexamethasone‐treated participants and saline‐treated controls (Wald 1995). A placebo‐controlled study of children eight weeks to 13 years of age with bacterial meningitis in Blantyre, Malawi, reported similar findings (Molyneux 2002).
Another placebo‐controlled blinded trial of dexamethasone given to infants and children six weeks to 12 years of age with meningitis was conducted in Costa Rica; investigators reported that overall neurological and audiological sequelae were significantly fewer among dexamethasone‐treated children (Odio 1991).
Of note, subgroup analysis was not carried out for children six to 12 weeks of age in the cited studies, which led to difficulty in applying study findings to the newborn period. This analysis could have been useful because immune characteristics and the range of pathogens causing sepsis at that age (four to 12 weeks) are usually similar to those of the newborn period.
Although corticosteroids have been reported effective in childhood bacterial meningitis in the developed world, the situation may be different in the developing world (Furyk 2011). This difference may be ascribed to the types of pathogens prevalent in the developing world, delay in the initiation of appropriate antibiotic treatment, partial treatment involving indiscriminate antibiotic use outside of hospitals or lack of facilities that can provide supportive care.
Mortality from neonatal meningitis has been reduced globally, but equally important is the need to reduce the proportion of infants who survive with daunting neurological disabilities. Therefore, in addition to antibiotics and other supportive therapeutic measures, corticosteroids are sometimes included in the management of childhood meningitis, in an effort to reduce inflammation and attendant neural tissue damage.
Treatment guidelines for neonatal meningitis do not commonly include adjuvant corticosteroids because the benefits of corticosteroids given on a short‐ or long‐term basis remain unclear.
Systematic reviews of adjuvant corticosteroid treatment in bacterial meningitis have focused only on children outside the neonatal period (Brouwer 2013), and review authors have described overall reduced mortality in S. pneumoniae meningitis but not in Haemophilus influenzae (H. influenzae) nor Neisseria meningitidis (N. meningitidis) meningitis. Corticosteroids have been reported to reduce severe hearing loss among children with H. influenzae meningitis but not among those with meningitis due to non‐Haemophilus species.
In high‐income countries, corticosteroids have been reported to reduce severe hearing loss and short‐term neurological sequelae, but investigators have observed no beneficial effects of corticosteroid therapy in low‐income countries.
Given that response to treatment might be different among patients of different ages, a systematic review of adjuvant corticosteroid treatment of newborns with bacterial meningitis has become necessary, to provide the evidence base required to update guidelines on clinical management of neonatal bacterial meningitis.
Objectives
To assess the efficacy and safety of adjuvant corticosteroids in reducing death and neurological sequelae in neonates with bacterial meningitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials (individually) comparing adjunctive corticosteroid treatment in addition to standard antibiotics versus standard antibiotics alone (with or without placebo) for the management of neonatal bacterial meningitis. We did not include studies with a cross‐over design.
Types of participants
Newborn infants from birth to 28 days of age with bacteriologically confirmed diagnosis of bacterial meningitis or suspect meningitis. We excluded infants with tuberculous meningitis.
We considered studies in which bacteriologically confirmed diagnosis of bacterial meningitis was defined using CSF microscopy, culture, PCR or a combination; and those in which suspect meningitis was defined as deranged CSF parameters such as leucocyte count greater than 32/mm3 or CSF protein greater than 150 mg/dL or CSF glucose less than 50% of simultaneously determined random blood glucose, in the absence of positive CSF culture.
Types of interventions
Intervention
Adjunctive parenteral corticosteroid (at any dose and for any duration of treatment). Corticosteroids of interest included dexamethasone, hydrocortisone, betamethasone and methylprednisolone administered by the intravenous route.
Control
Appropriate antibiotic therapy alone or in combination with placebo.
By "adjunctive" treatment, we mean that all babies in the trial must have received parenteral antibiotics of a class and at a dose considered sufficient for the treatment of neonatal meningitis. Classes of antibiotics considered included the third‐generation cephalosporins, penicillin, vancomycin and aminoglycoside: penicillin alone or with aminoglycoside and cephalosporin alone or with aminoglycoside and at a dose considered sufficient (doses vary with the drugs used, but high doses are generally considered anti‐meningitic).
Types of outcome measures
Primary outcomes
-
Mortality.
All‐cause death until hospital discharge.
All‐cause death during first year of life.
Presence of severe neurological deficits or developmental delay between one and two years of age (a neurological deficit was defined as a functional abnormality of a body area that is observed as the result of an abnormality in the function of the brain, spinal cord, muscles or nerves; developmental delay was defined as any significant lag in a child's physical or motor, cognitive, behavioural, emotional or social development, in comparison with other children of the same age and sex within similar environments; formal evaluation tools were used to assess neurological deficits and developmental delay). Examples of neurological deficits include mental retardation, cerebral palsy, epilepsy, blindness and behavioural disorders. We considered evaluation tools such as Bayley's Infant Scale or Griffith's Mental Development Scale (for neurodevelopmental deficits), the Gross Motor Functions Scale or the Movement Assessment Battery for Children (for cerebral palsy), the Sonken‐Silver visual acuity test (for blindness), distraction tests (for behavioural disorders) and electroencephalography (for epilepsy) ‐ all applied between one and two years of age. We also accepted other measures used by individual trialists to evaluate and document neurological deficits in their respective trials.
-
Sensorineural hearing loss.
Presence of sensorineural hearing loss at four to 10 weeks after discharge.
Presence of sensorineural hearing at one year of age (this was to be assessed by clinical examination and audiometry at one year of age).
Presence of sensorineural hearing loss at two years of age.
Secondary outcomes
Number of participants with seizures at any time.
Number of participants with seizures persisting beyond five days after treatment initiation.
