Abstract
Background
Vitamin D possesses immunomodulatory properties and has been implicated in the pathogenesis and severity of inflammatory bowel disease (IBD). Animal studies and emerging epidemiological evidence have demonstrated an association between vitamin D deficiency and worse disease activity. However, the role of vitamin D for the treatment of IBD is unclear.
Objectives
To evaluate the benefits and harms of vitamin D supplementation as a treatment for IBD.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was Jun 2023.
Selection criteria
We included randomised controlled trials (RCTs) in people of all ages with active or inactive IBD comparing any dose of vitamin D with another dose of vitamin D, another intervention, placebo, or no intervention.
We defined doses as: vitamin D (all doses), any‐treatment‐dose vitamin D (greater than 400 IU/day), high‐treatment‐dose vitamin D (greater than 1000 IU/day), low‐treatment‐dose vitamin D (400 IU/day to 1000 IU/day), and supplemental‐dose vitamin D (less than 400 IU/day).
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. clinical response for people with active disease, 2. clinical relapse for people in remission, 3. quality of life, and 4. withdrawals due to adverse events. Our secondary outcomes were 5. disease activity at end of study, 6. normalisation of vitamin D levels at end of study, and 7. total serious adverse events. We used GRADE to assess certainty of evidence for each outcome.
Main results
We included 22 RCTs with 1874 participants. Study duration ranged from four to 52 weeks. Ten studies enroled people with Crohn's disease (CD), five enroled people with ulcerative colitis (UC), and seven enroled people with CD and people with UC. Seventeen studies included adults, three included children, and two included both. Four studies enroled people with active disease, six enroled people in remission, and 12 enroled both.
We assessed each study for risk of bias across seven individual domains. Five studies were at low risk of bias across all seven domains. Ten studies were at unclear risk of bias in at least one domain but with no areas of high risk of bias. Seven studies were at high risk of bias for blinding of participants and assessors.
Vitamin D (all doses) versus placebo or no treatment
Thirteen studies compared vitamin D against placebo or no treatment.
We could not draw any conclusions on clinical response for UC as the certainty of the evidence was very low (risk ratio (RR) 4.00, 95% confidence interval (CI) 1.51 to 10.57; 1 study, 60 participants). There were no data on CD.
There may be fewer clinical relapses for IBD when using vitamin D compared to placebo or no treatment (RR 0.57, 95% CI 0.34 to 0.96; 3 studies, 310 participants). The certainty of the evidence was low.
We could not draw any conclusions on quality of life for IBD (standardised mean difference (SMD) −0.13, 95% CI −3.10 to 2.83 (the SMD value indicates a negligent decrease in quality of life, and the corresponding CIs indicate that the effect can range from a large decrease to a large increase in quality of life); 2 studies, 243 participants) or withdrawals due to adverse events for IBD (RR 1.97, 95% CI 0.18 to 21.27; 12 studies, 1251 participants; note 11 studies reported withdrawals but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 1 study rather than 12). The certainty of the evidence was very low.
High‐treatment‐dose vitamin D versus low‐treatment‐dose vitamin D
Five studies compared high treatment vitamin D doses against low treatment vitamin D doses.
There were no data on clinical response.
There may be no difference in clinical relapse for CD (RR 0.48, 95% CI 0.23 to 1.01; 1 study, 34 participants). The certainty of the evidence was low.
We could not draw any conclusions on withdrawals due to adverse events for IBD as the certainty of the evidence was very low (RR 0.89, 95% CI 0.06 to 13.08; 3 studies, 104 participants; note 2 studies reported withdrawals but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 1 study rather than 3).
The data on quality of life and disease activity could not be meta‐analysed, were of very low certainty, and no conclusions could be drawn.
Any‐treatment‐dose vitamin D versus supplemental‐dose vitamin D
Four studies compared treatment doses of vitamin D against supplemental doses.
There were no data on clinical response and relapse.
There were no data on quality of life that could be meta‐analysed.
We could not draw any conclusions on withdrawals due to adverse events for IBD as the certainty of the evidence was very low (RR 3.09, 95% CI 0.13 to 73.17; 4 studies, 233 participants; note 3 studies reported withdrawals but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 1 study rather than 4).
Authors' conclusions
There may be fewer clinical relapses when comparing vitamin D with placebo, but we cannot draw any conclusions on differences in clinical response, quality of life, or withdrawals, due to very low‐certainty evidence. When comparing high and low doses of vitamin D, there were no data for clinical response, but there may be no difference in relapse for CD. We cannot draw conclusions on the other outcomes due to very low certainty evidence. Finally, comparing vitamin D (all doses) to supplemental‐dose vitamin D, there were no data on clinical relapse or response, and we could not draw conclusions on other outcomes due to very low certainty evidence or missing data.
It is difficult to make any clear recommendations for future research on the basis of the findings of this review. Future studies must be clear on the baseline populations, the purpose of vitamin D treatment, and, therefore, study an appropriate dosing strategy. Stakeholders in the field may wish to reach consensus on such issues prior to new studies.
Keywords: Adult; Animals; Child; Humans; Colitis, Ulcerative; Colitis, Ulcerative/drug therapy; Crohn Disease; Crohn Disease/drug therapy; Neoplasm Recurrence, Local; Recurrence; Remission Induction; Vitamin D; Vitamin D/adverse effects
Plain language summary
Vitamin D for the treatment of inflammatory bowel disease
Key messages
The data we presently have for the use of vitamin D for the treatment of inflammatory bowel disease are of very low quality, and we do not know whether it works or if it is safe.
What is inflammatory bowel disease?
Inflammatory bowel disease is a life‐long disease that affects the gut. Its two main types are ulcerative colitis and Crohn's disease. Ulcerative colitis only affects the large intestine. Crohn's disease can affect any part of the gut, from mouth to bottom. Common symptoms include bloody poo, diarrhoea, stomach ache, fever, weight loss, and fatigue. We do not know exactly what causes it, but it is probably a mix of genes, problems with the immune system, bacteria in the gut, and something in the environment. There is no known cure, but the symptoms are usually managed with medicines, such as steroids and immune system medications, and sometimes surgery. Most people with inflammatory bowel disease have times when they have symptoms (called active disease) and other times when their symptoms are under control (called remission). When symptoms reappear after being in remission, it is called relapse.
What did we want to find out?
We wanted to find out if vitamin D works for the treatment of inflammatory bowel disease, and whether it is safe to use. Specifically, we looked at improvement of symptoms for people with active disease; relapse for people in remission; quality of life; and withdrawals from the trial because of side effects.
What did we do?
We searched for randomised controlled trials (studies where people are assigned to one of two or more treatment groups using a random method) comparing vitamin D with any other treatment, standard treatment, or different doses of vitamin D.
What did we find?
We found 22 trials with 1874 participants with inflammatory bowel disease. The studies lasted from four to 52 weeks. Ten studies were on Crohn's disease, five on ulcerative colitis, and seven on participants who had either of these. Seventeen studies were on adults, three on children, and two on both. Four included people with active disease, six in remission, and 12 on a mix of both. The studies included doses of vitamin D used to treat deficiency and doses given as supplements.
Thirteen studies compared vitamin D (all doses) against placebo (dummy treatment) or no other treatment. There was low‐quality evidence that there may be fewer clinical relapses when using vitamin D compared to placebo or no treatment. We cannot say anything about any of the other measures we looked at because the quality of the evidence was very low.
Five studies compared high‐treatment‐doses to low‐treatment‐doses of vitamin D. There were no data on improvement of symptoms. There was low‐quality evidence that there may be no difference on relapse in Crohn's disease, but there were no data on ulcerative colitis. We cannot say anything about any of the other measures we looked at because the quality of the evidence was very low.
Four studies compared treatment doses to supplement doses of vitamin D. There were no data on improvement of symptoms, relapses, or quality of life changes. We cannot say anything about any of the other measures we looked at because the quality of the evidence was very low.
What are the limitations of the evidence?
The evidence is mostly of very low and low quality. This is because of problems with the way the studies were carried out, and problems with how the results were reported. Additionally, the individual studies did not make the same measurements, meaning that we did not have enough numbers of people to strengthen the results of the measures we looked for.
How up‐to‐date is this review?
This review is up‐to‐date to June 2023.
Summary of findings
Summary of findings 1. Vitamin D (all doses) compared to placebo/no treatment for the treatment of inflammatory bowel disease.
| Vitamin D (all doses) compared to placebo/no treatment for the treatment of inflammatory bowel disease | ||||||
|
Patient or population: people with active or inactive inflammatory bowel disease of any age Setting: any inpatient or outpatient setting Intervention: vitamin D (all doses) Comparison: placebo/no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo/no treatment | Risk with vitamin D (all doses) | |||||
| Clinical response at end of study (4 weeks) | Study population | RR 4.00 (1.51 to 10.57) | 60 (1 study) | ⊕⊝⊝⊝ Very lowa | — | |
| 133 per 1000 | 533 per 1000 (201 to 1000) | |||||
| Clinical relapse at end of study (26–52) weeks | Study population | RR 0.57 (0.34 to 0.96) | 310 (3 studies) | ⊕⊕⊝⊝ Lowb | — | |
| 278 per 1000 | 159 per 1000 (95 to 267) | |||||
| Quality of life at end of study (26 weeks) | — | SMD 0.13 lower (3.10 lower to 2.83 higher) | — | 243 (2 studies) | ⊕⊝⊝⊝ Very lowc | SMD between 0.2 and 0.5 indicates a small effect; SMD between 0.5 and 0.8 indicates a moderate effect; SMD > 0.8 indicates a large effect. Raftery 2015 reported 'no significant difference' in quality of life measures between groups, but without corresponding numerical data suitable for analysis. |
| Withdrawals due to adverse events | Study population | RR 1.97 (0.18 to 21.27) | 1251 (12 studies) | ⊕⊝⊝⊝ Very lowd | 2/629 people from the vitamin D group withdrew due to an adverse event compared with 1/622 in the placebo/no treatment group. Note 11 studies reported withdrawals but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 1 study rather than 12. | |
| 2 per 1000 | 3 per 1000 (0 to 34) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD standard mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to very serious concerns with imprecision owing to low event numbers and one level due to serious concerns with risk of bias owing to selective reporting and other bias. bDowngraded two levels due to serious concerns with imprecision owing to low event numbers and serious concerns with risk of bias owing to unclear randomisation/allocation and other risk of bias. cDowngraded three levels due to very serious concerns regarding imprecision and heterogeneity. dDowngraded two levels due to serious concerns with imprecision owing to very low event numbers and one level owing to concerns with risk of bias in all areas.
Summary of findings 2. High‐treatment‐dose vitamin D (greater than 1000 IU/day) compared to low‐treatment‐dose vitamin D (400 IU/day to 1000 IU/day) for the treatment of inflammatory bowel disease.
| High‐treatment‐dose vitamin D (> 1000 IU/day) compared to low‐treatment‐dose vitamin D (400–1000 IU/day) for the treatment of inflammatory bowel disease | ||||||
| Patient or population: people with active or inactive inflammatory bowel disease of any age Setting: any inpatient or outpatient setting Intervention: high‐treatment‐dose vitamin D (> 1000 IU/day) Comparison: low‐treatment‐dose vitamin D (400–1000 IU/day) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with low‐treatment‐dose vitamin D | Risk with high‐treatment‐dose vitamin D | |||||
| Clinical response | — | — | — | — | No studies reported on this outcome | |
| Clinical relapse at end of study (52 weeks) | Study population | RR 0.48 (0.23 to 1.01) | 34 (1 study) | ⊕⊕⊝⊝ Lowa | — | |
| 688 per 1000 | 330 per 1000 (158 to 694) | |||||
| Quality of life | 1 study reported that quality of life, measured with the IBDQ, increased significantly in both groups, but the relevant data were not provided in the results. | — | 46 (1 study) |
⊕⊝⊝⊝ Very lowb | Results not reported in numerical method suitable for analysis. | |
| Withdrawals due to adverse events | Study population | RR 0.89 (0.06 to 13.08) | 104 (3 studies) | ⊕⊝⊝⊝ Very lowc | 1/53 person from the high‐treatment‐dose group withdrew due to an adverse event compared with 1/51 in the low‐treatment‐dose group. Note 2 studies reported withdrawals but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 1 study rather than 3. | |
| 20 per 1000 | 17 per 1000 (1 to 256) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IBDQ: Inflammatory Bowel Disease Questionnaire; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to serious concerns with imprecision owing to low event numbers. bDowngraded three levels due to serious concerns with imprecision and risk of bias. cDowngraded two levels due to serious concerns with imprecision owing to low event numbers and one level due to concerns with risk of bias owing to randomisation/allocation, blinding, and selective reporting.
Summary of findings 3. Any‐treatment‐dose vitamin D (greater than 400 IU/day) compared to supplemental‐dose vitamin D (less than 400 IU/day) for the treatment of inflammatory bowel disease.
| Any‐treatment‐dose vitamin D (> 400 IU/day) compared to supplemental‐dose vitamin D (< 400 IU/day) for the treatment of inflammatory bowel disease | ||||||
| Patient or population: people with active or inactive inflammatory bowel disease of any age Setting: any inpatient or outpatient setting Intervention: any‐treatment‐dose vitamin D (> 400 IU/day) Comparison: supplemental‐dose vitamin D (< 400 IU/day) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with supplemental‐dose vitamin D | Risk with any‐treatment‐dose vitamin D | |||||
| Clinical response | — | — | — | — | No studies reported this outcome. | |
| Clinical relapse | — | — | — | — | No studies reported this outcome. | |
| Quality of life | — | — | — | — | No studies reported this outcome; 1 study measured this outcome but did not report their results and so could not be included for meta‐analysis. | |
| Withdrawals due to adverse events | Study population | RR 3.09 (0.13 to 73.17) | 233 (4 studies) | ⊕⊝⊝⊝ Very lowb | 1/117 person in the any‐treatment‐ dose group withdrew due to an adverse event compared to 0/116 in the supplemental‐treatment‐dose group. Note 3 studies reported withdrawals but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 1 study rather than 4. | |
| 0 per 1000a | 1 per 1000 (0 to 73) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aA token number of 1 per 1000 was used to calculate the risk with any treatment of vitamin D. bDowngraded two levels due to serious concerns with imprecision due to low event numbers and one level due to concerns with risk of bias due to randomisation/allocation, blinding, and selective reporting.
Background
Description of the condition
Inflammatory bowel disease (IBD), primarily comprising Crohn's disease (CD) and ulcerative colitis (UC), is a chronic inflammatory disorder of the gastrointestinal tract. Clinical manifestations may include abdominal pain, cramping, diarrhoea, and blood in stools. People with CD may also manifest strictures, abscesses, fistulae, or a combination of these. The incidence and prevalence of IBD have been increasing worldwide with the highest rates in Europe and North America (Ng 2017). The highest age‐standardised prevalence rates of IBD are found in the USA (464.5, 95% uncertainty intervals (UI) 438.6 to 490.9 per 100,000 population), followed by the UK (449.6, 95% UI 420.6 to 481.6 per 100,000 population). By contrast, the lowest age‐standardised prevalence rates were observed in the Caribbean (6.7, 95% UI 6.3 to 7.2 per 100,000 population). Whereas incidence rates across North America and Europe had been increasing, more recent evidence suggests that there is stable or decreasing incidence in North America and Europe, and increasing incidence in newly industrialised countries (Alatab 2019). The mechanisms for the increase in IBD incidence rates over time are unclear, although some hypothesised reasons include lifestyle changes, urbanisation, medication exposure, and nutrition (Kaplan 2015; Molodecky 2012).
