Abstract
Rats and mice are used for studying neuronal circuits underlying recognition memory due to their ability to spontaneously remember the occurrence of an object, its place and an association of the object and place in a particular environment. A joint employment of lesions, pharmacological interventions, optogenetics and chemogenetics is constantly expanding our knowledge of the neural basis for recognition memory of object, place, and their association. In this review, we summarize current studies on recognition memory in rodents with a focus on the novel object preference, novel location preference and object-in-place paradigms. The evidence suggests that the medial prefrontal cortex- and hippocampus-connected circuits contribute to recognition memory for object and place. Under certain conditions, the striatum, medial septum, amygdala, locus coeruleus and cerebellum are also involved. We propose that the neuronal circuitry for recognition memory of object and place is hierarchically connected and constructed by different cortical (perirhinal, entorhinal and retrosplenial cortices), thalamic (nucleus reuniens, mediodorsal and anterior thalamic nuclei) and primeval (hypothalamus and interpeduncular nucleus) modules interacting with the medial prefrontal cortex and hippocampus.
Keywords: object recognition, spatial memory, medial prefrontal cortex, hippocampus, entorhinal cortex, thalamus, cell type specificity
1. Introduction
Recognition memory, i.e., the ability to retain and recognize stimuli or events, is fundamental for daily living and survival. Recognition memory consists of multiple associative components (what, where and when components), which allows deciding, if a stimulus is familiar or novel, and/or if it has previously been associated with other stimuli, contexts, or places.
Scientific exploration of recognition memory began with training rhesus monkeys to discriminate familiar from novel objects in reward-dependent matching (Gaffan, 1974) or nonmatching (Mishkin and Delacour, 1975) -to-sample tasks. In these tasks, an object was presented and then displaced. After a time delay, the familiar object was presented together with a novel object. The animals had to choose either the familiar (matching-to-sample) or the novel object (nonmatching-to-sample) to obtain food rewards. The nonmatching-to-sample paradigm was later adapted to measure recognition memory in rats (Aggleton, 1985; Mumby et al., 1990). Since these tasks require intensive training and repetitive reinforcements, scientists introduced new paradigms of spontaneous object exploration, which mimicked one-time incidental encoding and unexpected retrieval without necessarily involving rewards and the learning of rules.
The novel object preference (NOP) or object recognition test is a spontaneous object exploration paradigm designed for the measurement of recognition memory in animals (Ennaceur and Delacour, 1988). The NOP test utilizes the natural tendency of animals to preferentially explore novel objects relative to familiar ones. This exploration preference implies a mnemonic process, in which the object information is encoded, resulting in the discrimination of the familiar and novel objects. Variants of object recognition tests have been developed to serve different purposes, including assessing (1) where an object was located (novel location preference; NLP) (Ennaceur et al., 1997); (2) when an object was presented (temporal order) (Mitchell and Laiacona, 1998); (3) in which context an object was presented (Eacott and Norman, 2004); (4) the association between objects and their places (object-in-place memory; OiP) (Bussey et al., 2000); and (5) episodic-like (What-Where-When) memory (Dere et al., 2005; Kart-Teke et al., 2006; Good et al., 2007b; Good et al., 2007a).
Recognition memory incorporates two components: recollection and familiarity. The term “recollection” indicates the recognition of specific contextual details about a previous stimulus (“remembering”), while the discernment of “familiarity” involves the mere identification of a previous stimulus (“knowing”). Over decades, diffident views of the neuronal circuits underlying these components of recognition memory have been debated, predominantly focusing on two key neuronal substrates: the hippocampus (HPC) and perirhinal cortex (PRC) (Brown and Aggleton, 2001; Warburton and Brown, 2015). One view holds that the HPC and PRC account for recollection and familiarity, respectively (Brown and Aggleton, 2001). Another hypothesis states that both HPC and PRC are involved in the processing of recollection as well as familiarity (Squire et al., 2007). Both views were supported by a variety of studies involving electrophysiological recordings, lesions and neuroimaging (Eichenbaum et al., 2007; Sauvage et al., 2008; Smith et al., 2011; Merkow et al., 2015). In rodents, lesions of PRC, but (in most cases) not of HPC, impaired NOP memory (Warburton and Brown, 2015; Chao et al., 2020), which favors the view that the PRC plays a major role in mediating familiarity. However, a series of experiments on reversible inactivation of the mouse HPC has revealed that the HPC is also required for the processing of object recognition memory (Cohen et al., 2013). The present consensus seems to be that the HPC and PRC interdependently process both recollection and perception of familiarity in recognition memory.
Recognition memory requires multiple neuronal structures to support the complex dynamic process of memory formation and retrieval. Other brain regions besides the HPC and PRC support recognition memory. For example, the thalamus (Shaw and Aggleton, 1995) and hypothalamus (Schwartz and Teitelbaum, 1974) have been historically implicated, with the support of recent studies using virus-based genetic manipulations (see below). Human imaging studies indicated that the prefrontal cortex (PFC), parietal cortex, retrosplenial cortex (RSC) and parahippocampal cortex are involved as well (Rugg et al., 1999; Chen et al., 2017). This also holds for the striatum, amygdala, medial septum (MS), interpeduncular nucleus (IPN), locus coeruleus (LC) and cerebellum (see below).
It is important to identify the neuronal circuits that connect different brain regions for recognition memory. Classic methods of lesions and pharmacological interventions are invaluable for addressing these questions. However, they have limitations as to the possibility of specifically targeting the relevant cell types as well as disclosing the time dependency of their input. Progress depends on the ability to manipulate the relevant neuronal circuits directly and selectively to find answers to the following questions: (1) Which neurons in the individual circuits respond to mnemonic information? (2) How can neurons, which selectively encode recognition memory, be targeted without affecting others? (3) What are the neurobiological properties of these neurons? (4) How do these neurons interact with other neurons within (intra-regional connectivity) and outside the same brain region (inter-regional connectivity) during the individual memory states (learning, storage, or retrieval)? To answer these questions, it requires (1) behavioral paradigms, which allow accessing recognition memory with incidental encoding, long-term storage and unexpected retrieval in experimental animals, (2) functional mapping of circuits underlying recognition memory with high temporal (millisecond range) and spatial (single cell and dendritic spines) resolution, and (3) technologies to identify the subpopulation of neurons, which are selectively active during recognition memory processing, and to manipulate and monitor inter-neuronal transmission at the synaptic level. These criteria come within reach with the availability of advanced virus-based genetic tools of optogenetics and chemogenetics and in vivo Ca2+ imaging.
For the present review, a PUBMED (https://www.ncbi.nlm.nih.gov/pubmed) search was performed using the keywords “object memory” and “rat” or “mouse”. We present an overview of the studies employing NOP, NLP and OiP tests, as they assess the fundamental components of what and where as well as the what-where association in recognition memory. Moreover, we focus on the application of optogenetics and chemogenetics, followed by discussions of the molecular properties and the functional circuits of recognition memory. Studies of temporal order memory (Mitchell and Laiacona, 1998), object-context memory (Eacott and Norman, 2004) and episodic-like memory (Dere et al., 2005; Kart-Teke et al., 2006; Good et al., 2007b; Good et al., 2007a) are not included since the employed experimental paradigms are related to the concept of episodic memory and, therefore, should be independently discussed. Lastly, studies of recognition memory involving genetic mutations (a gene knockout, knockdown, or knock-in) are not included here.
2. Assessment of recognition memory for object and place using spontaneous object exploration
Spontaneous object exploration exploits the propensity of animals to explore objects with novel properties more than objects with familiar ones. Objects can be arranged according to variable purposes to assess different aspects of recognition memory, namely the identity (shape, size, texture) of objects (what; the NOP test), the location of objects (where; the NLP test) and their temporal or spatial relation (what-where association; the OiP test). Since spontaneous object exploration paradigms do not necessitate reinforcers applied to train an animal to learn a rule, they are economic as to time and labor, and unlikely to be confounded by the use of appetitive or aversive stimuli. As animals naturally prefer novel objects to familiar ones, the results of spontaneous object exploration implicate the formation of recognition memory for the familiar objects.
Recognition memory can be assessed with the following formula: (duration of exploring a novel object – duration of exploring a familiar object) / total duration of exploration. Similar exploration durations for the “novel” and the “familiar” objects lead to a value close to 0, indicating that no preference for object novelty has been formed. A positive value indicates a preference for object novelty, and thus, the formation of recognition memory (with respect to the familiar object). Inevitably, spontaneous object exploration tests involve novelty-seeking/preference behavior, which cannot be disentangled from memory processing. Given the operational definition of recognition memory assessed with the paradigm of spontaneous object exploration, it is difficult to judge whether animals recollect the information of the object shown previously or merely “sense” that something has changed, since either would lead to the preference for object novelty. Spontaneous object exploration paradigms, although holding many advantages in recognition memory research, have this limitation of commingling processes of recollection and representations of familiarity (Cole et al., 2019).
2.1. The novel object preference (NOP) test
The NOP test consists of a sample/learning and a test/retrieval trial, separated by a time delay. In the sample trial, the animal typically encounters two duplicates of novel objects in a habituated environment. In the test trial, the animal is presented with one previously shown object (familiar) and one new object (novel). The familiar and novel objects are placed at the same locations as in the sample trial, avoiding a change in their spatial locations (Figure 1). Normally, rodents explore the novel object more than the familiar one, indicating recognition memory for the previously explored object. This paradigm is considered a non-spatial memory test feasible to assess the information of object identity.
Figure 1.

Schematic diagrams of spontaneous object exploration paradigms of the novel object preference (NOP), novel location preference (NLP) and object-in-place preference (OiP). Circled objects are explored more than non-circled objects by rodents in the testing trial.
2.2. The novel location preference (NLP) test
The procedure of the NLP test is identical to the NOP test except that, in the test trial, one of the familiar objects is positioned at a novel location (Figure 1). Since the objects remain identical in the test trial, the property of “novelty” is not due to changes of object identity, but of location. Normally, rodents spend more time exploring the object at the novel location than the object in the familiar place, indicating recognition memory for the location, which the object had previously occupied. This paradigm is considered a spatial memory test for information of object location.
2.3. The object-in-place (OiP) test
In the OiP test four distinct objects located at four different locations are presented in the sample trial. After a time delay, the locations of two of the objects are interchanged, introducing an alteration of object configuration in the test trial (Figure 1). Normal rodents would explore the two objects with interchanged locations more than the objects that have been left at their previous locations. This paradigm is different from the NOP and NLP tests for the following reasons: (1) Instead of two identical objects, four distinct objects are applied, which can be assumed to require higher memory capacity; (2) The presence of distinct objects forms a specific inter-object relationship, which is changed by altering the object configuration (as to a detailed discussion of inter-object relationship see the review (Chao et al., 2020); (3) Since neither a novel object nor a new location is introduced, the novelty preference for the two displaced objects results from a change in the association between object and place. The OiP test, thus, allows for the assessment of the memory for the association between object and place.
Methodological details for the NOP, NLP and OiP tests can be found in the review (Chao et al., 2020).
3. Application of optogenetics and chemogenetics in behavioral neuroscience
A fundamental task in neuroscience is to understand how behavior is governed by neuronal activity in the respective anatomical circuits. In the past century, lesion models and pharmacological interventions have been widely used to uncover brain functions. The rationale behind these methods is that if a specific behavior is changed due to surgical or pharmacological intervention in a specific brain region, this region can be assumed to participate in the neuronal circuit(s) responsible for that specific function. To determine whether functional interactions of two regions contribute to a specific function, a procedure of disconnection can be applied as follows: a unilateral lesion of one of the regions in one hemisphere combined with a unilateral lesion of the other region in the opposite hemisphere. In this approach, the pathway connecting the two regions is disrupted at two different levels. If the interaction between the two regions is important for a specific behavior, the respective function should be impaired by the disconnection. Although the disconnection procedure assesses the necessity of functional interaction between two regions, it cannot determine the direction of interregional communication, nor can it exclude the effects of indirect disconnections between two regions. Pharmacological approaches also depend on many factors, e.g., agents’ doses, affinities to different receptors and pharmacokinetics. Local infusions of pharmacological agents, e.g., lidocaine, a voltage-dependent sodium channel blocker, and muscimol, a GABAA receptor agonist, are commonly used to transiently inactivate a brain region, while the effect depends on the extent of agent diffusion. Besides, both lesions and pharmacological interventions are limited in the possibility of specifically targeting the relevant cell types as well as disclosing the exact time dependency of their input. The invention of virus-based genetic methods of optogenetics and chemogenetics with cell type- and region-specific manipulations has greatly advanced our understanding of the neuronal bases for behaviors. A combination of the disconnection procedure and the contemporary methods will be particularly helpful to clarify the information flow between different brain regions underlying behavioral tasks. For instance, firstly, the functional necessity of an anatomical connection between two regions can be probed by its abolishment and, secondly, the functional directionality between two regions or circuits can be assessed by optogenetic or chemogenetic manipulations (e.g., manipulations of the axonal terminals in region Y stemming from region X – information flow from X to Y).
3.1. General background
Virus-based gene transfer techniques offer the possibility to selectively mediate and monitor neuronal activity from a specific population of cells with temporal precision. A variety of wild-type viruses has been adapted for this purpose, e.g., adenovirus, lentiviruses, herpes simplex virus, rabies virus and adeno-associated virus (AAV). Each virus has distinct properties and applications (Xu et al., 2020). With the help of these viruses, packaged genomic information can be transfected into specific cells, bypassing the methodological drawbacks of lesion and pharmacological interventions.
Optogenetic manipulation is achieved by expressing light-activated protein (opsins) in the targeted cells, allowing for neuronal depolarization or hyperpolarization with photostimulation (Deisseroth, 2015). For chemogenetic manipulation, designer receptors exclusively activated by designer drugs (DREADDs) are most commonly used for cell transfection (Roth, 2016). DREADDs are engineered G-protein coupled “designer” receptors that have a low affinity for their native ligands, but a high affinity for synthetic, otherwise inert, “designer” ligands. The “designer” ligands (e.g., clozapine-N-oxide and salvinorin B) can be administered locally or systemically to activate or inhibit neuronal activity (Armbruster et al., 2007; Vardy et al., 2015). Other types of engineered proteins have been developed, e.g., ligand-gated ion channels (Magnus et al., 2011). A designed chloride-permeable inhibitory PSAM4-glycine receptor, once activated by its ultrapotent agonist, induces neuronal inhibition (Magnus et al., 2019). Since the PSAM4-glycine receptor may cause neuronal activation on certain cell types (Gantz et al., 2021), cautions should be exercised when using such a chloride-permeable receptor.
Optogenetic and chemogenetic methods can target a specific cell type either via specific viral promoters or via recombination technologies. A relatively high spatial resolution is achieved either by the positioning of the optic fiber (optogenetics) or by intracranial microinjection of the designed ligands (chemogenetics) into a restricted brain region. Unlike permanent lesions, optogenetic and chemogenetic effects can be reversed once the actuator (light or designed ligand) is withdrawn. Importantly, dependent on the subtypes of opsins or G-protein coupled receptors, both methods can elicit neuronal excitation or inhibition. Although both tools share similar characteristics and advantages, their differences are apparent. Optogenetics manipulate neuronal activity in the range of milliseconds with controllable frequencies of light by the transmembrane movement of ions (Deisseroth et al., 2006), whereas chemogenetics are suitable for prolonged neuronal manipulations over minutes or even hours (Armbruster et al., 2007; Alexander et al., 2009). Whereas optogenetic manipulation is not physiological, DREADDs activations may be physiological (yet artificial) since they are based on G-protein-coupled receptor signaling. The application of chemogenetics is more convenient than that of optogenetics, as the former does not require a set of specialized hardware (e.g., optic fibers and LED generators). Moreover, the designed ligands can be flexibly delivered via systemic (injection, food, or water) or intracranial administration, thus avoiding the problem that some behaviors are difficult to study via optogenetics due to tethered implantation. Technological issues of optogenetics and chemogenetics are noted, in which the efficiency of both methods relies on the expression of transfected proteins (optogenetic opsins or chemogenetic receptors). In optogenetics, variable intensity and frequency of light pulses lead to different neuronal activations. Changes in temperature induced by light pulses can affect neurophysiological status (Owen et al., 2019). In chemogenetics, a high dose of clozapine-N-oxide induces side effects in behaviors because clozapine-N-oxide is metabolized into clozapine, an antipsychotic agent (Gomez et al., 2017). In addition, clozapine has a 100-fold greater sensitivity to muscarinic-based DREADDs than clozapine-N-oxide (Armbruster et al., 2007). The slow kinetics, metabolic concerns and potential side effects of clozapine-N-oxide challenge the use of DREADDs, although a novel ligand, deschloroclozapine, has been designed to minimize these problems (Nagai et al., 2020). Since the present review is not intended to discuss optogenetic and chemogenetic methodologies, the reader is referred to the following publications, which offer further information on these issues (Aston-Jones and Deisseroth, 2013; Deisseroth, 2015; Burnett and Krashes, 2016; Smith et al., 2016; Wiegert et al., 2017).
3.2. Strategies to manipulate neuronal activity
With virus-based genetic methods, specific neuronal types and circuits can be studied (Figure 2). Taking optogenetics or chemogenetics as an example, here, we present the key advancement of virus-based genetic tools in neuronal circuit studies, including anterograde and retrograde tracing viruses, and single- and dual-viral strategies to map out interregional connections. To simplify the presentation, we only illustrate the strategies for manipulating a model circuit from region X to region Y in wild-type animals (Figure 2B-D). Other methods can be found in the reviews (Hui et al., 2022; Zingg et al., 2022).
Figure 2.
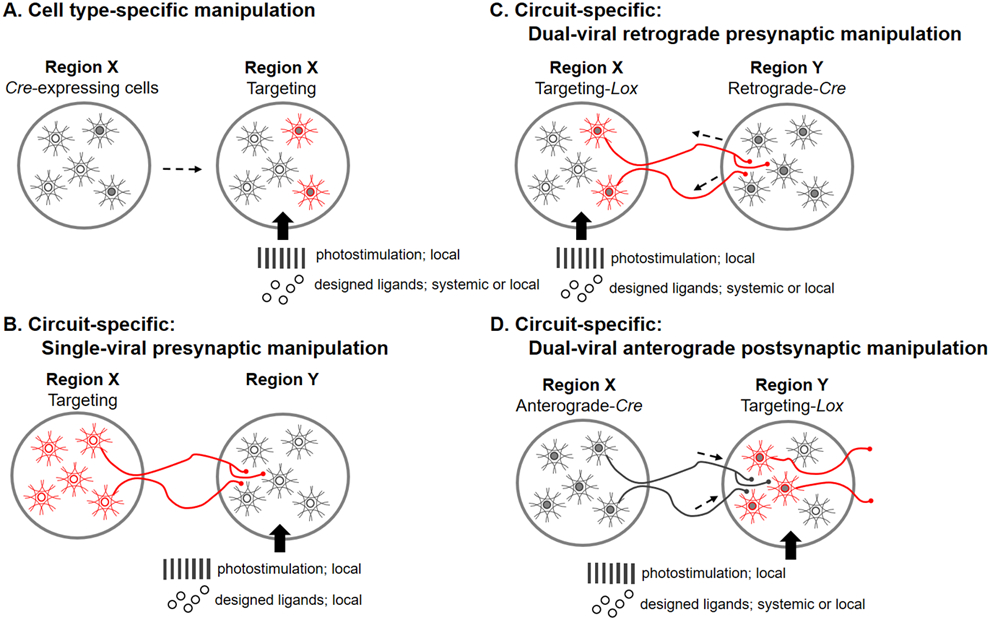
Strategies for viral vector-based genetic manipulations of neuronal activities. (A) Neurons expressing Cre (solid-gray dots) are targeted by a viral vector carrying DIO (red). Via the Cre-Lox recombination (as an example), this design enables bidirectional manipulation of a specific neuronal population in a brain region with optogenetic or chemogenetic approaches. (B) Neurons in source region X are targeted by a viral vector. Their axonal terminals in downstream region Y can be manipulated with optogenetic or chemogenetic stimulations. (C) A dual-viral retrograde targeting method to manipulate presynaptic neurons. A retrograde virus carrying Cre is injected into region Y, while another viral vector carrying DIO is injected into upstream region X. Via the Cre-Lox recombination, only neurons, which project to the Cre-injected region, are selectively manipulated. (D) A dual-viral anterograde targeting method to manipulate postsynaptic neurons. An anterograde virus carrying Cre is injected into region X, while another viral vector carrying DIO is injected into downstream region Y. Via the Cre-Lox recombination, only neurons, which receive projections from the Cre-injected region, are selectively manipulated. Neuronal manipulations can be achieved by photostimulation (optogenetics) or designed ligands (chemogenetics).
3.2.1. Cell type-specific manipulation
Cell type specificity can be obtained by capsid modification of viral vectors, by selection of promoters or enhancers, or by the employment of recombinase technologies, such as Cre-Lox or FLP-FRT recombination. For instance, the human synapsin (hSyn) promoter allows a high level of neuron-specific expression (Dashkoff et al., 2016). The calcium/calmodulin-dependent protein kinase II alpha (CaMKIIα) permits selective gene expression in excitatory neurons (Yaguchi et al., 2013). GABAergic interneurons can be targeted with the distal-less homeobox (Dlx) enhancer (Dimidschstein et al., 2016) or the glutamic acid decarboxylase 65 (GAD65) promoter (Hoshino et al., 2021). A major challenge of using AAV viral vectors for gene transfer is their limited packaging capacity (~4.7 kb) (Wu et al., 2010). To address this issue, truncated promoters have been developed, such as the miniature versions of Dlx and GAD65 used to transfect interneurons in the cerebral cortex (Dimidschstein et al., 2016; Hoshino et al., 2021). Another strategy to overcome the packaging limitation of AAV vectors is to employ recombinase technologies. For example, selective targeting of a specific cell type can be achieved by infusions of viral vectors carrying double-floxed inverted open reading frame (DIO) into transgenic animals of a specific Cre line, thereby producing Cre-Lox recombination and labeling Cre-expressing cells. This design enables bidirectional manipulation (excitation or inhibition) of a specific neuronal population in a brain region with optogenetic or chemogenetic approach (Figure 2A).
In addition to transient manipulation with optogenetics or chemogenetics, the same principle can be applied to cause chronic changes in the selected neurons. Cell inhibition or death can be induced by either viral-diphtheria toxin subunit A (DTA) which blocks protein synthesis, or viral-Casp3 which incurs cell apoptosis (Morgan et al., 2014). Another example is to inhibit neuronal activity with viral-tetanus toxin light chain (TeLC) that prevents vesicular neurotransmitter release (Sweeney et al., 1995). These methods do not require a designed actuator. Non-targeted regions or circuits may be affected by the ablation (DTA or Casp3) or sustained neuronal silencing (TeLC) of targeted regions. For instance, chronic silencing of excitatory neurons in the hippocampal subregion CA2 with TeLC disturbed the excitation/inhibition dynamic in the CA3 (Boehringer et al., 2017). Hence, the interpretation of such studies should be careful as to the effects exerted on the individual neuronal circuits. Nevertheless, the application of DTA, Casp3 or TeLC is suitable for chronic manipulation of a specific cell type, region, or pathway.
3.2.2. Single-viral targeting: Presynaptic manipulation
This approach transfects neurons in a brain region with a single viral vector carrying optogenetic or chemogenetic messages. The viral vector can target a specific population of neurons (e.g., with a promoter) in the source region X, and the nerve terminals of these neurons in the connected downstream region Y can be activated locally by photostimulation or designed ligands of DREADDs (Figure 2B). Ideally, such input-specific manipulations allow examination of how the information flows from region X to region Y as well as on associated behaviors. However, this approach has been questioned as to its specificity since the stimulation of nerve terminals might incur unwanted activation of axonal collaterals of other connected regions (Zingg et al., 2017). Light stimulation of nerve terminals has also been shown to cause backpropagation of electrical signals to the cell bodies in optogenetic studies (Grossman et al., 2013). Because of these concerns, transsynaptic tagging methods are being developed to better meet the requirement for pathway-specific interventions.
3.2.3. Dual-viral retrograde targeting: Presynaptic manipulation
The Cre-Lox system is a common strategy for transsynaptic tracing with two viral vectors. In this method, a viral vector carrying Cre is infused into one brain region, and another vector carrying DIO is infused into one of the connected regions. Depending on the property of the virus that carries Cre, anterograde or retrograde targeting can be attained.
In the retrograde approach, it serves to examine how the neuronal activity influences functions of the connected downstream region by direct manipulation of its upstream connected neurons. Retrograde transsynaptic viral tracers, e.g., canine adenovirus type 2 (Soudais et al., 2001) or retrograde AAV (Rothermel et al., 2013), carrying Cre are injected into region Y, while a tracer-dependent viral vector (e.g., with DIO) is performed at the upstream region X (Figure 2C). Via the Cre-Lox recombination, neurons in region X that send output to the target region are selectively labeled (Hui et al., 2022). By optogenetic or chemogenetic manipulation of the labeled neurons, this method is designed to examine the behavioral correlates to the specific presynaptic pathway. Yet, if the presynaptic neurons also send axonal collaterals to regions other than region Y, their activation likely affects regions beyond the targeted neuronal circuit.
3.2.4. Dual-viral anterograde targeting: Postsynaptic manipulation
This approach utilizes an anterograde transsynaptic viral tracer (e.g., AAV1-hSyn-Cre), which is injected into a source region X, while a tracer-dependent viral vector (e.g., containing DIO) is performed into the connected downstream region Y (Figure 2D). Through the Cre-Lox recombination, neurons in region Y that receive input from the source region are selectively labeled (Zingg et al., 2017). By optogenetic or chemogenetic manipulation of the neurons in the downstream region, this approach attempts to examine the functions of the X-to-Y pathway. This approach avoids the possibility of presynaptic manipulations leading to “co-activations” of other regions via axonal collaterals. In addition, this approach can be used to further investigate how the source region modulates a secondary downstream region by activating the axonal terminals of region Y neurons (Xiao et al., 2018; Zingg et al., 2022). Whereas AAV1-hSyn-Cre also contains the retrograde property, a mixture of pre- and postsynaptic targeting is possible if the source and target regions are reciprocally connected. Thus, AAV1-hSyn-Cre is not recommended to study reciprocally connected circuits (Zingg et al., 2017; Zingg et al., 2022).
To understand functions related to cell type and circuit specificity, these approaches should be applied in a complementary manner even in the same experimental setting. For instance, a comparison between manipulating the upstream and downstream regions would provide valuable information on how the activity of presynaptic and postsynaptic circuits regulate behaviors differently. This could also minimize an over-/misinterpretation of findings obtained with the individual approach.
3.3. Acute “off-target” effects by transient manipulations
It is critical to decipher whether observed effects are specifically related to the manipulated circuits or due to influences of otherwise remote ones. A landmark study reported that transient manipulations (pharmacological inactivation or optogenetic stimulation) of a brain region caused behavioral deficits that disappeared when the region was permanently lesioned (Otchy et al., 2015). Behavioral recovery is likely achieved by homeostatic regulation of neuronal activity involving other brain nuclei (Marder and Goaillard, 2006). This strongly challenges the prevailing assumption that the effects of transient manipulations of a brain region or circuit directly reflect the functions of this region or circuit, implying that the underlying processing does not necessarily require the transmitted information produced or stored by this region or circuit. Interestingly, the behavioral deficits induced by transient manipulations may persist for hours after the infliction of the permanent lesions (Otchy et al., 2015). Although permanent lesions may be assumed to acutely perturb remote circuits, they offer time for the brain to form a homeostatic adjustment systematically. Thus, the neuronal network underlying the impaired function may enter a new state of functionality, which contrasts the previous state of physiological unbalance as induced by the transient manipulations (Otchy et al., 2015; Vaidya et al., 2019). On account of this fact, transient perturbation of a local area in the brain generally incurs more severe effects than permanent lesions (Talwar et al., 2001; Wilke et al., 2010; Cohen and Stackman, 2015). Unlike permanent lesions that reveal the necessity of a region- or circuit-function relationship, transient manipulations may not recapitulate the effects of permanent lesions as incurring changes of remote circuits (“off-target” effects). Therefore, it is proposed to classify the role of a region or circuit either as “permissive”, if it is acutely necessary, but may be bypassed in the long run, or as “instructive”, if it provides essential input, which is not available from others (Otchy et al., 2015). Combining transient and chronic neuronal manipulations might help to distinguish the “permissive” or “instructive” role of circuits, which is not facile given the complexity of the highly interconnected brain (Wolff and Olveczky, 2018). Moreover, permanent lesions under some conditions might also incur “off-target” effects (Albasser et al., 2007; Dumont et al., 2012), which should be carefully examined. Nevertheless, the distinction between “permissive” and “instructive” brain regions/circuits should be kept in mind in addition to the pursuit of cell type- and pathway-specific targeting (Figure 3). Taken together, a perspective is recommended, which combines transient manipulations, chronic interventions, and neuronal monitoring, as a triple validation (Vaidya et al., 2019). In addition, neuronal monitoring (e.g., electrophysiological recordings, Ca2+ imaging, and/or immediate early genes (IEGs) expression (Albasser et al., 2007; Dumont et al., 2012) should be employed to examine the properties of neuronal functions, in order to prevent any misinterpretation of findings based on a single approach.