Fever clearance time (time between onset of treatment and sustained resolution of fever without recurrence during the same illness).
Duration of hospitalisation (in days).
Serious adverse events (leading to death, disability or prolonged hospitalisation), for example, secondary fever and gastrointestinal bleeding. We defined adverse effects as unfavourable outcomes that occur during or after use of an intervention but not necessarily caused by it. Serious adverse events were events that led to death, disability or prolonged hospitalisation.
Other adverse events.
Number of participants with ventriculitis (neuroimaging with evidence of intraventricular debris, pus and enhanced ventricular lining during hospitalisation).
Number of participants with hydrocephalus (clinically diagnosed with or without ultrasound confirmation of ventricular dilation occurring during hospitalisation or within one year of treatment).
Number of participants with SIADH at one month post treatment (rapid weight gain, decreased urine output, serum sodium < 130 mmol/L, plasma osmolality < 270 mOsm/kg, urinary osmolality > 100 mOsm/kg and urinary sodium > 40 mmol/L during hospitalisation).
Number of participants with bleeding diatheses at one month post treatment (external bleeding including oozing from puncture sites, purpura and petechiae, as well as evidence of internal bleeding such as haematuria and haematemesis occurring during hospitalisation).
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of The Cochrane Collaboration and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register). We conducted a comprehensive search of databases such as the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 7); MEDLINE via PubMed (1996 to 31 July 2015); EMBASE (1980 to 31 July 2015); the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 31 July 2015); and African Index Medicus (up to January 2015) using the following search terms: ((Corticosteroids or steroids or dexamethasone or methylprednisolone or betamethasone or hydrocortisone) AND meningitis), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies used for each database). We applied no language restrictions. When we identified a study reported in abstract format only, we evaluated it for possible inclusion in the review and attempted to contact the study authors for information needed to include or exclude the study. We performed a handsearch of the reference lists of articles for which we obtained the full text. We searched clinical trial registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform ‐ www.whoint/ictrp/search/en/; the International Standard Randomised Controlled Trial Number Registry ‐ ISRCTN Registry), and we searched the metaRegister of Controlled Trials (mRCT).
Searching other resources
We contacted researchers in the field to ask about ongoing studies, and we handsearched the abstracts of neonatology conferences to identify additional trials.
Data collection and analysis
Selection of studies
Two review authors (TAO and CCO) independently screened results (titles and abstracts) of the literature search for potentially relevant trials. We retrieved full reports of potentially relevant trials and independently determined whether they met the review inclusion criteria by using a pre‐tested eligibility form. For each step of the review, we resolved contentious issues by discussion. We worked upon the advice of Editors within the Cochrane Neonatal Group. We tried to contact trial authors for further information regarding trial eligibility in cases that appeared unclear. We listed all excluded studies, along with reasons for exclusion. We ensured that trials with multiple publications were included only once, but we considered for inclusion multiple publications that included different but relevant outcomes. We included all citations from the same study under the main reference.
Data extraction and management
Two review authors (TAO and CCO) independently extracted data using a pre‐tested data extraction form. One review author (CCO) entered the data into Review Manager (RevMan 2011), and a second review author (TAO) cross‐checked the data for completeness and accuracy. Extracted data included numbers of participants randomly assigned and numbers analysed in each group for each reported outcome.
We extracted data for dichotomous outcomes by recording the total number of participants randomly assigned, numbers of participants experiencing events and numbers of participants included in each treatment group.
For continuous outcomes, we extracted the numbers of participants for each treatment arm, arithmetic means and standard deviations (SDs). We did not encounter data with skewed distribution but had planned that if we did encounter this, or when data were reported as geometric means, we would extract geometric means and SDs on the log scale, or as medians and ranges when medians were used. For rate and count outcomes (such as participants with outcomes that occur more than once over the period of trial), we planned to extract numbers of events or episodes experienced in each trial arm and person‐time over which these events were experienced by each group. We planned to extract hazard ratios and SDs for time‐to‐event outcomes, and we extracted data on reported adverse events.
We attempted to contact trial authors when relevant details were not recorded or were unclear. When disagreements regarding data extraction arose, we resolved them by discussion and by seeking the opinion of the third review author (OTO).
Assessment of risk of bias in included studies
We assessed quality by using the Cochrane risk of bias assessment tool and the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system (Higgins 2011; Guyatt 2011).
We independently assessed risk of bias for every eligible study by using the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We resolved disagreements through discussion.
We independently assessed risk of bias within each included study in relation to five domains (allocation sequence generation, allocation concealment, blinding, handling of incomplete outcome data and selective outcome reporting) by assigning ratings of 'Yes' (low risk of bias), 'No' (high risk of bias) and 'Unclear' (uncertain risk of bias).
Specific assessments are detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Allocation sequence generation
We described for each included study the method used to generate the allocation sequence, to allow assessment of whether it should produce comparable groups.
We graded these methods in the following way.
Low risk of bias: truly random processes such as use of table of randomisation or computer‐generated random numbers.
High risk of bias: non‐random processes such as use of hospital record numbers or dates of birth.
Unclear risk of bias: Insufficient detail about the sequence generation process was available; therefore, we could not grade high or low risk of bias.
Allocation concealment
We described for each included study the method used to conceal allocation to interventions before assignment and assessed whether intervention allocation could have been predicted before or changed after recruitment. We graded these methods in the following way.
Low risk of bias: Participants and investigators could not forsee which arm of the study the patient would be assigned to because an appropriate method of allocation concealment was used, e.g. use of opaque sealed allocation envelopes or central allocation.
High risk of bias: Participants and investigators could forsee which arm of the study the patient would be assigned to because an inappropriate method of allocation with an open random allocation schedule was used, e.g. list of random numbers, assignment envelopes without appropriate safeguards, or use of the date of birth or case record number.