Description of the intervention
Vitamin D is a fat‐soluble hormone that is derived through sunlight exposure or oral consumption. The amount of vitamin D synthesis from sunlight (or ultraviolet B) exposure depends on several factors, such as duration of exposure, percentage of body surface area exposed, skin tone, latitude, season, and cloud cover (Webb 2006). Oral consumption of vitamin D may include dietary sources or pharmacological supplementation. Foods rich in vitamin D include some fish, beef liver, and vitamin D‐fortified food products. In the US, the mean daily intake from the diet is 200 international units (IU) to 240 IU; by comparison, the Institute of Medicine recommends a daily intake of 600 IU for all people aged 70 years or less and 800 IU for people aged above 70 years (Ross 2011), whilst the UK National Health Service recommends taking 10 μg supplements of vitamin D during the winter months from October to March for all adults (NHS 2020). Vitamin D supplementation is thus often needed to maintain normal vitamin D concentrations (i.e. greater than 30 ng/mL) (Bailey 2010). Vitamin D is often included in multivitamins, ranging from 50 IU to 1000 IU per tablet. Typical non‐prescription and prescription formulations may range from 400 IU per day to 50,000 IU per week. In IBD where intestinal malabsorption, dietary restrictions, and lifestyle changes may occur, the need for vitamin D supplementation may be even greater, although not clearly defined (Pappa 2008).
How the intervention might work
Vitamin D has traditionally been known for its prominent role in calcium and phosphorus homeostasis, although it has been more recently implicated in immune function. At the molecular level, vitamin D participates in regulating immune cell differentiation and proliferation (Chen 2007; Jeffery 2009; Manolagas 1986; Tsoukas 1984). In turn, vitamin D deficiency has been associated with the pathogenesis of several autoimmune diseases, such as experimental autoimmune encephalomyelitis, rheumatoid arthritis, and multiple sclerosis (Merlino 2004; Munger 2006). Similarly, in IBD, mice with a vitamin D receptor knockout have been shown to develop severe gastrointestinal inflammation (Froicu 2003; Froicu 2006), while administration of exogenous vitamin D or an analogue reduces expression of proinflammatory cytokines and lymphocyte infiltration in the lamina propria of a dextran sodium sulphate‐induced colitis mouse model (Laverny 2010). In humans, epidemiological studies have additionally associated vitamin D deficiency with increased risk of incident disease, and more severe disease activity (Ananthakrishnan 2012; Blanck 2013; Limketkai 2014; Ulitsky 2011). Normalisation of vitamin D concentrations has been associated with a lower risk of surgery amongst people with CD (Ananthakrishnan 2013), although optimal 25‐hydroxyvitamin D (25(OH)D) concentrations for IBD are yet undefined.
Why it is important to do this review
Current data suggest that vitamin D deficiency may be associated with more severe IBD (Ananthakrishnan 2013; Frigstad 2017; Kabbani 2016), but it is unclear whether this is causative or a result of inflammation which occurs in IBD (Fletcher 2019). The interpretation of existing, mostly retrospective, data is significantly challenged by confounding and reverse causation (do low vitamin D concentrations lead to more severe disease activity or vice versa?). This study systematically reviewed randomised controlled trials (RCTs) that evaluated the effects of vitamin D supplementation on IBD activity. Results from this review can help determine whether current data support the use of vitamin D as a potential economical, low‐risk, adjunctive treatment for IBD.
Objectives
To evaluate the benefits and harms of vitamin D supplementation as a treatment for IBD.
Methods
Criteria for considering studies for this review
Types of studies
We included all published, unpublished, and ongoing RCTs. We considered cross‐over and cluster‐RCTs for inclusion. We considered studies published as full text, abstract, and unpublished data provided by the author upon request.
Types of participants
We included people of all ages with active or inactive IBD.
Types of interventions
We included trials which included all forms of vitamin D, including vitamin D‐only and combination formulations, with or without drugs to treat IBD.
We considered any control interventions including placebo, any other type of intervention, or no intervention. We considered any dose and study duration.
We made the following comparisons.
Vitamin D (all doses) versus placebo
High‐treatment‐dose vitamin D (defined as greater than 1000 IU/day) versus low‐treatment‐dose vitamin D (defined as 400 IU/day to 1000 IU/day)
Any‐treatment‐dose vitamin D (defined as greater than 400 IU/day) versus supplemental‐dose vitamin D (defined as less than 400 IU/day).
Types of outcome measures
We considered both dichotomous and continuous outcomes. If both dichotomous and continuous measures were available for the same outcomes, we analysed and reported them separately.
We reported outcomes at the end of the study follow‐up period, with no restriction on the timing of these follow‐up periods.
Primary outcomes
The primary outcomes based on disease activity.
Clinical response for people with active IBD at end of study, as defined by the primary studies (e.g. a predefined decrease when lower scores indicate lower disease activity, or increase when lower numbers indicate higher disease activity, in an internationally recognisable disease activity scoring system such as Crohn's Disease Activity Index (CDAI), Harvey‐Bradshaw Index (HBI), Mayo score, etc.) (dichotomous outcome)
Clinical relapse for people in remission at end of study, as defined by the primary studies (e.g. a predefined increase above a certain threshold when lower scores indicate lower disease activity, or decrease when lower numbers indicate higher disease activity, in an internationally recognisable disease activity scoring system such as CDAI, HBI, Mayo score, etc.) (dichotomous)
For all participants.
Quality of life measures at end of study, as defined by the primary studies (e.g. the end of study scores or change scores in an internationally recognisable quality of life scale for IBD, such as the Inflammatory Bowel Disease Questionnaire (IBDQ)) (continuous outcome)
Withdrawals due to adverse events (dichotomous outcome)
Secondary outcomes
Disease activity at end of study, as defined by the primary studies (e.g. the end of study scores or change scores in an internationally recognisable disease activity scoring system such as CDAI, HBI, Mayo score, etc.) (continuous outcome)
Normalisation of vitamin D levels at end of study, as defined by the primary studies (e.g. the end of study scores or change scores in vitamin D levels, generally measured in serum) (dichotomous or continuous outcome)
Total serious adverse events (dichotomous outcome)
Search methods for identification of studies
Electronic searches
On 13 July 2020, 8 August 2021, and 10 June 2023, we searched the following sources.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Library (Issue 6, 2023) (Appendix 1); CENTRAL includes Cochrane Gut's Specialized Register
MEDLINE via OvidSP (1946 to 9 June 2023) (Appendix 2)
Embase via OvidSP (1974 to 2023 week 23) (Appendix 3)
ClinicalTrials.gov (clinicaltrials.gov/) (to 10 June 2023) (Appendix 4)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (trialsearch.who.int/) (to 10 June 2023) (Appendix 5)
There were no restrictions on time, document type, publication status, or language (Aali 2021).
Searching other resources
As complementary search methods, we scrutinised the reference lists of studies included in our review and relevant systematic reviews. We sought results of unpublished trials by contacting the trial investigators or study sponsors.
We obtained translations of papers when necessary.
Data collection and analysis
We conducted data collection and analysis according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a).
Selection of studies
Two review authors (CW, MG, VS, or BNL) independently screened each of the titles and abstracts identified during the literature search, using Covidence (Covidence). We discarded studies that clearly did not meet the inclusion criteria. We obtained the full report of studies that appeared to meet our inclusion criteria, or for which there was insufficient information to make a final decision. Two review authors (CW, MG, VS, or BNL) independently assessed the reports of each study to establish whether the studies met the inclusion criteria. A third review author (MG or BNL) resolved disagreements. We recorded studies rejected at this or subsequent stages in the Characteristics of excluded studies table, and recorded the main reason for exclusion. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (PRISMA 2020).
Where studies had multiple publications, we identified and exclude duplicates, and collated the reports of the same study so that each study, rather than each report, was the unit of interest for the review; in these cases, we assigned a single identifier with multiple references.
Data extraction and management
Two review authors (CW, MG, VS, or BNL) independently carried out data extraction for each study using piloted data extraction forms. Disagreements were resolved by a third review author (MG or BNL). We extracted relevant data from full‐text articles that met the inclusion criteria including:
methods: country and study design;
participant characteristics: state of disease, disease type, age, sex, site of disease;
eligibility criteria: inclusion and exclusion criteria;
intervention, comparator, and study duration;
participant outcomes: outcome definition, unit of measurement, and time of collection;
results: number of participants allocated to each group, missing participants, outcome results;
funding source and conflicts of interest;
author contact information.
When a trial reported multiple arms, we included only the relevant arms in the analyses; however, we listed all treatment arms in the Characteristics of included studies table. One review author (BNL) manually copied data into Review Manager Web, and another review author (CW) double‐checked the copied data (RevMan Web 2022). In the case of unclear or incomplete information or data, we contacted the study authors to request clarification.
Assessment of risk of bias in included studies
Following data extraction, two review authors (CW, MG, VS, or BNL) independently assessed each of the included studies for their risk of bias, using the Cochrane RoB 1 tool and criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following domains.
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other bias
We judged the studies to be at low, high, or unclear risk of bias for each domain assessed, based on the original risk of bias guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
After data extraction, the two review authors (CW, MG, VS, or BNL) compared the extracted data from each study to discuss and resolve discrepancies before transferring them into the Characteristics of included studies table.
We contacted study authors in order to clarify unclear judgements.
We identified no cluster‐RCTs and no special considerations had to be made for such RCTs.
Measures of treatment effect
For dichotomous outcomes, we expressed treatment effect as risk ratios (RR) with corresponding 95% confidence intervals (CIs). For continuous outcomes, we expressed the treatment effect as mean difference (MD) with 95% CI if studies used the same scales and methods. If studies assessed the same continuous outcome using different methods, we estimated the treatment effect using the standardised mean difference (SMD) with 95% CIs. We presented SMDs as standard deviation (SD) units and interpreted them as follows: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect, as outlined in Section 15.5.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b).
Unit of analysis issues
The participant was the unit of analysis. For studies comparing more than two intervention groups, we made multiple pair‐wise comparisons between all possible pairs of intervention groups. To avoid double counting, we divided shared intervention groups evenly amongst the comparisons. For dichotomous outcomes, we divided both the number of events and the total number of participants. For continuous outcomes, we divided the total number of participants, and left the means and SDs unchanged.
We planned to include cross‐over studies if data were separately reported before and after cross‐over and to only use data from the first phase for our analysis. We identified no cluster‐RCTs and no special considerations had to be made for such RCTs.
Dealing with missing data
We contacted study authors when there were missing data, or studies did not report data in sufficient detail. If studies reported variance other than standard variation, we attempted to convert them when possible, using relevant statistical tools and calculators recommended in Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b). We judged studies that failed to report measures of variance as being at high risk of selective reporting bias.
Assessment of heterogeneity
We scrutinised studies to ensure that they were clinically homogeneous in terms of participants, intervention, comparator, and outcome. To test for statistical heterogeneity, we used a Chi² test. A P value of less than 0.1 indicated the presence of heterogeneity. We quantified and represented inconsistencies with the I² statistic. We interpreted the thresholds as follows (Higgins 2021a):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%; may represent substantial heterogeneity;
75% to 100%: may represent considerable heterogeneity.
Assessment of reporting biases
We minimised most reporting biases by using an inclusive search strategy. We planned to investigate publication bias using a funnel plot if there were 10 or more studies, and by determining the magnitude of publication bias by visually inspecting the asymmetry of the funnel plot and by undertaking a linear regression of the intervention effect estimate against its standard error, weighted by the inverse of the variance of the intervention effect estimate (Egger 1997).
Data synthesis
To summarise the study characteristics, we undertook a narrative synthesis of all included studies. This included key summary data of characteristics of participants within included studies. We performed meta‐analysis for all outcomes with at least one study with data suitable for meta‐analysis. We synthesised data using the random‐effects model in Review Manager Web (RevMan Web 2022). We combined effect estimates of studies that reported data in a similar way in the meta‐analysis. We pooled RRs for dichotomous outcomes and MDs or SMDs for continuous outcomes with 95% CIs.
When meta‐analysis of effect estimates was not possible, we summarised effect estimates (e.g. range and distribution of observed effects), combined P values (e.g. evidence that there is an effect in at least one study), or vote count, based on the direction of effect (e.g. was there any evidence of an effect? (Higgins 2021a)).
Whilst recognising that vitamin D dosing regimens are a matter of debate (Fletcher 2019), for outcome analysis purposes, we defined dosages as:
less than 400 IU/day = prophylactic/supplemental dose;
401 IU/day to 1000 IU/day = low dose;
greater than 1001 IU/day = high dose.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses of potential effect modifiers if there were sufficient data available. We preplanned to perform subgroup analyses by disease type (CD or UC), disease activity (active or inactive), disease severity, age, long‐term (26 weeks or greater) or short‐term (less than 26 weeks) study duration, and vitamin D type.
Sensitivity analysis
When possible, we undertook sensitivity analyses for all outcomes, to assess whether the findings of the review were robust to the decisions made during the review process.
Our preplanned sensitivity analyses were:
investigation of whether the choice of model (fixed‐effect versus random‐effects) impacted the results;
analyses only including studies at low risk of bias across all risk of bias items;
analyses only including studies that had no risk of bias items rated as high risk;
analyses only including studies with reported and estimated SDs, excluding studies with converted SDs;
analyses excluding cluster‐RCTs.
Summary of findings and assessment of the certainty of the evidence
Two review authors (CW, MG, VS, or BNL) independently assessed the certainty of the evidence for each result; we resolved disagreements by consulting and reaching consensus with a third review author (MG or VS) (Schünemann 2021). We presented the primary outcomes for the following comparison in the summary of findings tables, including those where there were no data or no conclusions could be drawn.
Vitamin D (any dose) versus placebo (Table 1)
High‐treatment‐dose vitamin D (defined as greater than 1000 IU/day) versus low‐treatment‐dose vitamin D (defined as 400 IU/day to 1000 IU/day) (Table 2)
Any‐treatment‐dose vitamin D (defined as greater than 400 IU/day) versus supplemental‐dose vitamin D (defined as less than 400 IU/day) (Table 3)
We exported each comparison and all outcomes to GRADEpro GDT software to assess the certainty of the evidence (GRADEpro GDT). Based on risk of bias, inconsistency, imprecision, indirectness, and publication bias, we rated the certainty of the evidence for each outcome as high, moderate, low, or very low. The ratings were defined as follows.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect, and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect, and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
We justified all decisions to downgrade the certainty of the evidence using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
The electronic search strategy generated 895 records after removal of duplicates (Figure 1). Of these, we excluded 825 records after screening the titles and abstracts. A total of 70 records met the inclusion criteria for full‐text review. We excluded eight studies (eight records; Characteristics of excluded studies table). Twelve studies are ongoing (13 records; Characteristics of ongoing studies table). Sixteen studies are awaiting classification (18 records; Characteristics of studies awaiting classification table). We contacted the investigators for information on outcome data, but we received no information. It is possible some of them are still ongoing.
1.
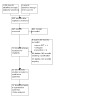
Study flow diagram detailing the steps in the screening process and number of studies at each point.
Twenty‐two studies (31 records) met the criteria for inclusion in this systematic review.
Included studies
There were 22 published RCTs (1874 participants) included in the qualitative analysis. Nineteen trials published in peer‐reviewed journals included 1697 participants (Ahamed 2019; Arihiro 2019; Bafutto 2020; Bendix 2020; Dadaei 2015; de Bruyn 2020; El Amrousy 2021; Jing 2019; Jorgensen 2010; Karimi 2020; Mathur 2017; Narula 2017; Pappa 2012; Pappa 2014; Raftery 2015; Sharifi 2016; Tan 2018; Vogelsang 1995; Wingate 2014). Three trials published in abstract form included 177 participants (Boothe 2011; Dash 2019; Sassine 2020).
The first peer‐reviewed trials were published in 1995 (Vogelsang 1995) and 2010 (Jorgensen 2010), followed by six trials between 2012 and 2016 (Dadaei 2015; Pappa 2012; Pappa 2014; Raftery 2015; Sharifi 2016; Wingate 2014). There has been an acceleration in the number of studies on this topic, where over half of peer‐reviewed trials included in this systematic review were published in 2017 and thereafter (Ahamed 2019; Arihiro 2019; Bafutto 2020; Bendix 2020; de Bruyn 2020; El Amrousy 2021; Jing 2019; Karimi 2020; Mathur 2017; Narula 2017; Tan 2018), and two recent abstracts are pending publication (Dash 2019; Sassine 2020).