Figure 3.

Common methods for studying brain function are classified by their specificity (X-axis) and temporal (Y-axis) profiles. In terms of specificity, viral-based genetic methods can target a specific cell type or circuit (blue fonts), while other methods are limited (neurotoxins can target a certain type of neurons but are difficult to apply for specific circuits). In contrast to chronic manipulations, transient manipulations offer high temporal precision and are reversible. They may, however, incur “off-target” effects (green background). DREADDs: designer receptors exclusively activated by designer drugs; DTA: diphtheria toxin subunit A; tDCS: transcranial direct current stimulation; TeLC: tetanus toxin light chain; TMS: transcranial magnetic stimulation.
Below we discuss findings from the NOP, NLP and OiP tests in rodent models after chronic lesions, pharmacological inactivation, or virus-based genetic manipulations (mainly optogenetics and chemogenetics).
4. Brain regions involved in recognition memory for object and place
The medial temporal lobe has been the main target in memory studies since bilateral temporal lobe resection in the patient H.M. resulted in severe amnesia (Scoville and Milner, 1957; Squire, 2009). The medial temporal lobe contains several structures, including the HPC, PRC, parahippocampal/postrhinal cortex and entorhinal cortex (EC), which are engaged in spatial navigation and episodic memory. Furthermore, the medial prefrontal cortex (mPFC), which receives massive projections from the anterior HPC (ventral HPC in rodents), interacts with this region during episodic encoding and long-term recognition memory (Eichenbaum, 2017; Chao et al., 2020). Since patients with lesions of the diencephalon displayed memory impairments, the thalamus is also implied in memory function (Shaw and Aggleton, 1995; Caulo et al., 2005). Apart from this, multiple regions including the insular cortex, hypothalamus, IPN, striatum, MS, amygdala, and LC, even the cerebellum, have been evidenced to play a role in the processing of recognition memory.
4.1. Hippocampus
4.1.1. Memory for object
Lesions of the HPC in rodents have produced equivocal results on object recognition memory. Some studies showed deficits in the NOP test induced by the HPC lesions (Clark et al., 2000; Broadbent et al., 2004; Ainge et al., 2006; Broadbent et al., 2010), but not in the majority (Gaskin et al., 2003; Winters et al., 2004; Forwood et al., 2005; Mumby et al., 2005; Albasser et al., 2010; Langston and Wood, 2010; Barker and Warburton, 2011; Albasser et al., 2012).
Pharmacological inactivation (lidocaine or muscimol) of the rodent HPC disrupted encoding, storage and recall in the NOP test (Hammond et al., 2004; Cohen et al., 2013; Asgeirsdottir et al., 2020), but see (Oliveira et al., 2010; Haettig et al., 2011; de Landeta et al., 2020). The disparate findings may be concluded that participation of the HPC in NOP memory likely depends on multiple factors, including the length of the inter-trial intervals (Cohen and Stackman, 2015; Asgeirsdottir et al., 2020), allocentric signals (Langston and Wood, 2010), contextual properties in the testing environment (Piterkin et al., 2008; Oliveira et al., 2010; Kim et al., 2014; Yi et al., 2016) and strength of memory (Cinalli et al., 2020). So far, it has not been systematically examined whether the HPC plays a “permissive” or “instructive” role in object recognition memory.
It is ambiguous whether chemogenetic manipulation of the HPC influences the formation of object recognition memory (Chao et al., 2020). Chemogenetic excitation or inhibition of the dorsal hippocampal neurons had no effects in the NOP test when the memory was tested 24 h after the sample trial (Lopez et al., 2016; Tuscher et al., 2018). Likewise, chemogenetic manipulations of excitatory or inhibitory neurons in the HPC CA1 subregion had no effects on object recognition (Lopez et al., 2016; Tuscher et al., 2018; Wang et al., 2018). This also holds for activation of the dentate gyrus granule neurons (Kahn et al., 2019). When chemogenetic receptors were activated after the learning trial, neither inhibiting excitatory nor exciting inhibitory neurons in the dorsal HPC affected object recognition in the NOP test with a 24 h inter-trial delay (Tuscher et al., 2018; Yu et al., 2018). In contrast, chemogenetic inhibition (KORD, but not hM4Di) of the dorsal hippocampal excitatory neurons after the sample trial disrupted NOP memory after a 24 h delay (Tuscher et al., 2018), indicating that the dorsal HPC is associated with the consolidation of NOP memory.
Optogenetic inhibition of the CA2/3 glutamatergic terminals in the dorsal CA1 (the trisynaptic circuit) caused short-term (5 min) object recognition deficits in the NOP test when photostimulation was performed during the test trial. No effects were observed when the CA2/3 terminals were activated in the posterior CA1 or dorsolateral septum (Raam et al., 2017). This indicates that the CA2/3 to CA1 glutamatergic circuit is critical for the retrieval of object recognition memory. In contrast, the ventral CA1 projecting to the dorsal CA3 (the opposite direction of the trisynaptic circuit) is critical for encoding information on the presented objects. This is evidenced by the finding that NOP memory was impaired by chemogenetic inhibition of the ventral CA1 neurons that selectively connected with the dorsal CA3, administered before the sample trial (Lin et al., 2021). Thus, distinct circuits between CA1 and CA3 are recruited in different stages of object memory, with the trisynaptic circuit relevant for object retrieval and its antidromic interconnection relevant for object encoding. As to the CA2, chronic inactivation of its excitatory neurons with TeLC did not affect the outcome of the NOP test (1 h delay) but affected social recognition (Hitti and Siegelbaum, 2014), which can be mediated by the medial septal diagonal band of Broca (MSDB) cholinergic circuit (Pimpinella et al., 2021). Optogenetic inhibition of excitatory neurons of the ventral HPC during the test trial of the NOP test did not influence the short-term (10 min) object recognition (Sun et al., 2020). Neither chemogenetic excitation of the ventral HPC neurons selectively projecting to the mPFC (Phillips et al., 2019) nor optogenetic inhibition of the ventral HPC nerve terminals at the nucleus accumbens (NAc) (Okuyama et al., 2016) affected NOP recall. However, this does not necessarily exclude any participation of the ventral HPC-connected circuits in the retrieval of recognition memory, since the ventral HPC–mPFC (Phillips et al., 2019) or ventral HPC–NAc circuit (Okuyama et al., 2016) is critical for the processing of social recognition memory. Furthermore, the ventral HPC may provide input to the mPFC to encode concurrent contextual information (Twining et al., 2020) and information on a previously explored social conspecific (Okuyama et al., 2016). Moreover, “anxiety cells” in the ventral HPC activated by anxiogenic environments (Jimenez et al., 2018) responded to signals of safety and negatively correlated with freezing behavior (Meyer et al., 2019). Thus, the ventral HPC-connected circuits may be important for memory recall when emotion and/or social behavior is involved.
At least 20 different types of inhibitory interneurons have been described in the CA1 (Klausberger and Somogyi, 2008), which may participate in mnemonic processes. One example is corticotropin-releasing hormone (CRH) interneurons (Hooper et al., 2018). DTA ablation or chemogenetic inhibition of these neurons in the dorsal HPC impaired NOP memory after a 3 h, but not a 24 h, delay. The CRH interneurons project from CA1 to CA3 principal neurons and thereby regulate the trisynaptic circuit activity via feedback inhibition (Hooper et al., 2018). The soma of CA1 oriens lacunosum-moleculare (OLM) interneurons is located in the stratum oriens, while their axons project to the distal dendrites of pyramidal cells in the stratum lacunosum-moleculare (Leão et al., 2012). Interestingly, DTA ablation of the ventral HPC OLM interneurons did not affect the outcome of the NOP test (Haam et al., 2018). A subpopulation of CA1 OLM interneurons is defined by the expression of nicotinic α2 acetylcholine receptor (OLMα2). Optogenetic excitation of the OLMα2 cells impaired NOP memory (both 1 and 24 h delays), in which mice also showed increased exploration of the object previously paired with light stimulation. Conversely, optogenetic inhibition of the OLMα2 cells facilitated memory and decreased exploration of the object previously paired with light stimulation. These effects were not observed during the consolidation, but during the encoding phase of memory, indicative of a role of intermediate CA1 OLMα2 cells in object learning. Moreover, inhibition on the dorsal HPC OLMα2 cells did not induce the same effects, probably due to differential cholinergic modulation along the dorsoventral axis of the HPC, as nicotine preferentially depolarized the OLMα2 cells in the intermediate, but not in the dorsal subregion (Siwani et al., 2018). Acetylcholine activated the HPC OLM interneurons and, by augmenting feedback inhibition of the CA1 pyramidal neurons, suppressed the information flow from CA1 to the deep-layer EC neurons (Haam et al., 2018). These findings suggest that the CA1 OLMα2 interneurons bidirectionally regulate CA1 encoding of object information. Taken together, different subtypes of hippocampal interneurons are associated with different stages or strengths of NOP memory: while the CRH cells are required for object memory within hours, but not over 1 day, the OLM cells are necessary for NOP learning, but not consolidation. Interestingly, chemogenetic excitation of parvalbumin-positive interneurons in the dorsal HPC did not influence the outcome of the NOP test (Zou et al., 2016; Wang et al., 2018; Zeidler et al., 2020). Since transient perturbation of the excitatory or inhibitory neurons “globally” might mask mnemonic information on objects processed by specific subtypes of interneurons in the HPC, more studies are required to unravel the mechanism.
4.1.2. Memory for place
Compatible with the role of HPC in spatial cognition (Hartley et al., 2014), both lesions and pharmacological inactivation of the HPC disturbed the encoding, storage and retrieval of NLP memory (Chao et al., 2020). This reinforces that the integrity of the HPC, particularly its dorsal subregions, is essential for the formation and regulation of spatial memory.
Inhibition of either all or only glutamatergic neurons in the dorsal HPC before the sample trial impaired NLP memory (Haettig et al., 2013; Lopez et al., 2016; Tuscher et al., 2018; Wang et al., 2018). In contrast, excitation of either all or only excitatory neurons in the dorsal HPC before the sample trial facilitated NLP memory after a 24 h delay (Lopez et al., 2016). Likewise, inhibition of GABAergic neurons in the dorsal HPC before the sample trial disrupted the NLP memory after a 24 h delay (Haettig et al., 2013). Optogenetic excitation of the hippocampal interneurons that contain neuronal nitric oxide synthase (nNOS) and have long-range projections impaired the long-term (24 h) NLP, but not NOP, memory (Wick et al., 2019). These results suggest that inhibitory GABAergic input is important for the processing of spatial information on objects. This apparently, was not the case for the parvalbumin-positive interneurons (Wang et al., 2018; Zeidler et al., 2020). Evidence suggests that the dorsal HPC excitatory neurons bidirectionally mediate information on object location, which can be enhanced or suppressed by excitation or inhibition of glutamatergic neurons, respectively. Furthermore, the role of the dorsal HPC excitatory neurons in the processing of information on object location was not state-dependent since pre-sample plus pre-test chemogenetic inhibition led to NLP deficits. The same treatment targeting the ventral HPC did not produce the same effects, indicating that the dorsal HPC is more involved in spatial function than the ventral one (Kahn et al., 2020). The dorsal HPC is also engaged in the consolidation of NLP, but not NOP, memory via the PL glucocorticoid system. Pharmacological inhibition (PD98059, a MEK inhibitor) of the dorsal HPC blocked the facilitating effects incurred by post-sample intra-PL infusions of a glucocorticoid receptor agonist in the NLP (24 h), but not NOP, test (Barsegyan et al., 2019), suggesting that the dorsal HPC interacts with the PL in regulating glucocorticoid actions on memory consolidation for object location.
The CA1 sends efferent input to the subiculum. Optogenetic inhibition of the CA1 pyramidal neuronal terminals in the subiculum blunted spatial recognition in a novel place preference task (Trouche et al., 2019), which suggests that the CA1-subiculum circuit mediates spatial novelty. Intriguingly, the subiculum not only receives projections from the CA1 but also sends efferent input back to this region, forming reciprocal connections (Sun et al., 2014; Xu et al., 2016). In this subiculum-CA1 circuit, chemogenetic inhibition of the subiculum excitatory neurons before the sample trial did not affect NOP memory after a 24 h delay. Strikingly, the same chemogenetic inhibition of the subiculum-CA1 circuit impaired long-term (24 h) NLP memory. Furthermore, optogenetic excitation of a subpopulation of subiculum neurons, which selectively project to CA1, potentiated object location memory after a 24 h delay (Sun et al., 2019). These findings indicate that the subiculum–CA1 circuit is bidirectionally recruited for spatial, but not non-spatial, processing of long-term object memory. Inhibition of the excitatory neurons in the dorsal HPC after the sample trial impaired NLP memory tested 4 h later (Tuscher et al., 2018). Both excitation and inhibition of GABAergic neurons in the dorsal HPC after the sample trial impaired object location memory after a 24 h delay (Yu et al., 2018). Thus, both excitatory and inhibitory neurons in the dorsal HPC are involved in the consolidation of information about object location. Also, the dorsal dentate gyrus in the HPC is engaged in the retrieval of object location memory. Excitation or inhibition of glutamatergic neurons as well as excitation of granule neurons in the dorsal dentate gyrus before the test trial impaired NLP memory after a 24 h delay (Kahn et al., 2019). Such a function of the dentate gyrus can be modulated by the input from the supramammillary nucleus (SuM) of the hypothalamus. Optogenetic excitation and inhibition of the SuM axonal terminals located at the dentate gyrus enhanced and disrupted the retrieval of NLP memory, respectively (Li et al., 2020a). By contrast, such manipulations did not affect the encoding of NLP memory. Thus, the hypothalamic SuM, particularly via its glutamate neurotransmission, can mediate the dentate gyrus-dependent recall of object location memory (Li et al., 2020a).
The role of the ventral HPC in object location memory remains unclear. Inhibition of the ventral HPC excitatory neurons impaired NLP memory (Wang et al., 2018), but see (Kahn et al., 2020). The discrepancy might arise from differences in experimental settings between the individual studies.
4.1.3. Memory for object-place association
Lesions of the HPC impaired the OiP performance in rats (Barker et al., 2007; Good et al., 2007b; Barker and Warburton, 2011, 2015), which can be explained by the processing of spatial signals and by the formation and representation of the object-place association in the HPC (Cowell et al., 2010). Either way, the HPC underlies recognition memory when the information involves a spatial component (Barker and Warburton, 2011).
4.2. Medial prefrontal cortex
4.2.1. Memory for object
Although earlier studies using delayed nonmatching to sample tasks implied the involvement of PFC (including anterior cingulate cortex [ACC], ventral mPFC, prelimbic cortex [PL] and infralimbic cortex [IL]) in object recognition memory (Kolb et al., 1994; Kesner et al., 1996; Ragozzino et al., 2002), lesions of the mPFC did not affect the performance in the NOP test (Ennaceur et al., 1997; Mitchell and Laiacona, 1998; Yee, 2000; Barker et al., 2007; Barker and Warburton, 2011; Cross et al., 2013). The inconsistent findings could result from differences in lesion methods (damages to passing fibers or not) as well as in experimental paradigms (reinforcement vs. spontaneous behaviors). Hence, it may be stated that the mPFC is not necessarily recruited in the processing of NOP memory.
Pharmacological inhibition (muscimol) on the mPFC after the sample trial disrupted NOP memory (with a 24 h delay), indicating the mPFC plays a role in the storage of object memory (de Landeta et al., 2021). Chemogenetic inhibition of the mPFC glutamatergic neurons after the sample trial impaired long-term (24 h) NOP memory (Tuscher et al., 2018), indicating that these excitatory neurons are involved in the long-term consolidation of object information. In contrast, optogenetic excitation of the mPFC glutamatergic neurons after the sample trial did not affect short-term (5 min) NOP memory (Benn et al., 2016). These findings are not necessarily contradictory because different species (rat vs. mouse), sexes (male vs. female), methods (optogenetic excitation vs. chemogenetic inhibition), and inter-trial intervals (min vs. hours) were employed. In addition, the stress-relevant glucocorticoid hormone in the PL modulates the consolidation of NOP memory, as intra-PL infusions of a glucocorticoid receptor agonist and antagonist after the sample trial facilitated and impaired NOP memory (24 h), respectively (Barsegyan et al., 2019). Thus, it may be inferred that the mPFC engages in the consolidation of object memory under certain conditions and that excitatory neurons in the mPFC play a major role.
4.2.2. Memory for place
In several studies, no involvement of mPFC was observed in NLP performance in rats (Ennaceur et al., 1997; Barker et al., 2007; Cross et al., 2013). Also, optogenetic stimulation of glutamatergic neurons in the mPFC did not influence short-term (5 min) NLP consolidation in rats (Benn et al., 2016). In mice, however, chemogenetic inhibition of the mPFC glutamatergic neurons impaired spatial memory consolidation (4 h) in the NLP test (Tuscher et al., 2018). Intra-PL infusions of a glucocorticoid receptor agonist or antagonist after the sample trial enhanced or disrupted NLP (24 h) memory, respectively (Barsegyan et al., 2019), suggesting that glucocorticoid receptor action in the PL bidirectionally mediates the consolidation of long-term NLP memory. Overall, the mPFC is not required for short-term object location memory, but to some degree, for the long-term consolidation of spatial information.
4.2.3. Memory for object-place association
Lesions and pharmacological inactivation of glutamatergic and cholinergic systems in the rodent mPFC caused impairments in the OiP test (Bussey et al., 2000; Barker and Warburton, 2009, 2015; Sabec et al., 2018; Chao et al., 2020), whereas no significant deficits were observed in the NOP and NLP paradigms.
Optogenetic stimulation of the mPFC glutamatergic neurons after the sample trial potentiated OiP performance, when tested 5 min later (Benn et al., 2016), suggesting that these neurons facilitate the stored information about the object-place association. This indicates that the mPFC is required for OiP memory and excitatory neurons in the mPFC are involved in the consolidation of the associative memory for object and place.
4.3. Perirhinal cortex
4.3.1. Memory for object
The PRC, which reciprocally connects with the HPC and mPFC in rats (Burwell et al., 1995; Burwell and Amaral, 1998b), has been proposed to account for the processing of object/item perception and recognition memory (Winters et al., 2008). Glutamatergic and cholinergic neurons in the PRC are considered to play a major role (Warburton et al., 2003; Warburton et al., 2013; Warburton and Brown, 2015). Lesions or pharmacological inactivation of the PRC impaired object recognition in humans (Buffalo et al., 1998), monkeys (Zola-Morgan et al., 1989; Gaffan, 1994) and rats (Ennaceur et al., 1996; Ennaceur and Aggleton, 1997; Bussey et al., 1999; Kesner et al., 2001; Hannesson et al., 2004; Winters et al., 2004; Hannesson et al., 2005; Winters and Bussey, 2005; Barker et al., 2007; Bartko et al., 2007b; Winters and Reid, 2010; Barker and Warburton, 2011; Chao et al., 2016a). The PRC is required for the encoding, consolidation and retrieval of NOP memory (Winters and Bussey, 2005), particularly when the memory trace is weak (Cinalli et al., 2020). Lesions of the PRC affected object recognition, especially after a long, but not after a short (seconds) time delay (Wiig and Burwell, 1998; Buffalo et al., 1999; Winters et al., 2004), but see (Bartko et al., 2007a; Albasser et al., 2015). PRC-lesioned rats also showed deficits in short-term object recognition, when the presented objects were similar (Norman and Eacott, 2004; Bartko et al., 2007a). Lesions of the PRC disrupted object recognition memory for both 2D and 3D items (Meunier et al., 1993; Murray and Richmond, 2001; Prusky et al., 2004). Moreover, damage to the PRC impaired object recognition when visual information was available, whereas olfactory and tactile recognition remained intact when studies were conducted in the dark (Albasser et al., 2011). Lesions or pharmacological inactivation (lidocaine) of the PRC also impaired the performance in a modified object exploration paradigm, which required the animals in the learning and test trials to use either tactile or visual information (exploration of objects under red light [tactile mode] in the learning trial and exploration of objects barred with transparent barriers [visual mode] in the test trial, or vice versa (Winters and Reid, 2010; Jacklin et al., 2016). In addition, PRC-lesioned rats showed impaired object recognition when the stimuli to be discriminated had many features in common (Bartko et al., 2007a). Expression of activity-regulated cytoskeleton-associated protein (Arc), an IEG serving as a marker for neuronal activation, was enhanced in the PRC area 35 (layer III to V) during exploring both landmark- and subtle-featured objects, while the PRC area 36 responded specifically to subtle-featured objects (Sethumadhavan et al., 2022). Thus, the PRC might encode the information on different object features in a layer-specific manner. These findings suggest that the PRC is important for the perceptual processing of complex information with the input from visual and tactile systems. This is in line with the widespread association between the PRC and neocortical areas (Bota et al., 2015).
In this context, it is worth mentioning that the PRC and postrhinal cortex play crucial parts in 2D object discrimination (Meunier et al., 1993; Prusky et al., 2004; Furtak et al., 2012). Activation of a portion of postrhinal cortical neurons with a constitutive protein kinase C (PKC), which promotes visual object discrimination, facilitated the encoding of newly learned visual stimuli (Zhang et al., 2005; Zhang et al., 2010; Zhang et al., 2019). The enhancement of visual learning and memory by PKC activation was attenuated by silencing the postrhinal circuits connected with the PRC or ventral temporal association area (Nagayach et al., 2021). Thus, both circuits (postrhinal cortex-PRC and -ventral temporal association area) are relevant for visual object learning and memory. The exploration of 3D objects increased the level of Arc mRNA in both the PRC (caudal area 35) and the caudal postrhinal cortex (Sethumadhavan et al., 2020).
Optogenetic and chemogenetic manipulation of PRC neurons can tune the signals of object familiarity and novelty in the context of visual recognition memory. In the test trial of a 2D object recognition test, 10-15 Hz optogenetic excitation of the PRC neurons coupled with the presentation of a novel image decreased the animal’s preference for novelty, while it had no effect when coupled with the presentation of a familiar image. Thus, the 10-15 Hz stimulation made the novel image appear like a familiar one. In contrast, 30-40 Hz stimulation of the PRC neurons coupled with the presentation of a familiar image in the sample trial increased the animal’s exploration of the familiar image, while it had no effect when coupled with the presentation of a novel image. Thus, in this setting, the 30-40 Hz stimulation made the animal treat the familiar image as a novel one (Ho et al., 2015). From this follows that the PRC regulates visual recognition memory in a frequency-dependent manner. In addition, pre-test chemogenetic excitation of the PRC neurons promoted retrieval in the NOP test after a delay of 7 days, which was not the case without stimulation, indicating that the activation of PRC neurons is sufficient to “restore” long-term object recognition memory (Nomura et al., 2019). More studies are needed to clarify how different subtypes of PRC neurons are involved in recognition memory.
4.3.2. Memory for place
Lesions of the PRC did not impair performance in the NLP test in rats (Barker et al., 2007; Barker and Warburton, 2011), consistent with the findings of the PRC playing a minor role in spatial processing (Wiig and Bilkey, 1994; Glenn and Mumby, 1998; Liu and Bilkey, 2001; Winters et al., 2004; Ramos, 2013). This is also in line with the double dissociation or predominant view that the HPC and PRC are mainly processing spatial and non-spatial information, respectively (Aggleton et al., 1997; Bussey et al., 1999; Winters et al., 2004; Chao et al., 2016a).
4.3.3. Memory for object-place association
The PRC processes associative object-place information together with the HPC and mPFC in the OiP test. In line with the essential role of the PRC in NOP memory, lesioning or pharmacological interventions in this region affected OiP memory (Barker et al., 2007; Barker and Warburton, 2008, 2011). Moreover, PRC lesions had mild yet significant effects on other spatial memory paradigms (Mumby and Glenn, 2000; Liu and Bilkey, 2001; Ramos and Vaquero, 2005; Abe et al., 2009), indicating that the PRC may support certain forms of spatial capabilities.
When contextual features are altered (Eacott and Norman, 2004; Barker and Warburton, 2020b), the PRC interacts with the postrhinal cortex in the processing of object exploration (Heimer-McGinn et al., 2017). As a result, the observed memory deficits for the object-place association in the PRC-lesioned rats may be accounted for by deficient processing of information related to the inter-object change of locations, rather than by an impairment of unitary object recognition.
4.4. Entorhinal cortex
The EC receives afferents from the HPC and, conversely, is a major source of (both excitatory and inhibitory) efferent projections to the HPC (Melzer et al., 2012). Importantly, the EC together with the postrhinal cortex mediate impulse traffic between the neocortex and HPC (de Curtis and Pare, 2004). Lesions of the EC affected the outcome in various versions of NOP and NLP tests. Different subdivisions of the EC (e.g., the lateral vs. medial compartment) were involved and the test results were determined by the complexity of environmental stimuli (Rodo et al., 2017). The lateral entorhinal cortex (LEC) receives most of its afferent from the PRC, insular cortex and mPFC (PL and IL), while the medial entorhinal cortex (MEC) receives projections mainly from the postrhinal, occipital and parietal cortices (Burwell and Amaral, 1998b, a; Witter et al., 2000). Electrophysiological recordings showed that the LEC neurons responded to objects in context (Fyhn et al., 2004; Deshmukh and Knierim, 2011, 2013; Tsao et al., 2013) and time (Tsao et al., 2018), while MEC neuronal firing correlated predominantly with spatial modularity (Fyhn et al., 2004; Hafting et al., 2005). Therefore, the LEC and MEC may be responsible for the performance in the NOP (non-spatial) and NLP (spatial) tests, respectively. Yet, LEC and MEC can operate synergistically (Save and Sargolini, 2017; Chao et al., 2020) and be both recruited for the processing of spatial information (Neunuebel et al., 2013; Knierim et al., 2014).
4.4.1. Memory for object
Lesions of the LEC did not cause deficits in the classic NOP test (Van Cauter et al., 2013; Wilson et al., 2013b; Wilson et al., 2013a; Kuruvilla and Ainge, 2017). However, NOP memory in LEC-lesioned rats was disrupted if three or four distinct objects were used as stimuli (Hunsaker et al., 2013; Kuruvilla and Ainge, 2017). Furthermore, the LEC was recruited in a memory-load-dependent manner in a delayed nonmatch-to-sample task based on 5 or 10 odors (Ku et al., 2017). The LEC sends GABAergic projections targeting hippocampal neurons. Optogenetic inhibition of these GABAergic terminals in the dorsal HPC attenuated NOP memory after a 10 min-delay, suggesting that the processing of NOP memory in the dorsal HPC is gated by GABAergic signals from the LEC (Basu et al., 2016). Silencing of the LEC fan cells also impaired object recognition when the location and context of objects were altered (Vandrey et al., 2020), suggesting that the LEC fan cells are necessary to link object information with environmental cues. Lesions of the MEC, on the other hand, did not affect the performance in the NOP test, irrespective of the type of objects (identical or distinct) (Hunsaker et al., 2013; Van Cauter et al., 2013; Hales et al., 2014; Kuruvilla and Ainge, 2017; Hales et al., 2018). However, pharmacological inhibition (muscimol) of the MEC after the sample trial disrupted long-term (24 h) NOP memory, suggesting that the MEC contributes to the consolidation of object memory (de Landeta et al., 2021). Hence, the LEC, but not MEC, is required for the processing of associative memory for distinct objects (inter-object relationship), while neither region is obligatory for single item recognition per se (yet the involvement of MEC in consolidation of NOP memory).