Unclear risk of bias: Insufficient detail about the process of allocation concealment was available; therefore, we could not judge high or low risk of bias.
Blinding of participants and researchers
We described for each included study methods used to blind study participants and researchers from knowledge of which intervention a participant received. We classified blinded studies and studies in which non‐blinding was not likely to significantly affect results as having low risk of bias. We classified non‐blinded studies as having high risk of bias.
Incomplete outcome data
We described for each included study methods used to account for incomplete outcome data with regard to the quantity, nature and handling of incomplete outcome data. When studies did not report complete outcome data, we planned to obtain missing data by contacting study authors. We extracted and reported data on attrition and exclusions, as well as the numbers involved (compared with the total randomly assigned); reasons for attrition/exclusion when reported or obtained from investigators; and re‐inclusions in analyses performed by review authors. We defined unbiased follow‐up as when at least 80% of participants could continue to be followed up. On this basis, we judged whether researchers dealt with incomplete data. We rated risk as follows: 'yes' (low risk of bias), 'no' (high risk of bias) and 'unclear' (uncertain risk of bias).
Selective outcome reporting
We attempted to assess the possibility of selective outcome reporting by investigators in the included trials; when available, we planned to look at study protocols; on this basis, we judged whether reports of the study were free from the suggestion of selective outcome reporting.
We rated as follows: 'yes' (low risk of bias), 'no' (high risk of bias) and 'unclear' (uncertain risk of bias).
We explored other sources of bias, particularly funding sources of included studies and other study peculiarities.
Measures of treatment effect
Continuous data
When means and SDs were available, we analysed continuous data. We planned to extract and utilise these data in the analysis, irrespective of means and SDs if mean differences were provided. We were interested in post‐intervention values. We planned to re‐calculate the SD when the standard error was reported. We planned to extract data from studies that reported adequately on skewed continuous data as medians rather than as means. We planned to report these data separately when appropriate.
Binary data
We analysed binary outcomes by calculating risk ratio (RR), risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH), along with 95% confidence intervals (95% CIs).
Unit of analysis issues
We planned to describe for each included study observations on participants at selected time points. We planned to analyse follow‐up data available at the point of discharge from the hospital, as well as at the age of one month, three months, six months and one year, as we planned to assess some outcomes at these different time points. We planned to adjust for clustering by applying the intra‐cluster correlation co‐efficient when cluster trials were identified. However, these issues did not arise in the conduct of the present review.
Dealing with missing data
We tried to contact the study authors(s) to supply unreported data (e.g. group means and SDs, details of dropouts, details of interventions received by the control group). We asked for data from assessments that probably were done but were not discussed in the trial reports. We planned that If a study reported outcomes only for participants completing the trial or only for participants who followed the protocol, we would ask for additional information to facilitate an intention‐to‐treat analysis, and when this was not possible, we would perform a complete case analysis.
Assessment of heterogeneity
We assessed statistical heterogeneity by examining the I2 statistic (Higgins 2002; Higgins 2003) ‐ a quantity that describes approximately the proportion of total variation that is due to variation between studies. In addition, we used a Chi2 test of homogeneity at the 10% level of statistical significance to determine the strength of evidence against the hypothesis that all studies include the same population. As a rough guide, we regarded an I2 statistic less than 25% as representing no heterogeneity, between 25% and 49% as representing low heterogeneity, from 50% to 74% as representing moderate heterogeneity and above 75% as indicating high heterogeneity. We inspected forest plots, as poor overlap may be due to significant heterogeneity.
Assessment of reporting biases
We planned to prepare funnel plots (estimated treatment effects against standard error) to explore publication bias when more than 10 trials were included in a comparison. Asymmetry could be due to publication bias but also could be due to a relationship between trial size and effect size. We found too few trials to perform this analysis.
Data synthesis
We conducted meta‐analyses for trials with similar characteristics. We used the fixed‐effect model and presented all results with 95% CIs. We planned to calculate RRs, RDs, NNTBs and NNTHs for dichotomous outcomes, and weighted mean differences (WMDs) for continuous outcomes ‐ all with 95% CIs.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses to assess the benefit or otherwise of adjunctive corticosteroid treatment.
We planned to perform subgroup analyses to examine:
the efficacy of adjuvant corticosteroids in infants with gestational age < 37 weeks (preterm infants) and ≥ 37 weeks (term infants);
the efficacy of corticosteroids as adjuvant treatment in different antibiotic classes (comparison was to be made for penicillin with or without aminoglycoside vs cephalosporin with or without aminoglycoside);
the efficacy of adjuvant corticosteroids based on the causative bacterial agent (comparison was to be made for GBS and other Gram‐positive bacteria vs Gram‐negative bacteria);
the impact of the time of initiation of adjunctive corticosteroid treatment (comparison was to be made for pre‐antibiotic (up to one hour before commencement of antibiotics) and post‐antibiotic (simultaneously with antibiotics or after commencement of antibiotics));
the impact of the duration of adjunctive corticosteroid treatment on outcomes of neonatal bacterial meningitis (comparison was to be made for duration < 4 days and ≥ 4 days);
the impact of corticosteroids on outcomes of studies conducted in developed countries versus developing countries; and
the impact of corticosteroids on outcomes among infants with confirmed meningitis (positive CSF culture or PCR) versus suspect meningitis (deranged cellular or chemical constituents of CSF without positive CSF culture or PCR).
We planned to assess important clinical heterogeneity by comparing the distribution of important clinical heterogeneity factors (study participants, study setting, types of interventions and co‐interventions and baseline antibiotic treatment) and methodological heterogeneity factors (randomisation, allocation concealment, blinding of outcome assessment, losses to follow‐up).
