Abstract
Traditional methods for determining superoxide dismutase (SOD) content and catalase (CAT) activity rely on measuring the absorbance of individual tissue (biological) samples using a cuvette and spectrophotometer, rather than cell cultures. Although there are kits available for SOD and CAT assays, these allow for high-throughput analysis of samples and might be too expensive for research laboratories in countries from the Global South, such as South Africa. This paper describes a simple and cost-effective method to determine SOD content and CAT activity in mammalian cell cultures following exposure to environmental chemical mixtures by measuring absorbance in 96-well microplates. Moreover, the equipment used for this method is considered standard for cell culture laboratories, while the reagents and consumables are easily obtainable.
-
•
Antioxidant enzyme levels can be measured in vitro in cell cultures.
-
•
The supernatant obtained can be used to determine protein concentration, SOD content, and CAT activity.
-
•
This method is simple and affordable, allowing for the analysis of multiple samples (up to 32 samples per microplate).
Method name: Cell-based SOD and CAT bioassays
Keywords: 96-well microplate, Absorbance, Antioxidant enzymes, Cell cultures, Hydrogen peroxide decomposition, In vitro bioassay, Pyrogallol autoxidation, Titration with potassium permanganate
Graphical abstract
Specifications Table
| Subject area: | Agricultural and Biological Sciences |
| More specific subject area: | Mammalian cell cultures; environmental samples; pure compounds |
| Name of your method: | Cell-based SOD and CAT bioassays |
| Name and reference of original method: |
Titration with an excess of KMnO4(Superoxide dismutase content) G. Cohen, D. Dembiec, J. Marcus, J, Measurement of catalase activity in tissue extracts, Anal. Biochem. 34 (1970) 30–38, doi: 10.1016/0003-2697(70)90083-7 Inhibition of pyrogallol autoxidation (Catalase activity) R. Del Maestro, W. McDonald, Distribution of superoxide dismutase, glutathione peroxidase and catalase in developing rat brain, Mech. aging Dev. 41 (1987) 29–38, doi: 10.1016/0047-6374(87)90051-0 S. Marklund, G. Marklund, Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47 (1974) 469–474, doi: 10.1111/j.1432-1033.1974.tb03714.x |
| Resource availability: | Not applicable |
Method details
General background
Anthropogenic activities cause complex mixtures of chemicals to end up in the environment. One example is the extensive use of agrochemicals to reduce crop losses. Although beneficial in ensuring food security, water-soluble chemicals can move towards off-site locations via spray-drift, run-off and/or leaching following application [1]. When bioavailable, these compounds can induce potential health effects in humans and wildlife. Toxicological effects associated with exposure to xenobiotics include oxidative stress–an imbalance between the production of reactive oxygen species (ROS) and a biological system's antioxidative capabilities [2]. The overproduction of ROS enhances inflammatory responses and has been implicated in the pathogenesis and/or progression of several human diseases including bronchial asthma, systemic sclerosis and Alzheimer's disease [3], [4], [5]. The first line of defense antioxidant enzymes, superoxide dismutase (SOD) and catalase (CAT), are responsible for preventing oxidative stress by detoxifying ROS. Superoxide dismutase converts superoxide radicals (O2•−) into hydrogen peroxide (H2O2) and molecular oxygen (O2) [6], while the resulting H2O2 is further neutralised by conversion into water (H2O) and O2 by CAT [7]. Here we describe a cell-based bioassay for SOD and CAT to determine whether pure compounds or environmental samples induce oxidative stress.
The original methods for the determination of SOD content [8] and CAT activity [9] are based on measuring the absorbance of tissue samples one at a time using a spectrophotometer and cuvette. In addition, the methods described for SOD content and CAT activity were developed for brain tissue and liver homogenates, respectively [8], [9], [10]. The major alteration compared to the traditional protocols is that our method allows for the determination of SOD content and CAT activity in mammalian cell cultures following exposure to mixtures of unknown contaminants extracted from environmental samples to determine their combined effect. The concentration and identity of the constituents in the mixture are unknown, yet their influence on SOD content and CAT activity can be determined. Moreover, SOD and CAT activities of multiple samples can be measured using 96-well cell culture microplates. The method we describe here is more efficient and offers a greater throughput of samples since the absorbance of many samples can be read simultaneously—up to 32 samples loaded in triplicate. This method describes the conditions for exposing adherent and immortalised cells and consequently measuring SOD content and CAT activity. However, specific exposure conditions may vary depending on the type of cell line used. Protein content represents the number of cells per well and is used to normalize SOD and CAT bioassay data as is done during the original methods where organ tissues were analysed.
Materials and methods
Equipment
-
•
Cell Culture CO2 incubator that is suitable for tissue culture.
-
•
Centrifuge that can cool to 4°C and reach 10,000 g.
-
•
pH meter.