4.4.2. Memory for place
Lesions of the LEC did not affect performance in the classic NLP test (Van Cauter et al., 2013; Wilson et al., 2013a). However, the LEC seemed to be involved in spatial recognition when distinct objects were used as stimuli in the sample trial (Van Cauter et al., 2013; Wilson et al., 2013a; Kuruvilla and Ainge, 2017), but see (Rodo et al., 2017). Likewise, lesions of the MEC did not affect the performance in the NLP test (Hales et al., 2014), but disrupted spatial recognition if numerous distinct objects were presented (Van Cauter et al., 2013; Rodo et al., 2017). Interestingly, several subtypes of MEC neurons are involved in the processing of NLP information. Inactivation of the stellate cells with TeLC in the MEC disrupted NLP (but not NOP) memory, indicative of a role of these neurons in the processing of object location (Tennant et al., 2018). It should be noted that only a portion of stellate cells belonged to grid cells (~25%), suggesting that some stellate cells in the MEC might have functions different from grid cells (Rowland et al., 2018). In sum, both LEC and MEC mediate spatial recognition when the diversity of objects is increased, while the MEC stellate cells are specifically required for the processing of spatial information.
Given these roles of the LEC and MEC in object and place recognition (Rodo et al., 2017), the OiP performance is likely dependent on both regions, although direct evidence is lacking.
4.5. Retrosplenial cortex
The RSC has received attention in studies of memory (Aggleton, 2010; Kaboodvand et al., 2018; Sato, 2021) because it has dense inter-connections with the anterior thalamic nucleus (ATN), PFC, HPC and parahippocampal regions (Wyss and Van Groen, 1992; Van Groen and Wyss, 2003). Moreover, it is implied in contextual memory (Keene and Bucci, 2008; Corcoran et al., 2011; Katche et al., 2013), spatial and goal-directed navigation (Vann and Aggleton, 2002; Vedder et al., 2017), sensory preconditioning (Robinson et al., 2014) and systems consolidation (de Sousa et al., 2019).
4.5.1. Memory for object
No significant effects on NOP memory were observed in RSC-lesioned rats (Ennaceur et al., 1997; Vann and Aggleton, 2002; Powell et al., 2017; Hayashi et al., 2020). However, when the objects were presented either in the dark (tactile/olfactory mode) or in the light behind a transparent barrier (visual mode) in the sample trial, followed by a testing trial with cross-modal combinations (cue conditions switched from dark to light or vice versa), lesions in the dysgranular RSC selectively impaired cross-modal recognition when cue conditions switched from dark to light between the initial sampling and testing (Hindley et al., 2014). When both granular and dysgranular RSC were lesioned, the impairment was no longer restricted to the transfer of information from tactile/olfactory to visual mode. This suggests a role of the RSC in the integration of multiple sensory information.
Either post-sample or pre-test, but not pre-sample, microinjections of muscimol, a selective GABAA receptor agonist, into the anterior RSC disrupted NOP memory after a 24 h, but not after a 3 h, delay (de Landeta et al., 2020). Combined pre- and post-sample muscimol infusions, however, did not influence NOP memory (de Landeta et al., 2020). Thus, the anterior RSC may be involved in long-term NOP consolidation and/or retrieval. Furthermore, the anterior RSC interacts with other brain regions in the establishment of NOP consolidation. Unilateral muscimol inhibition of the anterior RSC plus of the mPFC, PRC, ATN or MEC in the same hemispheres after the sample trial disrupted long-term (24 h) NOP memory in rats. Likewise, the consolidation was impaired by unilateral inhibition of the anterior RSC plus that of the ACC in the opposite hemispheres. The crosstalk between the anterior RSC and dorsal HPC was not involved in this processing (de Landeta et al., 2021). These findings demonstrate that the anterior RSC together with the mPFC, PRC, ATN, MEC, and particularly the ACC, are required for the consolidation of NOP memory. It remains to be explored how exactly the individual portions of the RSC (anterior and posterior) contribute to object recognition.
4.5.2. Memory for place
Lesions in the RSC appear to affect spatial recognition under certain conditions. Extensive lesions of RSC plus ACC disrupted the performance in the NLP test (Ennaceur et al., 1997), while lesions of the RSC alone failed to do so (Hayashi et al., 2020). Moreover, spatial recognition was impaired in RSC-lesioned rats, when distinct objects were presented during the sample trial (Parron and Save, 2004). Hence, like the EC, the RSC might play a role in spatial processing.
Post-sample muscimol infusions into the anterior RSC disrupted long-term (24 h) NLP memory (de Landeta et al., 2020), suggesting that the anterior RSC is crucial for the consolidation of spatial information.
4.5.3. Memory for object-place association
The impaired performance of RSC-lesioned rats in the OiP test (Vann and Aggleton, 2002) reflects that this region is relevant for object-place associative memory. This result is in line with the role of the RSC in location recognition when multiple distinct objects were present (Parron and Save, 2004). The default mode network involving the interaction of cortical (including the posterior cingulate cortex and RSC) and subcortical areas (including the medial temporal lobe) is relevant for episodic memory. The relevance of the RSC in episodic memory indicates its role as a memory gateway between the cortical default mode network and the medial temporal lobe (Kaboodvand et al., 2018).
4.6. Insular cortex
The insular cortex is engaged in the recognition of taste as well as in social and object memory (Bermudez-Rattoni, 2014; Cavalcante et al., 2017). The insular cortex reciprocally connects with other cerebral cortices (e.g., the mPFC) and subcortical regions (e.g., the thalamus), while its connectivity with subcortical regions is often unidirectional (predominant cortical-to-subcortical or subcortical-to-cortical projections). In addition, different input-output connections have been demonstrated between the anterior and medial-posterior insular cortex, suggesting functional distinction between the two subdivisions (Gehrlach et al., 2020).
4.6.1. Memory for object
Post-sample microinjections of scopolamine, a muscarinic cholinergic receptor antagonist, into the insular cortex impaired NOP memory after a 24 h delay (Bermudez-Rattoni et al., 2005), indicating the involvement of insular cortical cholinergic systems in the storage of object memory. Likewise, infusions of anisomycin, a protein synthesis blocker, into the insular cortex disrupted long-term (24 h), but not short-term (1.5 h), NOP memory (Balderas et al., 2008). Inhibition of histone deacetylase (HDAC) promoted the expression of brain-derived neurotrophic factor (BDNF) (Sada et al., 2020), which is associated with neuroprotection, synaptic plasticity and cognition (Brigadski and Leßmann, 2020). Microinjections of sodium butyrate, a HDAC inhibitor, into the anterior insular cortex after the sample trial facilitated NOP memory (Roozendaal et al., 2010; Chen et al., 2018), implying that NOP consolidation may be related to the activation of BDNF signaling (Ramirez-Mejia et al., 2021). The anterior insular cortex also interacts with the PL glucocorticoid receptors in the consolidation of NOP memory, in which pharmacological inhibition (PD98059) of the anterior insular cortex blocked the facilitation of NOP (24 h) memory induced by intra-PL infusions of a glucocorticoid receptor agonist after the sample trial (Barsegyan et al., 2019). These findings suggest that the insular cortex is essential for the consolidation of long-term object recognition memory with the engagement of cholinergic neurotransmission, histone acetylation and PL glucocorticoid receptor action.
4.6.2. Memory for place
Post-sample infusions of sodium butyrate into the anterior insular cortex did not affect NLP memory (Roozendaal et al., 2010; Chen et al., 2018), consistent with an earlier study reporting no significant effects on recognizing objects in different contexts after post-sample infusion of anisomycin into the insular cortex (Balderas et al., 2008). Thus, the insular cortex may not be involved in the storage of spatial memory for objects.
The role of the insular cortex in the OiP performance has not yet been investigated.
4.7. Thalamus
The thalamus is a key structure for cognitive functions (Wolff and Vann, 2019). It comprises various segregated nuclei receiving distinct afferents and sending widespread efferents throughout the brain and can be categorized into hierarchical modalities, which receive and relay peripheral and subcortical (first-order) or cortical (higher-order) information (Guillery, 1995; Sherman, 2007). The reticular nucleus regulates thalamic functions via inhibition of first-order and higher-order thalamic nuclei distinctly (Li et al., 2020b). Among them, ATN, mediodorsal thalamic nucleus (MD) and nucleus reuniens (NR) are associated with recognition memory.
4.7.1. Memory for object
The ATN is strongly interconnected with the dorsal subiculum (Meibach and Siegel, 1977; Christiansen et al., 2016) and the HPC (Wolff et al., 2015). ATN lesions did not have significant effects on single item recognition memory (Parker and Gaffan, 1997; Wilton et al., 2001; Mitchell and Dalrymple-Alford, 2005; Dumont and Aggleton, 2013; Nelson and Vann, 2014), indicating that the formation of NOP memory does not necessarily involve the ATN. However, the ATN is involved in NOP consolidation as pharmacological inhibition (muscimol) on the ATN after the sample trial impaired the NOP memory (de Landeta et al., 2021).
The MD is reciprocally connected with the PFC and is important for PFC-dependent cognitive functions (Funahashi, 2013; Halassa and Kastner, 2017; Parnaudeau et al., 2018). Lesions in the MD impaired recognition memory in humans and non-human primates (Aggleton and Mishkin, 1983; Zola-Morgan and Squire, 1985; Parker et al., 1997; Isaac et al., 1998). Also, deficits in a delayed nonmatching-to-sample task were exhibited in MD-lesioned rats (Mumby et al., 1993), although the NOP performance was unaffected by MD lesions (Kornecook et al., 1999; Mitchell and Dalrymple-Alford, 2005; Cross et al., 2013). Like the mPFC, the MD is not required for the NOP test.
The NR is connected with the PFC, HPC and PRC (Hoover and Vertes, 2012), which highlights its role in the modulation of PFC and HPC functions. Lesions, pharmacological inactivation (muscimol) or optogenetic excitation of the NR resulted in deficits in spatial working memory (Hallock et al., 2013; Duan et al., 2015), contextual fear memory (Ramanathan et al., 2018) and spatial memory (Loureiro et al., 2012). NR-lesioned rats did not display impaired performance in the NOP test (Barker and Warburton, 2018), indicating that the NR is not directly involved in the network of NOP memory.
4.7.2. Memory for place
As noted, the ATN is anatomically and functionally associated with the HPC, particularly with the dorsal subiculum (Nelson et al., 2020; Frost et al., 2021). The ATN fired synchronously with the HPC in theta rhythm (Vertes et al., 2001), and linked to HPC-dependent spatial learning and memory (Warburton et al., 1997; Warburton and Aggleton, 1999; Mitchell and Dalrymple-Alford, 2006; Jankowski et al., 2013). Consistently, chemogenetic inhibition on the ATN or its axonal terminals located in the dorsal subiculum impaired spatial working memory (Nelson et al., 2020). Lesions in the ATN also abrogated spatial firing (responding to place, head-direction, border and grids) in the subiculum, while not greatly affecting the HPC CA1 place cells (Frost et al., 2021), but lesions in the mammillothalamic tract innervating the ATN altered oscillatory theta and gamma activities in the HPC (Dillingham et al., 2019). Disconnecting fornix (HPC commissure) and ATN impaired performance in the NLP, but not in the NOP, test, suggesting that the functional association between ATN and HPC is important for the processing of object-location (Warburton et al., 2000; Okada and Okaichi, 2006). Still, the role of the ATN in NLP memory remains to be elucidated.
MD lesions in rats did not affect NLP performance either after a 5 min or after a 3 h delay (Cross et al., 2013), indicating that the MD is not relevant for the processing of object-location memory.
In NR-lesioned rats, no effects were observed on NLP test performance after a 3 h delay (Barker and Warburton, 2018). In contrast, another study in NR-lesioned mice showed an impairment of the NLP performance after a 1 h delay and altered firing patterns of CA1 place cells upon a change of environment cues (Jung et al., 2019). This inconsistency could be due to differences in species (rat vs. mouse), lesion methods (excitotoxic vs. electrolytic), lesion extent and experimental setup. On the other hand, chemogenetic inhibition of the NR glutamatergic neurons, either before or after the sample trial, impaired NLP memory (with a 4 h delay) in female mice (Schwabe et al., 2021), suggesting the NR is involved in the encoding and consolidation of object-location memory. Thus, it may be inferred that the NR participates in the processing of information on object location under certain conditions.
4.7.3. Memory for object-place association
Lesions of the ATN impaired OiP memory (Parker and Gaffan, 1997; Wilton et al., 2001). Several studies have shown a positive correlation between memory retrieval and hippocampal theta oscillations (Klimesch, 1999). The ATN was related to theta synchrony (Ketz et al., 2015), which, in particular, may indicate a role of the ATN-HPC/mPFC pathway in forming and retrieving complex associations between object and place.
Similar to the effects of mPFC lesions, MD-lesioned animals exhibited an impairment of OiP memory (Cross et al., 2013), suggesting that the MD processes complex mnemonic information. This is consistent with clinical studies, showing that patients with MD stroke exhibited deficits in recollection, but not in the perception of familiarity (Danet et al., 2017). Therefore, the MD likely participates in the mPFC-relevant circuits for the memory of object-place associations (Cross et al., 2013; Browning et al., 2015; Wolff et al., 2015).
Either pre-sample or pre-test infusions of muscimol into the NR disrupted OiP performance in rats after a 3 h, but not after a 5 min, delay. Infusion of the protein synthesis inhibitor anisomycin perturbed OiP memory tested after a 24 h, but not after a 3 h, delay (Barker and Warburton, 2018). These findings suggest that, firstly, the NR mediates the formation and retrieval of long-term, but not short-term, object-place associative memory; secondly, protein synthesis in the NR is required for long-term OiP memory. Taken together, the NR may coordinate the information flow between PFC and HPC (Ferraris et al., 2018; Hauer et al., 2019).
4.8. Hypothalamus
Despite its early implication in Alzheimer’s disease and memory (Schwartz and Teitelbaum, 1974; Saper and German, 1987; Adamantidis and de Lecea, 2009), the hypothalamus has been largely underrated as a neuronal substrate for object recognition.
4.8.1. Memory for object
A study with fiber photometry demonstrated that Ca2+ signals from the neurons expressing melanin-concentrating hormone (MCH) in the lateral hypothalamus responded specifically to novel, but not familiar, objects in freely behaving mice (Kosse and Burdakov, 2019). Moreover, optogenetic inhibition of the hypothalamic MCH neurons during the sample trial disrupted NOP memory (Kosse and Burdakov, 2019). Hence, the lateral hypothalamus may be important for the encoding of object novelty.
4.8.2. Memory for place
The hypothalamic SuM, which sends strong inputs to the hippocampal dentate gyrus, increasingly responded during the NLP retrieval phase. Furthermore, the neuronal response of SuM synchronized the hippocampal dentate gyrus activity in the NLP test, measured with in vivo Ca2+ fiber photometry (Li et al., 2020a). Thus, the neuronal correlation between the SuM and dentate gyrus may indicate their impacts on spatial memory retrieval. Pre-test chemogenetic excitation and inhibition of SuM neurons facilitated and impaired NLP memory, respectively. Such manipulations, in contrast, did not affect performance in the NOP test (Li et al., 2020a). The fact that the SuM can bidirectionally mediate the retrieval of object location memory highlights the role of the SuM-dentate gyrus circuit in spatial memory (Li et al., 2020a).
The role of the hypothalamus in object-place associative memory has not been examined.
4.9. Interpeduncular nucleus
The IPN is primarily a GABAergic area located in the midbrain. It receives dense projections from the medial habenula and sends efferents to a variety of brain regions, including the lateral hypothalamus, lateral habenula, ventral tegmental area (VTA) and HPC (Herkenham and Nauta, 1979).
4.9.1. Memory for object
Despite being recognized as a site relevant for mood regulation (McLaughlin et al., 2017), the IPN was implied in novelty preference behavior. In tests with both social (living mice) and non-social (unmovable object) stimuli, the IPN responded to exposure to familiar, but not novel, stimuli (Molas et al., 2017). Optogenetic excitation of interpeduncular GABAergic neurons decreased exploration of the novel object during the test trial in the NOP test, whereas optogenetic inhibition of these neurons increased exploration of the familiar object (Molas et al., 2017). This study suggests that the IPN encodes signals of novelty suppression in a bidirectional manner.
It remains to be determined whether the IPN participates in the NLP or OiP memory.
4.10. Striatum
4.10.1. Memory for object
Striatum and striatal dopamine (DA) contribute profoundly to object memory. Although lesions of the dorsal striatum did not affect object recognition, object memory was impaired when geometric cues were changed (Poulter et al., 2020) or when the two familiar objects were simultaneously replaced by novel ones (Korol et al., 2019). In addition, inactivation with lentivirus mediated TeLC in the dorsal medial striatum, but not in the NAc, impaired long-term (24 h) NOP memory (Qiao et al., 2017). Pre-sample infusions of an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX) or N-methyl-D-aspartate (NMDA) receptor antagonist 2-amino-5-phosphonopentanoic acid (AP5) into the NAc disrupted the performance of object recognition (Usiello et al., 1998; Sargolini et al., 2003), but see (Sargolini et al., 1999; Coccurello et al., 2012). Also, infusions of AP5, but not DNQX, into the NAc after the sample trial impaired the object recognition test (Sargolini et al., 2003), suggesting that the consolidation of object memory is mediated by NMDA, but not by AMPA, receptors in the NAc.
The striatal mediation of recognition memory likely also engages GABAergic medium spiny neurons, which comprise about 95% of striatal innervation (Tepper and Bolam, 2004) with nearly each medium spiny neuron receiving dopaminergic input from the VTA (Yao et al., 2008). NOP memory was impaired in mice after intra-striatal application of neurotoxic 6-hydroxydopamine (6-OHDA) (Masini et al., 2017), but see (Zurkovsky et al., 2013). Likewise, NOP memory was inhibited by injections of 6-OHDA into the rat NAc (Nelson et al., 2010). DA administered into the dorsolateral striatum of DA-deficient mice counteracted their deficits in NOP memory (Darvas and Palmiter, 2009, 2010), suggesting that the striatal DA is both necessary and sufficient for object recognition memory (De Leonibus et al., 2005; Darvas and Palmiter, 2009; Coccurello et al., 2012).
Collectively, the dorsal striatum is involved in object recognition under certain conditions, while the NAc plays a key role in the consolidation and perhaps in the formation of object recognition memory.
4.10.2. Memory for place
Application of 6-OHDA to the rat NAc induced NLP deficits (Nelson et al., 2010). Also, neuronal inactivation with TeLC in the dorsal medial striatum of mice disrupted the spatial recognition (Qiao et al., 2017), suggesting that the dorsal medial striatum is involved in object-location memory. Likewise, infusions of DNQX or AP5 into the NAc disrupted the performance of spatial novelty (Sargolini et al., 1999; Coccurello et al., 2012). Moreover, infusions of sub-effective doses of AP5+SCH23390 (a DA D1 receptor antagonist) or DNQX+sulpiride (a DA D2 receptor antagonist) into the NAc impaired the spatial novelty test as well (Coccurello et al., 2012). Thus, spatial recognition is mediated by co-activation of NMDA/DA D1 and AMPA/DA D2 receptors in the NAc.
Infusions of AP5 into the dorsal striatum after the sample trial impaired spatial recognition, when animals were placed into the arena at the same location, but not at a different location (De Leonibus et al., 2005). This suggests that the dorsal striatum is involved in the storage of spatial information by an egocentric, but not an allocentric, strategy. In contrast, microinjections of AP5 into the NAc impaired the consolidation of spatial recognition irrespective of the animal’s entering points (De Leonibus et al., 2005), which implied that the NAc, in contrast to the dorsal striatum, stores spatial information with both egocentric and allocentric strategies. Interestingly, the consolidation of spatial memory was dependent on activation of the NMDA, but not AMPA/kainate, receptor in the NAc, whereas the opposite was found concerning memory retrieval (Roullet et al., 2001). Since inactivation of the NAc and the contralateral ventral subiculum impaired the consolidation of spatial memory, it can be inferred that the interhemispheric connection between the NAc and ventral subiculum is required for this function (Torromino et al., 2019).
In sum, both dorsal striatum and NAc are involved in the mediation of spatial information on objects. NMDA receptor activation in the NAc is essential for the consolidation of object-location information, while AMPA receptor activation is important for the retrieval of spatial information.
4.11. Medial septum
The MS/MSDB sends projections to the HPC (Mesulam et al., 1983; Senut et al., 1989) and PFC (Henny and Jones, 2008). It coordinates the hippocampal theta rhythmic activity via acetylcholine (Lee et al., 1994; Vandecasteele et al., 2014; Dannenberg et al., 2015) and influences HPC-dependent spatial learning and memory (Everitt and Robbins, 1997; Solari and Hangya, 2018; Dashniani et al., 2020), including context-place memory (Easton et al., 2011).
4.11.1. Memory for object
Selective lesions of cholinergic neurons in the MS did not affect the recognition of different objects (Cai et al., 2012; Dashniani et al., 2015; Okada et al., 2015). Also, inactivation of the MSDB cholinergic neurons with TeLC did not affect the NOP performance, but social memory (Pimpinella et al., 2021). Pre-test chemogenetic inhibition of MS neurons that project to the dorsal HPC or ACC did not affect the outcome of the NOP test (Jin et al., 2020), indicating that the MS–HPC and MS–ACC circuits are not required for the retrieval of object memory. Hence, the MS/MSDB does not directly contribute to object recognition memory.
4.11.2. Memory for place
As mentioned earlier, the MS interacts with the HPC in mediating spatial learning and memory. Lesions of cholinergic neurons in the MS impaired spatial recognition of objects (Cai et al., 2012; Okada et al., 2015), but see (Dashniani et al., 2015). These findings were consistent with the effects of cholinergic lesions in the MS and nucleus basalis of Meynert (Paban et al., 2005). However, since the MS was involved in the regulation of exploratory behavior (Köhler and Srebro, 1980; Poucet, 1989; Gangadharan et al., 2016), the results from object exploration paradigms must be interpreted with caution.
Chemogenetic inhibition of cholinergic neurons in the MSDB before the test trial impaired the NLP performance (Pimpinella et al., 2021). Also, chemogenetic inhibition of the MS–HPC, but not MS–ACC, circuit before the test trial disrupted object location memory (Jin et al., 2020). Thus, the MS–HPC, but not the MS–ACC, circuit is crucial for the recollection of NLP memory. Hence, the MS/MSDB cholinergic system, in close relation with the dorsal HPC, plays an intricate role in spatial memory.
4.11.3. Memory for object-place association
Pre-test inhibition of MS neurons that selectively connected with the dorsal HPC impaired the performance in the OiP test, whereas no detrimental effect was observed with pre-test inhibition of MS neurons that connected with the ACC (Jin et al., 2020), suggesting that the MS-dorsal HPC, but not MS-ACC, circuit is engaged in the retrieval of the object-place association. Since the MS/MSDB-HPC circuit is required for the NLP recall, this, at least, holds for the retrieval of the spatial component.
4.12. Amygdala
4.12.1. Memory for object
The amygdala is a crucial region for the modulation of emotion-arousal memories. Although the infliction of lesions (Moses et al., 2005) or the induction of pharmacological inactivation by anisomycin (Balderas et al., 2008) had no effects on NOP memory in rats, chemogenetic excitation and inhibition of CRH neurons in the central amygdala, respectively, impaired and improved NOP memory after a 1 h delay (Paretkar and Dimitrov, 2018). This suggests that the CRH neurons in the amygdala bidirectionally regulate NOP performance. As excitation and inhibition of CRH neurons in the amygdala also elicited anxiogenic and anxiolytic behaviors, respectively (Paretkar and Dimitrov, 2018), the observed effects on NOP memory may be associated with different levels of anxiety.
Substantial evidence shows that the amygdala and the norepinephrine (NE) system act together to support NOP consolidation involving low emotion-arousal states. Infusions of NE or clenbuterol, a β-adrenoceptor agonist, into the rat basolateral amygdala (BLA) after the sample trial potentiated NOP memory function after a 24 h delay, whereas infusions of propranolol, a β-adrenoceptor antagonist, compromised NOP memory (Roozendaal et al., 2008; McReynolds et al., 2014; Beldjoud et al., 2015), but see (Mello-Carpes and Izquierdo, 2013). The enhancement of NOP consolidation induced by systemic administration of corticosterone was blocked by intra-BLA infusion of propranolol in rats, when they were tested under emotion-arousal conditions (without prior habitation to the testing environment) (Roozendaal et al., 2006). Conversely, intra-BLA microinjections of propranolol counteracted NOP deficits in rats with no previous habituation to the testing arena (Maroun and Akirav, 2008). These findings indicate that the β-adrenoceptor in the BLA modulates NOP memory depending on emotion-arousal levels. Since propranolol application in the BLA blocked the memory-enhancing effects of systemic or intra-insular cortex infusion of sodium butyrate (Chen et al., 2018), intra-BLA infusion of neuropeptide S (Han et al., 2014), and intra-mPFC (PL, but not IL) infusions of the glucocorticoid receptor agonist RU 28362 (Barsegyan et al., 2019), it may be inferred that β-adrenoceptor activation in the BLA is critical for NOP consolidation. Moreover, the NE system in the BLA interacted with the PL, anterior insular cortex, dorsal HPC and NR in the enhancement of glucocorticoid receptor-mediated object memory (Barsegyan et al., 2019). Intra-BLA propranolol infusions blocked the enhancing effects incurred by intra-PL infusions of RU 28362 after the sample trial in the NOP test (with a 24 h delay). Activation of the glucocorticoid receptor in the PL altered neuronal activity (c-Fos) in the anterior insular cortex, dorsal HPC and NR, which were “corrected” by intra-BLA propranolol infusion, suggesting that the NE system in the BLA is essential for enabling glucocorticoid actions on memory consolidation within the PL, anterior insular cortex, dorsal HPC and NR (Barsegyan et al., 2019).
The LC is one of the key regions of the NE system. Chemogenetic excitation of central amygdala neurons selectively projecting to the LC before the sample trial potentiated NOP memory after a 1 h delay (Paretkar and Dimitrov, 2018). Further, selective activation of the subpopulation of amygdala neurons linked to the LC was sufficient to enhance object recognition memory, indicating a significant role of the central amygdala-LC circuit in NOP memory (Paretkar and Dimitrov, 2018).
4.12.2. Memory for place
Similar to the effect on NOP memory, infusions of propranolol into the BLA abolished the facilitation of NLP memory induced by post-sample systemic or intra-insular cortex infusions of sodium butyrate (Chen et al., 2018) and intra-PL infusions of RU 28362 (Barsegyan et al., 2019). Intra-BLA propranolol infusions blocked the facilitation of NLP (24 h) memory induced by intra-PL infusions of a glucocorticoid receptor agonist after the sample trial (Barsegyan et al., 2019). It may be inferred that the BLA also regulates the consolidation of NLP memory via the NE system.
So far, no studies have been conducted on the function of the amygdala in OiP memory.
4.13. Locus coeruleus
The LC, located below the cerebellum and lateral to the fourth ventricle, is the source of norepinephrinergic innervation, which is responsible for a variety of behavioral and physiological functions (Benarroch, 2009; Schwarz and Luo, 2015). The LC has been considered a structurally and functionally homogeneous region for over 50 years. However, this concept is challenged by recent evidence that segregated clusters of efferent neurons in the LC may be related to distinct functions (Poe et al., 2020).
4.13.1. Memory for object
Post-sample microinjections of muscimol and NMDA (low dose) into the LC impaired or facilitated NOP memory, respectively (Mello-Carpes and Izquierdo, 2013; Mello-Carpes et al., 2016), indicating a role of the LC in bidirectional modulation of memory consolidation. Furthermore, chemogenetic inhibition of NE activity in the LC elevated α2 adrenoreceptor density, particularly in the EC, indicative of compensatory responses driven by the decrease of NE activity (Hamlett et al., 2020). Thus, the NE activity in the LC is critical for object recognition memory. Chemogenetic inhibition, but not excitation, of dopamine β-hydroxylase neurons in the LC impaired object recognition memory (Fortress et al., 2015; Hamlett et al., 2020). Although the LC-BLA circuit modulated anxiety-like and aversive behaviors induced by chronic pain (Llorca-Torralba et al., 2019), neither chemogenetic excitation nor inhibition of the nerve terminals in the BLA stemming from the tyrosine hydroxylase positive neurons in the LC influenced the performance in the NOP test. Thus, the tyrosine hydroxylase positive neurons in the LC are required for NOP memory, whereas their projections to the BLA are dispensable for NOP memory in normal conditions.