Seven studies were performed in North America: US (Boothe 2011; Mathur 2017; Pappa 2012; Pappa 2014) and Canada (Narula 2017; Sassine 2020; Wingate 2014). Five studies were performed in Asia: India (Ahamed 2019; Dash 2019), China (Jing 2019; Tan 2018), and Japan (Arihiro 2019). Five studies were performed in Europe: Denmark (Bendix 2020; Jorgensen 2010), Ireland (Raftery 2015), Austria (Vogelsang 1995), and in both the Netherlands and Belgium (de Bruyn 2020). Three studies were performed in Iran (Dadaei 2015; Karimi 2020; Sharifi 2016). One study was performed in Brazil (Bafutto 2020), and one study was performed in Egypt (El Amrousy 2021). See Characteristics of included studies for full details.
Participants
Ten studies involving 523 participants only enroled people with CD (Bafutto 2020; Bendix 2020; Boothe 2011; de Bruyn 2020; Jorgensen 2010; Narula 2017; Raftery 2015; Sassine 2020; Vogelsang 1995; Wingate 2014). Five studies involving 361 participants only enroled people with UC (Ahamed 2019; Dash 2019; Karimi 2020; Mathur 2017; Sharifi 2016). The remaining seven studies involving 976 participants enroled both people with CD and people with UC (Arihiro 2019; Dadaei 2015; El Amrousy 2021; Jing 2019; Pappa 2012; Pappa 2014; Tan 2018). These studies did not differentiate between CD or UC in the description of participants, interventions, or outcomes.
Disease activity at baseline also differed across studies. Four studies only enroled people with active disease (Ahamed 2019; Bafutto 2020; Bendix 2020; Karimi 2020). Six studies only enroled people in remission (de Bruyn 2020; Jorgensen 2010; Narula 2017; Raftery 2015; Sharifi 2016; Wingate 2014). The remaining 12 studies did not discriminate between active or inactive disease (Arihiro 2019; Boothe 2011; Dadaei 2015; Dash 2019; El Amrousy 2021; Jing 2019; Mathur 2017; Pappa 2012; Pappa 2014; Sassine 2020; Tan 2018; Vogelsang 1995).
Amongst the included studies, three were performed in children with mean ages ranging from 13.2 to 14.3 years (El Amrousy 2021; Sassine 2020; Wingate 2014). Ten studies were performed in adults with mean ages ranging from 32.0 to 44.9 years (Ahamed 2019; Arihiro 2019; de Bruyn 2020; Jorgensen 2010; Karimi 2020; Mathur 2017; Narula 2017; Raftery 2015; Sharifi 2016; Tan 2018). Two studies by the same group of authors enroled people aged between 5 and 21 years (Pappa 2012; Pappa 2014). Seven studies did not indicate the recruitment age, although participants in four of these studies were likely adults as the mean ages ranged between 35.0 and 41.9 years (Dadaei 2015; Dash 2019; Jing 2019; Vogelsang 1995).
There were approximately 53.3% males and 46.7% females in all the studies.
Intervention
All studies either compared vitamin D at different doses or with a non‐vitamin D control. There was substantial heterogeneity in the vitamin D doses used, ranging from the equivalent of 285 IU/day (Bafutto 2020; Pappa 2012) to a single dose of 300,000 IU of vitamin D3 (Sharifi 2016). When specified, most studies used vitamin D3 (cholecalciferol) as an intervention, while only two studies by the same group of authors reported using vitamin D2 (ergocalciferol) (Pappa 2012; Pappa 2014). The duration of treatment was similarly heterogeneous, ranging from 4 to 52 weeks. One study evaluated vitamin D in conjunction with infliximab (Bendix 2020).
Outcomes
One study reported the primary outcome of clinical response in people with active UC (Ahamed 2019).
Four studies examined clinical relapse following vitamin D therapy using mixed populations and mixed measures to determine clinical relapse (de Bruyn 2020; El Amrousy 2021; Jorgensen 2010; Narula 2017). de Bruyn 2020 and Jorgensen 2010 both used CDAI scores to monitor relapse, with de Bruyn 2020 defining relapse as a CDAI score of more than 220 at any point during follow‐up, and Jorgensen 2010 defining relapse as a score of more than 150 or an increase of more than 70 during the one‐year follow‐up. El Amrousy 2021 used the Paediatric Ulcerative Colitis Activity Index (PUCAI) score, but did not specify how relapse was defined other than to state that a score of less than 10 denoted remission. Narula 2017 used the HBI score to monitor relapse, defining relapse as a score of 5 or more with an increase of more than 3 points from baseline, or if there was an introduction or escalation of therapy.
Another seven studies investigated the impact of vitamin D therapy on quality of life using a mixture of validated quality of life measures (Bafutto 2020; Dash 2019; de Bruyn 2020; El Amrousy 2021; Karimi 2020; Mathur 2017; Raftery 2015). However, Bafutto 2020, Dash 2019, Karimi 2020, and Raftery 2015 were not included in these meta‐analyses as the data were not presented in useable numerical formats. A variety of instruments were used to measure psychometric data. The IBDQ and Short IBDQ were the most commonly used instruments (Bafutto 2020; Dash 2019; Karimi 2020; Mathur 2017; Raftery 2015). Other studies relied on a well‐being score (Ahamed 2019), EuroQol or 36‐item Short Form (SF‐36) (de Bruyn 2020), or IMPACT III (El Amrousy 2021).
Eighteen studies reported data for withdrawals due to adverse events (Ahamed 2019; Arihiro 2019; Bafutto 2020; Bendix 2020; Dadaei 2015; de Bruyn 2020; El Amrousy 2021; Jing 2019; Jorgensen 2010; Karimi 2020; Narula 2017; Pappa 2012; Pappa 2014; Raftery 2015; Sharifi 2016; Tan 2018; Vogelsang 1995; Wingate 2014).
For disease activity, 14 studies used disease activity scores, although the data available to estimate response or relapse rates varied broadly across studies (Ahamed 2019; Arihiro 2019; Boothe 2011; Dadaei 2015; Dash 2019; El Amrousy 2021; Jorgensen 2010; Karimi 2020; Mathur 2017; Narula 2017; Raftery 2015; Tan 2018; Vogelsang 1995; Wingate 2014). Fifteen studies reported data on inflammation biomarkers, such as faecal calprotectin or C‐reactive protein (CRP) (Bafutto 2020; Bendix 2020; Dadaei 2015; El Amrousy 2021; Jing 2019; Karimi 2020; Mathur 2017; Narula 2017; Pappa 2012; Pappa 2014; Raftery 2015; Sharifi 2016; Tan 2018; Vogelsang 1995; Wingate 2014). One study reported corticosteroid‐free remission as its sole outcome of disease activity (Sassine 2020). Two studies used endoscopic endpoints to assess disease activity (Bendix 2020; de Bruyn 2020).
Normalisation of vitamin D concentrations was generally reported as the mean concentrations at the time of follow‐up or the change from baseline. Nine studies measured vitamin D concentrations at the end of follow‐up (Bafutto 2020; Dadaei 2015; El Amrousy 2021; Narula 2017; Pappa 2012; Raftery 2015; Sharifi 2016; Tan 2018; Wingate 2014), whilst five studies measured change in vitamin D levels over the course of the study (Mathur 2017; Pappa 2012; Sassine 2020; Tan 2018; Vogelsang 1995). Jorgensen 2010 reported dichotomous data on the number of participants with vitamin D deficiency (defined as less than 50 nmol/L) at the end of the study, and Pappa 2014 presented data on the number of participants who maintained a level greater than 32 nmol/L at each follow‐up visit. Four studies collected and presented data on changes in vitamin D concentration in graphical form, but without corresponding numerical data with which to perform meta‐analysis (Arihiro 2019; Bendix 2020; Jorgensen 2010; Karimi 2020). Boothe 2011 did not state the numbers randomised to each group and so data on vitamin D concentrations could not be used in meta‐analysis.
Serious adverse events were generally reported as the number of adverse events that occurred in each intervention arm. Laboratory changes in the setting of possible vitamin D toxicity, such as hypercalcaemia or hyperphosphatemia, were also noted.
Funding
Non‐profit organisations or research foundations funded five studies (de Bruyn 2020; Jorgensen 2010; Narula 2017; Pappa 2014; Raftery 2015).
Governmental organisations funded two studies (Arihiro 2019; Tan 2018), and universities funded four studies (Karimi 2020; Mathur 2017; Sharifi 2016; Wingate 2014).
The remaining eleven studies did not state any means of funding (Ahamed 2019; Bafutto 2020; Bendix 2020; Boothe 2011; Dadaei 2015; Dash 2019; El Amrousy 2021; Jing 2019; Pappa 2012; Sassine 2020; Vogelsang 1995).
Conflicts of interest
Three studies stated that they had received the medication used in their trial from pharmaceutical companies, but stated no other conflicts of interest or industry involvement (de Bruyn 2020; Narula 2017; Wingate 2014).
One study stated that one author was the co‐director of the Clinical Investigator Training Program which was sponsored by Harvard University, Massachusetts Institute of Technology, Pfizer, and Merck (Pappa 2012).
Ten studies stated that they had no conflicts of interest (Ahamed 2019; Arihiro 2019; Bafutto 2020; Jorgensen 2010; Karimi 2020; Mathur 2017; Pappa 2014; Raftery 2015; Sharifi 2016; Tan 2018).
The remaining eight studies did not make any statement about conflicts of interest (Bendix 2020; Boothe 2011; Dadaei 2015; Dash 2019; El Amrousy 2021; Jing 2019; Sassine 2020; Vogelsang 1995).
Study details can be found in Table 4 and the Characteristics of included studies table.
1. Study details.
| Study | Publication status | Population | Comparisons | Duration | Outcomes assesseda |
| Ahamed 2019 | Full publication | Active/UC |
Group 1: nano liquid formulation of vitamin D3 60,000 IU/day for 8 days (n = 30) Group 2: similar appearing plus tasting syrup for 8 days (n = 30) |
4 weeks | 1a, 1d, 2c |
| Arihiro 2019 | Full publication | Active and inactive/CD and UC |
Group 1: vitamin D3 500 IU/day (n = 119) Group 2: placebo (n = 118) |
26 weeks | 1d, 2a, 2b, 2c |
| Bafutto 2020 | Full publication | Active/CD |
Group 1: vitamin D 2000 IU/week for 8 weeks (n = 10) Group 2: vitamin D 10,000 IU/week for 8 weeks (n = 10) Group 3: vitamin D 50,000 IU/week for 8 weeks (n = 10) |
52 weeks | 1c, 1d, 2b, 2c |
| Bendix 2020 | Full publication | Active/CD |
Group 1: high‐dose vitamin D (200,000 IU at baseline followed by 20,000 IU/day) plus infliximab (n = 8) Group 2: placebo plus infliximab (n = 8) Group 3: high‐dose vitamin D plus placebo (n = 16) Group 4: placebo plus placebo (n = 8) |
6 weeks | 1d, 2a, 2c |
| Boothe 2011 | Abstract | Unknown/CD |
Group 1: vitamin D 1000 IU/day Group 2: vitamin D 10,000 IU/day |
26 weeks | 2a, 2b, 2c |
| Dadaei 2015 | Full publication | Active and inactive/CD and UC |
Group 1: vitamin D3 50,000 IU/week (n = 53) Group 2: none (n = 55) |
12 weeks | 1d, 2a, 2b, 2c |
| Dash 2019 | Abstract | Unknown/UC |
Group 1: low‐dose vitamin D (dose not specified) (n = 76) Group 2: no intervention (n = 76) |
N/A | 1c, 2a, 2c |
| de Bruyn 2020 | Full publication | Inactive/post‐operative CD |
Group 1: vitamin D3 25,000 IU/week (n = 72) Group 2: comparable placebo vials (n = 71) |
26 weeks | 1b, 1c, 1d, 2c |
| El Amrousy 2021 | Full publication | Active and inactive/CD and UC |
Group 1: vitamin D3 2000 IU/day (n = 50) Group 2: placebo (n = 50) |
26 weeks | 1c, 1d, 2a, 2b, 2c |
| Jing 2019 | Full publication | Unknown/CD and UC |
Group 1: vitamin D 400 IU/day (n = 99) Group 2: no intervention (n = 99) |
4 weeks | 1d, 2b, 2c |
| Jorgensen 2010 | Full publication | Inactive/CD |
Group 1: vitamin D3 1200 IU/day plus calcium 1200 mg/day (n = 46) Group 2: calcium 1200 mg/day (n = 48) |
52 weeks | 1b, 1d, 2b, 2c |
| Karimi 2020 | Full publication | Active/UC |
Group 1: vitamin D 1000 IU/day (n = 25) Group 2: vitamin D 2000 IU/day (n = 25) |
12 weeks | 1c, 1d, 2a, 2c |
| Mathur 2017 | Full publication | Active and inactive/UC |
Group 1: vitamin D3 2000 IU/day (n = 8) Group 2: vitamin D3 4000 IU/day (n = 10) |
12 weeks | 1c, 1d, 2a, 2b, 2c |
| Narula 2017 | Full publication | Inactive/CD |
Group 1: vitamin D3 1000 IU/day (n = 16) Group 2: vitamin D3 10,000 IU/day (n = 18) |
52 weeks | 1b, 1d, 2b, 2c |
| Pappa 2012 | Full publication | Active and inactive/CD and UC |
Group 1: A: vitamin D2 2000 IU/day (n = 24) Group 2: B: vitamin D3 2000 IU/day (n = 24) Group 3: C: vitamin D2 50,000 IU/week (n = 23) |
6 weeks | 1d, 2b, 2c |
| Pappa 2014 | Full publication | Active and inactive/CD and UC |
Group 1: vitamin D2 400 IU/day (n = 32) Group 2: vitamin D2 1000 IU/day (between May 1 and October 31) plus 2000 IU/day (between November 1 and April 30) (n = 31) |
52 weeks | 1d, 2b, 2c |
| Raftery 2015 | Full publication | Inactive/CD |
Group 1: vitamin D3 2000 IU/day (n = 13) Group 2: placebo (n = 14) |
12 weeks | 1c, 1d, 2a, 2b, 2c |
| Sassine 2020 | Abstract | Inactive or mildly active/CD |
Group 1: vitamin D3 3000 IU/day (< 40 kg participant) or 4000 IU/day (≥ 40 kg participant) for 4 weeks, then 2000 IU/day for 48 weeks (n = 12) Group 2: vitamin D3 800 IU/day for 52 weeks (n = 13) |
52 weeks | 2b, 2c |
| Sharifi 2016 | Full publication | Inactive/UC |
Group 1: vitamin D3 300,000 IU intramuscularly (n = 46) Group 2: normal saline intramuscularly (n = 44) |
12 weeks | 1d, 2b, 2c |
| Tan 2018 | Full publication | Active and inactive/CD and UC |
Group 1: vitamin D 150,000 IU every 3 months plus elemental calcium 200 mg 3 times daily (CD: n = 23; UC: n = 25) Group 2: elemental calcium 200 mg 3 times daily (CD: n = 23; UC: n = 24) Group 3: "vehicle control group" (CD: n = 25; UC: n = 25) |
52 weeks | 1d, 2a, 2b, 2c |
| Vogelsang 1995 | Full publication | Active and inactive/CD |
Group 1: vitamin D3 1000 IU/day (n = 37) Group 2: no supplementation (n = 38) |
52 weeks | 1d, 2a, 2b, 2c |
| Wingate 2014 | Full publication | Inactive/CD |
Group 1: vitamin D3 400 IU/day (n = 40) Group 2: vitamin D3 2000 IU/day (n = 43) |
26 weeks | 1d, 2a, 2c |
CD: Crohn's disease; IU: international unit; n: number of participants; UC: ulcerative colitis.
aOutcomes:
- 1a. Clinical response in people with active disease, as defined by the primary studies
- 1b. Clinical relapse in people in remission
- 1c. Quality of life measures included changes in the standard Inflammatory Bowel Disease Questionnaire score or Short Inflammatory Bowel Disease Questionnaire score (continuous)
- 1d. Withdrawals due to adverse events (dichotomous)
- 2a. Disease activity at study end (continuous)
- 2b. Normalisation of vitamin D levels (dichotomous)
- 2c. Total serious adverse events (dichotomous)
Contact with authors
We attempted to contact 15 study authors for clarifications. We received responses from five, which provided us with unpublished information alongside what was published in their papers (El Amrousy 2021; Karimi 2020; Mathur 2017; Sharifi 2016; Tan 2018). Contact with the others failed either due to lack of response (Arihiro 2019; Dadaei 2015; Pappa 2012; Pappa 2014), or due to contact information that was no longer valid or lack of contact information (Ahamed 2019; Boothe 2011; Dash 2019; Jing 2019; Sassine 2020; Vogelsang 1995).