-
•
Ultrasonic bath.
-
•
Microplate reader that can measure absorbance at different wavelengths in 96-well microplates.
Consumables
-
•
24-Well, clear, flat-bottom cell culture microplates.
-
•
3 mL Plastic Pasteur pipettes (non-sterile).
-
•
2 mL Microcentrifuge tubes.
-
•
96-Well, clear, flat bottom cell culture microplates.
Chemicals and reagents
-
•
Dulbecco's phosphate-buffered saline (DPBS).
-
•
Trypsin-EDTA (100X).
-
•
Dipotassium hydrogen phosphate (K2HPO4).
-
•
Potassium dihydrogen phosphate (KH2PO4).
-
•
Bovine serum albumin (BSA).
-
•
Bradford's reagent.
-
•
Diethylenetriaminepentaacetic acid (DTPA).
-
•
Tris-buffer [tris(hydroxymethyl)aminomethane].
-
•
Pyrogallol.
-
•
Hydrogen chloride (HCl) (32%).
-
•
Hydrogen peroxide (H2O2) (30%).
-
•
Sulphuric acid (H2SO4) (≥97%).
-
•
Potassium permanganate (KMnO4).
Working solutions
-
•
DPBS (pH 7.4).
-
•
Trypsin-EDTA (diluted in DPBS; 1:9, v/v).
-
•
Potassium phosphate buffer (0.09 M K2HPO4 adjusted to pH 7.4 with 0.09 M KH2PO4). Note: This solution should be kept on ice.
-
•
DTPA/tris-buffer solution (1:49, v/v) (1 nM DTPA and 50 mM tris-buffer). Note: Aerate this solution for 20 min before use and adjust the pH to 8.2 with HCl.
-
•
Pyrogallol solution (24 nM pyrogallol in 10 mM HCl-acidified deionised water). Note: This solution should be prepared, used, and stored in the dark since pyrogallol is light-sensitive.
-
•
H2O2 (6 mM, prepared in DPBS).
-
•
H2SO4 (6 M, prepared in deionised water).
-
•
KMnO4 (1.9 mM, prepared in deionised water). Note: This solution should be prepared, used, and store in the dark since KMnO4 is light-sensitive.
Cells
-
•
HuTu-80 (HTB-40™), human duodenum epithelial adenocarcinoma (American Type Culture Collection, Virginia, United States of America).
-
•
H4IIE-luc, rat liver epithelial hepatoma (gifted from the University of Saskatchewan, Canada).
Methods
Harvesting of cells
To determine the SOD content, CAT activity and protein concentrations, the cell content must be harvested following exposure to samples.
-
1.
Seed 1 mL of cells into a 24-well, clear, flat-bottom microplate. The seeding density will depend on the specific cell line used.
-
2.
Incubate the cells at 37°C in humidified air supplemented with 5% CO2 for 24 h.
-
3.
Following attachment (±24 h), expose the cells to the samples in triplicate for 24 h. Note: Although the different methods of sample preparation are outside of the scope of this paper, it is worth mentioning that the cells can be exposed to samples either via directly dosing into the cell culture medium or by replacing the initial nutrient medium with a medium containing the samples.
-
4.
After 24 h of exposure, remove the initial nutrient media of the cells by inversion of the microplate.
-
5.
Wash the cells three times with 500 µL DPBS.
-
6.
Add 150 µL trypsin-EDTA to the cells and incubate at 37°C in 5% CO2 for 3 min (cell-specific).
-
7.
Add 500 µL DPBS to stop trypsin activity.
-
8.
Harvest the cells and transfer the cell suspension of individual wells to a 2 mL microcentrifuge tube using a plastic Pasteur pipette. Note: The microcentrifuge tubes should be labelled in such a way that it corresponds to the well number of the 24-well microplate.
-
9.
Centrifuge the cell suspension at 1000 g for 4 min at room temperature (± 25°C).
-
10.
Discard the supernatant (be careful not to discard the cell pellet).
-
11.
Re-suspend the cell pellet in 270 µL ice-cold potassium phosphate buffer.
-
12.
Sonicate the microcentrifuge tubes containing the cell suspension at medium intensity for 30 s.
-
13.
Centrifuge at 10,000 g for 10 min at 4 °C.
-
14.
The resulting supernatant is used for the determination of protein and SOD content, as well as CAT activity. Note: This supernatant can be frozen for up to seven days at −20°C. Thaw the supernatant on ice before determining SOD content and CAT activity.
Protein determination
Total protein concentration is used to normalize SOD content and CAT activity data. It was determined according to the method described by Bradford [11] using BSA as the standard.
-
1.
Transfer 5 µL supernatant to a 96-well, flat-bottom microplate in triplicate. Note: From here on the rest of the assay is performed in the dark since Bradford's reagent is light-sensitive.