4.13.2. Memory for place
The LC sends norepinephrinergic (Poe et al., 2020) and dopaminergic (Kempadoo et al., 2016; Takeuchi et al., 2016) projections to the dorsal HPC. Optogenetic excitation of the LC dopaminergic terminals in the dorsal HPC facilitated NLP memory (Kempadoo et al., 2016). This effect depended on the hippocampal DA D1/5, but not β-adrenergic, receptors, indicating that the dopaminergic LC-dorsal HPC circuit is crucial for the enhancement of spatial signals encoded in the HPC.
The role of the LC in the OiP performance has not been examined.
4.14. Cerebellum
The cerebellum is important for executing smooth and continuous movements. Emerging evidence also suggests that the cerebellum is involved in non-motor functions such as the expectation of reward (Kostadinov et al., 2019; Sendhilnathan et al., 2020), social behavior (Carta et al., 2019; Chao et al., 2021), working memory (Deverett et al., 2019), fear conditioning (Ernst et al., 2019) and spatial navigation (Babayan et al., 2017; Locke et al., 2018; Lefort et al., 2019). The cerebellum directly projects to several subcortical regions, including the thalamus and VTA, thereby reaching multiple inter-connected cerebral cortices, e.g., the PFC (Wagner and Luo, 2020).
4.14.1. Memory for object
Neither optogenetic nor chemogenetic excitation of Purkinje neurons in the cerebellar vermis (lobules 4/5) affected performance in the NOP test in mice (Zeidler et al., 2020; Chao et al., 2021). However, chemogenetic activation of Purkinje neurons in the vermis lobule 4/5 disrupted social behaviors and social memory, associated with a disorganized modular structure of the brain network by the cerebellar perturbation (Chao et al., 2021). Thus, the cerebellum may participate in the complex mnemonic processing of social and emotional information (Hoche et al., 2016; Chao et al., 2021), but play a minimal role in object recognition.
4.14.2. Memory for place
Optogenetic stimulation of Purkinje neurons in lobule 4/5 hampered the retrieval of NLP memory in mice (Zeidler et al., 2020). The cerebellar manipulation altered Ca2+ activity in the hippocampal CA1 neurons, providing evidence for the cerebellar impact on the HPC-dependent spatial learning and memory (Rochefort et al., 2011; Lefort et al., 2019). Given that no significant monosynaptic projections from the cerebellum to the HPC have been identified (Bohne et al., 2019; Fujita et al., 2020), the cerebellum–HPC functional connection is likely mediated by polysynaptic pathways, e.g., the cerebello-thalamo-cortical circuits (Bohne et al., 2019; Watson et al., 2019; Kelly et al., 2020; Pisano et al., 2021), oscillating in a frequency-specific manner (McAfee et al., 2019).
It remains unknown whether the cerebellum is involved in object-place association memory.
5. Neuronal circuits involved in recognition memory for object and place
The major brain regions involved in NOP, NLP and OiP memory are listed in Table 1. As lesions and pharmacological inactivation have been extensively reviewed (Warburton and Brown, 2015; Aggleton and Nelson, 2020; Chao et al., 2020), we here focus on the optogenetic and chemogenetic perturbations. Importantly, neurons in a brain region never function in isolation and are organized into circuits to process specific information. The cell type- and circuit-specific manipulations in the key brain regions related to recognition memory for object and place are summarized in Table 2.
Table 1.
Neuroanatomy assessed in NOP, NLP and OiP memory paradigms. C: conditional. X: general. Gray background indicates the involvement of memory consolidation. Blank cells indicate that either no significant effects have been found or, so far, no studies have been conducted in the respective context. # The cerebellum directly projects to the thalamic nuclei. NOP: novel object preference; NLP: novel location preference; OiP: object-in-place; HPC: hippocampus; mPFC: medial prefrontal cortex; PRC: perirhinal cortex; LEC: lateral entorhinal cortex; MEC: medial entorhinal cortex; RSC: retrosplenial cortex; IC: insular cortex; ATN: anterior thalamus; MD: mediodorsal thalamus; NR: nucleus reuniens; LH: lateral hypothalamus; SuM: supramammillary nucleus; IPN: interpeduncular nucleus; dSTR: dorsal striatum; NAc: nucleus accumbens; MS: medial septum; MSDB: diagonal band of Broca; LC: locus coeruleus; CBL4/5: cerebellum lobules 4/5; DA: dopamine; ACh: acetylcholine; NE: norepinephrine.
| NOP | NLP | OiP | |||
|---|---|---|---|---|---|
| HPC | C | X | X | mPFC-HPC system | |
| mPFC | X | ||||
| PRC | X | X | Cortical module | ||
| LEC | C | C | |||
| MEC | C | ||||
| RSC | C | X | |||
| IC | |||||
| ATN | X | Thalamic module | |||
| MD | X | ||||
| NR | C | X | |||
| LH | X | Primeval module | |||
| SuM | X | ||||
| IPN | X | ||||
| dSTR | C | C | DA | ||
| NAc | C | C | DA | ||
| MS | X | C | ACh | ||
| Amygdala | NE | ||||
| LC | C | NE&DA | |||
| CBL4/5 | X | # |
Table 2a.
Cell-type specific manipulations in the hippocampus in object exploration tests. AAV: adeno-associated virus or recombinant adeno-associated virus; CCK: cholecystokinin; C.N.E.: compatible with no important effect; CNO: clozapine-N-oxide; DG: dentate gyrus; dHPC: dorsal hippocampus; DTA: diphtheria toxin subunit A; h: hours; ITI: inter-trial interval; min: minutes; OLM: oriens lacunosum moleculare; OVX: ovariectomy; PV: parvalbumin; s: seconds; SALB: salvinorin B; TeLC: tetanus toxin light chain; vHPC: ventral hippocampus; (+) excitation; (−) inhibition. *The control animals also showed the deficits. **Decreased exploration of the old object.
| Animal | Treatment | Time of activation | Encoding | ITI | Findings | Reference |
|---|---|---|---|---|---|---|
| NOP test: | ||||||
| Chrna2-Cre male mice |
Ablation of vHPC OLM interneurons AAV9-FLEX-DTA into vHPC |
Not required | 10 min | 20 min | C.N.E. | Haam et al., 2018 |
| Amigo2-Cre male mice |
(−) CA2 excitatory neurons AAV5-EF1α-FLEX-TeLC into CA2 |
Not required | 5 min 5 min x4 |
1 h 1 h |
C.N.E. | Hitti & Siegelbaum, 2014 |
| BAC CRH-Cre mice both sexes |
Ablation of dHPC CRH neurons AAV-FLEX-DTA into dHPC |
Not required | 5 minx3 | 3 h 24 h |
Impaired C.N.E. |
Hooper et al., 2018 |
| PV-Cre male mice |
(+) vHPC PV neurons AAV-EF1a-DIO-hM3Dq into vHPC |
Pre-sample CNO 1.0 mg/kg, i.p. |
10 min | 30 min | C.N.E. | Wang et al., 2018 |
| CaMKII2α-Cre male mice |
(−) vHPC excitatory neurons AAV-EF1a-DIO-hM4Di into vHPC |
Pre-sample CNO 1.0 mg/kg, i.p. |
10 min | 30 min | C.N.E. | Wang et al., 2018 |
| BAC CRH-Cre mice both sexes |
(−) dHPC CRH neurons AAV8-hSyn-DIO-hM4Di into dHPC |
Pre-sample CNO 10 mg/kg, i.p. |
5 minx3 | 3 h 24 h |
Impaired* C.N.E. |
Hooper et al., 2018 |
| PV-Cre male mice |
(+) dDG PV neurons Lenti-EF1a-DIO-hM3Dq into dDG |
Pre-sample CNO 0.5 mg/kg, .i.p. |
10min 10min |
1h 24h |
C.N.E. C.N.E. |
Zou et al., 2016 |
| C57BL/6J male mice |
(+) dHPC neurons AAV2/8-hSyn-hM3Dq into dHPC |
Pre-sample CNO 3 mg/kg, i.p. |
3 min | 24h | C.N.E. | Lopez et al., 2016 |
| C57BL/6J male mice |
(+) dHPC excitatory neurons AAV2/8-CaMKIIα-hM3Dq into dHPC |
Pre-sample CNO 3 mg/kg, i.p. |
3 min | 24h | C.N.E. | Lopez et al., 2016 |
| C57BL/6J male mice |
(−) dHPC neurons AAV2/8-hSyn-hM4Di into dHPC |
Pre-sample CNO 3 mg/kg, i.p. |
10 min | 24h | C.N.E. | Lopez et al., 2016 |
| C57BL/6J male mice |
(−) dHPC excitatory neurons AAV2/8-CaMKIIα-hM4Di into dHPC |
Pre-sample CNO 3 mg/kg, i.p. |
10 min | 24h | C.N.E. | Lopez et al., 2016 |
| C57BL/6 | (−) dHPC excitatory neurons female OVX mice |
Pre-sample AAV8-CaMKIIα-hM4Di into dHPC |
reach 30s CNO 2 mg/kg, i.p. |
24 h | C.N.E. | Tuscher et al., 2018 |
| Chrna2-Cre mice both sexes |
(+) intermediate CA1 OLMα2 neurons AAV-EF1a-DIO-ChR2 into intermediate HPC |
During the sample trial 8 Hz only on one obj. |
10 min | 1 h 24 h |
Impaired Impaired |
Siwani et al., 2018 |
| Chrna2-Cre mice both sexes |
(+) intermediate CA1 OLMα2 neurons AAV-EF1a-DIO-ChR2 into intermediate HPC |
During the sample trial 8 Hz if not exploring obj. |
10 min | 1 h | C.N.E. | Siwani et al., 2018 |
| Chrna2-Cre mice both sexes |
(−) dorsal HPC OLMα2 neurons AAV9-FLEX-Arch into dorsal HPC |
During the sample trial 8 Hz only on one obj. |
10 min | 1 h | C.N.E. | Siwani et al., 2018 |
| Chrna2-Cre mice both sexes |
(−) intermediate CA1 OLMα2 neurons AAV9-FLEX-Arch into intermediate HPC |
During the sample trial 8Hz only on one obj. |
10 min | 1 h | Facilitated** | Siwani et al., 2018 |
| GAD65-Cre male mice |
(+) dHPC GABAergic neurons AAV2-CAG-DIO-hM3Dq into dHPC |
Post-sample CNO 0.5 mg/kg, i.p. |
10 min | 24 h | C.N.E. | Yu et al., 2018 |
| Chrna2-Cre mice both sexes |
(+) intermediate CA1 OLMα2 neurons AAV-EF1a-DIO-ChR2 into intermediate HPC |
Post-sample 8 Hz for 10 min |
10 min | 24 h | C.N.E. | Siwani et al., 2018 |
| C57BL/6 female OVX mice |
(−) dHPC excitatory neurons AAV8-CaMKIIα-hM4Di into dHPC |
Post-sample CNO 2-8 mg/kg, i.p. |
reach 30s | 24 h | C.N.E. | Tuscher et al., 2018 |
| C57BL/6 female OVX mice |
(−) dHPC excitatory neurons AAV8-CaMKIIα-KORD into dHPC |
Post-sample SALB 10 mg/kg, i.p. |
reach 30s | 24 h | Impaired | Tuscher et al., 2018 |
| POMC-Cre mice both sexes |
(+) dDG granule neurons AAV5-hSyn-DIO-hM3Dq into dDG |
Pre-test CNO 0.3 mg/kg, i.p. |
15 min | 24 h | C.N.E. | Kahn et al., 2019 |
| C57BL/6 male mice |
(−) vHPC excitatory neurons AAV-CaMKII-NpHR3.0 into vHPC |
During test trial constant light |
4 min | 10 min | C.N.E. | Sun et al., 2020 |
| Chrna2-Cre mice both sexes |
(+) intermediate CA1 OLMα2 neurons AAV-EF1a-DIO-ChR2 into intermediate HPC |
During the sample + test 8 Hz only on one obj. |
10 min | 1 h | C.N.E. | Siwani et al., 2018 |
| Dlx5/6-Flp: nNOS-Cre both sexes |
(+) HPC nNOS long-projecting neurons AAV-DJ-Con/Fon-ChR2 into dHPC |
During sample + test 7 Hz |
10 min | 24 h | C.N.E. | Wick et al., 2019 |
| PV-Cre: ChR2 both sexes |
(+) dHPC PV neurons Opto-fiber in dHPC |
During sample + test 7 Hz |
10 min | 24 h | C.N.E. | Zeidler et al., 2020 |
| NLP test: | ||||||
| Chrna2-Cre male mice |
Ablation of vHPC OLM interneurons AAV-FLEX-DTA into vHPC |
Not required | 10 min | 20 min | Impaired | Haam et al., 2018 |
| PV-Cre male mice |
(+) vHPC PV neurons AAV-EF1a-DIO-hM3Dq into vHPC |
Pre-sample CNO 1.0 mg/kg, i.p. |
10 min | 30 min | C.N.E. | Wang et al., 2018 |
| CaMKII2α-Cre male mice |
(−) vHPC excitatory neurons AAV-EF1a-DIO-hM4Di into vHPC |
Pre-sample CNO 1.0 mg/kg, i.p. |
10 min | 30 min | Impaired | Wang et al., 2018 |
| C57BL/6 female OVX mice |
(−) dHPC excitatory neurons AAV8-CaMKIIα-hM4Di into dHPC |
Pre-sample CNO 2 mg/kg, i.p. |
reach 30s | 4 h | Impaired | Tuscher et al., 2018 |
| C57BL/6J male mice |
(+) dHPC neurons AAV2/8-hSyn-hM3Dq into dHPC |
Pre-sample CNO 3 mg/kg, i.p. |
3 min | 24h | Facilitated | Lopez et al., 2016 |
| C57BL/6J male mice |
(+) dHPC excitatory neurons AAV2/8-CaMKIIα-hM3Dq into dHPC |
Pre-sample CNO 3 mg/kg, i.p. |
3 min | 24h | Facilitated | Lopez et al., 2016 |
| C57BL/6J male mice |
(−) dHPC neurons AAV2/8-hSyn-hM4Di into dHPC |
Pre-sample CNO 3 mg/kg, i.p. |
10 min | 24h | Impaired | Lopez et al., 2016 |
| C57BL/6J male mice |
(−) dHPC excitatory neurons AAV2/8-CaMKIIα-hM4Di into dHPC |
Pre-sample CNO 3 mg/kg, i.p. |
10 min | 24h | Impaired | Lopez et al., 2016 |
| Emx1-Cre: AlstR mice |
(−) dHPC excitatory neurons cannulae into dHPC |
Pre-sample allatostatin 1 μM/0.5 μl |
10 min | 24 h | Impaired | Haettig et al., 2013 |
| Dlx5/6-Cre: AlstR mice |
(−) dHPC GABAergic neurons cannulae into dHPC |
Pre-sample allatostatin 1 μM/0.5 μl |
10 min | 24 h | Impaired | Haettig et al., 2013 |
| C57BL/6 female OVX mice |
(−) dHPC excitatory neurons AAV8-CaMKIIα-hM4Di into dHPC |
Post-sample CNO 2 mg/kg, i.p. |
reach 30s | 4 h | Impaired | Tuscher et al., 2018 |
| C57BL/6 female OVX mice |
(−) dHPC excitatory neurons AAV8-CaMKIIα-KORD into dHPC |
Post-sample SALB 10 mg/kg, i.p. |
reach 30s | 4 h | Impaired | Tuscher et al., 2018 |
| GAD65-Cre male mice |
(+) dHPC GABAergic neurons AAV2-CAG-DIO-hM3Dq into dHPC |
Post-sample CNO 0.5 mg/kg, i.p. |
10 min | 24 h | Impaired | Yu et al., 2018 |
| C57BL/6N male mice |
(−) dHPC GABAergic neurons AAV2-VGAT-Cre + AAV2-EF1a-DIO-hM4Di |
Post-sample CNO 0.5 mg/kg, i.p. |
10 min | 24 h | Impaired | Yu et al., 2018 |
| Dlx5/6-Flp: nNOS-Cre both sexes |
(+) HPC nNOS long-projecting neurons AAV-DJ-Con/Fon-ChR2 into dHPC |
During sample + test 7 Hz |
10 min | 24 h | Impaired | Wick et al., 2019 |
| PV-Cre: ChR2 both sexes |
(+) HPC PV neurons Opto-fiber in HPC |
During sample + test 7 Hz |
5 min | 24 h | C.N.E. | Zeidler et al., 2020 |
| NLP test (3 objects): | ||||||
| C57BL/6 mice both sexes |
(+) dDG excitatory neurons AAV5-CaMKIIα-hM3Dq into dDG |
Pre-test CNO 0.3 mg/kg, i.p. |
6 min x3 | 24 h | Impaired | Kahn et al., 2019 |
| C57BL/6 mice both sexes | (−) dDG excitatory neurons AAV5-CaMKIIα-hM4Di into dDG |
Pre-test CNO 1.5 mg/kg, i.p. |
6 min x3 | 24 h | Impaired | Kahn et al., 2019 |
| POMC-Cre mice both sexes |
(+) dDG granule neurons AAV5-hSyn-DIO-hM3Dq into dDG |
Pre-test CNO 0.3 mg/kg, i.p. |
6 min x3 | 24 h | Impaired | Kahn et al., 2019 |
| C57BL/6 or wildtype both sexes mice |
(−) dHPC excitatory neurons AAV5-CaMKIIα-hM4Di into dHPC |
Pre-sample + Pre-test CNO 1.0 mg/kg, i.p. |
6 min x3 | 24 h | Impaired | Kahn et al., 2020 |
| C57BL or wildtype both sexes mice |
(−) vHPC excitatory neurons AAV5-CaMKIIα-KORD into vHPC |
Pre-sample + Pre-test SALB 1.0 mg/kg, s.c. |
6 min x3 | 24 h | C.N.E. | Kahn et al., 2020 |
5.1. Hippocampus and medial prefrontal cortex pathway
The mPFC and HPC are connected through direct (monosynaptic) and indirect (polysynaptic) projections (Varela et al., 2014). The dorsal and ventral HPC send axons to the mPFC, forming two direct pathways in rodents (Figure 4). The dorsal HPC (CA1 and dentate gyrus) directly projects to the ACC, PL and IL of mPFC (Xu and Südhof, 2013; DeNardo et al., 2015; Ye et al., 2017). This dorsal HPC-mPFC pathway is important for fear memory tested in the inhibitory avoidance task (Ye et al., 2017). In contrast, the ACC projects back to the dorsal HPC CA1 and CA3 (Rajasethupathy et al., 2015), but see (Andrianova et al., 2022). Optogenetic activation of the ACC-dorsal HPC circuit incurred the resurgence of a past fear experience (freezing) in a new context, suggesting that the ACC-dorsal HPC circuit is involved in the retrieval of fear memory (Rajasethupathy et al., 2015). The dorsal HPC CA2 subarea also connecting with the PL (Ye et al., 2017) is essential for the processing of social memory (Hitti and Siegelbaum, 2014).
Figure 4.

Neuroanatomy of recognition memory based on spontaneous object exploration tests in rodents. Blue and red lines show the mPFC- and HPC-connected circuits, respectively. Black lines show the projections sent from the LC and cerebellum to the dorsal HPC and thalamus, respectively. Note that other regions (IC, striatum, MS, hypothalamus, amygdala and IPN) are highly connected with the mentioned structures and contribute to recognition memory under certain conditions. The right panel shows a hypothetical model of recognition memory based on hierarchically organized, functionally distinct, yet complementary anatomical connectivity: Hypothalamus and IPN send information of familiarity and novelty (F/N signals) to the thalamic nuclei. The thalamic nuclei, as integrators, organize the incoming F/N signals and actively interact with the mPFC-HPC system and cortices. The HPC generates essential mnemonic information featured with spatial and contextual information, while the mPFC selects the “correct” memory dependent on environmental requirements; both interact with the reciprocally connected cortices that support the mPFC-HPC system. The mPFC-MD (blue) and HPC-ATN (red) circuits preferentially modulate information for memory guidance and formation, respectively, gated by the NR. Dashed lines represent possible connectivity. * The pathway from the mPFC to the HPC is debated (see Andrianova et al., 2022). mPFC: medial prefrontal cortex; HPC: hippocampus; RSC: retrosplenial cortex; PRC: perirhinal cortex; EC: entorhinal cortex (the lateral EC mutually connects with the mPFC and HPC); IC: insular cortex; MS: medial septum; IPN: interpeduncular nucleus; LC: locus coeruleus; MD: mediodorsal thalamus; NR: nucleus reuniens; ATN: anterior thalamic nucleus.
The indirect routes can be largely segregated into a cortical (support) module (RSC, PRC and LEC mutually connect with the mPFC-HPC system) and a thalamic (integration) module (MD, NR and ATN reciprocally convey information from the mPFC and HPC). Presumably, the mPFC-HPC system receives distinct, yet complementary, information about object identity (PRC) and object-context (RSC and LEC) by these modules, mediated by the thalamic nuclei (Figure 4). The functions of these networks are based on their anatomical connections. For instance, the IL sends efferents predominantly to the NR, while the ACC and PL preferentially project to the MD. Optogenetic inactivation of mPFC neurons projecting to the NR, but not to the MD, generalized fear memory in a novel context (Xu and Südhof, 2013). Subpopulations of mPFC neurons differentially project to the NR and PRC (mPFC layers V/VI project to the NR, and mPFC layers II/III project to the LEC/PRC (Schlecht et al., 2022). Sequence memory was impaired by chemogenetic inhibition of both mPFC-NR and mPFC-PRC circuits, but working memory was impaired by inhibition of the mPFC-NR circuit, and temporal context memory was impaired by inhibition of the mPFC-PRC circuit (Jayachandran et al., 2019). How the cortical and thalamic modules interact with the mPFC-HPC system via segregated circuits is a major topic in the field of recognition memory. A discussion of the indirect routes between the mPFC and HPC can be found in a review (Chao et al., 2020).
As to object exploration tests, disconnecting the mPFC-dorsal CA3 pathway did not have significant effects on the NOP and NLP tests (de Souza Silva et al., 2016). On the other hand, disconnection of the mPFC-dorsal CA1 pathway impaired spatial memory (Chao et al., 2017), in line with the notion that the mPFC-CA1 pathway is involved in spatial working memory (Churchwell and Kesner, 2011; O’Neill et al., 2013). Both the mPFC-CA1 and mPFC-CA3 pathways, however, are required for the integration of spatial and temporal information in support of episodic memory (de Souza Silva et al., 2016; Chao et al., 2017).
The necessity of the mPFC-HPC connections for OiP memory has been demonstrated by disconnecting the pathway in rats: permanent lesions impaired the performance in the OiP, but not in NOP and NLP, tests (Barker and Warburton, 2011), suggesting that functional interaction between the mPFC and HPC underlies the formation of an association of objects with their locations. Neuronal inhibition (Daun02 inactivation method (Koya et al., 2009; Cruz et al., 2013) of the intermediate CA1 neurons, selectively projecting to the mPFC, impaired the association between object and place (Barker et al., 2017). Since the functional disconnection of this circuit did not affect NOP and NLP performance, the mPFC-HPC interaction may not be required for the discrete processing of object identity and localization, but be necessary for transmitting information about the object-place association (Barker et al., 2017). In addition, optogenetic inhibition of the intermediate CA1 terminals in the mPFC disrupted the encoding, but not retrieval, of OiP memory, indicating that OiP memory recruits the intermediate CA1-mPFC circuit in learning the mnemonic association between object and place (Barker et al., 2021), consistent with the view that associative recognition memory engages different networks within the HPC-thalamo-cortical circuit (Barker et al., 2021).
Unilateral infusions of 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX), a potent AMPA receptor antagonist, or AP5 into the mPFC and HPC in the opposite hemispheres impaired the OiP performance tested with a 5 min or a 1 h delay. Crossed unilateral infusions of NBQX, but not AP5, into the mPFC and HPC impaired the retrieval of OiP memory. Disconnecting the mPFC-HPC circuit by infusions of NBQX or AP5, however, did not affect the performance in the NOP and NLP tests (Barker and Warburton, 2015). Thus, the glutamatergic system in the mPFC-HPC pathway is important for the encoding of the associative memory for object and place, while the retrieval of such memory is dependent on AMPA, but not NMDA, receptor signaling.
5.2. Hippocampus and perirhinal cortex pathway
Disconnection lesions of the HPC–PRC pathway impaired the performance in the OiP, but not in NOP and NLP tests, suggesting that the functional interaction between the HPC and PRC underlies the associative memory for objects and place. However, like the mPFC-HPC circuit, the HPC-PRC is not required for the discrete processing of object identity and localization (Barker and Warburton, 2011). Disconnecting the HPC–PRC circuit with infusions of NBQX impaired the encoding OiP memory after a 5 min or a 1 h delay (Barker and Warburton, 2015).
Functional disconnection achieved by NBQX infusions into the HPC and PRC also disrupted the retrieval of OiP memory. In contrast, unilateral infusions of AP5 into the HPC and PRC in the opposite hemispheres impaired the encoding of OiP memory after a 1 h, but not a 5 min, delay, while the retrieval of OiP memory was not affected. Likewise, crossed infusions of NBQX or AP5 into the HPC and PRC had no effects on the NOP and NLP tests (Barker and Warburton, 2015). Thus, the activation of AMPA receptors in the HPC–PRC pathway is crucial for encoding and retrieval of associative memory for object and place, whereas the activation of NMDA receptor is important for the encoding of long-term (1 h), but not short-term (5 min), OiP memory in the HPC–PRC pathway.
5.3. Perirhinal cortex and medial prefrontal cortex pathway
Disconnecting the mPFC–PRC pathway with lesions impaired OiP, but not NOP and NLP, memory in rats, indicating that the associative memory for object and place requires the functional interaction between the mPFC and PRC (Barker et al., 2007).
Crossed unilateral infusions of scopolamine into the mPFC and PRC impaired OiP performance after a 5 min or after a 1 h delay (Barker and Warburton, 2009). Furthermore, unilateral infusions of the AMPA receptor antagonist 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX) into the mPFC and PRC of opposite hemispheres impaired OiP performance. This suggests that, in addition to the muscarinic cholinergic receptor, the AMPA receptors in the mPFC–PRC circuit are required for the object-place association memory. Disconnecting the mPFC–PRC pathway with crossed unilateral infusions of AP5 caused deficits in long-term (1 h), but not short-term (5 min), OiP memory. In contrast, short-term (5 min) OiP memory was disrupted, when – in addition to unilateral AP5 in the mPFC – either CNQX or UBP302, a kainate (GLUK5) receptor antagonist, was infused into the PRC of the opposite hemispheres (Barker and Warburton, 2008). Thus, object-place association memory is dependent on NMDA receptor-mediated neurotransmission in the mPFC-PRC pathway, while AMPA and kainate, but not NMDA, receptors in the PRC are required for short-term memory.
5.4. Lateral entorhinal cortex and medial prefrontal cortex pathway
Disconnection lesions in the mPFC–LEC pathway impaired object recognition memory, when distinct, but not identical, objects had been presented in the sample trial. Likewise, disconnecting this pathway also impaired episodic-like and associative memories for recognizing distinct objects, location, and context, but not for the NOP and NLP tests that employed identical objects in the samples. Thus, the mPFC–LEC pathway is required for recognizing the association between distinct objects, but not for the discrete processing of object identity and localization (Chao et al., 2016b). Although OiP memory cannot be directly assessed by disconnecting the mPFC–LEC circuit, it likely recruits this pathway, since the mPFC–LEC disconnection disrupted the capability to recognize associations between distinct objects (Chao et al., 2016b). Collectively, the LEC may participate in the functional network of associative object-place memory by providing object-relevant information (Wilson et al., 2013b; Wilson et al., 2013a).