Excluded studies
We excluded eight studies. Four were not RCTs (JPRN‐UMIN000025961; Laing 2020; Mullin 2011; O'Sullivan 2019), and four used ineligible interventions (Kojecky 2020; Lee 2020; Sharifi 2020; Simek 2016). See Characteristics of excluded studies table.
Studies awaiting classification
We found 16 studies that are awaiting classification (Characteristics of studies awaiting classification table).
Ongoing studies
We found 12 ongoing studies (Characteristics of ongoing studies table).
Risk of bias in included studies
A summary of the risk of bias assessments for the included studies is summarised in Figure 2. Details for each risk of bias assessment are included in the Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Fifteen studies provided adequate information on how they randomised their sequence generation to be deemed at low risk of bias, primarily utilising computer‐generated random sequences (Ahamed 2019; Arihiro 2019; Bendix 2020; Dadaei 2015; El Amrousy 2021; Jing 2019; Jorgensen 2010; Mathur 2017; Narula 2017; Pappa 2012; Pappa 2014; Raftery 2015; Sharifi 2016; Vogelsang 1995; Wingate 2014). Seven studies were described as being randomised but gave insufficient information as to how a random sequence was generated, and so were deemed at unclear risk of bias (Bafutto 2020; Boothe 2011; Dash 2019; de Bruyn 2020; Karimi 2020; Sassine 2020; Tan 2018).
Twelve studies gave adequate information on their method of allocation concealment to be deemed at low risk of bias, utilising either third party central allocation or sealed opaque envelopes (Ahamed 2019; Arihiro 2019; Bendix 2020; El Amrousy 2021; Jorgensen 2010; Mathur 2017; Narula 2017; Pappa 2012; Pappa 2014; Raftery 2015; Vogelsang 1995; Wingate 2014). Ten studies did not provide information on how allocation concealment was achieved, and so were deemed at unclear risk of bias (Bafutto 2020; Boothe 2011; Dadaei 2015; Dash 2019; de Bruyn 2020; Jing 2019; Karimi 2020; Sassine 2020; Sharifi 2016; Tan 2018).
Blinding
Eleven studies described in sufficient detail their method of blinding participants and trial personnel to be deemed at low risk of performance bias (Ahamed 2019; Arihiro 2019; Bendix 2020; de Bruyn 2020; El Amrousy 2021; Jorgensen 2010; Karimi 2020; Mathur 2017; Narula 2017; Raftery 2015; Sassine 2020). Four studies stated that participants and personnel were blinded, but did not state how this was achieved, and so were deemed at unclear risk of bias (Bafutto 2020; Boothe 2011; Sharifi 2016; Wingate 2014). Seven studies either made no mention of blinding of participants and personnel, or openly stated that participants were not blinded to interventional arms, and so were deemed at high risk for performance bias (Dadaei 2015; Dash 2019; Jing 2019; Pappa 2012; Pappa 2014; Tan 2018; Vogelsang 1995).
Twelve studies described in sufficient detail their method of blinding outcome assessors to be deemed at low risk of detection bias (Ahamed 2019; Arihiro 2019; Bendix 2020; Boothe 2011; de Bruyn 2020; El Amrousy 2021; Jorgensen 2010; Karimi 2020; Mathur 2017; Narula 2017; Raftery 2015; Wingate 2014). Three studies were described as blinded, but did not describe how outcome assessors were blinded to participant allocation, and so were deemed at unclear risk of bias (Bafutto 2020; Sassine 2020; Sharifi 2016). Seven studies either made no mention of blinding of outcome assessors, or openly stated that assessors were not blinded to interventional arms, and so were deemed at high risk for detection bias (Dadaei 2015; Dash 2019; Jing 2019; Pappa 2012; Pappa 2014; Tan 2018; Vogelsang 1995).
Incomplete outcome data
Nineteen studies adequately reported their trial flow, with reasons given for withdrawals and balanced withdrawals across interventional arms, and were deemed at low risk for attrition bias (Ahamed 2019; Arihiro 2019; Bafutto 2020; Bendix 2020; Dadaei 2015; de Bruyn 2020; El Amrousy 2021; Jing 2019; Jorgensen 2010; Karimi 2020; Mathur 2017; Narula 2017; Pappa 2012; Pappa 2014; Raftery 2015; Sharifi 2016; Tan 2018; Vogelsang 1995; Wingate 2014).
Three studies did not provide sufficient information for attrition through the study process to be assessed, and were deemed at unclear risk of bias (Boothe 2011; Dash 2019; Sassine 2020).
Selective reporting
Twelve studies reported their outcomes appropriately per their trial registrations (Arihiro 2019; Dadaei 2015; de Bruyn 2020; El Amrousy 2021; Jorgensen 2010; Karimi 2020; Narula 2017; Pappa 2012; Pappa 2014; Raftery 2015; Tan 2018; Wingate 2014).
The other 10 studies either did not have trial registrations or did not fully appropriately report outcome data (Ahamed 2019; Bafutto 2020; Bendix 2020; Boothe 2011; Dash 2019; Jing 2019; Mathur 2017; Sassine 2020; Sharifi 2016; Vogelsang 1995).
Other potential sources of bias
Twenty studies were at low risk of bias for other bias as there were no baseline imbalances per group, or other imbalances affecting outcome data.
Only Jing 2019 and Mathur 2017 were rated at unclear risk, the first because it reported no baseline characteristics and the second for baseline imbalances between group disease activity scores.
Effects of interventions
See: Table 1; Table 2; Table 3
All outcome data can be found in Table 5 and Table 6.
2. Primary outcomes.
| Study ID | 1a. Clinical response in active disease | 1b. Clinical relapse | 1c. Quality of life measures | 1d. Withdrawals due to adverse events |
| Ahamed 2019 | Defined as reduction in UCDAI by > 3 points: Active: 16/30 Control: 4/30 |
Not reported | Not reported | Active: 0/30 Control: 0/30 |
| Arihiro 2019 | Not reported | Not reported | Not reported | Active: 0/119 Control: 0/118 |
| Bafutto 2020 | Not reported | Not reported | Studied but relevant data for meta‐analysis not provided | Group 1: 0/10 Group 2: 0/10 Group 3: 0/10 |
| Bendix 2020 | Not reported | Not reported | Not reported | Group 1: 1/8 Group 2: 0/8 Group 3: 0/16 Group 4: 0/8 |
| Boothe 2011 | Not reported | Not reported | Not reported | Not reported |
| Dadaei 2015 | Not reported | Not reported | Not reported | Active: 0/53 Control: 0/55 |
| Dash 2019 | Not reported | Not reported | Studied but relevant data for meta‐analysis not provided | Not reported |
| de Bruyn 2020 | Not reported | Defined as CDAI > 220 at any point during follow‐up: Active: 11/63 Control: 10/55 |
Change in IBDQ score at 26 weeks from baseline: Active: +24.9 (SD 3.7) Control: +31.1 (SD 3.8) |
Active: 2/72 Control: 1/71 |
| El Amrousy 2021 | Not reported | Relapse during study period: Active: 8/50 Control: 18/48 |
IMPACT‐III QoL Questionnaire Score: Active: 159.9 (SD 30.8) Control: 119.2 (SD 27.6) |
Active: 0/50 Control: 0/50 |
| Jing 2019 | Not reported | Not reported | Not reported | Active: 0/99 Control: 0/99 |
| Jorgensen 2010 | Not reported | Defined as a CDAI > 150 and an increase in CDAI of > 70 during the 1‐year follow‐up:
Active: 6/46 Control: 14/48 |
Not reported | Active: 0/46 Control: 0/48 |
| Karimi 2020 | Not reported | Not reported | Change in IBDQ score reported graphically but without corresponding data. | Active: 0/25 Control: 0/25 |
| Mathur 2017 | Not reported | Not reported | Change in SIBDQ score: Active: +1 (SD 1) Control: +0.1 (SD 1) |
Active: 0/10 Control: 0/8 |
| Narula 2017 | Not reported | Defined as HBI score ≥ 5 with an increase of > 3 points from baseline, or initiation or escalation of existing or new therapies: Active: 6/18 Control: 11/16 |
Not reported | Active: 1/18 Control: 1/16 |
| Pappa 2012 | Not reported | Not reported | Not reported | Group 1: 0/24 Group 2: 0/24 Group 3: 0/23 |
| Pappa 2014 | Not reported | Not reported | Not reported | Group 1: 0/32 Group 2: 1/31 |
| Raftery 2015 | Not reported | Not reported | Change in IBDQ score reported graphically but without corresponding data. | Active: 0/13 Control: 0/14 |
| Sassine 2020 | Not reported | Not reported | Not reported | Not reported |
| Sharifi 2016 | Not reported | Not reported | Not reported | Active: 0/46 Control: 0/44 |
| Tan 2018 | Not reported | Not reported | Not reported | UC Group 1: 0/25 UC Group 2: 0/24 UC Group 3: 0/25 CD Group 1: 0/23 CD Group 2: 0/23 CD Group 3: 0/25 |
| Vogelsang 1995 | Not reported | Not reported | Not reported | Active: 0/37 Control: 0/38 |
| Wingate 2014 | Not reported | Not reported | Not reported | Active: 0/43 Control: 0/40 |
CD: Crohn's disease; CDAI: Crohn's Disease Activity Index; HBI: Harvey‐Bradshaw Index; IBDQ: Inflammatory Bowel Disease Questionnaire; SD: standard deviation; SIBDQ: Short Inflammatory Bowel Disease Questionnaire; UC: ulcerative colitis; UCDAI: Ulcerative Colitis Disease Activity Index.
See Table 4 for information on interventions given to each group.
3. Secondary outcomes.
| Study ID | 2a. Disease activity at study end | 2b. Normalisation of vitamin D levels | 2c. Total serious adverse events |
| Ahamed 2019 | Not reported | Not reported | Group 1: 0/30 Group 2: 0/30 |
| Arihiro 2019 | UC (Lichtinger score): Active (n = 88): 3.24 (SD 0.16) Control (n = 80): 2.75 (SD 1.18) CD (CDAI score): Active (n = 27): 78.8 (SD 65.3) Control (n = 28): 65.3 (SD 44.6) |
Change in vitamin D levels reported graphically but without corresponding confidence interval data. | Active: 0/115 Control: 0/108 |
| Bafutto 2020 | Not reported | Vitamin D level at end of study: Group 1: 26 (SD 6.7) Group 2: 26 (SD 5.8) Group 3: 46.4 (SD 12.7) |
Group 1: 0/10 Group 2: 0/10 Group 3: 0/10 |
| Bendix 2020 | HBI score at study end reported graphically but without corresponding data. | Change in vitamin D level reported graphically but without corresponding data for groups 3 and 4. | Group 1: 1/8 Group 2: 1/8 Group 3: 1/16 Group 4: 1/8 |
| Boothe 2011 | No information on numbers randomised to each group so unable to include in meta‐analysis | No information on numbers randomised to each group so unable to include in meta‐analysis | Not reported |
| Dadaei 2015 | Data gathered but not presented | Vitamin D level at end of study: Active: 67.89 (SD 33.7) (n = 53) Control: 23.90 (SD 8.3) (n = 55) |
Active: 0/53 Control: 0/55 |
| Dash 2019 | No information on numbers randomised to each group so unable to include in meta‐analysis | Not reported | Not reported |
| de Bruyn 2020 | Not reported | Not reported | Active: 2/72 Control: 1/71 |
| El Amrousy 2021 | PCDAI at study end: Active (n = 27): 13.6 (SD 3.1) Control (n = 26): 27.5 (SD 3.5) PUCAI at study end: Active (n = 23): 11.1 (SD 2.4) Control (n = 22): 21.8 (SD 2.9) |
Vitamin D level at end of study: Active: 52.8 (SD 6.7) (n = 50) Control: 13.4 (SD 2.5) (n = 48) |
Active: 0/50 Control: 0/48 |
| Jing 2019 | |||
| Jorgensen 2010 | Not reported | Vitamin D deficiency (< 50 nmol/L) at end of study: Group 1: 15/46 Group 2: 14/48 Vitamin D level at end of study reported graphically but without corresponding data for group 2. |
Group 1: 0/46 Group 2: 0/48 |
| Karimi 2020 | Disease activity score at end of study reported graphically but without corresponding data. | Vitamin D level at end of study reported graphically but without corresponding data. | Group 1: 0/25 Group 2: 0/25 |
| Mathur 2017 | Mean change in partial Mayo score: Group 1: −0.5 (SD 1.5) Group 2: −1.3 (SD 2.9) |
Change in vitamin D level:
Group 1: 5.00 (SD 3.82) Group 2: 16.80 (SD 9.15) |
Group 1: 0/8 Group 2: 0/10 |
| Narula 2017 | Not reported | Vitamin D level at end of study: Group 1: 82.8 nmol/L (SD 26.3) Group 2: 160.8 nmol/L (SD 43.2) |
Group 1: 0/8 Group 2: 0/12 |
| Pappa 2012 | Not reported | Change in vitamin D from start to end of follow‐up: Group A: 9.3 (SD 1.8) Group B: 16.4 (SD 2.0) Group C: 25.4 (SD 2.5) Vitamin D level at end of study: Group A: 25.7 (SD 2.2) Group B: 31.5 (SD 1.9) Group C: 40.8 (SD 2.6) |
Group A: 0 Group B: 0 Group C: 0 |
| Pappa 2014 | Not reported | Not reported as change or as level at end of follow‐up. Reported as number of participants who maintained level > 32 at each follow‐up visit. | Group A: 0 Group B: 0 |
| Raftery 2015 | Disease activity score at end of study reported graphically but without corresponding data. | Vitamin D level at end of follow‐up: Active: 91.6 (75.5–107.6 nmol/L) Control: 40.4 (30.4–50.4 nmol/L) |
Active: 0 Control: 0 |
| Sassine 2020 | Not reported | Change in vitamin D from start to end of follow‐up: High dose: median 38.0 (IQR 34.0 to 49.0) Low dose: median 4.0 (IQR −1.5 to 12.0) |
Not reported |
| Sharifi 2016 | Not reported | Vitamin D at end of follow‐up: Active: 40.8 (SD 5.2) (n = 46) Control: 33.9 (SD 10.6) (n = 40) |
Active: 0 Control: 0 |
| Tan 2018 | Mayo score at end of follow‐up: UC Group A: 3.12 (SD 1.04) UC Group C: 3.04 (SD 1.54) CDAI at end of follow‐up: CD Group A: 92.87 (SD 36.65) CD Group C: 91.47 (SD 45.46) |
Change in vitamin D from start to end of follow‐up: UC Group A: 17.47 (SD 13.01) UC Group B: 5.30 (SD 6.28) UC Group C: 2.02 (SD 6.19) CD Group A: 12.47 (SD 9.15) CD Group B: 4.73 (SD 6.97) CD Group C: 1.36 (SD 4.75) Vitamin D level at end of follow‐up: UC Group A: 28.09 (SD 11.60) UC Group B: 17.83 (SD 6.62) UC Group C: 13.07 (SD 5.02) CD Group A: 23.04 (SD 9.66) CD Group B: 15.94 (SD 7.87) CD Group C: 13.30 (SD 4.58) |
Group A: 0 Group B: 0 Group C: 0 |
| Vogelsang 1995 | Change in CDAI score from start to end of follow‐up: Active: median −43 (−70 to +23) Control: median −2 (−36 to +22) |
Change in vitamin D from start to end of follow‐up: Active: median 2.0 (−6.7 to +13) (n=30) Control: median −2.7 (−10.1 to +5.5) (n=30) |
Active: 0 Control: 0 |
| Wingate 2014 | PCDAI < 10 at end of follow‐up: High dose: 32/43 Low dose: 29/40 |
Vitamin D level at end of follow‐up: High dose: 34.4 (SD 10.4) Low dose: 28.0 (SD 8.8) |
High dose: 0 Low dose: 0 |
CD: Crohn's disease; CDAI: Crohn's Disease Activity Index; HBI: Harvey‐Bradshaw Index; n: number of participants; PCDAI: Pediatric Crohn's Disease Activity Index; UC: ulcerative colitis.