However, this was not possible as available data were too scanty to allow meaningful conclusions on any of the issues investigated by the subgroup analyses.
Sensitivity analysis
We planned to conduct sensitivity analyses to explore the effects of the methodological quality of trials and to ascertain whether studies with high risk of bias overestimated the effects of treatment.
Results
Description of studies
Results of the search
We ran the initial search in June 2013 and retrieved 74 potentially relevant records after removing duplicate records. We ran updated searches in November 2014 and again in July 2015.
Included studies
Upon screening retrieved records, we found that only two studies (Daoud 1999; Mathur 2013) met our inclusion criteria (Characteristics of included studies). Both studies were conducted in developing countries (Jordan and India, respectively), and both examined the effects of adjuvant dexamethasone at a similar dose on clinical outcomes in neonatal meningitis. We retrieved no records for an ongoing trial.
Daoud 1999 randomly assigned 52 full‐term neonates to adjuvant dexamethasone or antibiotic treatment alone. All children in the trial were treated with cefotaxime and steroid, starting about 10 to 15 minutes before antibiotic administration. Dexamethasone was administered six‐hourly for four days at a dose of 0.15 mg/kg body weight.
Mathur 2013 randomly assigned 80 neonates to adjuvant dexamethasone or saline placebo. Neonates in the trial were treated with ceftriaxone and amikacin initially, with subsequent addition of meropenem for severely ill neonates.
Both trials were conducted in a neonatal intensive care/special baby care unit.
Mathur 2013 performed a power calculation and recruited enough participants to give the study 80% power to detect differences in effects between arms if present. Study authors aimed to recruit enough participants to give the study 80% power to detect differences in effects between arms if they existed, and they set the threshold at a reduction in mortality from 35% to 10%. Patients with major congenital malformations and those who received antibiotics for 24 hours or longer before presentation were excluded; children with microcephaly were eventually included, although they were reported as showing no differences between groups at baseline.
Participants in Daoud 1999 were suspected to have meningitis on the basis of the clinical picture and CSF abnormalities such as pleocytosis (>30 leucocytes/mL), raised protein (> 100 mg/100 mL) and blood sugar ratio reduced to less than 0.5.
After the initial period of hospitalisation, investigators in Daoud 1999 assessed participants at three‐monthly intervals and provided follow‐up for two years.
Participants in the Mathur study (Mathur 2013) were assessed at admission, 24+ or ‐6 hours later and at days 7, 14 and 21. Brain stem auditory evoked response was assessed at four to six weeks post discharge, and assessment was repeated after an additional four weeks, depending on results of the initial examination. We are uncertain as to the maximum duration of follow‐up, as researchers provided no data on participants after one year.
Both studies reported a similar microbiological spectrum causing meningitis. Most of the positive CSF cultures revealed Klebsiella pneumoniae. Other organisms isolated wereEnterobacter, S. aureus, Pseudomonas, E. coli, Acinetobacter, group BStreptococcus, Klebsiella oxytoca, meningococci and Staphylococcus epidermidis (which may have been a contaminant).
Both studies included mostly term neonates. Mathur 2013 reported mean gestational age of 37.1 and 37.6 weeks for the dexamethasone and control groups, respectively, and Daoud 1999 explicitly stated that only full‐term neonates were included.
Excluded studies
We excluded most of the retrieved studies because investigators did not study the effects of adjuvant steroids in neonates. Most studies examined adjunctive steroid use among children diagnosed with meningitis who were older than four weeks of age.
One trial randomly assigned 70 neonates with meningitis to receive 10 days (study group) or 14 days (control group) of antibiotics (Mathur 2015). The primary outcome measure studied was treatment failure in each group within 28 days of enrolment. Both groups received adjuvant steroids.
Risk of bias in included studies
The risk of bias in included studies was variable, as shown in Figure 1 and Figure 2. Mathur 2013 was described as an open randomised trial.
1.
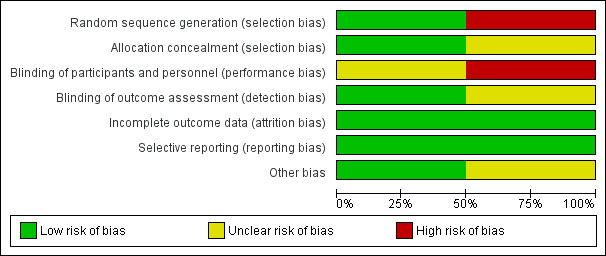
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
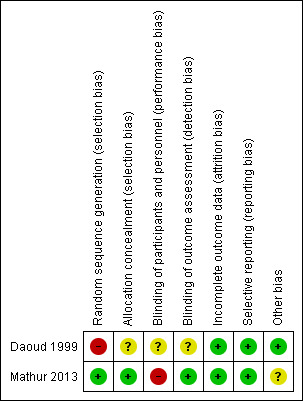
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Investigators in Mathur 2013 reported generation of allocation sequence as adequate, as trialists used a computer‐generated randomisation list. The method of sequence generation was inadequate in Daoud 1999, in which treatment was allocated in an alternate fashion, making it a quasi‐randomised trial.
Mathur 2013 used sealed opaque envelopes to conceal allocation, and allocation concealment was not reported in Daoud 1999.
Blinding
Daoud 1999 did not report blinding, and Mathur 2013 was an open (unblinded) trial. However, investigators who analysed CSF cytokine levels and brain stem evoked potentials in the Mathur 2013 trial were blinded.
Incomplete outcome data
In both trials, all or more than 90% of randomly assigned participants were included in the analysis, although some outcomes were assessed on the basis of the number of participants who were alive at the time the outcome was analysed. Trialists did not use an intention‐to‐treat principle in the analysis of trial outcomes, although no reports described cross‐over of participants or protocol violations during the conduct of the trial.