5.5. Thalamus-medial prefrontal cortex-hippocampus pathways
Crossed unilateral lesions of MD and mPFC caused deficits in OiP, but not NOP and NLP, memory after either a 5 min or a 3 h delay. This suggests that the MD-PFC pathway is recruited for associative object-place memory irrespective of the time delay (Cross et al., 2013). Consistently, unilateral lesions of the MD impaired learning of OiP scene discriminations in rhesus monkeys and disconnecting the MD-PFC pathway further deteriorated this ability (Browning et al., 2015), underscoring the importance of the MD-mPFC circuit in OiP memory.
Optogenetic inhibition of the NR terminals in the mPFC impaired the encoding of OiP memory, whereas optogenetic inhibition of the mPFC terminals in the NR disturbed OiP retrieval (Barker et al., 2021). Thus, the information flow from the mPFC to NR is crucial for the recall of OiP memory, while the opposite flow is required for learning the object-place association. Chemogenetic inhibition of the NR terminals in the dorsal and intermediate HPC disrupted the encoding of OiP memory, whereas chemogenetic inhibition of the NR terminals in the intermediate, but not dorsal, HPC impaired the retrieval of OiP memory (Barker et al., 2021). Thus, the NR mediates the learning and retrieval of object-place association by targeting either the dorsal plus intermediate or the intermediate HPC.
5.6. Dopamine, acetylcholine, and medial prefrontal cortex-hippocampus pathways
To investigate the functional interaction between the mPFC and midbrain DA system, unilateral NMDA lesions in the mPFC were combined with unilateral 6-OHDA lesions in the ipsi- or contralateral midbrain in rats. Irrespective of the side of 6-OHDA lesions, the combined lesions caused deficits in the NLP and OiP tests. In addition, disconnecting the mPFC-midbrain DA pathway specifically disrupted performance in the NOP test (Chao et al., 2013). Thus, recognition memory for object, place and their association require the interaction between the mPFC and midbrain DA system, although unilateral lesions of either mPFC or midbrain DA system alone would not affect NOP, NLP and OiP memory (Chao et al., 2013).
The functional relationship between the MS and HPC was examined using the OiP test. Crossed lesions of MS and either CA1 or CA3 disrupted the performance of spatial recognition. Thus, the MS-CA1 or MS-CA3 circuit is important for this function (Okada and Okaichi, 2010). Chemogenetic inhibition of MS neurons that selectively connect with the HPC, but not with the ACC, impaired the retrieval of NLP and OiP memory (Jin et al., 2020). These findings suggest that cholinergic neurotransmission from the MS to HPC is essential for the retrieval of information of place and/or object-place association.
5.7. The interpeduncular nucleus-connected pathways
The IPN receives cholinergic/glutamatergic and dopaminergic projections from the medial habenula and VTA, respectively. Optogenetic excitation or inhibition of the medial habenula nerve terminals in the IPN disrupted novelty preference in both social and non-social settings, suggesting that the medial habenula-IPN cholinergic/glutamatergic circuit is critical for novelty preference (Molas et al., 2017). In contrast, optogenetic excitation of the VTA dopaminergic terminals in the IPN impaired the preference for a novel conspecific, but not for a novel object. This deficit was counteracted by infusions of the DA D1 receptor antagonist, SCH39166, into the IPN. Thus, the VTA-IPN dopaminergic circuit specifically suppressed the perception of social novelty by DA D1 receptor activation (Molas et al., 2017). In sum, the medial habenula-IPN circuit controls the processing of novelty preference for both social and non-social stimuli, while the VTA-IPN circuit selectively regulates social novelty preference.
6. Molecular mechanisms for object recognition memory
It is generally believed that memory formation requires activity-dependent, long-lasting changes in the strength of information flow from presynaptic to postsynaptic neurons through neural circuits. Synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD), is mediated by molecular mechanisms underpinning structural and functional remodeling of network connectivity. Signaling pathways that are responsible for synaptic plasticity include activation of cell-surface receptors, e.g., receptor tyrosine kinases (RTKs), which lead to post-transcriptional modifications of synaptic proteins and activation of transcription factors. These cascade events alter gene expression and protein synthesis in neurons, and thereby impact synaptic structure and function, which ultimately underlie the consolidation of recognition memory (Martin et al., 2000; Citri and Malenka, 2008; Davis et al., 2010; Stacho and Manahan-Vaughan, 2022).
RTKs bind neurotrophins including BDNF, which induces receptor dimerization and tyrosine phosphorylation to activate several downstream signaling pathways. A well-known pathway underlying synaptic plasticity and recognition memory is the Ras mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) cascade (Davis and Laroche, 2006). The MAPK/ERK cascade transmits signals from cell-surface receptors to the nucleus via transcription factors and IEGs. Several IEGs and transcription factors, including c-Fos, Arc, Homer1a, early growth response 1 (Egr1; also known as Zif268), nuclear receptor 4a family (NR4a1 and NR4a2) and cAMP response element-binding protein (CREB), can be activated by object recognition (Zhu et al., 1995; Warburton et al., 2005; McNulty et al., 2012; Seoane et al., 2012; Barbosa et al., 2013; Beer et al., 2013; Hoang et al., 2018; Hoang et al., 2021). These mechanisms reflect the spontaneous object exploration protocols used for testing recognition memory because they involve a rapid response to stimuli during learning and long-lasting retention of information even after a single learning episode (usually within minutes).
The role of synaptic plasticity in object recognition memory with spatial or non-spatial information has been demonstrated for the hippocampal circuits. Exploring a novel environment induced robust LTP in the hippocampal CA and dentate gyrus regions (Kemp and Manahan-Vaughan, 2004, 2008; Hagena and Manahan-Vaughan, 2011), and increased Homer1a expression throughout the HPC (Hoang et al., 2021). The HPC LTP might be responsible for the construction of the global representation of space, associated with activation of IEGs in the whole HPC. Also, the exploration of novel or familiar objects, that had been moved to novel locations, could be related to HPC LTD (Manahan-Vaughan and Braunewell, 1999; Kemp and Manahan-Vaughan, 2004, 2008; Goh and Manahan-Vaughan, 2013), and increased Homer1a expression in the proximal CA3 (close to the dentate gyrus) and the lower blade of the dentate gyrus (Hoang et al., 2021). Thus, HPC LTD might play a role in certain forms of object-spatial information, linked to the localized activation of IEGs, in discrete hippocampal circuits: exploration of large landmark objects, that had been displaced, elicited LTD at the perforant path-dentate gyrus and mossy fiber-CA1 circuits (Kemp and Manahan-Vaughan, 2008; Hagena and Manahan-Vaughan, 2011), while that of small subtle objects incurred LTD at the Schaffer collateral-CA1 and commissural/asssociational-CA3 circuits (Manahan-Vaughan and Braunewell, 1999; Kemp and Manahan-Vaughan, 2004; Goh and Manahan-Vaughan, 2013). In addition, an alteration of the spatial object configuration of large landmark items elicited IEG (Arc and Homer1a) activation in the dentate gyrus and proximal CA3, while that of small-subtle items enhanced IEG expression in the distal CA1 (close to the subiculum) and proximal CA3 (Hoang et al., 2018). This may be relevant for the predominant projections of LEC and MEC to the distal CA1 and proximal CA1 (close to the dentate gyrus), respectively, via the lateral and medial perforant paths (Fyhn et al., 2004). Moreover, the lateral and medial perforant path inputs to the dentate gyrus induced LTD and LTP, respectively (Collitti-Klausnitzer et al., 2021). Thus, LTP and LTD might distinctly modulate the HPC in the processing of spatial and non-spatial information in a circuit-dependent manner. The HPC LTP (with contribution of the MEC circuit), which preferentially conveys spatial information and targets the dentate gyrus and proximal CA1, is involved in spatial representation and recognition of the locations of large-landmark items. In contrast, the HPC LTD (in association with the LEC circuit, which preferentially transfers item-relevant messages and targets the distal CA1), is implicated in the processing of subtle features and/or inter-object information. This circuit-dependent perspective on synaptic plasticity is in line with the circuit findings discussed and may explain the ambiguous role of the HPC in the NOP test, which mainly involves LTD, as characterized by more heterogeneous properties in the processing of object-associated spatial information compared to LTP (Stacho and Manahan-Vaughan, 2022).
The molecular mechanisms underlying synaptic plasticity have been extensively studied (Martin et al., 2000; Citri and Malenka, 2008; Davis et al., 2010; Stacho and Manahan-Vaughan, 2022). Synaptic plasticity in the mPFC also contributes to the mnemonic relationship between object and location. Nicotinic acetylcholine receptors α7 and α4β2 are believed to underlie LTP and LTD in the mPFC and are essential for the encoding and retrieval of OiP memory, respectively (Sabec et al., 2018). This is consistent with the role of mPFC mediating the association between object and place. The subtypes of nicotinic acetylcholine receptors modulate the different stages of OiP memory associated with IEGs. The effects of IEG activation on synaptic plasticity have been reviewed previously (Minatohara et al., 2015; Stacho and Manahan-Vaughan, 2022). As some IEGs activated by recognition memory tasks can be partially regulated by epigenetic modifications (Srivas and Thakur, 2019), epigenetic mechanisms of DNA methylation and histone acetylation likely play a role in object recognition memory (Stefanko et al., 2009; Roozendaal et al., 2010; Haettig et al., 2011; Ramirez-Mejia et al., 2021). NOP impairments along with increased expression of hippocampal histone H3K9me3 at the promoters of Arc and Egr1 were found in aged mice (Kushwaha and Thakur, 2020), suggesting that epigenetic modifications of IEGs are involved in age-related loss of object memory. In addition, HDAC inhibition in the insular cortex and HPC enhanced NOP and NLP memory, respectively, via CREB binding protein (Stefanko et al., 2009; Roozendaal et al., 2010; Haettig et al., 2011) and BDNF signaling (Ramirez-Mejia et al., 2021). Since similar HDAC inhibitions were applied, it may be concluded that the types of enhanced memory are region-specific. In the HPC, HDAC inhibition was mediated by CREB binding protein and enhanced LTP (Vecsey et al., 2007). Since BDNF was involved in CA1 theta-stimulation induced LTP and early LTD (<90 min), while the NLP test-induced LTD, but no robust LTP (Aarse et al., 2016), it may be inferred that BDNF signaling is critical for certain forms of synaptic plasticity in the HPC and HPC-dependent memory, including object-location recognition memory (Cunha et al., 2010; Aarse et al., 2016). Further studies are required to examine, how epigenetic modifications interact with the IEGs, CREB, and BDNF signaling pathways to affect synaptic plasticity in the neuronal circuits underlying object recognition memory.
Transcriptomes and proteomes are used to gain a systematic understanding of the molecular basis for object recognition memory. RNA sequencing data from the rat PRC after object recognition memory revealed reduced levels of small nucleolar RNAs (Scott et al., 2017). In addition, upregulated IEGs and transcription factors, including Arc, Egr1, Trps1 and heat shock proteins Hspa1b and Hapa5, were identified in the context of exploring novel objects. In terms of differences between exploring novel and familiar objects, upregulated transcriptional regulation and splicing factors of Sart1 and Gabpb2, calcium signaling related factors Pkd1l1 and Esyt1, and altered cell-cell communication and neuron outgrowth relevant factors Sema4c, Epha7 and Shtn1 were identified. Sema4c was particularly critical for dendrite complexity (Simonetti et al., 2021). In the rat PRC, genes related to neuronal action potentials and transcription, extracellular matrix and structure organization, and pathways for extracellular matrix receptor interaction and focal adhesion, were activated during recognition of familiar objects, while ribosome pathways were associated with the exploration of novel objects (Scott et al., 2017). The multiple-categorized genes activated by recognition of familiar objects in the PRC seem to underlie its strong role in the NOP test. The proteomes of hippocampal CA1 and CA3 subregions were characterized in mice at basal conditions and after the NOP or NLP test (von Ziegler et al., 2018). At basal conditions, CA1 protein expression was higher in ITPKA (a regulator related to the morphology of hippocampal dendritic spines), NTM (a protein related for neurite outgrowth and adhesion), EFHD2 (a regulator of synapse formation) and GAP43 (a factor associated with spine growth). In contrast, CA3 protein expression was higher in CPNE4 (a calcium-dependent phospholipid-binding protein), SYNPR (a protein of small synaptic vesicles), HPCAL1 and NCALD (calcium-binding proteins). The CA1 showed similar protein expressions induced by either the NOP or NLP test. In contrast, the CA3 displayed distinct protein clusters and dynamics depending on the type of recognition memory tests (von Ziegler et al., 2018). This is consistent with the more “general” role of CA1 playing in recognition memory compared to CA3, as CA1, being the output of HPC, receives convergent information from diverse circuits.
Transcriptomic and proteomic studies are limited by the difficulty to differentiate between cells that specifically respond to stimuli and cells that are non-responding. A way to overcome this limitation is to selectively label cells that are activated during a specific experience (in this context, object recognition memory). The responding cells are conceptualized as the memory engram: a population of cells, which is activated during learning, stores information and is later reactivated during memory recall (Han et al., 2007; Liu et al., 2012; Josselyn and Tonegawa, 2020). Transcriptome analysis on engram cells sheds light on gene expression “locked” by a specific memory. For instance, RNA sequencing of the engram cells in the hippocampal dentate gyrus after contextual fear conditioning identified CREB-dependent transcription factors of Atf3, Penk and Kcnq3, featuring the engram cells specifically engaging the CREB signaling for fear memory consolidation (Rao-Ruiz et al., 2019).
In addition to functional changes (LTP and LTD), object recognition memory involves genes and proteins that affect neuronal structure, e.g., dendritic spines (Scott et al., 2017; von Ziegler et al., 2018). Memory - information processed and stored in the brain - can be considered to occur at dendrites and dendritic spines, which are the loci of synaptic plasticity (Kandel et al., 2014; Holtmaat and Caroni, 2016). Learning and memory reorganize and stabilize spine dynamics (Hayashi-Takagi et al., 2015; Ma and Zuo, 2022). Shrinkage of new or recently potentiated dendritic spines was able to blunt memory strength (Hayashi-Takagi et al., 2015), whereas enhancement of spine turnover facilitated memory (Frank et al., 2018). Moreover, induction of LTP and LTD in the hippocampal CA1 led to de novo growth and shrinkage of spines, respectively (Nagerl et al., 2004), accompanied by actin modifications (Okamoto et al., 2004). Thus, it is not surprising that genes and proteins relevant to spine morphology are identified in object recognition memory tests, with a strong link between IEGs, synaptic plasticity and spine remodeling (Stacho and Manahan-Vaughan, 2022).
Taken together, molecular mechanisms underlying object recognition memory are associated with IEGs, transcription signaling and epigenetic modifications, which can reshape the structure and function of neuronal circuits connected through synapses. Systematic analysis of molecular characteristics of the engram cells within a neuronal circuit in a task-dependent manner will expand our knowledge of the fundamental form of recognition memory.
7. A hypothetical model of recognition memory
The mPFC-HPC system, assisted by the cortical and thalamic modules, is critical for processing mnemonic information on the association between object and place. The complex interactions between the mPFC, HPC, PRC, LEC, MD and NR (likely also RSC and ATN) account for the encoding and retrieval of OiP memory, which is mediated by glutamate, DA and acetylcholine neurotransmission. Specifically, in the mPFC-PRC circuit, OiP memory is mediated by NMDA receptor action. Muscarinic cholinergic receptors in the mPFC-PRC pathway underlie short- (minutes) and long-term (hours) memory for object-place association, while AMPA and kainate (not NMDA) receptors are activated for short-term (minutes) memory.
The interaction between the mPFC-HPC pathway and other modules is intricate. For instance, the dorsal CA1 pyramidal neurons send monosynaptic projections to the NAc fast-spiking parvalbumin neurons, forming a feedforward inhibition of the NAc medium spiny neurons for reward-based spatial memory (sucrose-conditioned place preference) (Trouche et al., 2019). This mechanism might be associated with the role of NAc in the storage of spatial information (Roullet et al., 2001; De Leonibus et al., 2005) as it requires the HPC-NAc interaction (Torromino et al., 2019). The neural basis of behavior consists of distinct functional patterns within the individual circuits (e.g., feedforward excitation/inhibition, feedback inhibition, lateral inhibition, and mutual inhibition); the exploration of the dynamic relationship between neuronal functions and neuronal circuits has just begun.
We propose a hypothetical model of recognition memory (Figure 5), which is composed of modules with distinct, but complementary functions hierarchically organized in interconnecting circuits. The neuroanatomy of recognition memory for object and place can be perceived as a neuronal network around the mPFC-HPC interconnection, which is enabled by the thalamic nuclei (integrative module) and the cerebral cortices (support module) (Table 1 & Figure 4). The IPN and lateral hypothalamus send signals to virtually all thalamic nuclei, providing basic discrimination of whether an encountered item is “new” or “familiar” (primeval module). The thalamus connects with the mPFC-HPC axis and actively integrates the external and internal information. The ATN-HPC and MD-PFC pathways gate complex information of object-place association (Cross et al., 2013; Ketz et al., 2015), and the NR serves as a functional hub for reciprocal communications with the PFC and HPC (Ferraris et al., 2018; Hauer et al., 2019). The HPC encodes the memory trace and transmits it further to the mPFC (memory formation), while the mPFC, as a top-down executor, guides the HPC to correctly retrieve the information according to the current demands (memory guidance).
Figure 5.
A hypothetical circuit of the associative memory for object-place. Arrow lines indicate that the interaction between the linked regions is essential for object-place association memory. The connections are color-coded for either encoding (red), retrieval (blue) or encoding plus retrieval (purple). Gray lines indicate functional interactions between the hypothetically linked regions, which, so far, have not been tested. * The interaction between the mPFC and LEC has not been directly examined in the object-in-place memory test; however, this pathway is likely to contribute to the association of object and place. Note that the NR functional circuits are mainly based on a preprint paper (Barker et al., 2021). The right panel depicts the mPFC-HPC system responsible for memory guidance and formation, the cortical module accounting for object (PRC), object-association (LEC) and place (RSC) information, and the thalamic module processing the mPFC-relevant (MD), HPC-relevant (ATN) and mPFC-HPC messages (NR), which can be modulated by DA and ACh (presumably via the medial septum connections). mPFC: medial prefrontal cortex; HPC: hippocampus; LEC: lateral entorhinal cortex; PRC: perirhinal cortex; RSC: retrosplenial cortex; MD: mediodorsal thalamus; NR: nucleus reuniens; ATN: anterior thalamus; DA: dopamine; ACh: acetylcholine; glutamate-R: glutamate receptor; mAChR: muscarinic acetylcholine receptor; AMPAR: AMPA receptor.
Regarding object-place association memory, this model pinpoints that the mPFC-HPC circuit is the main neuronal system for OiP memory (Eichenbaum, 2017; Chao et al., 2020). The mPFC-HPC system involves the cortical and thalamic modules and is mediated by dopaminergic and cholinergic neurotransmission (Figure 5). Although our model is similar to recent models of object-place association memory (Aggleton and Nelson, 2020; Barker and Warburton, 2020a), it is structured with different modules, each encompassing multiple interconnected brain regions. We speculate that information is micro-processed between regions and macro-processed between modules in a functionally distinct yet complementary manner. The functional mPFC-HPC system may be dependent on direct anatomical projections and/or indirect connectivity between cortical (support) and thalamic (integration) modules. In addition, we propose that the mPFC-HPC circuit is mediated by the dopaminergic and cholinergic systems for memory guidance and formation, respectively.
The circuitry model for recognition memory will evolve with a better understanding of the dynamic activation of certain circuits by different cognitive demands (object, place, and their association) and memory states (learning, storage, or retrieval). For instance, NOP memory mainly recruits the primeval module and PRC with the support from the HPC and LEC, whereas NLP memory is essentially processed by the dorsal HPC with the support from the EC, RSC, and SuM.
Other brain regions are also involved in recognition memory, including the postrhinal cortex, inferior temporal cortex, area TE, fornix, mammillary bodies, cingulate and VTA (Rossato et al., 2013; Aggleton and Nelson, 2020). However, the evidence for their roles in object recognition memory is not yet solid. Some regions can be merely viewed as a functional extension of the “default” system, e.g., the fornix as a major output region of the HPC. The cerebellum, as a complex structure involved in motor as well as cognitive functions (Wagner and Luo, 2020), is also engaged in NLP memory, likely via the cerebello-thalamo-cortical circuits (Pisano et al., 2021). The striatum, MS, amygdala, and LC are associated with either NOP or NLP memory (Roozendaal et al., 2008; Chen et al., 2018) mediated by dopaminergic, cholinergic and norepinephrinergic neurotransmission. These and other neurotransmitters, including glutamate and GABA, regulate different stages of recognition memory via region-specific and neurotransmitter-specific (inter)actions.
8. Conclusion
The conventional methods of lesions and pharmacological interventions, combined with advanced virus-based genetic tools of optogenetics and chemogenetics, have exceedingly increased our knowledge about the neuronal basis underlying recognition memory for object, place, and object-place association. A neuronal circuit can now be selectively and bidirectionally manipulated with spatiotemporal precision. With the development of safer and more efficient viral vectors, we will gain more insights into complex brain functions. Our current model suggests that recognition memory for object and place is regulated by a hierarchical network composed of the mPFC-HPC system for memory formation and guidance, the cortical module for memory support, the thalamic module for information integration, and the primeval module for mediating the signals of familiarity and novelty. Recognition memory for object-place association is based on a structured network involving the mPFC-HPC pathway, assisted by the cortical and thalamic modules in a functionally distinct manner. Understanding the neuronal structure and molecular basis of recognition memory may address the complexities of amnesia exhibited in dementia, epilepsy, and psychiatric disorders. Future efforts may be directed towards dissecting the individual modules with cell type- and pathway-specific targeting.
Table 2b.
Cell type-specific manipulations in other structures in object exploration tests. AAV: adeno-associated virus or recombinant adeno-associated virus; CB: cerebellum; CeA: central nucleus of amygdala; C.N.E.: compatible with no important effect; CNO: clozapine-N-oxide; CRH: corticotropin-releasing hormone; dmSTR: dorsomedial striatum; EC: entorhinal cortex; h: hours; IPN: interpeduncular nucleus; ISI: inter-sample interval; ITI: inter-trial interval; L4/5: lobules 4/5; LC: locus coeruleus; Lenti: lentiviruses; LH: lateral hypothalamus; min: minutes; MCH: melanin-concentrating hormone; mPFC: medial prefrontal cortex; MSDB: diagonal band of Broca; NAc: nucleus accumbens; NOP: novel object preference; NPP: novel place preference; NR: nucleus reuniens; OC: object context; OiP: object-in-place; OPC: object place context; OVX: ovariectomy; PRC: perirhinal cortex; s: seconds; SALB: salvinorin B; SuM: supramammillary nucleus; TeLC: tetanus toxin light chain; TH: tyrosine hydroxylase; TOM: temporal order memory; (+) excitation; (−) inhibition.
| Animal | Treatment | Test | Time of activation | Encoding | ITI | Findings | Reference |
|---|---|---|---|---|---|---|---|
| mPFC | |||||||
| Lister hooded male rats |
(+) mPFC excitatory neurons Lenti-CaMKIIa-ChR2 |
NOP | Post-sample 50 Hz |
reach 40s or 4 min |
5 min | C.N.E. | Benn et al., 2016 |
| C57BL/6 female OVX mice |
(−) mPFC excitatory neurons AAV8-CaMKIIα-hM4Di |
NOP | Post-sample CNO 2 mg/kg, i.p. |
reach 30s | 24 h | Impaired | Tuscher et al., 2018 |
| Lister hooded male rats |
(+) mPFC excitatory neurons Lenti-CaMKIIa-ChR2 |
NLP | Post-sample 50 Hz |
3 min | 5 min | C.N.E. | Benn et al., 2016 |
| C57BL/6 female OVX mice |
(−) mPFC excitatory neurons AAV8-CaMKIIα-hM4Di |
NLP | Post-sample CNO 2 mg/kg, i.p. |
reach 30s | 4 h | Impaired | Tuscher et al., 2018 |
| Lister hooded male rats |
(+) mPFC excitatory neurons Lenti-CaMKIIa-ChR2 |
OiP | Post-sample 50 Hz |
5 min | 5 min | Facilitated | Benn et al., 2016 |
| PRC | |||||||
| Long-Evans male rats |
(+) caudal PRC neurons Lenti-Synapsin-ChR2 |
NOP 2D |
During test trial 10-15 Hz at new obj. |
reach 15s or 5 min |
5 min | Decreased new image exploration |
Ho et al., 2015 |
| Long-Evans male rats |
(+) caudal PRC neurons Lenti-Synapsin-ChR2 |
NOP 2D |
During test trial 30-40 Hz at old obj. |
reach 15s or 5 min |
5 min | Increased old image exploration |
Ho et al., 2015 |
| C57BL/6J male mice |
(+) PRC neurons AAV-hSyn-hM3Dq |
NOP | Pre-test CNO 1.0 mg/kg, i.p. |
15 min | 7 d | Facilitated | Nomura et al., 2019 |
| LEC & MEC | |||||||
| Sim1-Cre mice both sexes |
(−) LEC fan cells AAV-FLEX-TeLC |
NOP | Not required | 3 min | 1 min | C.N.E. (Blunted) | Vandrey et al., 2020 |
| Sim1-Cre mice both sexes |
(−) MEC stella neurons AAV1/2-FLEX-TeLC |
NOP | Not required | 5 min | 3 min | C.N.E. | Tennant et al., 2018 |
| Sim1-Cre mice both sexes |
(−) MEC stella neurons AAV1/2-FLEX-TeLC |
NLP | Not required | 5 min | 3 min | Impaired | Tennant et al., 2018 |
| Subcortical and CB regions: | |||||||
| C57BL/6 male mice |
(−) dmSTR neurons Lenti-TeLC |
NOP | Not required | 10 min | 24 h | Impaired | Qiao et al., 2017 |
| C57BL/6 male mice |
(−) dmSTR neurons Lenti-TeLC |
NLP | Not required | 10 min | 24 h | Impaired | Qiao et al., 2017 |
| C57BL/6 male mice |
(−) NAc neurons Lenti-TeLC |
NOP | Not required | 10 min | 24 h | C.N.E. | Qiao et al., 2017 |
| ChAT-Cre male mice |
(−) MSDB choline neurons AAV-DJ-DIO-TeLC |
NOP | Not required | 10 min | 1 h | C.N.E. | Pimpinella et al., 2021 |
| CRH-Cre mice | (+) CeA CRH neurons AAV-hSyn-DIO-hM3Dq |
NOP | Pre-sample CNO 1.0 mg/kg, i.p. |
3-4 min x3 | 1 h | Impaired | Paretkar & Dimitrov, 2018 |
| CRH-Cre mice | (−) CeA CRH neurons AAV-hSyn-DIO-hM4Di |
NOP | Pre-sample CNO 1.0 mg/kg, i.p. |
3-4 min x3 | 1 h | Facilitated | Paretkar & Dimitrov, 2018 |
| Wildtype male mice |
(+) LC TH+ neurons AAV2/9-PRSx8-hM3Dq |
NOP | Pre-sample CNO 0.3 mg/kg, i.p. |
15 min | 1.5 h | C.N.E. | Fortress et al., 2015 |
| Wildtype male mice |
(−) LC TH+ neurons AAV2/9-PRSx8-hM4Di |
NOP | Pre-sample CNO 0.3 mg/kg, i.p. |
15 min | 1.5 h 24 h |
Impaired Impaired |
Hamlett et al., 2020 |
| MCH-Cre male mice |
(−) LH MCH neurons AAV8-FLEX-Arch into LH |
NOP | During sample trial exploring obj. |
reach 30 s | 1 h | Impaired | Kosse & Burdakov, 2019 |
| GAD65-Cre male mice |
(−) LH GABAergic neurons AAV8-FLEX-Arch into LH |
NOP | During sample trial exploring obj. |
reach 30 s | 1 h | Facilitated | Kosse & Burdakov, 2019 |
| Vgat-Cre mice both sexes |
(+) SuM neurons AAV5-hSyn-DIO-hM3Dq |
NOP | Pre-test 1 h CNO 1.0 mg/kg. i.p. |
5 min | 6 h | C.N.E. | Li et al., 2020a |
| Vgat-Cre mice both sexes |
(−) SuM neurons AAV5-hSyn-DIO-hM4Di |
NOP | Pre-test 1 h CNO 1.0 mg/kg. i.p. |
5 min | 6 h | C.N.E. | Li et al., 2020a |
| GAD2-Cre mice both sexes |
(+) IPN GABAergic neurons AAV2-Ef1a-DIO-ChR2 & opto-fiber in IPN |
NOP T-maze |
During test trial 20 Hz |
5 minx2 | 24 h | Decreased new obj. exploration |
Molas et al., 2017 |
| GAD2-Cre mice both sexes |
(−) IPN GABAergic neurons AAV2-Ef1a-DIO-eNpHR3 & opto-fiber in IPN |
NOP T-maze |
During test trial constant light |
5 minx2 | 24 h | Increased old obj. exploration |
Molas et al., 2017 |
| PCP-Cre: ChR2 both sexes |
(+) CB Purkinje neurons Opto-fiber in CB L4/5 or simplex |
NOP | During sample + test 7 Hz |
10 min | 24 h | C.N.E. | Zeidler et al., 2020 |
| C57BL/6J male mice |
(+) CB Purkinje neurons AAV8-Pcp2-hM3Dq into CB L4/5 |
NOP | Pre-sample CNO 1.0 mg/kg, i.p. |
7 min | 45 min | C.N.E. | Chao et al., 2021 |
| C57BL/6 female mice |
(−) NR excitatory neurons AAV8-CaMKIIα-KORD |
NLP | Pre-sample SALB 10 mg/kg, i.p. |
reach 30s | 4 h | Impaired | Schwabe et al., 2021 |
| C57BL/6 female mice |
(−) NR excitatory neurons AAV8-CaMKIIα-KORD |
NLP | Post-sample SALB 10 mg/kg, i.p. |
reach 30s | 4 h | Impaired | Schwabe et al., 2021 |
| Vgat-Cre mice both sexes |
(+) SuM neurons AAV5-hSyn-DIO-hM3Dq |
NLP | Pre-test 1 h CNO 1.0 mg/kg, i.p. |
5 min | 6 h | Facilitated | Li et al., 2020a |
| Vgat-Cre mice both sexes |
(−) SuM neurons AAV5-hSyn-DIO-hM4Di |
NLP | Pre-test 1 h CNO 1.0 mg/kg. i.p. |
5 min | 6 h | Impaired | Li et al., 2020a |
| ChAT-Cre male mice |
(−) MSDB choline neurons AAV-hSyn-DIO-hM4Di |
NLP | Pre-test 0.5 h CNO 3.0 mg/kg. i.p. |
10 min | 1 h | Impaired | Pimpinella et al., 2021 |
| PCP-Cre: ChR2 both sexes |
(+) CB Purkinje neurons Opto-fiber in CB L4/5 or simplex |
NLP | During sample + test 7 Hz |
5 min | 24 h | Impaired | Zeidler et al., 2020 |
Table 2c.