Vitamin D (all doses) versus placebo or no treatment
Thirteen studies compared the effect of vitamin D (all doses) against placebo or no treatment, when combining all doses of vitamin D as active treatment (Ahamed 2019; Arihiro 2019; Bendix 2020; Dadaei 2015; Dash 2019; de Bruyn 2020; El Amrousy 2021; Jing 2019; Jorgensen 2010; Raftery 2015; Sharifi 2016; Tan 2018; Vogelsang 1995).
Primary outcomes
Clinical response for people with active disease
One study compared the rates of clinical response in people with active disease when using vitamin D compared to placebo (Ahamed 2019). The rate of clinical response in active UC was 16/30 for vitamin D compared with 4/30 for placebo (RR 4.00, 95% CI 1.51 to 10.57; 1 study, 60 participants; very low‐certainty evidence; Analysis 1.1). No conclusions can be drawn due to very low certainty of this outcome owing to very serious imprecision and risk of bias (Table 1).
1.1. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 1: Clinical response
Clinical relapse for people in remission
Three studies compared the rates of clinical relapse in people in remission when using vitamin D compared to placebo or no treatment (de Bruyn 2020; El Amrousy 2021; Jorgensen 2010). There may be a difference in clinical relapse favouring vitamin D (25/159) when compared to placebo (42/151) for people with IBD (RR 0.57, 95% CI 0.34 to 0.96; 3 studies, 310 participants; low‐certainty evidence; Analysis 1.2). The certainty of the evidence was low due to serious concerns with imprecision and risk of bias (Table 1).
1.2. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 2: Clinical relapse (mixed IBD)
This result showed no difference following sensitivity analyses using a fixed‐effect model (Analysis 1.3), but sensitivity analysis for removal of risk of bias was not possible due to the small number of studies remaining when all studies at unclear risk of bias were removed.
1.3. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 3: Clinical relapse (mixed IBD) – sensitivity analysis (fixed‐effect)
Quality of life
Two studies reported quality of life measures at the end of follow‐up when comparing vitamin D to placebo or no treatment (de Bruyn 2020; El Amrousy 2021). They found no difference in quality of life scores between groups (SMD −0.13, 95% CI −3.10 to 2.83 (the SMD value indicates a negligent decrease in quality of life, and the corresponding CI indicates that the effect could range from a large decrease to a large increase in quality of life); 2 studies, 243 participants; very low‐certainty evidence; Analysis 1.4), but no conclusions could be drawn due to very low certainty of this outcome owing to very serious concerns with inconsistency and imprecision (Table 1).
1.4. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 4: Quality of life (QoL) (end of follow‐up/change in score)
A sensitivity analysis using a fixed‐effect analysis showed slightly different results (SMD −0.34, 95% CI −0.63 to −0.06 (the SMD value indicates a small decrease in quality of life, and the corresponding CIs indicate that the effect could range from a moderate to a negligible decrease in quality of life); 2 studies, 243 participants; very low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 5: QoL (end of follow‐up/change in score) – sensitivity analysis (fixed‐effect)
One study reported quality of life measures comparing vitamin D to no treatment, but did not present the relevant data in the results section and so could not be used for meta‐analysis (Dash 2019).
Another study reported change in quality of life measure scores when comparing vitamin D to placebo (Raftery 2015), but presented the data graphically with corresponding numerical data, and so could not be used for meta‐analysis.
Withdrawals due to adverse events
Twelve studies reported the number of withdrawals due to adverse events between vitamin D and placebo or no treatment (Ahamed 2019; Arihiro 2019; Bendix 2020; Dadaei 2015; de Bruyn 2020; El Amrousy 2021; Jing 2019; Jorgensen 2010; Raftery 2015; Sharifi 2016; Tan 2018; Vogelsang 1995). They found no difference in numbers of withdrawals between the vitamin D (2/629) and placebo or no treatment (1/622) groups (RR 1.97, 95% CI 0.18 to 21.27; 12 studies, 1251 participants; very low‐certainty evidence; note 11 studies reported withdrawals but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 1 study rather than 12; Analysis 1.6). The certainty of the evidence was very low due to serious concerns with imprecision owing to very low event numbers and serious concerns with risk of bias owing to unclear randomisation, allocation, and other sources of bias (Table 1).
1.6. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 6: Withdrawals due to adverse events
This result remained the same on sensitivity analysis using a fixed‐effect analysis (Analysis 1.7), and on sensitivity analysis for removal of studies at risk of bias the remaining studies did not report any withdrawals in either group and so no analysis could be performed (Analysis 1.8).
1.7. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 7: Withdrawals due to adverse events – sensitivity analysis (fixed‐effect)
1.8. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 8: Withdrawals due to adverse events – sensitivity analysis (removal of studies at risk of bias)
Secondary outcomes
Disease activity
Three studies compared disease activity at the end of follow‐up between the vitamin D and placebo or no treatment groups in people with CD (Arihiro 2019; El Amrousy 2021; Tan 2018). They found no difference in disease activity between the two groups (SMD −1.25, 95% CI −3.39 to 0.89; 156 participants (the SMD value indicates a large decrease in disease activity, and the corresponding CIs indicate that the effect could range from a large decrease to a large increase); 3 studies; very low‐certainty evidence; Analysis 1.9). The certainty of the evidence was very low due to very serious concerns with inconsistency, imprecision, and risk of bias.
1.9. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 9: Disease activity at end of follow‐up (Crohn's disease)
Sensitivity analysis using a fixed‐effect analysis showed a difference between the two groups favouring vitamin D (SMD −0.44, 95% CI −0.80 to −0.07; 156 participants (the SMD value indicates a small decrease in disease activity, and the corresponding CIs indicate that the effect could range from a large decrease to a negligent decrease); 3 studies; very low‐certainty evidence; Analysis 1.10). Sensitivity analysis for removal of studies at risk of bias was not possible due to the small number of studies remaining after removing those at unclear or high risk of bias.
1.10. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 10: Disease activity at end of follow‐up (Crohn's disease) – sensitivity analysis (fixed‐effect)
One study compared change in disease activity score from the beginning to the end of the follow‐up period when comparing vitamin D to placebo or no treatment for people with CD (Vogelsang 1995). They found a difference in change in disease activity score favouring vitamin D (MD −41.00, 95% CI −67.03 to −14.97; 1 study, 75 participants; very low‐certainty evidence; Analysis 1.11), but the certainty of this evidence was very low due to very serious concerns regarding imprecision owing to low participant numbers and serious concerns regarding risk of bias relating to blinding.
1.11. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 11: Disease activity at end of follow‐up – change in disease activity score (Crohn's disease)
Three studies compared disease activity at the end of follow‐up between the vitamin D and placebo or no treatment groups within the UC population (Arihiro 2019; El Amrousy 2021; Tan 2018). They found no difference in disease activity between the two groups (SMD −1.03, 95% CI −2.93 to 0.88 (the SMD value indicates a large decrease in disease activity, and the corresponding CI indicates that the effect could range from a large decrease to a large increase); 3 studies, 263 participants; very low‐certainty evidence; Analysis 1.12). The certainty of the evidence was very low due to very serious concerns with inconsistency, imprecision, and risk of bias.
1.12. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 12: Disease activity at end of follow‐up (ulcerative colitis)
These results did not change on sensitivity analysis using a fixed‐effect model (Analysis 1.13), and sensitivity analysis for removal of studies at risk of bias was not possible due to the small number of studies remaining after removing those at unclear or high risk of bias.
1.13. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 13: Disease activity at end of follow‐up (ulcerative colitis) – sensitivity analysis (fixed‐effect)
Two studies compared disease activity at the end of follow‐up between vitamin D and placebo (Bendix 2020; Raftery 2015), but reported data graphically without corresponding numerical data and so could not be used for meta‐analysis.
One study compared disease activity at the end of follow‐up between vitamin D and no treatment, but as information on numbers randomised to each group was not presented, we were unable to use this for meta‐analysis (Dash 2019).
One study compared disease activity at the end of follow‐up between vitamin D and no treatment, but these data were not presented in the results and so could not be used for meta‐analysis (Dadaei 2015).
Normalisation of vitamin D levels
Four studies compared the normalisation of vitamin D levels at the end of the study period between vitamin D and placebo or no treatment groups using continuous measures on participants with IBD (Dadaei 2015; El Amrousy 2021; Raftery 2015; Sharifi 2016). They found a difference between the two groups favouring vitamin D (MD 34.84, 95% CI 13.54 to 56.14; 4 studies, 319 participants; very low‐certainty evidence; Analysis 1.14). The certainty of the evidence was very low due to very serious concerns with inconsistency, imprecision, and risk of bias.
1.14. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 14: Normalisation of vitamin D levels – vitamin D levels at end of study period (continuous outcomes)
These results did not change on sensitivity analysis using a fixed‐effect analysis (Analysis 1.15), and sensitivity analysis for removal of studies at risk of bias was not possible due to the small number of studies remaining after removing those at unclear or high risk of bias.
1.15. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 15: Normalisation of vitamin D levels – vitamin D levels at end of study period (continuous outcomes) – sensitivity analysis (fixed effect)
One study compared vitamin D against placebo or no treatment for the normalisation of vitamin D levels as a dichotomous outcome finding no difference between groups (RR 1.12, 95% CI 0.61 to 2.05; 94 participants; low‐certainty evidence; Analysis 1.16) (Jorgensen 2010). The certainty of the evidence was low due to serious concerns with imprecision due to low event numbers.
1.16. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 16: Normalisation of vitamin D levels – numbers of people with vitamin D deficiency at end of study (dichotomous outcome)
Two studies compared normalisation of vitamin D levels between the vitamin D and placebo groups, but reported data graphically without corresponding numerical data and so could not be used for meta‐analysis (Arihiro 2019; Bendix 2020).
Total serious adverse events
Eleven studies compared the number of serious adverse events between the vitamin D and placebo or no treatment groups (Ahamed 2019; Arihiro 2019; Bendix 2020; Dadaei 2015; de Bruyn 2020; El Amrousy 2021; Jorgensen 2010; Raftery 2015; Sharifi 2016; Tan 2018; Vogelsang 1995). They found no difference between numbers of withdrawals between the vitamin D (3/526 participants withdrew) and placebo or no treatment (2/511 participants withdrew) groups (RR 1.07, 95% CI 0.18 to 6.24; 11 studies, 1037 participants; very low‐certainty evidence; Analysis 1.17). The certainty of the evidence was very low due to very serious concerns with imprecision due to low event numbers and serious concerns with risk of bias due to randomisation, allocation, blinding, selective reporting, and other sources of bias.
1.17. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 17: Total serious adverse events – mixed dose (mixed population)
This result remained the same on sensitivity analysis using a fixed‐effect analysis (Analysis 1.18), and on sensitivity analysis for removal of studies at risk of bias (Analysis 1.19).
1.18. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 18: Total serious adverse events – mixed dose (mixed population) – sensitivity analysis (fixed‐effect)
1.19. Analysis.

Comparison 1: Vitamin D (all doses) versus placebo or no treatment, Outcome 19: Total serious adverse events – mixed dose (mixed population) – sensitivity analysis (removal of studies at risk of bias)
High‐treatment‐dose vitamin D versus low‐treatment‐dose vitamin D
Five studies compared the effect of high‐treatment‐dose vitamin D against low‐treatment‐dose vitamin D (Bafutto 2020; Boothe 2011; Karimi 2020; Narula 2017; Sassine 2020).
Primary outcomes
Clinical response for people with active disease
None of the five studies reported on this outcome.
Clinical relapse for people in remission
One study compared the rates of clinical relapse when using high‐treatment‐dose vitamin D compared to low‐treatment‐dose vitamin D in participants with CD (Narula 2017). They found no difference in the rate of clinical relapse when taking high‐treatment‐dose vitamin D (6/18 participants relapsed) compared with low‐treatment‐dose vitamin D (11/16 participants relapsed) (RR 0.48, 95% CI 0.23 to 1.01; 34 participants; low‐certainty evidence; Analysis 2.1). The certainty of the evidence was low due to serious concerns with imprecision (Table 2).
2.1. Analysis.

Comparison 2: High‐treatment‐dose vitamin D (greater than 1000 IU/day) versus low‐treatment‐dose vitamin D (400 IU/day to 1000 IU/day), Outcome 1: Clinical relapse (Crohn's disease)
Quality of life
One study assessed change in quality of life measures when comparing high‐treatment‐dose vitamin D to low‐treatment‐dose vitamin D, but reported data graphically without corresponding numerical data, and so could not be used in meta‐analysis (Karimi 2020).
One study assessed quality of life measures when comparing high‐treatment‐dose vitamin D to low‐treatment‐dose vitamin D, but the relevant data were not provided in the results, and so could not be used for meta‐analysis (Bafutto 2020).
Withdrawals due to adverse events
Three studies compared the number of withdrawals due to adverse events between the high‐treatment‐dose vitamin D and low‐treatment‐dose vitamin D groups (Bafutto 2020; Karimi 2020; Narula 2017). The number of withdrawals due to adverse events was 1/53 in the high‐treatment‐dose vitamin D group compared to 1/51 in the low‐treatment‐dose vitamin D group (RR 0.89, 95% CI 0.06 to 13.08; 3 studies, 104 participants; very low‐certainty evidence; Analysis 2.2; note 2 studies reported withdrawals but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 1 study rather than 3). No conclusions could be drawn due to very low certainty of this outcome owing to very serious imprecision and risk of bias (Table 2).
2.2. Analysis.

Comparison 2: High‐treatment‐dose vitamin D (greater than 1000 IU/day) versus low‐treatment‐dose vitamin D (400 IU/day to 1000 IU/day), Outcome 2: Withdrawals due to adverse events – high‐dose vitamin D versus low‐dose vitamin D (mixed population)
Secondary outcomes
Disease activity
One study assessed disease activity scores at the end of the study period when comparing high‐treatment‐dose vitamin D to low‐treatment‐dose vitamin D, but reported data graphically without corresponding numerical data, and so could not be used in meta‐analysis (Karimi 2020).
Normalisation of vitamin D levels
Three studies compared vitamin D levels at the end of the study period between high‐treatment‐dose vitamin D and low‐treatment‐dose vitamin D groups (Bafutto 2020; Narula 2017). There was no difference between groups (MD 48.09, 95% CI −8.31 to 104.50; 2 studies, 54 participants; very low‐certainty evidence; Analysis 2.3), but no conclusions could be drawn due to very low certainty of this outcome owing to very serious imprecision and risk of bias.
2.3. Analysis.

Comparison 2: High‐treatment‐dose vitamin D (greater than 1000 IU/day) versus low‐treatment‐dose vitamin D (400 IU/day to 1000 IU/day), Outcome 3: Normalisation of vitamin D levels (vitamin D level at end of follow‐up) – high‐treatment‐dose vitamin D versus low‐treatment‐dose vitamin D (mixed measures)
One study assessed change in vitamin D levels (Sassine 2020). It found higher vitamin D levels for the high‐treatment‐dose (MD 34.00, 95% CI 25.69 to 42.31; 25 participants; 1 study; very low‐certainty evidence; Analysis 2.4), but no conclusions could be drawn due to very low certainty of this outcome owing to very serious imprecision and risk of bias.
2.4. Analysis.

Comparison 2: High‐treatment‐dose vitamin D (greater than 1000 IU/day) versus low‐treatment‐dose vitamin D (400 IU/day to 1000 IU/day), Outcome 4: Normalisation of vitamin D levels (change in vitamin D levels) – high‐treatment‐dose vitamin D versus low‐treatment‐dose vitamin D (mixed measures)
Total serious adverse events
Three studies compared the number of serious adverse events between the high‐treatment‐dose vitamin D and low‐treatment‐dose vitamin D groups (Bafutto 2020; Karimi 2020; Narula 2017). However, all studies had no serious adverse events and no results could be estimated. No conclusions could be drawn due to very low certainty of this outcome owing to very serious imprecision and risk of bias.