Selective reporting
Both trials reported outcomes identified in the methods. We had no access to the respective trial protocols, although we had no reason to suspect selective outcome reporting.
Other potential sources of bias
Daoud 1999 was funded by a University grant, and Mathur 2013 did not disclose the source of funding but stated that funding sources played no role in the design, analysis and reporting of the study.
Effects of interventions
See: Table 1
Adjuvant corticosteroid versus placebo or no treatment (Comparison 1)
We assessed the two included trials under this comparison and have presented results below and in the 'Summary of findings' table.
Primary outcomes
Available data enabled meta‐analytic evaluation of one of the primary outcomes of this review.
All‐cause death until hospital discharge
Daoud 1999 reported six deaths among 27 participants randomly assigned to dexamethasone and seven deaths among 25 participants randomly assigned to the control group. They reported no statistically significant differences.
Mathur 2013 reported five deaths among the 40 neonates randomly assigned to dexamethasone and 16 deaths among 40 neonates in the control group. This finding was statistically significant.
Meta‐analysis of the two studies showed that a reduction in the risk of all‐cause death until hospital discharge was associated with administration of adjuvant corticosteroids (typical RR 0.46, 95% CI 0.24 to 0.88; typical RD ‐0.19, 95% CI ‐0.33 to ‐0.04; NNTB = 6). We observed low heterogeneity for this outcome with I2 = 49% for RR, and moderate heterogeneity for RD with I2 = 51% (Mantel‐Haenszel (MH) fixed‐effect meta analysis; Analysis 1.1).
1.1. Analysis.
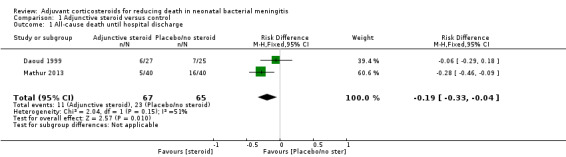
Comparison 1 Adjunctive steroid versus control, Outcome 1 All‐cause death until hospital discharge.
All‐cause death in the first year of life
We could not assess all‐cause death in the first year of life, as neither trial reported this outcome.
Severe neurological deficits or developmental delay between one and two years of age
Only one study (Daoud 1999) assessed this outcome in participants at about 2 years of age. Trialists calculated an "optimality score", which assessed components of both developmental and neurological development. Components of the score included tone, posture, spontaneous motility, elicited motility interaction and reflexes. Neonates who scored above 20 were considered normal, those scoring 17 to 20 were considered to have a mild deficit and those scoring less than 17 were considered to have a moderate to severe deficit. Although this score was not included in our protocol, it mirrored the assessment scales for this outcome as specified in our protocol. Six children in the dexamethasone group of 27 children (or 20 surviving children) versus seven children out of 25 (or 18 surviving children) in the control group had a diminished optimality score (RR 0.77, 95% CI 0.32 to 1.87; RD ‐0.09, 95% CI ‐0.39 to 0.21)Analysis 1.2. This finding was not statistically significant (test of heterogeneity not applicable).
1.2. Analysis.
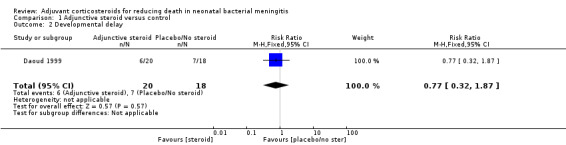
Comparison 1 Adjunctive steroid versus control, Outcome 2 Developmental delay.
Hearing loss at four to 10 weeks after discharge
Mathur 2013 reported that six of the 40 children (or 35 surviving children) randomly assigned to dexamethasone and 10 of the 40 (or 24 surviving children) randomly assigned to the control group had sensorineural deafness at four to 10 weeks after discharge (RR 0.41, 95% CI 0.17 to 0.98; RD ‐0.25, 95% CI ‐0.48 to ‐0.01) (test for heterogeneity not applicable; Analysis 1.3).
1.3. Analysis.
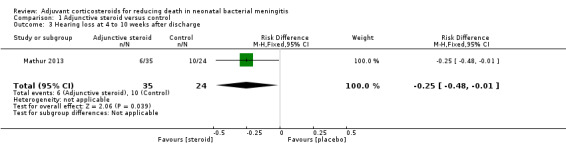
Comparison 1 Adjunctive steroid versus control, Outcome 3 Hearing loss at 4 to 10 weeks after discharge.
Sensorineural deafness at one year of age
This outcome was not clearly reported in the included studies.
Hearing loss at two years of age
One participant in the intervention arm of the Daoud 1999 study was lost to follow‐up. Daoud 1999 reported that two of the 20 remaining children randomly assigned to dexamethasone had sensorineural deafness at two years of age and one of the 18 children in the control group had sensorineural deafness (RR 1.8, 95% CI 0.18 to 18.21; RD 0.04, 95% CI ‐0.12 to 0.21) (test for heterogeneity not applicable; Analysis 1.4).
1.4. Analysis.
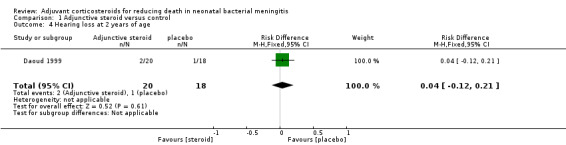
Comparison 1 Adjunctive steroid versus control, Outcome 4 Hearing loss at 2 years of age.
Secondary outcomes
-
Daoud 1999 reported sufficient data to enable assessment of only three of our secondary outcomes.
Number of participants with seizures at any time (two participants in the dexamethasone group vs one in the control group).
Number of participants with seizures persisting after five days of treatment (two in the dexamethasone group vs one in the control group).