Pathway-specific manipulations in object exploration tests. AAV: adeno-associated virus or recombinant adeno-associated virus; ACC: anterior cingulate cortex; BLA: basolateral amygdala; CeA: central nucleus amygdala; C.N.E.: compatible with no important effect; CNO: clozapine-N-oxide; DA: dopamine; DG: dentate gyrus; dHPC: dorsal hippocampus; DLS: dorsolateral septum; h: hours; iCA1: intermediate CA1; IPN: interpeduncular nucleus; ISI: inter-sample interval; ITI: inter-trial interval; LC: locus coeruleus; LEC: lateral entorhinal cortex; Lenti: lentiviruses; mHb: medial habenula; min: minutes; mPFC: medial prefrontal cortex; MS: medial septum; NAc: nucleus accumbens; OVX: ovariectomy; pdCA1: posterior dorsal CA1; s: seconds; SuM: supramammillary nucleus; TH: tyrosine hydroxylase; vHPC: ventral hippocampus; VTA: ventral tegmental area; (+) excitation; (−) inhibition. *Two novel objects were replaced out of four objects. **The manipulated group was still able to show a preference for the novel object.
| Animal | Treatment | Time of activation | Encoding | ITI | Findings | Reference |
|---|---|---|---|---|---|---|
| NOP test: | ||||||
| Lister Hooded male rats |
(−) pdCA1 neurons -< mPFC Lenti-lacZ into mPFC + cannule into pdCA1 |
Days before Daun02 4 μg/μl |
4 min x4 | 1 h | C.N.E. | Barker et al., 2017 |
| Lister Hooded male rats |
(−) iCA1 neurons -< mPFC Lenti-lacZ into mPFC + cannule into iCA1 |
Days before Daun02 4 μg/μl |
5 min | 1 h | C.N.E.* | Barker et al., 2017 |
| Gad2-Cre male mice |
(−) LEC GABAergic terminals in dCA1 AAV2/9-Syn-FLEX-PSAM into LEC, cannulae in dCA1 |
Pre-sample PSEM308 15 μM/0.5 μl |
10 min x2 | 10 min | Blunted** | Basu et al., 2016 |
| C57BL/6J male mice |
(+) selectively CeA neurons -< LC AAV-CAG-Cre into LC + AAV-hSyn-DIO-hM3Ds into CeA |
Pre-sample CNO 1 mg/kg, i.p. |
3-4 minx3 | 1 h | Facilitated | Paretkar & Dimitrov, 2018 |
| Long Evans male TH-Cre rats |
(−) LC TH terminals in BLA AAV2-hSyn-DIO-hM4Di into LC, cannulae in BLA |
Pre-sample CNO 3 μM/0.5 μl |
10 min | 3 h 24 h |
C.N.E. C.N.E. |
Llorca-Torralba et al., 2019 |
| Long Evans male TH-Cre rats |
(+) LC TH terminals in BLA AAV2-hSyn-DIO-hM3Ds into LC, cannulae in BLA |
Pre-sample CNO 3 μM/0.5 μl |
10 min | 3 h 24 h |
C.N.E. C.N.E. |
Llorca-Torralba et al., 2019 |
| C57BL/6J mice both sexes |
(−) selectively subiculum excitatory neurons -< CA1 CAV2-Cre into CA1 + AAV2-DIO-hM4Di into subiculum |
Pre-sample CNO 1.5 mg/kg, i.p. |
10 min | 24 h | C.N.E. | Sun et al., 2019 |
| C57BL/6J mice either sex |
(−) selectively CA1 neurons -< dorsal CA3 CAV2-Cre into CA3 + AAV2-DIO-hM4Di into CA1 |
Pre-sample CNO 5.0 mg/kg, i.p. |
10 min | 24 h | Impaired | Lin et al., 2021 |
| C57BL/6 mice female OVX |
(−) mFPC + dHPC excitatory neurons AAV8-CaMKIIα-hM4Di into mPFC AAV8-CaMKIIα-KORD into dHPC |
Post-sample CNO + SALB subdoses |
reach 30s | 24 h | Impaired | Tuscher et al., 2018 |
| C57BL/6 male mice |
(+) selectively vHPC neurons -< mPFC AAV2-Cre into mPFC + AAV8-DIO-hSyn-hM3Dq into vHPC |
Pre-test CNO 3 mg/kg, i.p. |
10 min x7 | 24 h | C.N.E. | Phillips et al., 2019 |
| C57BL/6J male mice |
(−) MS neurons -< dHPC AAV2-Cre into dHPC + AAV9-DIO-hM4Di into MS |
Pre-test CNO 1.0 mg/kg, i.p. |
5 min | 1 h | C.N.E. | Jin et al., 2020 |
| C57BL/6J male mice |
(−) MS neurons -< ACC AAV2-Cre into ACC + AAV9-DIO-hM4Di into MS |
Pre-test CNO 1.0 mg/kg, i.p. |
5 min | 1 h | C.N.E. | Jin et al., 2020 |
| C57BL/6 male mice |
(−) CA2/3 excitatory terminals in CA1 AAV5-CaMKIIα-eNpHR into CA2/3 & opto-fiber into CA1 |
During test trial | 5 min x3 | 5 min | Impaired | Raam et al., 2017 |
| C57BL/6 male mice |
(−) CA2/3 excitatory terminals in pCA1 AAV5-CaMKIIα-eNpHR into CA2/3 & opto-fiber into pCA1 |
During test trial | 5 min x3 | 5 min | C.N.E. | Raam et al., 2017 |
| C57BL/6 male mice |
(−) CA2/3 excitatory terminals in DLS AAV5-CaMKIIα-eNpHR into CA2/3 & opto-fiber into DLS |
During test trial | 5 min x3 | 5 min | C.N.E. | Raam et al., 2017 |
| Trpc4-Cre male mice |
(−) vCA1 excitatory terminals at NAc AAV9-hSyn-DIO-eArchT into vCA1 & opto-fiber into NAc |
During test trial constant light |
6 min x3 | 24 h | C.N.E. | Okuyama et al., 2016 |
| DAT-Cre mice both sexes |
(+) VTA DA terminals in IPN AAV2-Ef1a-DIO-ChR2 into VTA, opto-fiber in IPN |
During test trial 30 Hz |
5 minx2 | 24 h | C.N.E. | Molas et al., 2017 |
| Chat-Cre mice both sexes |
(+) mHb choline-/glutamatergic terminals in IPN AAV2-Ef1a-DIO-ChR2 into mHb, opto-fiber in IPN |
During test trial 20 Hz |
5 minx2 | 24 h | Impaired | Molas et al., 2017 |
| Chat-Cre mice both sexes |
(−) mHb choline-/glutamatergic terminals in IPN AAV2-Ef1a-DIO-NpHR3.0 into mHb, opto-fiber in IPN |
During test trial constant light |
5 minx2 | 24 h | Impaired | Molas et al., 2017 |
| NLP test: | ||||||
| Lister Hooded male rats |
(−) pdCA1 neurons -< mPFC Lenti-lacZ into mPFC + cannule into pdCA1 |
Days before Daun02 4 μg/μl |
3 min | 4 h | C.N.E. | Barker et al., 2017 |
| Lister Hooded male rats |
(−) iCA1 neurons -< mPFC Lenti-lacZ into mPFC + cannule into iCA1 |
Days before Daun02 4 μg/μl |
3 min | 4 h | C.N.E. | Barker et al., 2017 |
| TH-IRES-Cre mice both sexes |
(+) LC TH terminals in dHPC AAV2/5-EF1a-DIO-ChR2 into LC + optic fiber in dHPC |
During sample trial 20 Hz |
5 min | 24 h | Facilitated | Kempadoo et al., 2016 |
| Rosa-LSL-ChR2 both sexes |
(+) selectively subiculum neurons -< CA1 AAVrg-hSyn-Cre into CA1 + opto-fiber in subiculum |
During sample trial 6 Hz |
3 min | 24 h | Facilitated | Sun et al., 2019 |
| Vgat-Cre mice both sexes |
(+) SuM terminals in DG AAV5-EF1α-DIO-ChR2 into SuM + optic fiber in DG |
During sample trial 10 Hz |
5 min | 6 h | C.N.E. | Li et al., 2020a |
| Vgat-Cre mice both sexes |
(−) SuM terminals in DG AAV5-EF1α-DIO-Arch3.0 into SuM + optic fiber in DG |
During sample trial constant light |
5 min | 6 h | C.N.E. | Li et al., 2020a |
| C57BL/6J mice both sexes |
(−) selectively subiculum excitatory neurons -< CA1 CAV2-Cre into CA1 + AAV2-DIO-hM4Di into subiculum |
Pre-sample CNO 1.5 mg/kg, i.p. |
10 min | 24 h | Impaired | Sun et al., 2019 |
| C57BL/6J mice either sex |
(−) selectively CA1 neurons -< dorsal CA3 CAV2-Cre into CA3 + AAV2-DIO-hM4Di into CA1 |
Pre-sample CNO 5.0 mg/kg, i.p. |
10 min | 24 h | Impaired | Lin et al., 2021 |
| C57BL/6 mice female OVX |
(−) mFPC + dHPC excitatory neurons AAV8-CaMKIIα-hM4Di into mPFC + AAV8-CaMKIIα-KORD into dHPC |
Post-sample CNO + SALB subdoses |
reach 30s | 4 h | Impaired | Tuscher et al., 2018 |
| Vgat-Cre mice both sexes |
(+) SuM terminals in DG AAV5-EF1α-DIO-ChR2 into SuM + optic fiber in DG |
During test trial 10 Hz |
5 min | 6 h | Facilitated | Li et al., 2020a |
| Vgat-Cre mice both sexes |
(+) SuM terminals in CA2 AAV5-EF1α-DIO-ChR2 into SuM + optic fiber in CA2 |
During test trial 10 Hz |
5 min | 6 h | C.N.E. | Li et al., 2020a |
| Vgat-Cre mice both sexes |
(−) SuM terminals in DG AAV5-EF1α-DIO-Arch3.0 into SuM + optic fiber in DG |
During test trial constant light |
5 min | 6 h | Impaired | Li et al., 2020a |
| C57BL/6J male mice |
(−) MS neurons -< dHPC AAV2-Cre into dHPC + AAV9-DIO-hM4Di into MS |
Pre-test CNO 1.0 mg/kg, i.p. |
5 min | 1 h | Impaired | Jin et al., 2020 |
| C57BL/6J male mice |
(−) MS neurons -< ACC AAV2-Cre into ACC + AAV9-DIO-hM4Di into MS |
Pre-test CNO 1.0 mg/kg, i.p. |
5 min | 1 h | C.N.E. | Jin et al., 2020 |
| OiP test: | ||||||
| Lister Hooded male rats |
(−) pdCA1 neurons -< mPFC Lenti-lacZ into mPFC + cannule into pdCA1 |
Days before Daun02 4 μg/μl |
5 min | 1 h | C.N.E. | Barker et al., 2017 |
| Lister Hooded male rats |
(−) iCA1 neurons -< mPFC Lenti-lacZ into mPFC + cannule into iCA1 |
Days before Daun02 4 μg/μl |
5 min | 1 h | Impaired | Barker et al., 2017 |
| C57BL/6J male mice |
(−) MS neurons -< dHPC AAV2-Cre into dHPC + AAV9-DIO-hM4Di into MS |
Pre-test CNO 1.0 mg/kg, i.p. |
10 min | 1 h | Impaired | Jin et al., 2020 |
| C57BL/6J male mice |
(−) MS neurons -< ACC AAV2-Cre into ACC + AAV9-DIO-hM4Di into MS |
Pre-test CNO 1.0 mg/kg, i.p. |
10 min | 1 h | C.N.E. | Jin et al., 2020 |
Acknowledgments
We acknowledge funding support by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) grant R15NS112964 to YMY, the Winston and Maxine Wallin Neuroscience Discovery Fund to YMY, the Academic Investment Research Program-Individual Principal Investigator Award AIRP2-IND-67 to YMY, and the Brain & Behavior Research Foundation (BBRF) Young Investigator Grant (29192) to OYC.
References
- Aarse J, Herlitze S, Manahan-Vaughan D (2016) The requirement of BDNF for hippocampal synaptic plasticity is experience-dependent. Hippocampus 26:739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Ishida Y, Nonaka H, Iwasaki T (2009) Functional difference between rat perirhinal cortex and hippocampus in object and place discrimination tasks. Behav Brain Res 197:388–397. [DOI] [PubMed] [Google Scholar]
- Adamantidis A, de Lecea L (2009) A role for melanin-concentrating hormone in learning and memory. Peptides 30:2066–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP (1985) One-trial object recognition by rats. Q J Exp Psychol-B 37:279–294. [Google Scholar]
- Aggleton JP (2010) Understanding retrosplenial amnesia: insights from animal studies. Neuropsychologia 48:2328–2338. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Mishkin M (1983) Visual recognition impairment following medial thalamic lesions in monkeys. Neuropsychologia 21:189–197. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Nelson AJD (2020) Distributed interactive brain circuits for object-in-place memory: A place for time? Brain Neurosci Adv 4:2398212820933471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Keen S, Warburton EC, Bussey TJ (1997) Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res Bull 43:279–287. [DOI] [PubMed] [Google Scholar]
- Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER (2006) The role of the hippocampus in object recognition in rats: examination of the influence of task parameters and lesion size. Behav Brain Res 167:183–195. [DOI] [PubMed] [Google Scholar]
- Albasser MM, Poirier GL, Warburton EC, Aggleton JP (2007) Hippocampal lesions halve immediate-early gene protein counts in retrosplenial cortex: distal dysfunctions in a spatial memory system. Eur J Neurosci 26:1254–1266. [DOI] [PubMed] [Google Scholar]
- Albasser MM, Amin E, Lin TC, Iordanova MD, Aggleton JP (2012) Evidence that the rat hippocampus has contrasting roles in object recognition memory and object recency memory. Behav Neurosci 126:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser MM, Chapman RJ, Amin E, Iordanova MD, Vann SD, Aggleton JP (2010) New behavioral protocols to extend our knowledge of rodent object recognition memory. Learn Mem 17:407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser MM, Amin E, Iordanova MD, Brown MW, Pearce JM, Aggleton JP (2011) Separate but interacting recognition memory systems for different senses: the role of the rat perirhinal cortex. Learn Mem 18:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser MM, Olarte-Sanchez CM, Amin E, Brown MW, Kinnavane L, Aggleton JP (2015) Perirhinal cortex lesions in rats: Novelty detection and sensitivity to interference. Behav Neurosci 129:227–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL (2009) Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianova L, Yanakieva S, Margetts-Smith G, Kohli S, Brady ES, Aggleton JP, Craig MT (2022) No evidence from complementary data sources of a direct projection from the mouse anterior cingulate cortex to the hippocampal formation. Preprint:doi.org/ 10.1101/2022.1101/1125.477805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A 104:5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgeirsdottir HN, Cohen SJ, Stackman RW Jr. (2020) Object and place information processing by CA1 hippocampal neurons of C57BL/6J mice. J Neurophysiol 123:1247–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Deisseroth K (2013) Recent advances in optogenetics and pharmacogenetics. Brain Res 1511:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babayan BM, Watilliaux A, Viejo G, Paradis AL, Girard B, Rondi-Reig L (2017) A hippocampo-cerebellar centred network for the learning and execution of sequence-based navigation. Sci Rep 7:17812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F (2008) The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem 15:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FF, Santos JR, Meurer YS, Macedo PT, Ferreira LM, Pontes IM, Ribeiro AM, Silva RH (2013) Differential cortical c-Fos and Zif-268 expression after object and spatial memory processing in a standard or episodic-like object recognition task. Front Behav Neurosci 7:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC (2008) NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. J Neurosci 28:2837–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC (2009) Critical role of the cholinergic system for object-in-place associative recognition memory. Learn Mem 16:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC (2011) When is the hippocampus involved in recognition memory? J Neurosci 31:10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC (2015) Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: a critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefrontal cortices. Cereb Cortex 25:472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC (2020a) Multi-level analyses of associative recognition memory: the whole is greater than the sum of its parts. Curr Opin Behav Sci 32:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC (2007) Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 27:2948–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Tran S, Gilroy K, Bashir ZI, Warburton EC (2021) Encoding and retrieval of associative recogntion memory engage different sub-networks within a hippocampal-thalamo-cortical memory circuit. Preprint:doi.org/ 10.21203/rs.21203.rs-889826/v889821. [DOI] [Google Scholar]
- Barker GR, Banks PJ, Scott H, Ralph GS, Mitrophanous KA, Wong LF, Bashir ZI, Uney JB, Warburton EC (2017) Separate elements of episodic memory subserved by distinct hippocampal-prefrontal connections. Nat Neurosci 20:242–250. [DOI] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC (2018) A critical role for the nucleus reuniens in long-term, but not short-term associative recognition memory formation. J Neurosci 38:3208–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC (2020b) Putting objects in context: A prefrontal-hippocampal-perirhinal cortex network. Brain Neurosci Adv 4:2398212820937621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsegyan A, Mirone G, Ronzoni G, Guo C, Song Q, van Kuppeveld D, Schut EHS, Atsak P, Teurlings S, McGaugh JL, Schubert D, Roozendaal B (2019) Glucocorticoid enhancement of recognition memory via basolateral amygdala-driven facilitation of prelimbic cortex interactions. Proc Natl Acad Sci U S A 116:7077–7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ (2007a) Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci 27:2548–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ (2007b) Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learn Mem 14:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, Siegelbaum SA (2016) Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science 351:aaa5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer Z, Chwiesko C, Kitsukawa T, Sauvage MM (2013) Spatial and stimulus-type tuning in the LEC, MEC, POR, PrC, CA1, and CA3 during spontaneous item recognition memory. Hippocampus 23:1425–1438. [DOI] [PubMed] [Google Scholar]
- Beldjoud H, Barsegyan A, Roozendaal B (2015) Noradrenergic activation of the basolateral amygdala enhances object recognition memory and induces chromatin remodeling in the insular cortex. Front Behav Neurosci 9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE (2009) The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 73:1699–1704. [DOI] [PubMed] [Google Scholar]
- Benn A, Barker GR, Stuart SA, Roloff EV, Teschemacher AG, Warburton EC, Robinson ES (2016) Optogenetic stimulation of prefrontal glutamatergic neurons enhances recognition memory. J Neurosci 36:4930–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Rattoni F (2014) The forgotten insular cortex: its role on recognition memory formation. Neurobiol Learn Mem 109:207–216. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Okuda S, Roozendaal B, McGaugh JL (2005) Insular cortex is involved in consolidation of object recognition memory. Learn Mem 12:447–449. [DOI] [PubMed] [Google Scholar]
- Boehringer R, Polygalov D, Huang AJY, Middleton SJ, Robert V, Wintzer ME, Piskorowski RA, Chevaleyre V, McHugh TJ (2017) Chronic loss of CA2 transmission leads to hippocampal hyperexcitability. Neuron 94:642–655 e649. [DOI] [PubMed] [Google Scholar]
- Bohne P, Schwarz MK, Herlitze S, Mark MD (2019) A new projection from the deep cerebellar nuclei to the hippocampus via the ventrolateral and laterodorsal thalamus in mice. Front Neural Circuits 13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota M, Sporns O, Swanson LW (2015) Architecture of the cerebral cortical association connectome underlying cognition. Proc Natl Acad Sci U S A 112:E2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigadski T, Leßmann V (2020) The physiology of regulated BDNF release. Cell Tissue Res 382:15–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE (2004) Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A 101:14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE (2010) Object recognition memory and the rodent hippocampus. Learn Mem 17:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP (2001) Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2:51–61. [DOI] [PubMed] [Google Scholar]
- Browning PG, Chakraborty S, Mitchell AS (2015) Evidence for mediodorsal thalamus and prefrontal cortex interactions during cognition in macaques. Cereb Cortex 25:4519–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Reber PJ, Squire LR (1998) The human perirhinal cortex and recognition memory. Hippocampus 8:330–339. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM (1999) Dissociation between the effects of damage to perirhinal cortex and area TE. Learn Mem 6:572–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett CJ, Krashes MJ (2016) Resolving behavioral output via chemogenetic designer receptors exclusively activated by designer drugs. J Neurosci 36:9268–9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG (1998a) Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol 391:293–321. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG (1998b) Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol 398:179–205. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG (1995) Perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus 5:390–408. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Aggleton JP (1999) Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci 19:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, Aggleton JP (2000) Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res 111:187–202. [DOI] [PubMed] [Google Scholar]
- Cai L, Gibbs RB, Johnson DA (2012) Recognition of novel objects and their location in rats with selective cholinergic lesion of the medial septum. Neurosci Lett 506:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K (2019) Cerebellar modulation of the reward circuitry and social behavior. Science 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulo M, Van Hecke J, Toma L, Ferretti A, Tartaro A, Colosimo C, Romani GL, Uncini A (2005) Functional MRI study of diencephalic amnesia in Wernicke-Korsakoff syndrome. Brain 128:1584–1594. [DOI] [PubMed] [Google Scholar]
- Cavalcante LES, Zinn CG, Schmidt SD, Saenger BF, Ferreira FF, Furini CRG, Myskiw JC, Izquierdo I (2017) Modulation of the storage of social recognition memory by neurotransmitter systems in the insular cortex. Behav Brain Res 334:129–134. [DOI] [PubMed] [Google Scholar]
- Chao OY, Pum ME, Huston JP (2013) The interaction between the dopaminergic forebrain projections and the medial prefrontal cortex is critical for memory of objects: implications for Parkinson's disease. Exp Neurol 247:373–382. [DOI] [PubMed] [Google Scholar]
- Chao OY, Huston JP, Nikolaus S, de Souza Silva MA (2016a) Concurrent assessment of memory for object and place: Evidence for different preferential importance of perirhinal cortex and hippocampus and for promnestic effect of a neurokinin-3 R agonist. Neurobiol Learn Mem 130:149–158. [DOI] [PubMed] [Google Scholar]
- Chao OY, de Souza Silva MA, Yang YM, Huston JP (2020) The medial prefrontal cortex - hippocampus circuit that integrates information of object, place and time to construct episodic memory in rodents: Behavioral, anatomical and neurochemical properties. Neurosci Biobehav Rev 113:373–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao OY, Huston JP, Li JS, Wang AL, de Souza Silva MA (2016b) The medial prefrontal cortex-lateral entorhinal cortex circuit is essential for episodic-like memory and associative object-recognition. Hippocampus 26:633–645. [DOI] [PubMed] [Google Scholar]
- Chao OY, Nikolaus S, Lira Brandao M, Huston JP, de Souza Silva MA (2017) Interaction between the medial prefrontal cortex and hippocampal CA1 area is essential for episodic-like memory in rats. Neurobiol Learn Mem 141:72–77. [DOI] [PubMed] [Google Scholar]
- Chao OY, Zhang H, Pathak SS, Huston JP, Yang YM (2021) Functional Convergence of Motor and Social Processes in Lobule IV/V of the Mouse Cerebellum. Cerebellum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Gilmore AW, Nelson SM, McDermott KB (2017) Are there multiple kinds of episodic memory? An fMRI investigation comparing autobiographical and recognition memory tasks. J Neurosci 37:2764–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Barsegyan A, Nadif Kasri N, Roozendaal B (2018) Basolateral amygdala noradrenergic activity is required for enhancement of object recognition memory by histone deacetylase inhibition in the anterior insular cortex. Neuropharmacology 141:32–41. [DOI] [PubMed] [Google Scholar]
- Christiansen K, Dillingham CM, Wright NF, Saunders RC, Vann SD, Aggleton JP (2016) Complementary subicular pathways to the anterior thalamic nuclei and mammillary bodies in the rat and macaque monkey brain. Eur J Neurosci 43:1044–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Kesner RP (2011) Hippocampal-prefrontal dynamics in spatial working memory: interactions and independent parallel processing. Behav Brain Res 225:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinalli DA Jr., Cohen SJ, Guthrie K, Stackman RW Jr. (2020) Object recognition memory: distinct yet complementary roles of the mouse CA1 and perirhinal cortex. Front Mol Neurosci 13:527543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Malenka RC (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33:18–41. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR (2000) Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20:8853–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccurello R, Oliverio A, Mele A (2012) Dopamine-glutamate interplay in the ventral striatum modulates spatial learning in a receptor subtype-dependent manner. Neuropsychopharmacology 37:1122–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW Jr. (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW Jr. (2013) The rodent hippocampus is essential for nonspatial object memory. Curr Biol 23:1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole E, Simundic A, Mossa FP, Mumby DG (2019) Assessing object-recognition memory in rats: Pitfalls of the existent tasks and the advantages of a new test. Learn Behav 47:141–155. [DOI] [PubMed] [Google Scholar]
- Collitti-Klausnitzer J, Hagena H, Dubovyk V, Manahan-Vaughan D (2021) Preferential frequency-dependent induction of synaptic depression by the lateral perforant path and of synaptic potentiation by the medial perforant path inputs to the dentate gyrus. Hippocampus 31:957–981. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzman YF, Gao C, Jovasevic V, Guedea AL, Radulovic J (2011) NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci 31:11655–11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM (2010) Components of recognition memory: dissociable cognitive processes or just differences in representational complexity? Hippocampus 20:1245–1262. [DOI] [PubMed] [Google Scholar]
- Cross L, Brown MW, Aggleton JP, Warburton EC (2013) The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative recognition and recency but not item recognition. Learn Mem 20:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT (2013) New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci 14:743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL (2010) A simple role for BDNF in learning and memory? Front Mol Neurosci 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danet L, Pariente J, Eustache P, Raposo N, Sibon I, Albucher JF, Bonneville F, Peran P, Barbeau EJ (2017) Medial thalamic stroke and its impact on familiarity and recollection. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg H, Pabst M, Braganza O, Schoch S, Niediek J, Bayraktar M, Mormann F, Beck H (2015) Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks. J Neurosci 35:8394–8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD (2009) Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proc Natl Acad Sci U S A 106:14664–14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD (2010) Restricting dopaminergic signaling to either dorsolateral or medial striatum facilitates cognition. J Neurosci 30:1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashkoff J, Lerner EP, Truong N, Klickstein JA, Fan Z, Mu D, Maguire CA, Hyman BT, Hudry E (2016) Tailored transgene expression to specific cell types in the central nervous system after peripheral injection with AAV9. Mol Ther Methods Clin Dev 3:16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashniani MG, Burjanadze MA, Naneishvili TL, Chkhikvishvili NC, Beselia GV, Kruashvili LB, Pochkhidze NO, Chighladze MR (2015) Exploratory behavior and recognition memory in medial septal electrolytic, neuro- and immunotoxic lesioned rats. Physiol Res 64:755–767. [DOI] [PubMed] [Google Scholar]
- Dashniani MG, Burjanadze MA, Chkhikvishvili NC, Solomonia RO, Kandashvili M, Naneishvili TL, Beselia GV, Kruashvili LB, Chighladze MR (2020) Modulation of spatial memory and expression of hippocampal neurotransmitter receptors by selective lesion of medial septal cholinergic and GABAergic neurons. Exp Brain Res 238:2385–2397. [DOI] [PubMed] [Google Scholar]
- Davis S, Laroche S (2006) Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: a review. Genes Brain Behav 5 Suppl 2:61–72. [DOI] [PubMed] [Google Scholar]
- Davis S, Renaudineau S, Poirier R, Poucet B, Save E, Laroche S (2010) The formation and stability of recognition memory: what happens upon recall? Front Behav Neurosci 4:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Pare D (2004) The rhinal cortices: a wall of inhibition between the neocortex and the hippocampus. Prog Neurobiol 74:101–110. [DOI] [PubMed] [Google Scholar]
- de Landeta AB, Pereyra M, Medina JH, Katche C (2020) Anterior retrosplenial cortex is required for long-term object recognition memory. Sci Rep 10:4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Landeta AB, Pereyra M, Miranda M, Bekinschtein P, Medina JH, Katche C (2021) Functional connectivity of anterior retrosplenial cortex in object recognition memory. Neurobiol Learn Mem 186:107544. [DOI] [PubMed] [Google Scholar]
- De Leonibus E, Oliverio A, Mele A (2005) A study on the role of the dorsal striatum and the nucleus accumbens in allocentric and egocentric spatial memory consolidation. Learn Mem 12:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa AF, Cowansage KK, Zutshi I, Cardozo LM, Yoo EJ, Leutgeb S, Mayford M (2019) Optogenetic reactivation of memory ensembles in the retrosplenial cortex induces systems consolidation. Proc Natl Acad Sci U S A 116:8576–8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Silva MA, Huston JP, Wang AL, Petri D, Chao OY (2016) Evidence for a specific integrative mechanism for episodic memory mediated by AMPA/kainate receptors in a circuit involving medial prefrontal cortex and hippocampal CA3 region. Cereb Cortex 26:3000–3009. [DOI] [PubMed] [Google Scholar]
- Deisseroth K (2015) Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci 18:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ (2006) Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci 26:10380–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo LA, Berns DS, DeLoach K, Luo L (2015) Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing. Nat Neurosci 18:1687–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA (2005) Integrated memory for objects, places, and temporal order: evidence for episodic-like memory in mice. Neurobiol Learn Mem 84:214–221. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ (2011) Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci 5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ (2013) Influence of local objects on hippocampal representations: Landmark vectors and memory. Hippocampus 23:253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverett B, Kislin M, Tank DW, Wang SS (2019) Cerebellar disruption impairs working memory during evidence accumulation. Nat Commun 10:3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillingham CM, Milczarek MM, Perry JC, Frost BE, Parker GD, Assaf Y, Sengpiel F, O'Mara SM, Vann SD (2019) Mammillothalamic disconnection alters hippocampocortical oscillatory activity and microstructure: implications for diencephalic amnesia. J Neurosci 39:6696–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidschstein J et al. (2016) A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat Neurosci 19:1743–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan AR, Varela C, Zhang Y, Shen Y, Xiong L, Wilson MA, Lisman J (2015) Delta frequency optogenetic stimulation of the thalamic nucleus reuniens is sufficient to produce working memory deficits: relevance to schizophrenia. Biol Psychiatry 77:1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont JR, Aggleton JP (2013) Dissociation of recognition and recency memory judgments after anterior thalamic nuclei lesions in rats. Behav Neurosci 127:415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont JR, Amin E, Poirier GL, Albasser MM, Aggleton JP (2012) Anterior thalamic nuclei lesions in rats disrupt markers of neural plasticity in distal limbic brain regions. Neuroscience 224:81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Norman G (2004) Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci 24:1948–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A, Fitchett AE, Eacott MJ, Baxter MG (2011) Medial septal cholinergic neurons are necessary for context-place memory but not episodic-like memory. Hippocampus 21:1021–1027. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2017) Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci 18:547–558. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C (2007) The medial temporal lobe and recognition memory. Annual Review of Neuroscience 30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31:47–59. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP (1997) The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res 88:181–193. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP (1996) Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res 80:9–25. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP (1997) Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res 113:509–519. [DOI] [PubMed] [Google Scholar]