-
2.
Add 245 µL Bradford's reagent to each microplate well.
-
3.
Measure the absorbance at 590 nm using a microplate reader.
-
4.
Prepare a protein standard calibration curve by serially diluting BSA in deionised water (1:1) (0–2,000 µg/mL).
-
5.Plot the BSA protein concentration (µg/mL) (x-axis) against the corresponding absorbance value (y-axis) to obtain Eq. (1):
(1)
where y = relative response (absorbance), m = the gradient of the straight line, x = protein concentration (µg/mL), and c = the y intercept of the straight line.
-
6.
Substitute a sample's absorbance (y-value) into Eq. (1) to calculate the protein concentration (x-value) in µg/mL.
-
7.
After calculation, the total protein concentration of cells after exposure to samples is converted from µg/mL to mg/mL.
-
8.
Obtained values are used to normalize the SOD content and CAT activity data.
SOD content
The removal of O2•− by SOD activity is determined by measuring the reduction in the rate of autoxidation of the organic compound pyrogallol (2,3-dihydroxy phenol), which rapidly occurs in the presence of O2•−. The pyrogallol assay is based on the principle that SOD within cells will convert the O2•− into O2 and H2O2, and as a result, white pyrogallol will not autoxidise into a variety of yellow-brown oxidation products.
-
1.
Transfer 4 µL supernatant to a 96-well, flat-bottom microplate in triplicate.
-
2.
Add 245 µL DTPA/tris-buffer solution to the microplate wells.
Note: From here on the rest of the assay is performed in the dark since pyrogallol is light-sensitive.
-
3.
Add 4 µL pyrogallol solution to the microplate wells to initiate the reaction.
-
4.
Record the kinetic reaction by measuring absorbance at 560 nm every 30 s for 4.5 min starting at time zero using a microplate reader. There are 10 time points in total.
-
5.
For data calculation, determine the mean absorbance and reaction gradient.
-
6.
Calculate the reaction rate (change in absorbance over 4.5 min) and normalize the value to a percentage.
-
7.
One unit of SOD is defined as the amount of enzyme necessary to inhibit 50% of the reaction (pyrogallol autoxidation). Consequently, divide the %inhibition by 50 to obtain the SOD content in units.
-
8.
Multiply the amount of SOD in units by 125 to obtain ng SOD (one SOD unit equals 125 ng SOD).
-
9.
Multiply the amount of ng SOD by the dilution factor (i.e., 4) to obtain the fraction responsible for SOD content in µL.
-
10.
Convert ng SOD/µL to ng SOD/mL.
-
11.
Use the protein content values to normalize the obtained SOD values and express SOD content in ng SOD/mg protein.
CAT activity
The determination of CAT activity is based on the principle of measuring the enzyme-catalysed decomposition of H2O2 using titration with an excess KMnO4—a very strong oxidizing reagent.
-
1.
Transfer 4 µL supernatant to a 96-well, flat bottom, white-walled, microplate in triplicate.
-
2.
Add 93 µL H2O2 (6 mM) to the microplate wells and incubate at 37°C for 3 min.
-
3.
After incubation, stop the reaction by adding 19 µL H2SO4 (6 M) to the microplate wells.
Note: From here on the assay is performed in the dark since KMnO4 is light-sensitive.
-
4.
Add 130 µL KMnO4 (1.9 mM) to the microplate wells.
-
5.
To determine the residual KMnO4, immediately (within 30 to 60 s) measure absorbance at 490 nm using a microplate reader.
-
6.
Include a CAT blank comprised of only reagents (KMnO4, potassium phosphate buffer, and H2SO4) and no supernatant.
-
7.Under the conditions described the enzyme-catalysed decomposition of H2O2 by CAT follows first-order kinetics as given by Eq. (2):
where k = the first-order reaction rate constant, t = the time interval over which the reaction is measured (3 min), S0 = the substrate concentration at time zero, S3 = the substrate concentration at time 3 min, and 2.3 = the first-order kinetic conversion factor.(2) -
8.
S0 is the mean absorbance of the CAT standard.
-
9.
To obtain S3, subtract the absorbance values of individual wells from the S0.
-
10.
Calculate the first-order reaction rate constant, k.
-
11.
Normalize the obtained values against protein content.
-
12.
Convert the values to µM by multiplying with 1000.
-
13.
Express the results as µM H2O2/min/mg protein.