-
Hydrocephalus (one participant each in the dexamethasone and control groups).
As these were not reported by Mathur 2013, we could not do a meta‐analysis for these outcomes. The relatively low event rate in this study precluded clinically meaningful conclusions or subgroup analysis at this time.
-
Adverse events: Trialists in Mathur 2013 commented that no gastric bleed occurred; investigators in Daoud 1999 reported that no dexamethasone‐related side effects were observed among trial participants.
Both studies provided no data on the incidence of SIADH, bleeding diathesis or ventriculitis, and no data on fever clearance time and duration of hospitalisation.
We were not able to explore the a priori subgroup analysis because data were insufficient to allow a meaningful analysis.
Discussion
Summary of main results
The main objective of this review was to ascertain from reliable research the impact of adjunctive corticosteroids on important clinical outcomes among neonates with meningitis. It is biologically plausible to believe and indeed it has been demonstrated that beneficial effects are seen when adjunctive corticosteroids are used to treat older children and adults with bacterial meningitis, particularly that caused by Streptococcus pneumoniae. Thus, the standard of care includes adjunctive corticosteroid treatment for these patients. However, we decided to conduct this index review with the goal of generating an evidence‐based statement with regard to neonates, given their peculiarities with regard to physiology and the spectrum of likely infective organisms.
Adjunctive corticosteroid administration reduced the risk of death among neonates with bacterial meningitis; although data show a trend towards increased or worsened outcomes with regards to sensorineural deafness at two years of age, this difference is not statistically significant.
The only trial that examined long‐term effects on neurological sequelae showed no difference, as neonates in both arms of the trial had similar outcomes with regards to neurological development. Researchers reported a decrease in hearing loss at four to 10 weeks after discharge.
We were not able to perform meaningful assessment of the effects of adjunctive corticosteroids with regards to our secondary outcomes, as these were assessed in only one study and events were too few to permit meaningful conclusions.
Clinical heterogeneity was not a major issue in the meta‐analysis of these outcomes. Clinical heterogeneity factors centred mainly on the duration of adjunctive steroid administration, the addition of an aminoglycoside and the use of meropenem for treatment of severely ill neonates in the Mathur 2013 trial. This was reflected in the low statistical heterogeneity observed, with an I2 score = 49% for the primary outcome of all‐cause death until hospital discharge.
Overall completeness and applicability of evidence
Of note, Mathur 2013 had a much shorter period of follow‐up, which made it impossible to ascertain long‐term effects of the intervention among participants.
The included trials mainly investigated use of adjunctive steroids among full‐term neonates; these investigations were carried out in developing countries. Both trials gave third‐generation cephalosporins as baseline antibiotic treatment for these neonates.
In view of the paucity of reliable research data on this problem, it is too early to make far reaching conclusions based on the limited data available. We are not able to answer any of the questions that we set out to answer in our pre‐planned subgroup analyses.
Quality of the evidence
This review includes data from a randomised controlled trial and a quasi‐randomised trial with high risk of bias. Only Mathur 2013 reported allocation concealment. This was an unblinded study, although it is unlikely that this fact would greatly affect the assessment of primary outcomes, as some of the outcome assessors were blinded. Of note, however, the Mathur trial did not provide sufficient follow‐up to enable assessment of the effects of steroid treatment on long‐term neurological sequelae ‐ a key question among clinicians. This review has brought to light the paucity of reliable research evidence on this clinical problem.
Using the GRADE system to assess the quality of evidence, we rated the quality of evidence for the primary outcome of all‐cause death until hospital discharge as very low (Guyatt 2011). We rated evidence for the number of infants with sensorineural deafness at two years of age and the number of infants with developmental delay as low quality.
Potential biases in the review process
The paucity of studies on a disease condition as common as neonatal meningitis is clearly highlighted. Our review is limited by this fact and by the fact that one of the included studies is a quasi‐randomised clinical trial with potentially high risk of bias.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the first systematic review undertaken to examine this clinical question in the newborn. We are not able to comment on agreement or disagreement of our findings with those of other studies.
Authors' conclusions
Implications for practice.
Some reduction in death and hearing loss is evident when adjunctive steroids are used in the treatment of neonatal meningitis. This benefit is not seen with regards to improvement in other neurological sequelae.
However, given that the quality of evidence is very low as a result of the small numbers of studies and participants and the very low quality of available studies, adjuvant steroids should not be used routinely in the treatment of neonatal meningitis.
Implications for research.
This review shows that evidence on potential benefits/harms of using adjunctive corticosteroids for the treatment of neonatal meningitis is scanty. It highlights a significant gap in knowledge and lack of reliable data to inform evidence‐based clinical practice for a common disease. Well‐designed and appropriately powered randomised controlled trials are needed to investigate the clinical utility or otherwise of adjunctive steroid treatment for neonates with bacterial meningitis. These studies may answer more clinically relevant questions if investigators build into their design the intention to explore the clinical utility of steroids for long‐term outcomes such as neurodevelopmental sequelae and for prevention of neurological disabilities such as hearing loss. These studies may generate results with greater applicability if researchers explore other biological factors such as the microbiological spectrum that causes meningitis and the gestational age of participants, then build on a multi‐centre collaboration to enhance the generalisability of study findings.
Acknowledgements
We appreciate our home institutions ‐ the Obafemi Awolowo College of Health Sciences, Olabisi Onabanjo University, Sagamu, Nigeria, and the Nigeria Effective Health Care Research Programme, Institute of Tropical Diseases Research and Prevention, University of Calabar Teaching Hospital, Calabar, Nigeria, for various support provided, especially Professor Martin Meremikwu, Dr Emmanuel Effa and Mrs Olabisi Oduwole. This review was supported by a grant from the Reviews for Africa Programme. We are very grateful to all staff members of the Cochrane Neonatal Review Group Editorial Base, especially Yolanda Brosseau, Colleen Ovelman and Drs Roger Soll, Arne Ohlsson and J Horbar for advice and guidance throughout the review process.