- Ernst TM, Brol AE, Gratz M, Ritter C, Bingel U, Schlamann M, Maderwald S, Quick HH, Merz CJ, Timmann D (2019) The cerebellum is involved in processing of predictions and prediction errors in a fear conditioning paradigm. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (1997) Central cholinergic systems and cognition. Annu Rev Psychol 48:649–684. [DOI] [PubMed] [Google Scholar]
- Ferraris M, Ghestem A, Vicente AF, Nallet-Khosrofian L, Bernard C, Quilichini PP (2018) The nucleus reuniens controls long-range hippocampo-prefrontal gamma synchronization during slow oscillations. J Neurosci 38:3026–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Hamlett ED, Vazey EM, Aston-Jones G, Cass WA, Boger HA, Granholm AC (2015) Designer receptors enhance memory in a mouse model of Down syndrome. J Neurosci 35:1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ (2005) Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus 15:347–355. [DOI] [PubMed] [Google Scholar]
- Frank AC, Huang S, Zhou M, Gdalyahu A, Kastellakis G, Silva TK, Lu E, Wen X, Poirazi P, Trachtenberg JT, Silva AJ (2018) Hotspots of dendritic spine turnover facilitate clustered spine addition and learning and memory. Nat Commun 9:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost BE, Martin SK, Cafalchio M, Islam MN, Aggleton JP, O'Mara SM (2021) Anterior thalamic inputs are required for subiculum spatial coding, with associated consequences for hippocampal spatial memory. J Neurosci 41:6511–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Kodama T, du Lac S (2020) Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S (2013) Thalamic mediodorsal nucleus and its participation in spatial working memory processes: comparison with the prefrontal cortex. Front Syst Neurosci 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Ahmed OJ, Burwell RD (2012) Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination. Neuron 76:976–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB (2004) Spatial representation in the entorhinal cortex. Science 305:1258–1264. [DOI] [PubMed] [Google Scholar]
- Gaffan D (1974) Recognition impaired and association intact in the memory of monkeys after transection of the fornix. J Comp Physiol Psychol 86:1100–1109. [DOI] [PubMed] [Google Scholar]
- Gaffan D (1994) Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res 99:411–422. [DOI] [PubMed] [Google Scholar]
- Gangadharan G, Shin J, Kim SW, Kim A, Paydar A, Kim DS, Miyazaki T, Watanabe M, Yanagawa Y, Kim J, Kim YS, Kim D, Shin HS (2016) Medial septal GABAergic projection neurons promote object exploration behavior and type 2 theta rhythm. Proc Natl Acad Sci U S A 113:6550–6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz SC, Ortiz MM, Belilos AJ, Moussawi K (2021) Excitation of medium spiny neurons by 'inhibitory' ultrapotent chemogenetics via shifts in chloride reversal potential. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin S, Tremblay A, Mumby DG (2003) Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus 13:962–969. [DOI] [PubMed] [Google Scholar]
- Gehrlach DA, Weiand C, Gaitanos TN, Cho E, Klein AS, Hennrich AA, Conzelmann KK, Gogolla N (2020) A whole-brain connectivity map of mouse insular cortex. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Mumby DG (1998) Place memory is intact in rats with perirhinal cortex lesions. Behavioral Neuroscience 112:1353–1365. [DOI] [PubMed] [Google Scholar]
- Goh JJ, Manahan-Vaughan D (2013) Spatial object recognition enables endogenous LTD that curtails LTP in the mouse hippocampus. Cereb Cortex 23:1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M (2017) Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good MA, Hale G, Staal V (2007a) Impaired "episodic-like" object memory in adult APPswe transgenic mice. Behav Neurosci 121:443–448. [DOI] [PubMed] [Google Scholar]
- Good MA, Barnes P, Staal V, McGregor A, Honey RC (2007b) Context- but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behav Neurosci 121:218–223. [DOI] [PubMed] [Google Scholar]
- Grossman N, Simiaki V, Martinet C, Toumazou C, Schultz SR, Nikolic K (2013) The spatial pattern of light determines the kinetics and modulates backpropagation of optogenetic action potentials. J Comput Neurosci 34:477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW (1995) Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat 187 ( Pt 3):583–592. [PMC free article] [PubMed] [Google Scholar]
- Haam J, Zhou J, Cui G, Yakel JL (2018) Septal cholinergic neurons gate hippocampal output to entorhinal cortex via oriens lacunosum moleculare interneurons. Proc Natl Acad Sci U S A 115:E1886–E1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Sun Y, Wood MA, Xu X (2013) Cell-type specific inactivation of hippocampal CA1 disrupts location-dependent object recognition in the mouse. Learn Mem 20:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA (2011) HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem 18:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436:801–806. [DOI] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D (2011) Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb Cortex 21:2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Kastner S (2017) Thalamic functions in distributed cognitive control. Nat Neurosci 20:1669–1679. [DOI] [PubMed] [Google Scholar]
- Hales JB, Schlesiger MI, Leutgeb JK, Squire LR, Leutgeb S, Clark RE (2014) Medial entorhinal cortex lesions only partially disrupt hippocampal place cells and hippocampus-dependent place memory. Cell Rep 9:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales JB, Vincze JL, Reitz NT, Ocampo AC, Leutgeb S, Clark RE (2018) Recent and remote retrograde memory deficit in rats with medial entorhinal cortex lesions. Neurobiol Learn Mem 155:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL, Griffin AL (2013) Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behav Neurosci 127:860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlett ED, Ledreux A, Gilmore A, Vazey EM, Aston-Jones G, Boger HA, Paredes D, Granholm AE (2020) Inhibitory designer receptors aggravate memory loss in a mouse model of down syndrome. Neurobiol Dis 134:104616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW (2004) On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem 82:26–34. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA (2007) Neuronal competition and selection during memory formation. Science 316:457–460. [DOI] [PubMed] [Google Scholar]
- Han RW, Xu HJ, Zhang RS, Wang P, Chang M, Peng YL, Deng KY, Wang R (2014) Neuropeptide S interacts with the basolateral amygdala noradrenergic system in facilitating object recognition memory consolidation. Neurobiol Learn Mem 107:32–36. [DOI] [PubMed] [Google Scholar]
- Hannesson DK, Howland JG, Phillips AG (2004) Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci 24:4596–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesson DK, Howland JG, Pollock M, Mohapel P, Wallace AE, Corcoran ME (2005) Anterior perirhinal cortex kindling produces long-lasting effects on anxiety and object recognition memory. Eur J Neurosci 21:1081–1090. [DOI] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, O'Keefe J (2014) Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc Lond B Biol Sci 369:20120510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer BE, Pagliardini S, Dickson CT (2019) The reuniens nucleus of the thalamus has an essential role in coordinating slow-wave activity between neocortex and hippocampus. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H (2015) Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Oguro M, Sato N (2020) Involvement of the retrosplenial cortex in the processing of the temporal aspect of episodic-like memory in rats. Neurosci Res 154:52–55. [DOI] [PubMed] [Google Scholar]
- Heimer-McGinn VR, Poeta DL, Aghi K, Udawatta M, Burwell RD (2017) Disconnection of the perirhinal and postrhinal cortices impairs recognition of objects in context but not contextual fear conditioning. J Neurosci 37:4819–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE (2008) Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci 27:654–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ (1979) Efferent connections of the habenular nuclei in the rat. J Comp Neurol 187:19–47. [DOI] [PubMed] [Google Scholar]
- Hindley EL, Nelson AJ, Aggleton JP, Vann SD (2014) Dysgranular retrosplenial cortex lesions in rats disrupt cross-modal object recognition. Learn Mem 21:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA (2014) The hippocampal CA2 region is essential for social memory. Nature 508:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JW, Poeta DL, Jacobson TK, Zolnik TA, Neske GT, Connors BW, Burwell RD (2015) Bidirectional modulation of recognition memory. J Neurosci 35:13323–13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TH, Aliane V, Manahan-Vaughan D (2018) Novel encoding and updating of positional, or directional, spatial cues are processed by distinct hippocampal subfields: Evidence for parallel information processing and the "what" stream. Hippocampus 28:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TH, Boge J, Manahan-Vaughan D (2021) Hippocampal subfield-specific Homer1a expression is triggered by learning-facilitated long-term potentiation and long-term depression at medial perforant path synapses. Hippocampus 31:897–915. [DOI] [PubMed] [Google Scholar]
- Hoche F, Guell X, Sherman JC, Vangel MG, Schmahmann JD (2016) Cerebellar Contribution to Social Cognition. Cerebellum 15:732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Caroni P (2016) Functional and structural underpinnings of neuronal assembly formation in learning. Nat Neurosci 19:1553–1562. [DOI] [PubMed] [Google Scholar]
- Hooper A, Fuller PM, Maguire J (2018) Hippocampal corticotropin-releasing hormone neurons support recognition memory and modulate hippocampal excitability. PLoS One 13:e0191363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP (2012) Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Struct Funct 217:191–209. [DOI] [PubMed] [Google Scholar]
- Hoshino C, Konno A, Hosoi N, Kaneko R, Mukai R, Nakai J, Hirai H (2021) GABAergic neuron-specific whole-brain transduction by AAV-PHP.B incorporated with a new GAD65 promoter. Mol Brain 14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui Y, Zheng X, Zhang H, Li F, Yu G, Li J, Zhang J, Gong X, Guo G (2022) Strategies for Targeting Neural Circuits: How to Manipulate Neurons Using Virus Vehicles. Front Neural Circuits 16:882366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Chen V, Tran GT, Kesner RP (2013) The medial and lateral entorhinal cortex both contribute to contextual and item recognition memory: a test of the binding of items and context model. Hippocampus 23:380–391. [DOI] [PubMed] [Google Scholar]
- Isaac CL, Holdstock JS, Cezayirli E, Roberts JN, Holmes CJ, Mayes AR (1998) Amnesia in a patient with lesions limited to the dorsomedial thalamic nucleus. Neurocase 4:497–508. [Google Scholar]
- Jacklin DL, Cloke JM, Potvin A, Garrett I, Winters BD (2016) The dynamic multisensory engram: neural circuitry underlying crossmodal object recognition in rats changes with the nature of object experience. J Neurosci 36:1273–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Ronnqvist KC, Tsanov M, Vann SD, Wright NF, Erichsen JT, Aggleton JP, O'Mara SM (2013) The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front Syst Neurosci 7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran M, Linley SB, Schlecht M, Mahler SV, Vertes RP, Allen TA (2019) Prefrontal pathways provide top-down control of memory for sequences of events. Cell Rep 28:640–654 e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L, Paninski L, Hen R, Kheirbek MA (2018) Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97:670–683 e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Chen R, Shao M, Yang X, Ma L, Wang F (2020) Dorsal hippocampus- and ACC-projecting medial septum neurons differentially contribute to the recollection of episodic-like memory. FASEB J 34:11741–11753. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Tonegawa S (2020) Memory engrams: Recalling the past and imagining the future. Science 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Huh Y, Cho J (2019) The ventral midline thalamus mediates hippocampal spatial Information processes upon spatial cue changes. J Neurosci 39:2276–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaboodvand N, Backman L, Nyberg L, Salami A (2018) The retrosplenial cortex: A memory gateway between the cortical default mode network and the medial temporal lobe. Hum Brain Mapp 39:2020–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JB, Port RG, Anderson SA, Coulter DA (2020) Modular, circuit-based interventions rescue hippocampal-dependent social and spatial memory in a 22q11.2 deletion syndrome mouse model. Biol Psychiatry 88:710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JB, Port RG, Yue C, Takano H, Coulter DA (2019) Circuit-based interventions in the dentate gyrus rescue epilepsy-associated cognitive dysfunction. Brain 142:2705–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR (2014) The molecular and systems biology of memory. Cell 157:163–186. [DOI] [PubMed] [Google Scholar]
- Kart-Teke E, De Souza Silva MA, Huston JP, Dere E (2006) Wistar rats show episodic-like memory for unique experiences. Neurobiol Learn Mem 85:173–182. [DOI] [PubMed] [Google Scholar]
- Katche C, Dorman G, Gonzalez C, Kramar CP, Slipczuk L, Rossato JI, Cammarota M, Medina JH (2013) On the role of retrosplenial cortex in long-lasting memory storage. Hippocampus 23:295–302. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ (2008) Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behav Neurosci 122:1070–1077. [DOI] [PubMed] [Google Scholar]
- Kelly E et al. (2020) Regulation of autism-relevant behaviors by cerebellar-prefrontal cortical circuits. Nat Neurosci 23:1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D (2004) Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A 101:8192–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D (2008) The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex 18:968–977. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A 113:14835–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hunt ME, Williams JM, Long JM (1996) Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex 6:311–318. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Ravindranathan A, Jackson P, Giles R, Chiba AA (2001) A neural circuit analysis of visual recognition memory: role of perirhinal, medial, and lateral entorhinal cortex. Learn Mem 8:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketz NA, Jensen O, O'Reilly RC (2015) Thalamic pathways underlying prefrontal cortex-medial temporal lobe oscillatory interactions. Trends Neurosci 38:3–12. [DOI] [PubMed] [Google Scholar]
- Kim JM, Kim DH, Lee Y, Park SJ, Ryu JH (2014) Distinct roles of the hippocampus and perirhinal cortex in GABAA receptor blockade-induced enhancement of object recognition memory. Brain Res 1552:17–25. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P (2008) Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev 29:169–195. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP, Deshmukh SS (2014) Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc Lond B Biol Sci 369:20130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Srebro B (1980) Effects of lateral and medial septal lesions on exploratory behavior in the albino rat. Brain Res 182:423–440. [DOI] [PubMed] [Google Scholar]
- Kolb B, Buhrmann K, McDonald R, Sutherland RJ (1994) Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cereb Cortex 4:664–680. [DOI] [PubMed] [Google Scholar]
- Kornecook TJ, Anzarut A, Pinel JP (1999) Rhinal cortex, but not medial thalamic, lesions cause retrograde amnesia for objects in rats. Neuroreport 10:2853–2858. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gardner RS, Tunur T, Gold PE (2019) Involvement of lactate transport in two object recognition tasks that require either the hippocampus or striatum. Behav Neurosci 133:176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosse C, Burdakov D (2019) Natural hypothalamic circuit dynamics underlying object memorization. Nat Commun 10:2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostadinov D, Beau M, Blanco-Pozo M, Hausser M (2019) Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells. Nat Neurosci 22:950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT (2009) Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci 12:1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SP, Nakamura NH, Maingret N, Mahnke L, Yoshida M, Sauvage MM (2017) Regional specific evidence for memory-load dependent activity in the dorsal subiculum and the lateral entorhinal cortex. Front Syst Neurosci 11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla MV, Ainge JA (2017) Lateral entorhinal cortex lesions impair local spatial frameworks. Front Syst Neurosci 11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha A, Thakur MK (2020) Increase in hippocampal histone H3K9me3 is negatively correlated with memory in old male mice. Biogerontology 21:175–189. [DOI] [PubMed] [Google Scholar]
- Langston RF, Wood ER (2010) Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus 20:1139–1153. [DOI] [PubMed] [Google Scholar]
- Leão RN, Mikulovic S, Leao KE, Munguba H, Gezelius H, Enjin A, Patra K, Eriksson A, Loew LM, Tort AB, Kullander K (2012) OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci 15:1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G (1994) Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience 62:1033–1047. [DOI] [PubMed] [Google Scholar]
- Lefort JM, Vincent J, Tallot L, Jarlier F, De Zeeuw CI, Rondi-Reig L, Rochefort C (2019) Impaired cerebellar Purkinje cell potentiation generates unstable spatial map orientation and inaccurate navigation. Nat Commun 10:2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bao H, Luo Y, Yoan C, Sullivan HA, Quintanilla L, Wickersham I, Lazarus M, Shih YI, Song J (2020a) Supramammillary nucleus synchronizes with dentate gyrus to regulate spatial memory retrieval through glutamate release. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y et al. (2020b) Distinct subnetworks of the thalamic reticular nucleus. Nature 583:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Amalraj M, Blanton C, Avila B, Holmes TC, Nitz DA, Xu X (2021) Noncanonical projections to the hippocampal CA3 regulate spatial learning and memory by modulating the feedforward hippocampal trisynaptic pathway. PLoS Biol 19:e3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Bilkey DK (2001) The effect of excitotoxic lesions centered on the hippocampus or perirhinal cortex in object recognition and spatial memory tasks. Behav Neurosci 115:94–111. [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S (2012) Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca-Torralba M, Suarez-Pereira I, Bravo L, Camarena-Delgado C, Garcia-Partida JA, Mico JA, Berrocoso E (2019) Chemogenetic silencing of the locus coeruleus-basolateral amygdala pathway abolishes pain-induced anxiety and enhanced aversive learning in rats. Biol Psychiatry 85:1021–1035. [DOI] [PubMed] [Google Scholar]
- Locke TM, Soden ME, Miller SM, Hunker A, Knakal C, Licholai JA, Dhillon KS, Keene CD, Zweifel LS, Carlson ES (2018) Dopamine D1 receptor-positive neurons in the lateral nucleus of the cerebellum contribute to cognitive behavior. Biol Psychiatry 84:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AJ, Kramar E, Matheos DP, White AO, Kwapis J, Vogel-Ciernia A, Sakata K, Espinoza M, Wood MA (2016) Promoter-specific effects of DREADD modulation on hippocampal synaptic plasticity and memory formation. J Neurosci 36:3588–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro M, Cholvin T, Lopez J, Merienne N, Latreche A, Cosquer B, Geiger K, Kelche C, Cassel JC, Pereira de Vasconcelos A (2012) The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. J Neurosci 32:9947–9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Zuo Y (2022) Synaptic modifications in learning and memory - A dendritic spine story. Semin Cell Dev Biol 125:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM (2011) Chemical and genetic engineering of selective ion channel-ligand interactions. Science 333:1292–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Bonaventura J, Zemla R, Gomez JL, Ramirez MH, Hu X, Galvan A, Basu J, Michaelides M, Sternson SM (2019) Ultrapotent chemogenetics for research and potential clinical applications. Science 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH (1999) Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A 96:8739–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM (2006) Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 7:563–574. [DOI] [PubMed] [Google Scholar]
- Maroun M, Akirav I (2008) Arousal and stress effects on consolidation and reconsolidation of recognition memory. Neuropsychopharmacology 33:394–405. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711. [DOI] [PubMed] [Google Scholar]
- Masini D, Lopes-Aguiar C, Bonito-Oliva A, Papadia D, Andersson R, Fisahn A, Fisone G (2017) The histamine H3 receptor antagonist thioperamide rescues circadian rhythm and memory function in experimental parkinsonism. Transl Psychiatry 7:e1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee SS, Liu Y, Sillitoe RV, Heck DH (2019) Cerebellar Lobulus Simplex and Crus I Differentially Represent Phase and Phase Difference of Prefrontal Cortical and Hippocampal Oscillations. Cell Rep 27:2328–2334 e2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin I, Dani JA, De Biasi M (2017) The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation. J Neurochem 142 Suppl 2:130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty SE, Barrett RM, Vogel-Ciernia A, Malvaez M, Hernandez N, Davatolhagh MF, Matheos DP, Schiffman A, Wood MA (2012) Differential roles for Nr4a1 and Nr4a2 in object location vs. object recognition long-term memory. Learn Mem 19:588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Anderson KM, Donowho KM, McIntyre CK (2014) Noradrenergic actions in the basolateral complex of the amygdala modulate Arc expression in hippocampal synapses and consolidation of aversive and non-aversive memory. Neurobiol Learn Mem 115:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meibach RC, Siegel A (1977) Thalamic projections of the hippocampal formation: evidence for an alternate pathway involving the internal capsule. Brain Res 134:1–12. [DOI] [PubMed] [Google Scholar]
- Mello-Carpes PB, Izquierdo I (2013) The nucleus of the solitary tract --> nucleus paragigantocellularis --> locus coeruleus --> CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol Learn Mem 100:56–63. [DOI] [PubMed] [Google Scholar]
- Mello-Carpes PB, da Silva de Vargas L, Gayer MC, Roehrs R, Izquierdo I (2016) Hippocampal noradrenergic activation is necessary for object recognition memory consolidation and can promote BDNF increase and memory persistence. Neurobiol Learn Mem 127:84–92. [DOI] [PubMed] [Google Scholar]
- Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, Monyer H (2012) Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science 335:1506–1510. [DOI] [PubMed] [Google Scholar]
- Merkow MB, Burke JF, Kahana MJ (2015) The human hippocampus contributes to both the recollection and familiarity components of recognition memory. Proc Natl Acad Sci U S A 112:14378–14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI (1983) Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10:1185–1201. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA (1993) Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci 13:5418–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Odriozola P, Cohodes EM, Mandell JD, Li A, Yang R, Hall BS, Haberman JT, Zacharek SJ, Liston C, Lee FS, Gee DG (2019) Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minatohara K, Akiyoshi M, Okuno H (2015) Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front Mol Neurosci 8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Delacour J (1975) An analysis of short-term visual memory in the monkey. J Exp Psychol Anim Behav Process 1:326–334. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC (2005) Dissociable memory effects after medial thalamus lesions in the rat. Eur J Neurosci 22:973–985. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC (2006) Lateral and anterior thalamic lesions impair independent memory systems. Learn Mem 13:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JB, Laiacona J (1998) The medial frontal cortex and temporal memory: tests using spontaneous exploratory behaviour in the rat. Behav Brain Res 97:107–113. [DOI] [PubMed] [Google Scholar]
- Molas S, Zhao-Shea R, Liu L, DeGroot SR, Gardner PD, Tapper AR (2017) A circuit-based mechanism underlying familiarity signaling and the preference for novelty. Nat Neurosci 20:1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CW, Julien O, Unger EK, Shah NM, Wells JA (2014) Turning on caspases with genetics and small molecules. Methods Enzymol 544:179–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses SN, Cole C, Driscoll I, Ryan JD (2005) Differential contributions of hippocampus, amygdala and perirhinal cortex to recognition of novel objects, contextual stimuli and stimulus relationships. Brain Res Bull 67:62–76. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Glenn MJ (2000) Anterograde and retrograde memory for object discriminations and places in rats with perirhinal cortex lesions. Behav Brain Res 114:119–134. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Pinel JPJ, Wood ER (1990) Nonrecurring-items delayed nonmatching-to-sample in rats - a new paradigm for testing nonspatial working memory. Psychobiology 18:321–326. [Google Scholar]
- Mumby DG, Pinel JPJ, Dastur FN (1993) Mediodorsal thalamic lesions and object recognition in rats. Psychobiology 21:27–36. [Google Scholar]
- Mumby DG, Tremblay A, Lecluse V, Lehmann H (2005) Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus 15:1050–1056. [DOI] [PubMed] [Google Scholar]
- Murray EA, Richmond BJ (2001) Role of perirhinal cortex in object perception, memory, and associations. Curr Opin Neurobiol 11:188–193. [DOI] [PubMed] [Google Scholar]
- Nagai Y et al. (2020) Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci 23:1157–1167. [DOI] [PubMed] [Google Scholar]
- Nagayach A, Ghafari M, Zhao Y, Collins GS, Singh A, Geller AI (2021) Connected neurons in multiple neocortical areas, comprising parallel circuits, encode essential information for visual shape learning. J Chem Neuroanat 118:102024. [DOI] [PubMed] [Google Scholar]
- Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T (2004) Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 44:759–767. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Vann SD (2014) Mammilliothalamic tract lesions disrupt tests of visuo-spatial memory. Behav Neurosci 128:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJ, Thur KE, Marsden CA, Cassaday HJ (2010) Dissociable roles of dopamine within the core and medial shell of the nucleus accumbens in memory for objects and place. Behav Neurosci 124:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD, Kinnavane L, Amin E, O'Mara SM, Aggleton JP (2020) Deconstructing the direct reciprocal hippocampal-anterior thalamic pathways for spatial learning. J Neurosci 40:6978–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, Yoganarasimha D, Rao G, Knierim JJ (2013) Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J Neurosci 33:9246–9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H et al. (2019) Central histamine boosts perirhinal cortex activity and restores forgotten object memories. Biol Psychiatry 86:230–239. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ (2004) Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behav Brain Res 148:79–91. [DOI] [PubMed] [Google Scholar]
- O'Neill PK, Gordon JA, Sigurdsson T (2013) Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J Neurosci 33:14211–14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Okaichi H (2006) [The functional cooperation of the hippocampus and anterior thalamus via the fimbria-fornix in spatial memory in rats]. Shinrigaku Kenkyu 77:261–270. [DOI] [PubMed] [Google Scholar]