Any‐treatment‐dose vitamin D (high and low dose) versus supplemental‐dose vitamin D
Four studies compared the effect of any‐treatment dose (high and low doses) of vitamin D against supplemental‐dose vitamin D (Bafutto 2020; Pappa 2012; Pappa 2014; Wingate 2014).
Primary outcomes
Clinical response for people with active disease
None of the four studies reported on this outcome.
Clinical relapse for people in remission
None of the four studies reported on this outcome.
Quality of life
One study assessed quality of life measured when comparing any‐treatment‐dose vitamin D with supplemental‐dose vitamin D, but the relevant data were not provided in the results and so could not be used for meta‐analysis (Bafutto 2020).
Withdrawals due to adverse events
Four studies reported withdrawals due to adverse events (Bafutto 2020; Pappa 2012; Pappa 2014; Wingate 2014). There was no difference between groups (RR 3.09, 95% CI 0.13 to 73.17; 4 studies, 233 participants; very low‐certainty evidence; Analysis 3.1; note 3 studies reported withdrawals but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 1 study rather than 4), but no conclusions could be drawn due to very low certainty of this outcome due to very serious concerns with imprecision and risk of bias (Table 3).
3.1. Analysis.

Comparison 3: Any‐treatment‐dose (greater than 400 IU/day) versus supplemental‐dose vitamin D (less than 400 IU/day), Outcome 1: Withdrawals due to adverse events
Secondary outcomes
Disease activity
One study compared disease activity at the end of follow‐up (Wingate 2014). There was no difference between groups (RR 1.03, 95% CI 0.79 to 1.33; 1 study, 83 participants; 1 study; very low certainty evidence; Analysis 3.2), but no conclusions could be drawn. The certainty of the evidence was very low due to serious concerns with imprecision and risk of bias.
3.2. Analysis.

Comparison 3: Any‐treatment‐dose (greater than 400 IU/day) versus supplemental‐dose vitamin D (less than 400 IU/day), Outcome 2: Disease activity at end of follow‐up – any‐treatment‐dose vitamin D versus supplemental‐dose vitamin D (Crohn's disease – Pediatric Crohn's Disease Activity Index < 10 at end of follow‐up)
Normalisation of vitamin D levels
Two studies compared the normalisation of vitamin D levels at the end of the study period (Bafutto 2020; Wingate 2014). There was no difference between groups (SMD 1.19, 95% CI −0.04 to 2.41 (the SMD value indicates a large increase in vitamin D levels, and the corresponding CIs indicate that the effect could range from a negligent decrease to a large increase); 2 studies, 103 participants; very low‐certainty evidence; Analysis 3.3), but no conclusions could be drawn due to very low certainty of this evidence owing to very serious concerns with imprecision and risk of bias.
3.3. Analysis.

Comparison 3: Any‐treatment‐dose (greater than 400 IU/day) versus supplemental‐dose vitamin D (less than 400 IU/day), Outcome 3: Normalisation of vitamin D levels (vitamin D level at end of follow‐up) – any‐treatment‐dose vitamin D versus supplemental‐dose vitamin D (mixed measures)
One study compared the normalisation of vitamin D levels as change in vitamin D levels (Pappa 2012). Vitamin D levels were higher in the any‐treatment‐dose group than the supplemental‐dose group (MD 16.1, 95% CI 14.85 to 17.35; 47 participants; 1 study; very low certainty evidence; Analysis 3.4), but no conclusions could be drawn due to very low certainty of this evidence due to very serious concerns with imprecision and risk of bias.
3.4. Analysis.

Comparison 3: Any‐treatment‐dose (greater than 400 IU/day) versus supplemental‐dose vitamin D (less than 400 IU/day), Outcome 4: Normalisation of vitamin D levels (change in vitamin D levels) – any‐treatment‐dose vitamin D versus supplemental‐dose vitamin D (mixed measures)
Total serious adverse events
Four studies reported number of serious adverse events (Bafutto 2020; Pappa 2012; Pappa 2014; Wingate 2014). All studies had no serious adverse events and results could not be estimated. No conclusions could be drawn due to very low certainty of this evidence owing to very serious imprecision and risk of bias.
Discussion
Summary of main results
The review included 22 RCTs with 1874 participants. Study duration ranged from 4 to 52 weeks. Ten studies enroled participants with CD, five enroled participants with UC, and seven enroled participants with CD or UC. Seventeen studies included adults, three included children, and two included both. Participants in four RCTs had active disease, six were in remission, and 12 had a mix of both.
The most researched comparison was vitamin D (all doses) against placebo or no treatment with 13 studies. The only reported outcomes where the certainty of the evidence was not very low was clinical relapse for IBD, where we found low‐certainty evidence that there may be fewer relapses when using vitamin D compared to placebo or no treatment, and normalisation of vitamin D levels in participants with CD, where we found low‐certainty evidence that there may be no difference between vitamin D and placebo or no treatment, when it was measured as a dichotomous outcome.
Five studies compared high‐treatment‐dose vitamin D against low‐treatment‐dose vitamin D. The only reported outcome where the certainty of the evidence was not very low was clinical relapse for participants with CD, where we found low‐certainty evidence that there may be no difference between treatment doses.
Four studies compared any‐treatment‐dose vitamin D against supplemental doses. All reported outcomes had very low‐certainty evidence.
Overall completeness and applicability of evidence
The evidence is incomplete in a number of ways. The lack of clear consensus on the goal of vitamin D treatment in IBD is pervasive. This is vital to ensure consistency of research and certainty of future studies and would suggest the need for perhaps a core outcome set or other consensus approach by stakeholders.
The disease state of patients and prior experience are key clinical factors in the context of chronic remitting disease. Given that studies varied in disease activity, disease form, and especially the vitamin D status of participants at baseline, the applicability of any results from the evidence base was implicitly limited.
A particular area of note was the range of vitamin D dosing. There are contexts within the journey of people with IBD that support study of homogeneous populations (Gjuladin‐Hellon 2019a; Gjuladin‐Hellon 2019b), and allow identification of key findings from such populations (Gordon 2021a; Iheozor‐Ejiofor 2019). However, as the studies included did not explicitly discuss or contextualise their trials in a manner that was homogeneous, as stated above, it is difficult to judge the rationale and as such the mechanism of potential action being proposed by study authors to justify such specific dosing choices. These ranged from prophylactic to high replacement or treatment doses.
These issues are complex, but the interplay between participant demographics and treatment dosing are key to interpreting the purpose and likely outcomes of treatment and so such issues in the evidence further limit the applicability of findings to practise.
Finally, sample size of trials has resulted in issues with precision in most GRADE analyses in this review. This is a pervasive issue within the field (Iheozor‐Ejiofor 2021), with a need for adequate sample size calculations using published resources (Gordon 2021b).
Quality of the evidence
The studies were thoroughly reviewed for quality and bias assessed. Only four studies were judged at low risk of bias in all areas. This is common in large reviews due to shifts in reporting stands with time. In this review, the evidence base was relatively contemporaneous, with all but two studies published since 2012. As such, these issues with poor reporting of bias elements is more stark. Of particular note were issues with unclear bias due to allocation concealment or selective reporting, both seen in 10 studies each, representing almost half the cohort.
The certainty of the evidence on GRADE analysis was exclusively low or very low, with both the impact of risk of bias and imprecision a key factor impacting the certainty. This was exacerbated by the methodological and clinical heterogeneity issues mentioned above that did not appear purposeful or related to planned study of specific populations or treatments. As such, this has reduced the overall certainty of evidence further.
Reporting of adverse events was also very sparse and so this was reflected in the GRADE analysis.
Potential biases in the review process
Gaps in information to judge risk of bias were pervasive, as discussed above. Given the relatively contemporaneous nature of the evidence, the review team considered it prudent to contact primary authors to request clarification or additional information. Many did not respond and, as such, judgements could not be changed and remained as they were, based on the published forms of the studies.
We will include the data that may become available in future updates, but this could represent a source of bias in the review, with 12 ongoing studies identified in the review process. Conversely, the use of such unpublished data can also be seen as a source of bias.
We are aware of the possibility of industry funding for the validity of the results. Funding from manufacturing companies or any conflicts of interests from both primary studies and the review team have been reported.
Agreements and disagreements with other studies or reviews
Major international guidelines do not discuss the role of vitamin D in IBD (Feuerstein 2020; Feuerstein 2021; Torres 2020). The 2019 UK guidelines do suggest people with IBD should achieve normal dietary levels of vitamin D, but they do not propose how this is achieved, the specific role for vitamin therapy, or the dosing that could be employed (Lamb 2019).
There have been a number of recent systematic reviews on the topic of vitamin D for treatment of IBD. However, they tend to make conclusions without considering the GRADE certainty of their results. Valvano 2022 included 12 RCTs, and concluded that vitamin D supplementation can reduce the risk of clinical relapse in people with CD but not people with UC. Guzman‐Prado 2020, which included a mix of RCTs and observational studies, found a decrease in HBI scores and concluded that indicates clinical improvement. Guo 2021, which included 17 RCTs, found no difference in disease activity or relapse rates. Sun 2023 investigated the effect of vitamin D on children with IBD. It included five studies, a mix of observational and RCTs, in their meta‐analysis for clinical remission, and concluded that vitamin D supplementation can improve disease activity. Another systematic review focussing on children, Rigterink 2019, reported on the heterogeneity of the study design, inclusion and exclusion criteria, baseline demographics, and treatment strategies of their 10 included observational and RCT studies, and did not reach any conclusions on the effect of vitamin D on clinical activity. Głąbska 2021 performed a qualitative systematic review on the effects of vitamin D on mental health for a mix of people with IBD and irritable bowel syndrome. It concluded that vitamin D has positive effects on anxiety, depression, and quality of life, despite the heterogeneity of the reporting, and that only four of its included studies were on people with IBD, of which two were RCTs.
Authors' conclusions
Implications for practice.
There may be fewer clinical relapses when comparing vitamin D with placebo, but we cannot draw any conclusions on differences in clinical response, quality of life, withdrawals, serious adverse events, disease activity, or normalisation of vitamin D levels due to very low‐certainty evidence.
When comparing high‐ and low‐treatment doses of vitamin D, there were no data for clinical response, but there may be no difference in relapse for Crohn's disease. We cannot draw conclusions on the other outcomes due to very low‐certainty evidence.
Finally, comparing vitamin D at treatment doses to supplemental doses, there are no data on clinical relapse or response, and we cannot draw conclusions on other outcomes due to very low‐certainty evidence or missing data.
Implications for research.
It is difficult to make any clear recommendations for future research on the basis of the findings of this review.
The evidence has demonstrated major issues with clinical and methodological heterogeneity that reflect a lack of consensus amongst the core researching community regarding the doses of vitamin D, the disease state to employ such treatments, and the goals of therapy and associated outcomes for study.
It is recommended that a consensus is reached on these issues prior to any further research. In particular, defining a specific group of people with IBD, the rationale for vitamin D and, as such, the proposal of a clear dosing regimen to achieve a given outcome is key. This will ensure future studies are focussed on these areas of interest and will enhance certainty in these areas.
Within all such studies, reporting in a manner that is consistent with clarity for risk of bias judgements is vital.
History
Protocol first published: Issue 7, 2015
Acknowledgements
Cochrane Gut Group supported the review authors in the development of this review.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Grigorios Leontiadis, Cochrane Gut Group.
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Sam Hinsley, Central Editorial Service.
Editorial Assistant (conducted editorial policy checks and supported editorial team): Sara Hales‐Brittain, Central Editorial Service.
Copy Editor (copy editing and production): Anne Lawson, Cochrane Central Production Service.
Peer‐reviewers (provided comments and recommended an editorial decision): Walter Fries, Department of Clinical and Experimental Medicine, UOC Gastroenterologia e Malattie Intestinali Croniche, Messina, Italy (clinical/content review); Brian Duncan, Cochrane Consumer Group (consumer review); Jennifer Hilgart, Cochrane (methods review); Farhad Shokraneh, Systematic Review Consultants LTD, Nottingham, UK (search review); Margaret Anderson, Centre for Public Health, Queen's University Belfast, Belfast, UK (search review). One additional peer reviewer provided clinical/content peer review but chose not to be publicly acknowledged.
The search was designed and run by Yuhong Yuan (Information Specialist at the Cochrane Gut Group). This search was peer‐reviewed, revised, and re‐run (twice) by Farhad Shokraneh (Systematic Review Consultants Ltd).
Appendices
Appendix 1. CENTRAL search strategy
10/06/2023 04:43:54
#1 ([mh "Inflammatory bowel diseases"] or inflammatory bowel disease* or crohn* or ulcerative colitis or ulcerative colorectitis or ulcerative proctocolitis or ulcerative enteritis or regional enteritis or IBD) and ([mh "Vitamin D"] or vitamin D* or vitamin D2* or vitamin D3* or Vit‐D* or Vita‐D* or Ergocalciferol* or Cholecalciferol* or Alfacalcidol or calcitriol or calcidiol or calcifediol or calciferol or calciol or calderol or dihydrotachysterol or dedrogyl or dihydrotachysterol or dihydroxycolecalciferol or dihydroxycholecalciferol or dihydroxyvitamin D* or doxercalciferol or eldecalcitol or ercalcidiol or hidroferol or hydroxycalciferol or hydroxycolecalciferol or hydroxycholecalciferol or hydroxyergocalciferol* or hydroxyvitamin D* or paricalcitol or tachystin or 25 OHD or 25ohd or "25(OH)D") with Cochrane Library publication date Between Aug 2021 and Jun 2023, in Trials 17
Appendix 2. MEDLINE via OvidSP search strategy
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations and Daily <1946 to June 09, 2023>
1 exp Inflammatory bowel diseases/ or (inflammatory bowel disease* or crohn* or ulcerative colitis or ulcerative colorectitis or ulcerative proctocolitis or ulcerative enteritis or regional enteritis or IBD).tw,kw. (138431)
2 exp Vitamin D/ or (vitamin D* or vitamin D2* or vitamin D3* or Vit‐D* or Vita‐D* or Ergocalciferol* or Cholecalciferol* or Alfacalcidol or calcitriol or calcidiol or calcifediol or calciferol or calciol or calderol or dihydrotachysterol or dedrogyl or dihydrotachysterol or dihydroxycolecalciferol or dihydroxycholecalciferol or dihydroxyvitamin D* or doxercalciferol or eldecalcitol or ercalcidiol or hidroferol or hydroxycalciferol or hydroxycolecalciferol or hydroxycholecalciferol or hydroxyergocalciferol* or hydroxyvitamin D* or paricalcitol or tachystin or 25 OHD or 25ohd or "25(OH)D").tw,kw. (108766)
3 ((Randomized Controlled Trial or Controlled Clinical Trial).pt. or Random*.mp. or (Placebo or Trial or Groups).ab. or Drug Therapy.fs.) not (exp Animals/ not Humans.sh.) (5266629)
4 and/1‐3 (461)
5 limit 4 to ed=20210806‐20230609 (56)
6 limit 4 to dt=20210806‐20230609 (54)
7 5 or 6 (72)
Note: Line 3. RCT filter, we used the "Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format”.We made the following minor revision: we used “random*” instead of “randomized.ab” or “randomly.ab.” to capture word variations such as “randomised, randomization, random”.