Appendices
Appendix 1. Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Data and analyses
Comparison 1. Adjunctive steroid versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause death until hospital discharge | 2 | 132 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.19 [‐0.33, ‐0.04] |
| 2 Developmental delay | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.32, 1.87] |
| 3 Hearing loss at 4 to 10 weeks after discharge | 1 | 59 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.25 [‐0.48, ‐0.01] |
| 4 Hearing loss at 2 years of age | 1 | 38 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.12, 0.21] |
| 5 Seizures at any time | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.18, 19.19] |
| 6 Seizures persisting after 5 days of treatment | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.18, 19.19] |
| 7 Hydrocephalus | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 14.03] |
1.5. Analysis.
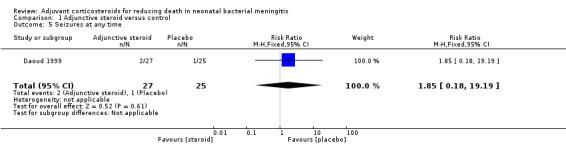
Comparison 1 Adjunctive steroid versus control, Outcome 5 Seizures at any time.
1.6. Analysis.
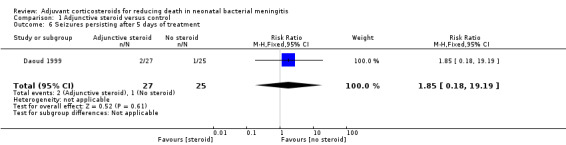
Comparison 1 Adjunctive steroid versus control, Outcome 6 Seizures persisting after 5 days of treatment.
1.7. Analysis.
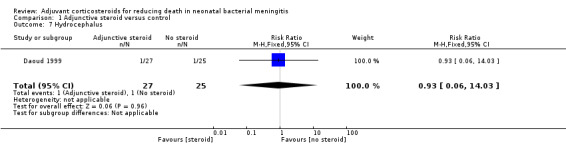
Comparison 1 Adjunctive steroid versus control, Outcome 7 Hydrocephalus.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Daoud 1999.
| Methods | Quasi‐randomised clinical trial, conducted in Jordan between January 1993 and December 1995 Alternate allocation was used |
|
| Participants | 52 full‐term neonates. Diagnosis of meningitis was suspected on the basis of clinical picture and CSF abnormalities: pleocytosis (> 30 leucocytes/mL), raised protein (> 100 mg/100 mL) and reduced CSF to blood sugar ratio < 0.5 | |
| Interventions | Dexamethasone administered at a dose of 0.15 mg per kg every 6 hours. Duration of administration was 4 days Control group did not receive placebo. Both groups were treated with cefotaxime Steroids were administered about 10 to 15 minutes before antibiotics |
|
| Outcomes | Number of all‐cause deaths until hospital discharge, number of infants with sensorineural deafness at two years of age, number of participants with developmental delay, number of participants with seizures at any time, number of participants with hydrocephalus | |
| Notes | Ethical approval was obtained from the Jordan University of Science and Technology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation was used |
| Allocation concealment (selection bias) | Unclear risk | This was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant was lost to follow‐up; 9 were excluded afterwards, as they were thought to have aseptic meningitis |
| Selective reporting (reporting bias) | Low risk | We have no reason to think that investigators selectively reported outcomes |
| Other bias | Low risk | This study was funded by a government university grant |
Mathur 2013.
| Methods | Randomised controlled trial conducted in India between February 2008 and January 2009 | |
| Participants | 80 neonates with mean gestational age of about 37 weeks. Neonates with major congenital malformations and those who had received antibiotics for 24 hours were excluded from the study | |
| Interventions | Dexamethasone was administered at a dose of 0.15 mg/kg per dose at 6‐hourly intervals. Duration of administration of antibiotics was 48 hours. Control group received placebo (saline). Both groups received antibiotics ‐ mainly ceftriaxone and Amikacin ‐ although severely ill participants were treated with meropenem | |
| Outcomes | Mortality, progression of systemic inflammatory response syndrome up to 48 hours, brain stem auditory evoked response after 4 to 6 weeks and again 4 weeks later for participants with initial abnormalities and CSF cytokine levels (TNFalpha and IL‐1beta after 24 ± 6 hours) | |
| Notes | This study provided a power calculation. Study authors aimed to recruit enough participants to give the study 80% power to detect differences in effect between arms if they existed; they set the threshold at a reduction in mortality from 35% to 10% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Investigators reported using sealed opaque envelopes to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an unblinded trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Some outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis and were accounted for |
| Selective reporting (reporting bias) | Low risk | We have no reason to think that selective outcome reporting occurred |
| Other bias | Unclear risk | Researchers do not report the funding source, although they state that funders played no role in analysis and reporting of the study |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Mathur 2015 | This was a randomised controlled trial conducted at a referral neonatal unit. Participants were 70 neonates with meningitis randomly assigned to receive 10 days (study group) or 14 days (control group) of antibiotics. The primary outcome measure studied was treatment failure in each group within 28 days of enrolment. Both groups received adjuvant steroids |
Differences between protocol and review
We were not able to search the Science Citation Index and Latin American Caribbean Health Sciences Literature (LILACS) databases.
We added two outcomes that were not specified in the protocol.
Hearing loss at two years of age.
Hearing loss at four to 10 weeks after discharge.
We changed the order of outcomes, as suggested by external reviewers.
The authors of one trial described an optimality score, which they used to document neurological deficit and developmental delay as sequelae of meningitis among trial participants.