- Okada K, Okaichi H (2010) Functional cooperation between the hippocampal subregions and the medial septum in unreinforced and reinforced spatial memory tasks. Behav Brain Res 209:295–304. [DOI] [PubMed] [Google Scholar]
- Okada K, Nishizawa K, Kobayashi T, Sakata S, Kobayashi K (2015) Distinct roles of basal forebrain cholinergic neurons in spatial and object recognition memory. Sci Rep 5:13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y (2004) Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci 7:1104–1112. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S (2016) Ventral CA1 neurons store social memory. Science 353:1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R (2010) Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem 17:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otchy TM, Wolff SB, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SM, Olveczky BP (2015) Acute off-target effects of neural circuit manipulations. Nature 528:358–363. [DOI] [PubMed] [Google Scholar]
- Owen SF, Liu MH, Kreitzer AC (2019) Thermal constraints on in vivo optogenetic manipulations. Nat Neurosci 22:1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paban V, Chambon C, Jaffard M, Alescio-Lautier B (2005) Behavioral effects of basal forebrain cholinergic lesions in young adult and aging rats. Behav Neurosci 119:933–945. [DOI] [PubMed] [Google Scholar]
- Paretkar T, Dimitrov E (2018) The central amygdala corticotropin-releasing hormone (CRH) neurons modulation of anxiety-like behavior and hippocampus-dependent memory in mice. Neuroscience 390:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A, Gaffan D (1997) The effect of anterior thalamic and cingulate cortex lesions on object-in-place memory in monkeys. Neuropsychologia 35:1093–1102. [DOI] [PubMed] [Google Scholar]
- Parker A, Eacott MJ, Gaffan D (1997) The recognition memory deficit caused by mediodorsal thalamic lesion in non-human primates: a comparison with rhinal cortex lesion. Eur J Neurosci 9:2423–2431. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S, Bolkan SS, Kellendonk C (2018) The mediodorsal thalamus: an essential partner of the prefrontal cortex for cognition. Biol Psychiatry 83:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parron C, Save E (2004) Comparison of the effects of entorhinal and retrosplenial cortical lesions on habituation, reaction to spatial and non-spatial changes during object exploration in the rat. Neurobiol Learn Mem 82:1–11. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Robinson HA, Pozzo-Miller L (2019) Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpinella D, Mastrorilli V, Giorgi C, Coemans S, Lecca S, Lalive AL, Ostermann H, Fuchs EC, Monyer H, Mele A, Cherubini E, Griguoli M (2021) Septal cholinergic input to CA2 hippocampal region controls social novelty discrimination via nicotinic receptor-mediated disinhibition. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano TJ, Dhanerawala ZM, Kislin M, Bakshinskaya D, Engel EA, Hansen EJ, Hoag AT, Lee J, de Oude NL, Venkataraju KU, Verpeut JL, Hoebeek FE, Richardson BD, Boele HJ, Wang SS (2021) Homologous organization of cerebellar pathways to sensory, motor, and associative forebrain. Cell Rep 36:109721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piterkin P, Cole E, Cossette MP, Gaskin S, Mumby DG (2008) A limited role for the hippocampus in the modulation of novel-object preference by contextual cues. Learn Mem 15:785–791. [DOI] [PubMed] [Google Scholar]
- Poe GR, Foote S, Eschenko O, Johansen JP, Bouret S, Aston-Jones G, Harley CW, Manahan-Vaughan D, Weinshenker D, Valentino R, Berridge C, Chandler DJ, Waterhouse B, Sara SJ (2020) Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci 21:644–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucet B (1989) Object exploration, habituation, and response to a spatial change in rats following septal or medial frontal cortical damage. Behav Neurosci 103:1009–1016. [DOI] [PubMed] [Google Scholar]
- Poulter SL, Kosaki Y, Sanderson DJ, McGregor A (2020) Spontaneous object-location memory based on environmental geometry is impaired by both hippocampal and dorsolateral striatal lesions. Brain Neurosci Adv 4:2398212820972599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AL, Vann SD, Olarte-Sanchez CM, Kinnavane L, Davies M, Amin E, Aggleton JP, Nelson AJD (2017) The retrosplenial cortex and object recency memory in the rat. Eur J Neurosci 45:1451–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM, Nelson L, Shabanpoor A, Sutherland RJ (2004) Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. Proc Natl Acad Sci U S A 101:5064–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Wang X, Ma L, Li S, Liang J (2017) Functional inactivation of dorsal medial striatum alters behavioral flexibility and recognition process in mice. Physiol Behav 179:467–477. [DOI] [PubMed] [Google Scholar]
- Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A (2017) Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Commun 8:2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP (2002) The effects of prelimbic and infralimbic lesions on working memory for visual objects in rats. Neurobiol Learn Mem 77:29–43. [DOI] [PubMed] [Google Scholar]
- Rajasethupathy P, Sankaran S, Marshel JH, Kim CK, Ferenczi E, Lee SY, Berndt A, Ramakrishnan C, Jaffe A, Lo M, Liston C, Deisseroth K (2015) Projections from neocortex mediate top-down control of memory retrieval. Nature 526:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan KR, Ressler RL, Jin J, Maren S (2018) Nucleus reuniens is required for encoding and retrieving precise, hippocampal-dependent contextual fear memories in rats. J Neurosci 38:9925–9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Mejia G, Gil-Lievana E, Urrego-Morales O, Soto-Reyes E, Bermudez-Rattoni F (2021) Class I HDAC inhibition improves object recognition memory consolidation through BDNF/TrkB pathway in a time-dependent manner. Neuropharmacology 187:108493. [DOI] [PubMed] [Google Scholar]
- Ramos JM (2013) Differential contribution of hippocampus, perirhinal cortex and postrhinal cortex to allocentric spatial memory in the radial maze. Behav Brain Res 247:59–64. [DOI] [PubMed] [Google Scholar]
- Ramos JM, Vaquero JM (2005) The perirhinal cortex of the rat is necessary for spatial memory retention long after but not soon after learning. Physiol Behav 86:118–127. [DOI] [PubMed] [Google Scholar]
- Rao-Ruiz P, Couey JJ, Marcelo IM, Bouwkamp CG, Slump DE, Matos MR, van der Loo RJ, Martins GJ, van den Hout M, van IWF, Costa RM, van den Oever MC, Kushner SA (2019) Engram-specific transcriptome profiling of contextual memory consolidation. Nat Commun 10:2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, Bucci DJ (2014) Chemogenetic silencing of neurons in retrosplenial cortex disrupts sensory preconditioning. J Neurosci 34:10982–10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Arabo A, Andre M, Poucet B, Save E, Rondi-Reig L (2011) Cerebellum shapes hippocampal spatial code. Science 334:385–389. [DOI] [PubMed] [Google Scholar]
- Rodo C, Sargolini F, Save E (2017) Processing of spatial and non-spatial information in rats with lesions of the medial and lateral entorhinal cortex: Environmental complexity matters. Behav Brain Res 320:200–209. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL (2006) Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A 103:6741–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL (2008) Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem 90:576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA (2010) Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci 30:5037–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Radiske A, Kohler CA, Gonzalez C, Bevilaqua LR, Medina JH, Cammarota M (2013) Consolidation of object recognition memory requires simultaneous activation of dopamine D1/D5 receptors in the amygdala and medial prefrontal cortex but not in the hippocampus. Neurobiol Learn Mem 106:66–70. [DOI] [PubMed] [Google Scholar]
- Roth BL (2016) DREADDs for Neuroscientists. Neuron 89:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel M, Brunert D, Zabawa C, Diaz-Quesada M, Wachowiak M (2013) Transgene expression in target-defined neuron populations mediated by retrograde infection with adeno-associated viral vectors. J Neurosci 33:15195–15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet P, Sargolini F, Oliverio A, Mele A (2001) NMDA and AMPA antagonist infusions into the ventral striatum impair different steps of spatial information processing in a nonassociative task in mice. J Neurosci 21:2143–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland DC, Obenhaus HA, Skytoen ER, Zhang Q, Kentros CG, Moser EI, Moser MB (2018) Functional properties of stellate cells in medial entorhinal cortex layer II. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Chua PM, Dolan RJ (1999) The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. Neuroimage 10:520–529. [DOI] [PubMed] [Google Scholar]
- Sabec MH, Wonnacott S, Warburton EC, Bashir ZI (2018) Nicotinic acetylcholine receptors control encoding and retrieval of associative recognition memory through plasticity in the medial prefrontal cortex. Cell Rep 22:3409–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada N, Fujita Y, Mizuta N, Ueno M, Furukawa T, Yamashita T (2020) Inhibition of HDAC increases BDNF expression and promotes neuronal rewiring and functional recovery after brain injury. Cell Death Dis 11:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, German DC (1987) Hypothalamic pathology in Alzheimer's disease. Neurosci Lett 74:364–370. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Roullet P, Oliverio A, Mele A (1999) Effects of lesions to the glutamatergic afferents to the nucleus accumbens in the modulation of reactivity to spatial and non-spatial novelty in mice. Neuroscience 93:855–867. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Roullet P, Oliverio A, Mele A (2003) Effects of intra-accumbens focal administrations of glutamate antagonists on object recognition memory in mice. Behav Brain Res 138:153–163. [DOI] [PubMed] [Google Scholar]
- Sato N (2021) Episodic-like memory of rats as retrospective retrieval of incidentally encoded locations and involvement of the retrosplenial cortex. Sci Rep 11:2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage MM, Fortin NJ, Owens CB, Yonelinas AP, Eichenbaum H (2008) Recognition memory: opposite effects of hippocampal damage on recollection and familiarity. Nat Neurosci 11:16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Sargolini F (2017) Disentangling the Role of the MEC and LEC in the Processing of Spatial and Non-Spatial Information: Contribution of Lesion Studies. Front Syst Neurosci 11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht M, Jayachandran M, Rasch GE, Allen TA (2022) Dual projecting cells linking thalamic and cortical communication routes between the medial prefrontal cortex and hippocampus. Neurobiol Learn Mem 188:107586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe MR, Lincoln CM, Ivers MM, Frick KM (2021) Chemogenetic inactivation of the nucleus reuniens impairs object placement memory in female mice. Neurobiol Learn Mem 185:107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Teitelbaum P (1974) Dissociation between learning and remembering in rats with lesions in the lateral hypothalamus. J Comp Physiol Psychol 87:384–398. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Luo L (2015) Organization of the locus coeruleus-norepinephrine system. Curr Biol 25:R1051–R1056. [DOI] [PubMed] [Google Scholar]
- Scott H, Rogers MF, Scott HL, Campbell C, Warburton EC, Uney JB (2017) Recognition memory-induced gene expression in the perirhinal cortex: A transcriptomic analysis. Behav Brain Res 328:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendhilnathan N, Semework M, Goldberg ME, Ipata AE (2020) Neural correlates of reinforcement learning in mid-lateral cerebellum. Neuron 106:1055. [DOI] [PubMed] [Google Scholar]
- Senut MC, Menetrey D, Lamour Y (1989) Cholinergic and peptidergic projections from the medial septum and the nucleus of the diagonal band of Broca to dorsal hippocampus, cingulate cortex and olfactory bulb: a combined wheatgerm agglutinin-apohorseradish peroxidase-gold immunohistochemical study. Neuroscience 30:385–403. [DOI] [PubMed] [Google Scholar]
- Seoane A, Tinsley CJ, Brown MW (2012) Interfering with Fos expression in rat perirhinal cortex impairs recognition memory. Hippocampus 22:2101–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethumadhavan N, Hoang TH, Strauch C, Manahan-Vaughan D (2020) Involvement of the Postrhinal and Perirhinal Cortices in Microscale and Macroscale Visuospatial Information Encoding. Front Behav Neurosci 14:556645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethumadhavan N, Strauch C, Hoang TH, Manahan-Vaughan D (2022) The perirhinal cortex engages in area and layer-specific encoding of item dimensions. Front Behav Neurosci 15:744669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C, Aggleton JP (1995) Evidence for the independence of recognition and recency memory in amnesic subjects. Cortex 31:57–71. [DOI] [PubMed] [Google Scholar]
- Sherman SM (2007) The thalamus is more than just a relay. Curr Opin Neurobiol 17:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti M, Paldy E, Njoo C, Bali KK, Worzfeld T, Pitzer C, Kuner T, Offermanns S, Mauceri D, Kuner R (2021) The impact of Semaphorin 4C/Plexin-B2 signaling on fear memory via remodeling of neuronal and synaptic morphology. Mol Psychiatry 26:1376–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwani S, Franca ASC, Mikulovic S, Reis A, Hilscher MM, Edwards SJ, Leao RN, Tort ABL, Kullander K (2018) OLMalpha2 cells bidirectionally modulate learning. Neuron 99:404–412 e403. [DOI] [PubMed] [Google Scholar]
- Smith CN, Wixted JT, Squire LR (2011) The hippocampus supports both recollection and familiarity when memories are strong. Journal of Neuroscience 31:15693–15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV (2016) DREADDS: Use and application in behavioral neuroscience. Behav Neurosci 130:137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari N, Hangya B (2018) Cholinergic modulation of spatial learning, memory and navigation. Eur J Neurosci 48:2199–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudais C, Laplace-Builhe C, Kissa K, Kremer EJ (2001) Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J 15:2283–2285. [DOI] [PubMed] [Google Scholar]
- Squire LR (2009) The legacy of patient H.M. for neuroscience. Neuron 61:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE (2007) Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci 8:872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivas S, Thakur MK (2019) Correction to: Epigenetic regulation of neuronal immediate early genes is associated with decline in their expression and memory consolidation in scopolamine-induced amnesic mice. Mol Neurobiol 56:6669–6672. [DOI] [PubMed] [Google Scholar]
- Stacho M, Manahan-Vaughan D (2022) The Intriguing Contribution of Hippocampal Long-Term Depression to Spatial Learning and Long-Term Memory. Front Behav Neurosci 16:806356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA (2009) Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A 106:9447–9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Li X, Li A, Zhang J, Ding Z, Gong H, Luo Q (2020) Ventral hippocampal-prefrontal interaction affects social behavior via parvalbumin positive neurons in the medial prefrontal cortex. iScience 23:100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D, Choi J, Callaway EM, Xu X (2014) Cell-type-specific circuit connectivity of hippocampal CA1 revealed through Cre-dependent rabies tracing. Cell Rep 7:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin S, Lin X, Chen L, Qiao X, Jiang L, Zhou P, Johnston KG, Golshani P, Nie Q, Holmes TC, Nitz DA, Xu X (2019) CA1-projecting subiculum neurons facilitate object-place learning. Nat Neurosci 22:1857–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ (1995) Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14:341–351. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernandez G, Deisseroth K, Greene RW, Morris RG (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar SK, Musial PG, Gerstein GL (2001) Role of mammalian auditory cortex in the perception of elementary sound properties. J Neurophysiol 85:2350–2358. [DOI] [PubMed] [Google Scholar]
- Tennant SA, Fischer L, Garden DLF, Gerlei KZ, Martinez-Gonzalez C, McClure C, Wood ER, Nolan MF (2018) Stellate cells in the medial entorhinal cortex are required for spatial learning. Cell Rep 22:1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP (2004) Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14:685–692. [DOI] [PubMed] [Google Scholar]
- Torromino G, Autore L, Khalil V, Mastrorilli V, Griguoli M, Pignataro A, Centofante E, Biasini GM, De Turris V, Ammassari-Teule M, Rinaldi A, Mele A (2019) Offline ventral subiculum-ventral striatum serial communication is required for spatial memory consolidation. Nat Commun 10:5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Koren V, Doig NM, Ellender TJ, El-Gaby M, Lopes-dos-Santos V, Reeve HM, Perestenko PV, Garas FN, Magill PJ, Sharott A, Dupret D (2019) A hippocampus-accumbens tripartite neuronal motif guides appetitive memory in space. Cell 176:1393-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A, Moser MB, Moser EI (2013) Traces of experience in the lateral entorhinal cortex. Curr Biol 23:399–405. [DOI] [PubMed] [Google Scholar]
- Tsao A, Sugar J, Lu L, Wang C, Knierim JJ, Moser MB, Moser EI (2018) Integrating time from experience in the lateral entorhinal cortex. Nature 561:57–62. [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Taxier LR, Fortress AM, Frick KM (2018) Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol Learn Mem 156:103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Lepak K, Kirry AJ, Gilmartin MR (2020) Ventral hippocampal input to the prelimbic cortex dissociates the context from the cue association in trace fear memory. J Neurosci 40:3217–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, Sargolini F, Roullet P, Ammassari-Teule M, Passino E, Oliverio A, Mele A (1998) N-methyl-D-aspartate receptors in the nucleus accumbens are involved in detection of spatial novelty in mice. Psychopharmacology (Berl) 137:175–183. [DOI] [PubMed] [Google Scholar]
- Vaidya AR, Pujara MS, Petrides M, Murray EA, Fellows LK (2019) Lesion studies in contemporary neuroscience. Trends Cogn Sci 23:653–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter T, Camon J, Alvernhe A, Elduayen C, Sargolini F, Save E (2013) Distinct roles of medial and lateral entorhinal cortex in spatial cognition. Cereb Cortex 23:451–459. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM (2003) Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol 463:249–263. [DOI] [PubMed] [Google Scholar]
- Vandecasteele M, Varga V, Berenyi A, Papp E, Bartho P, Venance L, Freund TF, Buzsaki G (2014) Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc Natl Acad Sci U S A 111:13535–13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey B, Garden DLF, Ambrozova V, McClure C, Nolan MF, Ainge JA (2020) Fan cells in layer 2 of the lateral entorhinal cortex are critical for episodic-like memory. Curr Biol 30:169–175 e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP (2002) Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav Neurosci 116:85–94. [PubMed] [Google Scholar]
- Vardy E et al. (2015) A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY, Wilson MA (2014) Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct 219:911–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA (2007) Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci 27:6128–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder LC, Miller AMP, Harrison MB, Smith DM (2017) Retrosplenial cortical neurons encode navigational cues, trajectories and reward locations during goal directed navigation. Cereb Cortex 27:3713–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Albo Z, Viana Di Prisco G (2001) Theta-rhythmically firing neurons in the anterior thalamus: implications for mnemonic functions of Papez's circuit. Neuroscience 104:619–625. [DOI] [PubMed] [Google Scholar]
- von Ziegler LM, Selevsek N, Tweedie-Cullen RY, Kremer E, Mansuy IM (2018) Subregion-specific proteomic signature in the hippocampus for recognition processes in adult mice. Cell Rep 22:3362–3374. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Luo L (2020) Neocortex-cerebellum circuits for cognitive processing. Trends Neurosci 43:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liang J, Chen L, Shen Y, Zhao J, Xu C, Wu X, Cheng H, Ying X, Guo Y, Wang S, Zhou Y, Wang Y, Chen Z (2018) Pharmaco-genetic therapeutics targeting parvalbumin neurons attenuate temporal lobe epilepsy. Neurobiol Dis 117:149–160. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Aggleton JP (1999) Differential deficits in the Morris water maze following cytotoxic lesions of the anterior thalamus and fornix transection. Behav Brain Res 98:27–38. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Brown MW (2015) Neural circuitry for rat recognition memory. Behav Brain Res 285:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Baird AL, Aggleton JP (1997) Assessing the magnitude of the allocentric spatial deficit associated with complete loss of the anterior thalamic nuclei in rats. Behav Brain Res 87:223–232. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Barker GR, Brown MW (2013) Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology 74:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Baird AL, Morgan A, Muir JL, Aggleton JP (2000) Disconnecting hippocampal projections to the anterior thalamus produces deficits on tests of spatial memory in rats. Eur J Neurosci 12:1714–1726. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW (2003) Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron 38:987–996. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Glover CPJ, Massey PV, Wan HM, Johnson B, Bienemann A, Deuschle U, Kew JNC, Aggleton JP, Bashir ZI, Uney J, Brown MW (2005) CAMP responsive element-binding protein phosphorylation is necessary for perirhinal long-term potentiation and recognition memory. Journal of Neuroscience 25:6296–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TC, Obiang P, Torres-Herraez A, Watilliaux A, Coulon P, Rochefort C, Rondi-Reig L (2019) Anatomical and physiological foundations of cerebello-hippocampal interaction. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick CZ, Tetzlaff MR, Krook-Magnuson E (2019) Novel long-range inhibitory nNOS-expressing hippocampal cells. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert JS, Mahn M, Prigge M, Printz Y, Yizhar O (2017) Silencing neurons: tools, applications, and experimental constraints. Neuron 95:504–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig KA, Bilkey DK (1994) The effects of perirhinal cortical lesions on spatial reference memory in the rat. Behav Brain Res 63:101–109. [DOI] [PubMed] [Google Scholar]
- Wiig KA, Burwell RD (1998) Memory impairment on a delayed non-matching-to-position task after lesions of the perirhinal cortex in the rat. Behav Neurosci 112:827–838. [DOI] [PubMed] [Google Scholar]
- Wilke M, Turchi J, Smith K, Mishkin M, Leopold DA (2010) Pulvinar inactivation disrupts selection of movement plans. J Neurosci 30:8650–8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DI, Watanabe S, Milner H, Ainge JA (2013a) Lateral entorhinal cortex is necessary for associative but not nonassociative recognition memory. Hippocampus 23:1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DI, Langston RF, Schlesiger MI, Wagner M, Watanabe S, Ainge JA (2013b) Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus 23:352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton LA, Baird AL, Muir JL, Honey RC, Aggleton JP (2001) Loss of the thalamic nuclei for "head direction" impairs performance on spatial memory tasks in rats. Behav Neurosci 115:861–869. [PubMed] [Google Scholar]
- Winters BD, Bussey TJ (2005) Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci 25:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Reid JM (2010) A distributed cortical representation underlies crossmodal object recognition in rats. J Neurosci 30:6253–6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ (2008) Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev 32:1055–1070. [DOI] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ (2004) Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci 24:5901–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T (2000) Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci 911:1–24. [DOI] [PubMed] [Google Scholar]
- Wolff M, Vann SD (2019) The cognitive thalamus as a gateway to mental representations. J Neurosci 39:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, Alcaraz F, Marchand AR, Coutureau E (2015) Functional heterogeneity of the limbic thalamus: From hippocampal to cortical functions. Neurosci Biobehav Rev 54:120–130. [DOI] [PubMed] [Google Scholar]
- Wolff SB, Olveczky BP (2018) The promise and perils of causal circuit manipulations. Curr Opin Neurobiol 49:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yang H, Colosi P (2010) Effect of genome size on AAV vector packaging. Mol Ther 18:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, Van Groen T (1992) Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus 2:1–11. [DOI] [PubMed] [Google Scholar]
- Xiao L, Bornmann C, Hatstatt-Burkle L, Scheiffele P (2018) Regulation of striatal cells and goal-directed behavior by cerebellar outputs. Nat Commun 9:3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Südhof TC (2013) A neural circuit for memory specificity and generalization. Science 339:1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Sun Y, Holmes TC, Lopez AJ (2016) Noncanonical connections between the subiculum and hippocampal CA1. J Comp Neurol 524:3666–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Holmes TC, Luo MH, Beier KT, Horwitz GD, Zhao F, Zeng W, Hui M, Semler BL, Sandri-Goldin RM (2020) Viral vectors for neural circuit mapping and recent advances in trans-synaptic anterograde tracers. Neuron 107:1029–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi M, Ohashi Y, Tsubota T, Sato A, Koyano KW, Wang N, Miyashita Y (2013) Characterization of the properties of seven promoters in the motor cortex of rats and monkeys after lentiviral vector-mediated gene transfer. Hum Gene Ther Methods 24:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Spealman RD, Zhang J (2008) Dopaminergic signaling in dendritic spines. Biochem Pharmacol 75:2055–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kapeller-Libermann D, Travaglia A, Inda MC, Alberini CM (2017) Direct dorsal hippocampal-prelimbic cortex connections strengthen fear memories. Nat Neurosci 20:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK (2000) Cytotoxic lesion of the medial prefrontal cortex abolishes the partial reinforcement extinction effect, attenuates prepulse inhibition of the acoustic startle reflex and induces transient hyperlocomotion, while sparing spontaneous object recognition memory in the rat. Neuroscience 95:675–689. [DOI] [PubMed] [Google Scholar]
- Yi JH, Park HJ, Kim BC, Kim DH, Ryu JH (2016) Evidences of the role of the rodent hippocampus in the non-spatial recognition memory. Behav Brain Res 297:141–149. [DOI] [PubMed] [Google Scholar]
- Yu JY, Fang P, Wang C, Wang XX, Li K, Gong Q, Luo BY, Wang XD (2018) Dorsal CA1 interneurons contribute to acute stress-induced spatial memory deficits. Neuropharmacology 135:474–486. [DOI] [PubMed] [Google Scholar]
- Zeidler Z, Hoffmann K, Krook-Magnuson E (2020) HippoBellum: acute cerebellar modulation alters hippocampal dynamics and function. J Neurosci 40:6910–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GR, Zhao H, Choi EM, Svestka M, Wang X, Nagayach A, Singh A, Cook RG, Geller AI (2019) An identified ensemble within a neocortical circuit encodes essential information for genetically-enhanced visual shape learning. Hippocampus 29:710–725. [DOI] [PubMed] [Google Scholar]
- Zhang GR, Wang X, Kong L, Lu XG, Lee B, Liu M, Sun M, Franklin C, Cook RG, Geller AI (2005) Genetic enhancement of visual learning by activation of protein kinase C pathways in small groups of rat cortical neurons. J Neurosci 25:8468–8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GR, Cao H, Kong L, O'Brien J, Baughns A, Jan M, Zhao H, Wang X, Lu XG, Cook RG, Geller AI (2010) Identified circuit in rat postrhinal cortex encodes essential information for performing specific visual shape discriminations. Proc Natl Acad Sci U S A 107:14478–14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, McCabe BJ, Aggleton JP (1995) Effects of the novelty or familiarity of visual stimuli on the expression of the immediate early gene c-fos in rat brain. Neuroscience 69:821–829. [DOI] [PubMed] [Google Scholar]
- Zingg B, Dong HW, Tao HW, Zhang LI (2022) Application of AAV1 for anterograde transsynaptic circuit mapping and input-dependent neuronal cataloging. Curr Protoc 2:e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, Zhang LI (2017) AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR (1985) Amnesia in monkeys after lesions of the mediodorsal nucleus of the thalamus. Ann Neurol 17:558–564. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA (1989) Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci 9:4355–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Chen L, Deng D, Jiang D, Dong F, McSweeney C, Zhou Y, Liu L, Chen G, Wu Y, Mao Y (2016) DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction. Curr Mol Med 16:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Bychkov E, Tsakem EL, Siedlecki C, Blakely RD, Gurevich EV (2013) Cognitive effects of dopamine depletion in the context of diminished acetylcholine signaling capacity in mice. Dis Model Mech 6:171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]