Appendix 3. Embase via OvidSP search strategy
Database: Embase <1974 to 2023 Week 23>
1 exp inflammatory bowel disease/ or (inflammatory bowel disease* or crohn* or ulcerative colitis or ulcerative colorectitis or ulcerative proctocolitis or ulcerative enteritis or regional enteritis or IBD).tw,kw. (241108)
2 exp vitamin D/ or (vitamin D* or vitamin D2* or vitamin D3* or Vit‐D* or Vita‐D* or Ergocalciferol* or Cholecalciferol* or Alfacalcidol or calcitriol or calcidiol or calcifediol or calciferol or calciol or calderol or dihydrotachysterol or dedrogyl or dihydrotachysterol or dihydroxycolecalciferol or dihydroxycholecalciferol or dihydroxyvitamin D* or doxercalciferol or eldecalcitol or ercalcidiol or hidroferol or hydroxycalciferol or hydroxycolecalciferol or hydroxycholecalciferol or hydroxyergocalciferol* or hydroxyvitamin D* or paricalcitol or tachystin or 25 OHD or 25ohd or "25(OH)D").tw,kw. (202115)
3 (random*.tw. or placebo*.mp. or double‐blind*.tw.) not (exp animal/ not human/) (2042171)
4 and/1‐3 (436)
5 limit 4 to em=202131‐202323 (49)
Lines 3, RCT filter, we used the "Hedge Best balance of sensitivity and specificity filter for identifying randomized trials in Embase". https://hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx
Appendix 4. ClinicalTrials.gov search strategy
This search included only the terms that retrieved at least one relevant randomised controlled trial.
Advanced Search
Condition or disease: Inflammatory Bowel Diseases OR Crohn OR Ulcerative Colitis
Study type: Interventional Studies (Clinical Trials)
Intervention/treatment: Vitamin D OR Vitamin D2 OR Vitamin D3 OR Ergocalciferol OR Cholecalciferol OR Alfacalcidol OR Calcitriol OR Calcidiol OR Calcifediol OR Calderol OR Dedrogyl OR Hidroferol OR Hydroxycholecalciferol OR Hydroxyvitamin
First Posted: From 08/08/2021 To 06/10/2023 (MM/DD/YYYY)
Appendix 5. WHO ICTRP search strategy
This search included only the terms that retrieved at least one relevant randomised controlled trial. The date was limited to 1st January 2021 instead of 8th August 2021 because of possible indexing delays between supplying records from the original trial registers and processing and adding them to WHO ICTRP.
Advanced Search
Inflammatory Bowel Diseases OR Crohn OR Ulcerative Colitis in the Condition
Vitamin D OR Vitamin D3 OR Ergocalciferol OR Cholecalciferol OR Calcitriol OR Hydroxycholecalciferol in the Intervention
Recruitment status is ALL
Date of registration is between 01/01/2021 and 10/06/2023
Data and analyses
Comparison 1. Vitamin D (all doses) versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Clinical response | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 4.00 [1.51, 10.57] |
| 1.1.1 Ulcerative colitis | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 4.00 [1.51, 10.57] |
| 1.2 Clinical relapse (mixed IBD) | 3 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.34, 0.96] |
| 1.3 Clinical relapse (mixed IBD) – sensitivity analysis (fixed‐effect) | 3 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.36, 0.89] |
| 1.4 Quality of life (QoL) (end of follow‐up/change in score) | 2 | 243 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐3.10, 2.83] |
| 1.4.1 Any‐treatment‐dose‐vitamin D (mixed measures of quality of life – mixed population) | 2 | 243 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐3.10, 2.83] |
| 1.5 QoL (end of follow‐up/change in score) – sensitivity analysis (fixed‐effect) | 2 | 243 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.63, ‐0.06] |
| 1.5.1 Any‐treatment‐dose‐vitamin D (mixed measures of QoL – mixed population) | 2 | 243 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.63, ‐0.06] |
| 1.6 Withdrawals due to adverse events | 12 | 1251 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.18, 21.27] |
| 1.6.1 Any‐treatment‐dose vitamin D (mixed population) | 8 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.18, 21.27] |
| 1.6.2 Low‐treatment‐dose vitamin D (Crohn's disease) | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.6.3 Supplemental‐dose vitamin D (mixed population) | 2 | 435 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.7 Withdrawals due to adverse events – sensitivity analysis (fixed‐effect) | 12 | 1251 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.18, 21.27] |
| 1.7.1 Any‐treatment‐dose vitamin D (mixed population) | 8 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.18, 21.27] |
| 1.7.2 Low‐treatment‐dose vitamin D (Crohn's disease) | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7.3 Supplemental‐dose vitamin D (mixed population) | 2 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.8 Withdrawals due to adverse events – sensitivity analysis (removal of studies at risk of bias) | 4 | 382 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.9 Disease activity at end of follow‐up (Crohn's disease) | 3 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐3.39, 0.89] |
| 1.9.1 All‐treatment‐dose vitamin D (mixed measures at end of treatment) | 2 | 101 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.04 [‐6.13, 2.06] |
| 1.9.2 Supplemental‐dose vitamin D (Crohn's Disease Activity Index score) | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.29, 0.77] |
| 1.10 Disease activity at end of follow‐up (Crohn's disease) – sensitivity analysis (fixed‐effect) | 3 | 156 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.80, ‐0.07] |
| 1.10.1 All‐treatment‐dose vitamin D (mixed measures at end of treatment) | 2 | 101 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐1.50, ‐0.52] |
| 1.10.2 Supplemental‐dose vitamin D (Crohn's Disease Activity Index score) | 1 | 55 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.29, 0.77] |
| 1.11 Disease activity at end of follow‐up – change in disease activity score (Crohn's disease) | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐41.00 [‐67.03, ‐14.97] |
| 1.11.1 Low‐treatment‐dose vitamin D (change in Crohn's Disease Activity Index score) | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐41.00 [‐67.03, ‐14.97] |
| 1.12 Disease activity at end of follow‐up (ulcerative colitis) | 3 | 263 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐2.93, 0.88] |
| 1.12.1 All‐treatment‐dose vitamin D (mixed scores) | 2 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.92 [‐5.86, 2.01] |
| 1.12.2 Supplemental‐dose vitamin D (Lichtinger score) | 1 | 168 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.28, 0.90] |
| 1.13 Disease activity at end of follow‐up (ulcerative colitis) – sensitivity analysis (fixed‐effect) | 3 | 263 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.08, 0.45] |
| 1.13.1 All ‐treatment‐dose vitamin D (mixed scores) | 2 | 95 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐1.32, ‐0.35] |
| 1.13.2 Supplemental‐dose vitamin D (Lichtinger score) | 1 | 168 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.59 [0.28, 0.90] |
| 1.14 Normalisation of vitamin D levels – vitamin D levels at end of study period (continuous outcomes) | 4 | 319 | Mean Difference (IV, Random, 95% CI) | 34.84 [13.54, 56.14] |
| 1.14.1 All‐treatment‐dose vitamin D (mixed measures) | 4 | 319 | Mean Difference (IV, Random, 95% CI) | 34.84 [13.54, 56.14] |
| 1.15 Normalisation of vitamin D levels – vitamin D levels at end of study period (continuous outcomes) – sensitivity analysis (fixed effect) | 5 | 394 | Mean Difference (IV, Fixed, 95% CI) | 30.41 [28.77, 32.04] |
| 1.15.1 All‐treatment‐dose vitamin D (mixed measures) | 4 | 319 | Mean Difference (IV, Fixed, 95% CI) | 32.50 [30.80, 34.20] |
| 1.15.2 Low‐treatment‐dose vitamin D (change from start to end of follow‐up) | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 4.70 [‐1.27, 10.67] |
| 1.16 Normalisation of vitamin D levels – numbers of people with vitamin D deficiency at end of study (dichotomous outcome) | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.61, 2.05] |
| 1.16.1 Low‐treatment‐dose vitamin D (vitamin D deficiency at end of follow‐up) | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.61, 2.05] |
| 1.17 Total serious adverse events – mixed dose (mixed population) | 11 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.18, 6.24] |
| 1.17.1 All‐treatment‐dose vitamin D (mixed population) | 8 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.18, 6.24] |
| 1.17.2 Low‐treatment‐dose vitamin D (mixed population) | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.17.3 Supplemental‐dose vitamin D (mixed population) | 1 | 223 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.18 Total serious adverse events – mixed dose (mixed population) – sensitivity analysis (fixed‐effect) | 11 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.21, 6.07] |
| 1.18.1 All‐treatment‐dose vitamin D (mixed population) | 8 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.21, 6.07] |
| 1.18.2 Low‐treatment‐dose vitamin D (mixed population) | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.18.3 Supplemental‐dose vitamin D (mixed population) | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.19 Total serious adverse events – mixed dose (mixed population) – sensitivity analysis (removal of studies at risk of bias) | 4 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.04, 7.00] |
Comparison 2. High‐treatment‐dose vitamin D (greater than 1000 IU/day) versus low‐treatment‐dose vitamin D (400 IU/day to 1000 IU/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Clinical relapse (Crohn's disease) | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.23, 1.01] |
| 2.2 Withdrawals due to adverse events – high‐dose vitamin D versus low‐dose vitamin D (mixed population) | 3 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.06, 13.08] |
| 2.3 Normalisation of vitamin D levels (vitamin D level at end of follow‐up) – high‐treatment‐dose vitamin D versus low‐treatment‐dose vitamin D (mixed measures) | 2 | 54 | Mean Difference (IV, Random, 95% CI) | 48.09 [‐8.31, 104.50] |
| 2.4 Normalisation of vitamin D levels (change in vitamin D levels) – high‐treatment‐dose vitamin D versus low‐treatment‐dose vitamin D (mixed measures) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 34.00 [25.69, 42.31] |
Comparison 3. Any‐treatment‐dose (greater than 400 IU/day) versus supplemental‐dose vitamin D (less than 400 IU/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Withdrawals due to adverse events | 4 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.13, 73.17] |
| 3.1.1 Any‐treatment‐dose vitamin D (mixed population) | 4 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.13, 73.17] |
| 3.1.2 Low‐treatment‐dose vitamin D (Crohn's disease) | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.2 Disease activity at end of follow‐up – any‐treatment‐dose vitamin D versus supplemental‐dose vitamin D (Crohn's disease – Pediatric Crohn's Disease Activity Index < 10 at end of follow‐up) | 1 | 83 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.79, 1.33] |
| 3.3 Normalisation of vitamin D levels (vitamin D level at end of follow‐up) – any‐treatment‐dose vitamin D versus supplemental‐dose vitamin D (mixed measures) | 2 | 103 | Std. Mean Difference (IV, Random, 95% CI) | 1.19 [‐0.04, 2.41] |
| 3.4 Normalisation of vitamin D levels (change in vitamin D levels) – any‐treatment‐dose vitamin D versus supplemental‐dose vitamin D (mixed measures) | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 16.10 [14.85, 17.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahamed 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: India |
|
| Participants |
State of disease/disease type: active UC Inclusion criteria: adults with active UC (UCDAI ≥ 3 and recent increase in stool frequency) and vitamin D deficiency (< 40 ng/mL) Exclusion criteria: acute severe colitis requiring hospitalisation Age: median: 29 years (Group 1); 37.5 years (Group 2) Sex (male/female): 16/14 (Group 1); 20/10 (Group 2) Site of disease: not stated Number randomised: 30 (Group 1); 30 (Group 2) Number analysed: 30 (Group 1); 30 (Group 2) |
|
| Interventions |
Group 1: nano liquid formulation of vitamin D3 60,000 IU/day for 8 days Group 2: similar appearing and tasting syrup for 8 days |
|
| Outcomes | Duration of 4 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: not stated Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence developed by individual not involved in study. |
| Allocation concealment (selection bias) | Low risk | The pharmacy provided serially numbered bottles with an active ingredient or similar looking and tasting placebo in identical sealed containers in accordance with the random sequence. 8 bottles with the same serial number were placed in identical‐looking opaque serially labelled sealed boxes by the pharmacy which was provided to the participants. The bottles were brown to prevent degradation of the ingredients. The actual allocation was not available to any of the investigators until completion of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants, investigators, laboratory personnel, and clinical outcome assessors were blinded regarding allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical outcomes assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes reported appropriately. CRP and ESR data were collected, but insufficiently reported (via graph/figure). |
| Other bias | Low risk | No baseline imbalances. No other concerns. |
Arihiro 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Japan |
|
| Participants |
State of disease/disease type: active and inactive CD and UC Inclusion criteria: adults (aged 18–80 years) with CD or UC in a stable condition and those with no contraindication to treatment Exclusion criteria: people with influenza, history of urinary stones, having taken vitamin D supplementation, or allergic to vitamin D supplements Age: mean: 44.1 years (Group 1); 45.4 years (Group 2) Sex (male): 60.9% (Group 1); 61.1% (Group 2) Number randomised: 119 (Group 1); 118 (Group 2) Number analysed: 115 (Group 1); 108 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 500 IU/day Group 2: placebo |
|
| Outcomes |
Duration of follow‐up: 6 months Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Ministry of Education, Culture, Sports, Science, and Technology in the Japan‐Supported Program for the Strategic Research Foundation at Private Universities; Department of Gastroenterology and Hepatology at Jikei University of Medicine (Tokyo, Japan) Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence developed by individual who did not see study participants. |
| Allocation concealment (selection bias) | Low risk | Concealed by numbering applied by staff member uninvolved with study participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical bottles with similar appearance and taste of interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes recorded by office secretaries who had no clinical involvement in the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was low and relatively balanced across treatment arms. Group 1: 4/119 withdrawals for withdrawal of consent (3) and loss to follow‐up (1). Group 2: 10/118 withdrawals for withdrawal of consent (5), termination by investigator (1), and loss to follow‐up (4). |
| Selective reporting (reporting bias) | Low risk | Pretrial registration on UMIN Clinical Trials Registry. All outcomes reported. |
| Other bias | Low risk | Balanced baseline characteristics and no other concerns. |
Bafutto 2020.
| Study characteristics | ||
| Methods |
Study design: RCT (abstract) Setting: Brazil |
|
| Participants |
State of disease/disease type: active CD Inclusion criteria: adults (aged 18–70 years) with moderate‐to‐severe CD on anti‐TNF therapy and 25(OH)D < 30 ng/mL Exclusion criteria: pregnancy, chronic kidney or liver disease, sarcoidosis, tuberculosis, hyper‐ or hypoparathyroidism, neoplasia, use of anticonvulsants, received calcium or vitamin D supplementation within preceding 6 months Age: mean: 41 years (Group 1); 37 (Group 2); 33 years (Group 3) Sex (males): 20% (Group 1); 80% (Group 2); 50% (Group 3) Number randomised: 10 (Group 1); 10 (Group 2); 10 (Group 3) Number analysed: not stated |
|
| Interventions |
Group 1: vitamin D 2000 IU/week for 8 weeks Group 2: vitamin D 10,000 IU/week for 8 weeks Group 3: vitamin D 50,000 IU/week for 8 weeks |
|
| Outcomes |
Duration of follow‐up: 52 weeks Outcomes
|
|
| Notes |
Funding source: none Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, although method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information to assess allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as double‐blind trial, although method of blinding was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported as double‐blind trial, although method of blinding was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed intervention period of 8 weeks. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods reported, no clinical trial registration. |
| Other bias | Low risk | Balanced baseline characteristics and no other concerns. |
Bendix 2020.
| Study characteristics | ||
| Methods |
Study design: RCT (abstract) Setting: Denmark |
|
| Participants |
State of disease/disease type: active CD Inclusion criteria: adults (aged 18–80 years) with active CD (HBI > 4 and faecal calprotectin > 100 mg/kg, CRP > 8 mg/L, or a combination of these; CDEIS ≥ 5) Exclusion criteria: pregnancy, ongoing infection, tuberculosis, 25(OH)D > 40 ng/mL, treatment with biological therapy or change of azathioprine dose within 3 months, hypercalcaemia or hypercalciuria or both, pseudohypoparathyroidism, prior calcium‐containing nephrolithiasis, disorders of renal calcium and phosphate excretion, breastfeeding, vaccinated with live vaccine within 4 weeks, untreated abscess, oral prednisone use, budesonide > 3 mg/day, other allergies, rare diseases, and specific treatments Age: median: 28 years (Group 1); 26 years (Group 2); 35 years (Group 3); 30 years (Group 4) Sex (males): 50% (Group 1); 50% (Group 2); 50% (Group 3); 38% (Group 4) Number randomised: 8 (Group 1); 8 (Group 2); 16 (Group 3); 8 (Group 4) Number analysed: 7 (Group 1); 8 (Group 2); 16 (Group 3); 8 (Group 4) |
|
| Interventions |
Group 1: high‐dose vitamin D (200,000 IU at baseline followed by 20,000 IU/day) plus infliximab Group 2: placebo plus infliximab Group 3: high‐dose vitamin D plus placebo Group 4: placebo plus placebo |
|
| Outcomes |
Duration of follow‐up: 6 weeks Outcomes
|
|
| Notes |
Funding source: not stated Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised by hospital pharmacy. |
| Allocation concealment (selection bias) | Low risk | Central randomisation with allocations held in concealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Allocation concealed from participants, prepared by unblinded nurse. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced withdrawals and unblindings. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported per methods, but no trial registration. |
| Other bias | Low risk | Balanced baseline characteristics and no other concerns. |
Boothe 2011.