Contributions of authors
TAO initiated and designed the review. CCO contributed to the design of the review. All review authors interpreted the analysed data and made substantial intellectual contributions to the writing of this review.
Sources of support
Internal sources
-
Effective Health Care Research Programme, Nigeria.
Provided support and mentorship for preparation of the review
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C, USA.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C
-
Nigerian Branch of the South African Cochrane Centre, Nigeria.
Reviews for Africa Programme (RAP) Fellowship 2012
World Health Organization, Switzerland.
Declarations of interest
None.
New
References
References to studies included in this review
Daoud 1999 {published data only}
- Daoud AS, Batieha A, Al‐Sheyyab M, Abueikteish F, Obeidat A, Mahafza T. Lack of effectiveness of dexamethasone in neonatal bacterial meningitis. European Journal of Pediatrics 1999;158(3):230‐3. [PUBMED: 10094445] [DOI] [PubMed] [Google Scholar]
Mathur 2013 {published data only}
- Mathur NB, Garg A, Mishra TK. Role of dexamethasone in neonatal meningitis: a randomized controlled trial. Indian Journal of Pediatrics 2013;80(2):102‐7. [PUBMED: 23054852] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Mathur 2015 {published data only}
- Mathur NB, Kharod P, Kumar S. Evaluation of duration of antibiotic therapy in neonatal bacterial meningitis: a randomized controlled trial. Journal of Tropical Pediatrics 2015;61(2):119‐25. [PUBMED: 25681965] [DOI] [PubMed] [Google Scholar]
Additional references
Airede 2008
- Airede KI, Adeyemi O, Ibrahim T. Neonatal bacterial meningitis and dexamethasone adjunctive usage in Nigeria. Nigerian Journal of Clinical Practice 2008;11(3):235‐45. [PUBMED: 19140361] [PubMed] [Google Scholar]
Brouwer 2010
- Brouwer MC, McIntyre P, Gans J, Prasad K, Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD004405.pub3] [DOI] [PubMed] [Google Scholar]
Brouwer 2013
- Brouwer MC, McIntyre P, Prasad K, Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD004405.pub4] [DOI] [PubMed] [Google Scholar]
Chang 2003
- Chang CJ, Chang WN, Huang LT, Huang SC, Chang YC, Hung PL, et al. Neonatal bacterial meningitis in southern Taiwan. Pediatric Neurology 2003;29(4):288‐94. [PUBMED: 14643389] [DOI] [PubMed] [Google Scholar]
Delouvois 1991
- Louvois J, Blackbourn J, Hurley R, Harvey D. Infantile meningitis in England and Wales: a two year study. Archives of Disease in Childhood 1991;66(5):603‐7. [PUBMED: 2039250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furyk 2011
- Furyk JS, Swann O, Molyneux E. Systematic review: neonatal meningitis in the developing world. Tropical Medicine and International Health 2011;16(6):672‐9. [PUBMED: 21395927] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles. Journal of Clinical Epidemiology 2011;64(4):380‐2. [PUBMED: 21185693] [DOI] [PubMed] [Google Scholar]
Harvey 1999
- Harvey D, Holt D, Bedford H. Bacterial meningitis in the newborn: a prospective study of mortality and morbidity. Seminars in Perinatology 1999;23(3):218‐25. [PUBMED: 10405191] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta analyses. British Medical Journal 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Laving 2003
- Laving AM, Musoke RN, Wasunna AO, Revathi G. Neonatal bacterial meningitis at the newborn unit of Kenyatta National Hospital. East African Medical Journal 2003;80(9):456‐62. [PUBMED: 14640166] [DOI] [PubMed] [Google Scholar]
McIntyre 1997
- McIntyre PB, Berkey CS, King SM, Schaad UB, Kilpi T, Kanra GY, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta‐analysis of randomized clinical trials since 1988. JAMA 1997;278(11):925‐31. [PUBMED: 9302246] [DOI] [PubMed] [Google Scholar]
Molyneux 2002
- Molyneux EM, Walsh AL, Forsyth H, Tembo M, Mwenechanya J, Kayira K, et al. Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomized controlled trial. Lancet 2002;360(9328):211‐8. [PUBMED: 12133656] [DOI] [PubMed] [Google Scholar]
Mustafa 1990
- Mustafa MM, Ramilo O, Saez Liorens X, Olsen KD, Magness RR, McCracken GH Jr. Cerebrospinal fluid prostaglandins, interleukins 1 beta, and tumor necrosis factor in bacterial meningitis. Clinical and laboratory correlations in placebo treated and dexamethasone treated patients. American Journal of Diseases of Children 1990;144(8):883‐7. [PUBMED: 2116086] [DOI] [PubMed] [Google Scholar]
Odio 1991
- Odio CM, Faingezicht I, Paris M, Nassar M, Baltodano A, Rogers J, et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. New England Journal of Medicine 1991;324(22):1525‐31. [PUBMED: 2027357] [DOI] [PubMed] [Google Scholar]
Rang 2003
- Rang HP, Dale MM, Ritter JM, Moore PK. The pituitary and adrenal cortex. In: Hunter I editor(s). Pharmacology. 5th Edition. Edinburgh: Churchill Livingstone, 2003. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Stoll 1997
- Stoll B. The global impact of neonatal infection. Clinics in Perinatology 1997;24(1):1‐21. [PUBMED: 9099499] [PubMed] [Google Scholar]
Wald 1995
- Wald ER, Kaplan SL, Mason EO Jr, Sabo D, Ross L, Arditi M, et al. Dexamethasone therapy for children with bacterial meningitis. Meningitis Study Group. Pediatrics 1995;95(1):21‐8. [PUBMED: 7770303] [PubMed] [Google Scholar]