Method validation
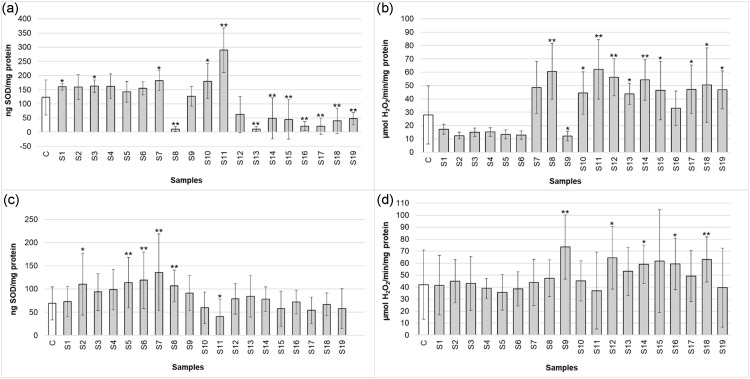
To ensure the validity of the method, human duodenum epithelial adenocarcinoma (HuTu-80) and rat liver epithelial hepatoma (H4IIE-luc) cells were exposed to environmental samples, followed by the determination of SOD content and CAT activity. Cells were seeded (80,000 cells/mL) in 24-well microplates and incubated for 24 h at 37°C in a humidified atmosphere with 5% CO2 and 95% air. After adherent growth, the cells were exposed to agricultural soil samples (83 mg soil equivalents/mL) for 24 h. The cell content was harvested, and the resulting supernatant was used to determine SOD content and CAT activity in 96-well microplates. Fig. 1 shows statistically significant (*p ≤ 0.05 and **p ≤ 0.01) responses between control cells (i.e., untreated) and those exposed to the soil samples for both SOD content and CAT activity. These results indicate that this method is reliable and can be used to measure antioxidant responses in different adherent and immortalised mammalian cell lines.
Fig. 1.
SOD content in (a) HuTu-80 and (c) H4IIE-luc cells; and CAT activity in (b) HuTu-80 and (d) H4IIE-luc cells. Data are expressed as mean ± standard deviation (n = 9). The Mann-Whitney U test was used to determine statistically significant (*p ≤ 0.05 and **p ≤ 0.01) responses compared to the control (C). S1–S19: Sample 1–19.
Ethics statements
No human or animal subjects were used during this study. Based on the review by the Faculty of Natural and Agricultural Sciences Ethics Committee (FNASREC) of the North-West University (NWU), South Africa, this study was cleared as having no ethical risk (Ethics number: NWU-01690-20-A9).
CRediT authorship contribution statement
Ilzé Engelbrecht: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization, Project administration. Suranie Horn: Conceptualization, Resources, Writing – review & editing, Visualization, Supervision, Funding acquisition. John P. Giesy: Methodology, Writing – review & editing. Rialet Pieters: Conceptualization, Resources, Writing – review & editing, Visualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Department of Science and Technology (DST) and the National Research Foundation (NRF) of South Africa in the form of both project funding (grant number: 146150) and a PhD scholarship (grant number: 121713); and the Water Research Commission (WRC) of South Africa (project number: C2020/2021-00195) in the form of project funding.
Data availability
Data will be made available on request.
References
- 1.Horak I., Horn S., Pieters R. Agrochemicals in freshwater systems and their potential as endocrine disrupting chemicals: a South African context. Environ. Pollut. 2021;268 doi: 10.1016/j.envpol.2020.115718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jabłońska-Trypuć A., Wołejko E., Wydro U., Butarewicz A. The impact of pesticides on oxidative stress level in human organism and their activity as an endocrine disruptor. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes. 2017;52(7):483–494. doi: 10.1080/03601234.2017.1303322. [DOI] [PubMed] [Google Scholar]
- 3.Vasconcelos L.H.C., Ferreira S.R.D., Silva M.D.C.C., Ferreira P.B., de Souza I.L.L., Cavalcante F.D.A., Silva B.A.D. Uncovering the role of oxidative imbalance in the development and progression of bronchial asthma. Oxid. Med. Cell. Longev. 2021 doi: 10.1155/2021/6692110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doridot L., Jeljeli M., Chêne C., Batteux F. Implication of oxidative stress in the pathogenesis of systemic sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol. 2019 doi: 10.1016/j.redox.2019.101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S., Puli L., Patil C.R. Role of reactive oxygen species in the progression of Alzheimer's disease. Drug Discov. Today. 2020;26:794–803. doi: 10.1016/j.drudis.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 6.McCord J.M., Fridovich I. Superoxide dismutase - an enzymatic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244(22):6049–6055. doi: 10.1016/S0021-9258(18)63504-5. [DOI] [PubMed] [Google Scholar]
- 7.Chance B. The enzyme-substrate compounds of catalase and peroxides. Nature. 1948;161:914–917. doi: 10.1038/161914a0. [DOI] [PubMed] [Google Scholar]
- 8.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen G., Dembiec D., Marcus J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 10.Del Maestro R., McDonald W. Distribution of superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Mech. Ageing Dev. 1987;41:29–38. doi: 10.1016/0047-6374(87)90051-0. [DOI] [PubMed] [Google Scholar]
- 11.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.