| Study characteristics | ||
| Methods |
Study design: RCT (abstract) Setting: USA |
|
| Participants |
State of disease/disease type: unknown CD Inclusion criteria: people with CD and 25(OH)D < 30 ng/mL Exclusion criteria: not stated Age: not stated Sex: not stated Number randomised: 15 Number analysed: not stated |
|
| Interventions |
Group 1: vitamin D 1000 IU/day Group 2: vitamin D 10,000 IU/day |
|
| Outcomes |
Duration of follow‐up: 26 weeks Outcomes
|
|
| Notes |
Funding source: not stated Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, although method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information to assess allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as blinded trial, although method of blinding was not reported, and not clearly stated how participants were blinded to treatment arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported as blinded trial, although method of blinding was not reported, stated that physicians were blinded to intervention arms. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information to assess attrition. |
| Selective reporting (reporting bias) | Unclear risk | Not yet published although abstract was presented in 2011. |
| Other bias | Low risk | No evidence that other significant sources of bias exist |
Dadaei 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Iran |
|
| Participants |
State of disease/disease type: active and inactive IBD Inclusion criteria: IBD diagnosis confirmed by gastroenterologist Exclusion criteria: 25(OH)D > 30 ng/mL, confirmed coeliac disease, renal disease requiring dialysis, polycystic kidney disease, pregnant Age: mean: 37.3 years (Group 1); 38.7 years (Group 2) Sex (male): 49.1% (Group 1); 41.8% (Group 2) Number randomised: 53 (Group 1); 55 (Group 2) Number analysed: 53 (Group 1); 55 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 50,000 IU/week Group 2: none |
|
| Outcomes |
Duration of follow‐up: 12 weeks Outcomes
|
|
| Notes |
Funding source: not stated Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | No information given regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported for participants, no treatment given to control group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding reported for outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals reported, note the higher levels of withdrawals from control group. |
| Selective reporting (reporting bias) | Low risk | Trial prospectively registered in Iranian Registry of Clinical Trials. |
| Other bias | Low risk | Balanced baseline characteristics and no other concerns. |
Dash 2019.
| Study characteristics | ||
| Methods |
Study design: RCT (abstract) Setting: India |
|
| Participants |
State of disease/disease type: unknown UC Inclusion criteria: newly diagnosed UC Exclusion criteria: not stated Age: mean: 39.68 (SD 10.07) years Sex: not stated Number randomised: 76 (Group 1); 76 (Group 2) Number analysed: not stated |
|
| Interventions |
Group 1: low‐dose vitamin D (dose not specified) Group 2: no intervention |
|
| Outcomes |
Duration of follow‐up: not stated Outcomes
|
|
| Notes |
Funding source: not stated Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, although method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information to assess allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information to assess attrition. |
| Selective reporting (reporting bias) | Unclear risk | Not yet published although abstract was presented in 2019. |
| Other bias | Low risk | No evidence that other significant sources of bias exist. |
de Bruyn 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: multicentre in the Netherlands and Belgium |
|
| Participants |
State of disease/disease type: inactive postoperative CD Inclusion criteria: adults (aged ≥ 18 years) who underwent a first or second ileocaecal or ileocolonic resection with ileocolonic anastomosis for CD or underwent closure of a loop ileostomy after a previous ileocaecal or ileocolonic resection, normal serum calcium levels not exceeding the upper limit of normal, and within 14 days after surgery Exclusion criteria: macroscopic evidence of CD at the proximal or distal resection margin, ileorectal anastomosis, active perianal fistulae, extensive small bowel resection (> 60 cm removed), additional stricturoplasty or other small bowel resections, postoperative definite stoma, primary hyperparathyroidism, sarcoidosis, tuberculosis, or pregnant/breastfeeding Age: median: 31 years (Group 1); 33 years (Group 2) Sex (male): 39% (Group 1); 41% (Group 2) Number randomised: 72 (Group 1); 71 (Group 2) Number analysed: 63 (Group 1); 55 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 25,000 IU/week Group 2: comparable placebo vials |
|
| Outcomes |
Duration of follow‐up: 26 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: BROAD Medical Research Program – Crohn's and Colitis Foundation and the International Organization for Inflammatory Bowel Diseases Conflicts of interest: vitamin D and placebo vials were provided by SMB Pharma. Authors disclosed no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, although method of randomisation was not reported. Quote: "Randomization was performed at the pharmacy of the Amsterdam University Medical Center within 2 weeks after surgery, and subjects were stratified by baseline 25‐OH vitamin D level (<75 or 75 nmol/L)." |
| Allocation concealment (selection bias) | Unclear risk | No information to assess allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Interventions were comparable in appearance. Quote: "comparable placebo vials". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Central reading of endoscopies, all study personnel blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were relatively balanced across treatment arms. Group 1: 14/72: withdrawals for adverse event (2), loss to follow‐up (3), pregnancy (1), refusal of colonoscopy (1), relapse (3), non‐compliance (2), and other (2) Group 2: 16/70: withdrawals for adverse event (1), loss to follow‐up (4), refusal of colonoscopy (4), relapse (3), non‐compliance (1), and other (3) |
| Selective reporting (reporting bias) | Low risk | Trial preregistered in ClinicalTrials.gov. Listed outcomes were reported. |
| Other bias | Low risk | Balanced baseline characteristics, no other concerns. |
El Amrousy 2021.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Egypt |
|
| Participants |
State of disease/disease type: active and inactive CD and UC Inclusion criteria: children (aged 1–18 years) with IBD, 25(OH)D < 20 ng/mL, and on stable dose of IBD medication for ≥ 3 months before enrolment Exclusion criteria: recent systematic corticosteroids for diseases other than IBD, antibiotic use within 60 days, drugs that interfere with metabolism of vitamin D, change in IBD therapy within last 3 months, history of gut surgery or irradiation, BMI > 25, chronic diseases (e.g. diabetes mellitus, renal, cardiac, endocrine, connective tissue, or hepatic disease) Age: mean: 13.4 years (Group 1); 13.0 years (Group 2) Sex (men): 29 (Group 1); 26 (Group 2) Number randomised: 50 (Group 1); 50 (Group 2) Number analysed: 50 (Group 1); 48 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 2000 IU/day Group 2: placebo |
|
| Outcomes |
Duration of follow‐up: 6 months Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: not stated Conflicts of interest: not stated Additional data received from author after email contact in 2021
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes and sequential numbers. Participants, treating physicians, and investigators were blinded to group assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, treating physicians, and investigators were blinded to group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, treating physicians, and investigators were blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were relatively balanced across treatment arms. Group 1: 0/50 withdrawals Group 2: 2/50 withdrawals, both for loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Trial preregistered in Pan African Clinical Trials Registry. Listed outcomes were reported |
| Other bias | Low risk | No baseline imbalances. No other concerns. |
Jing 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: China |
|
| Participants |
State of disease/disease type: unknown CD and UC Inclusion criteria: confirmed diagnosis of IBD Exclusion criteria: intestinal surgery, non‐steroidal drugs within past month, long‐term smoker, pregnancy, hepatic disease, renal disease, calcium or vitamin D supplementation Age: mean: 41.9 years Sex (male:female): 104:94 Number randomised: 99 (Group 1); 99 (Group 2) Number analysed: 99 (Group 1); 99 (Group 2) |
|
| Interventions |
Group 1: vitamin D 400 IU/day Group 2: no intervention |
|
| Outcomes |
Duration of follow‐up: 1 month Outcomes
|
|
| Notes |
Funding source: not stated Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as randomised, although method of randomisation was not reported. Quote: "randomly divided". |
| Allocation concealment (selection bias) | Unclear risk | No information to assess allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported per methods but no trial registration. |
| Other bias | Unclear risk | No baseline characteristics reported. |
Jorgensen 2010.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Denmark |
|
| Participants |
State of disease/disease type: inactive CD Inclusion criteria: adults (aged ≥ 18 years) with CD in remission (CDAI < 150 with biochemical signs of quiescent CD through CRP and serum albumin within normal range), no use of corticosteroids within 4 weeks of enrolment, normal serum calcium Exclusion criteria: pregnancy, short bowel syndrome Age: mean: 36 years (Group 1); 38 years (Group 2) Sex (female): 72% (Group 1); 60% (Group 2) Number randomised: 46 (Group 1); 48 (Group 2) Number analysed: 46 (Group 1); 48 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 1200 IU/day plus calcium 1200 mg/day Group 2: calcium 1200 mg/day |
|
| Outcomes |
Duration of follow‐up: 12 months Primary outcome
Secondary outcome
|
|
| Notes |
Funding source: Danish Colitis Crohn Foundation, Hørslev Foundation Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation in groups of 10, vials selected randomly, and randomisation list only known to central pharmacy. |
| Allocation concealment (selection bias) | Low risk | Randomisation and allocation of assignments were performed centrally by a third party. Assignments were stored in a sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical matching placebos in coded medication containers were provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessors were blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were relatively balanced across treatment arms Group 1: 9/46 withdrawals for non‐adherence Group 2: 7/48 withdrawals for non‐adherence |
| Selective reporting (reporting bias) | Low risk | Preregistered in ClinicalTrials.gov. Listed outcomes were reported, although CDAI definition of relapse was changed from > 220 to ≥ 150. |
| Other bias | Low risk | Balanced baseline characteristics, no other concerns. |
Karimi 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Iran |
|
| Participants |
State of disease/disease type: active UC Inclusion criteria: adults (aged ≥ 18 years) with mild‐to‐moderate UC based on histopathological evidence, colonoscopic findings, and clinical signs and symptoms; BMI 18.5–30 kg/m2 Exclusion criteria: other diseases and intestinal disorders; pregnancy; lactation; use of oral contraceptive pill; supplementation with vitamin D, omega‐3, multivitamins, polyphenol, or antioxidants; use of anticoagulant, non‐steroidal anti‐inflammatory drug, aspirin, antihistamine, and calcium channel antagonist; relapse of disease Age: mean: 39.7 years (Group 1); 34.0 years (Group 2) Sex (male): 50% (Group 1); 54.2% (Group 2) Number randomised: 25 (Group 1); 25 (Group 2) Number analysed: 22 (Group 1); 24 (Group 2) |
|
| Interventions |
Group 1: low‐dose vitamin D (1000 IU/day) Group 2: high‐dose vitamin D (2000 IU/day) |
|
| Outcomes |
Duration of follow‐up: 12 weeks Outcomes
|
|
| Notes |
Funding source: Shahid Beheshti University of Medical Sciences Conflicts of interest: none Additional information received from author after email contact: Quote: "Serum concentrations of TNF‐α decreased in 15 patients in high dose group and in 1 patient in low dose group at the end of the study compared to the beginning of the study. Serum concentrations of hs‐CRP decreased in 17 patients in high dose group and in 7 patients in low dose group at the end of the study. Serum vitamin D increased in 22 patients in high dose group and in 10 patients in low dose group at the end of the study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that participants were randomised but no details of randomisation or sequence generation given. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was given in identical 'pearl' to vitamin D 'pearl'. Boxes were labelled A, B, and C, but contents unknown to participants or researcher. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Boxes labelled by external person uninvolved in study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study flow adequately described, balanced withdrawals. |
| Selective reporting (reporting bias) | Low risk | Trial registered in Iranian Registry of Clinical Trials after study initiation. |
| Other bias | Low risk | Balanced baseline characteristics and no other concerns. |
Mathur 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: US |
|
| Participants |
State of disease/disease type: active and inactive UC Inclusion criteria: adults (aged ≥ 18 years) with UC and 25(OH)D < 30 ng/mL within 1 year of enrolment Exclusion criteria: receiving vitamin D supplementation > 2000 IU/day Age: mean: 41.1 years (Group 1); 40.2 years (Group 2) Sex (male): 88% (Group 1); 60% (Group 2) Number randomised: 8 (Group 1); 10 (Group 2) Number analysed: 8 (Group 1); 10 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 2000 IU/day Group 2: vitamin D3 4000 IU/day |
|
| Outcomes |
Duration of follow‐up: 90 days Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Central California Faculty Medical Group Conflicts of interest: none Additional information provided by author after email contact: after request for further information regarding disease activity scores for each group, the study author responded that 3 participants in each group had Mayo scores of 0. These data were not used in meta‐analysis as the study did not feature in any of our comparisons. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. |
| Allocation concealment (selection bias) | Low risk | Assignments were stored in a sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment was relabelled and packaged for blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study investigators were blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study flow described, all randomised patients completed the study. |
| Selective reporting (reporting bias) | Unclear risk | Prospective trial registration, however trial registration did not give details on outcomes being studied. |
| Other bias | Unclear risk | The mean Partial Mayo UC score at baseline was lower for Group 1 (1.4) than Group 2 (4.0) (P = 0.03). |
Narula 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Canada |
|
| Participants |
State of disease/disease type: inactive CD Inclusion criteria: adults (aged 18–70 years) with CD in remission (HBI ≤ 4) for ≥ 28 days Exclusion criteria: current or anticipated pregnancy; short bowel syndrome; any condition that could predispose to vitamin D toxicity (e.g. renal insufficiency, sarcoidosis, hyperparathyroidism, malignancy); concomitant therapy with thiazide diuretics, barbiturates, digitalis; use of supplements containing vitamin D Age: mean: 35 years (Group 1); 33 years (Group 2) Sex (female): 63% (Group 1); 56% (Group 2) Number randomised: 16 (Group 1); 18 (Group 2) Number analysed: 8 (Group 1); 12 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 1000 IU/day Group 2: vitamin D3 10,000 IU/day |
|
| Outcomes |
Duration of follow‐up: 12 months Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Canadian Association of Gastroenterology Conflicts of interest: vitamin supplements and placebo tablets were provided by Jamieson Laboratories. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated that participants were randomised prior to enrolment by a third party. |
| Allocation concealment (selection bias) | Low risk | Randomisation and allocation of assignments were performed centrally by a third party. Assignments were stored in a sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebos in coded medication containers were provided, participants and researchers unaware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors unaware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study flow described with balanced withdrawals. |
| Selective reporting (reporting bias) | Low risk | Prospective trial registration and outcomes matched registration. |
| Other bias | Low risk | No evidence that other significant sources of bias existed. |
Pappa 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: US |
|
| Participants |
State of disease/disease type: active and inactive CD and UC Inclusion criteria: children and adults (aged 5–21 years) with IBD and 25(OH)D ≤ 20 ng/mL within 8 weeks of enrolment Exclusion criteria: liver failure, kidney failure, on anticonvulsant metabolised through cytochrome P450, pregnancy, inability to take oral medications, attendance at tanning salons once weekly or more, on treatment for hypovitaminosis D Age: mean: 15.9 years (Group 1); 14.7 years (Group 2); 16.3 years (Group 3) Sex (male): 58% (Group 1); 42% (Group 2); 61% (Group 3) Number randomised: 24 (Group 1); 24 (Group 2); 23 (Group 3) Number analysed: 20 (Group 1); 21 (Group 2); 20 (Group 3) |
|
| Interventions |
Group 1: A: vitamin D2 2000 IU/day Group 2: B: vitamin D3 2000 IU/day Group 3: C: vitamin D2 50,000 IU/week |
|
| Outcomes |
Duration of follow‐up: 6 weeks Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: not stated Conflicts of interest: 1 author is the co‐director of the Clinical Investigator Training Program (sponsored by Harvard University, Massachusetts Institute of Technology, Pfizer, and Merck) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators blinded to the next treatment assignment". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study flow described with balanced withdrawals. |
| Selective reporting (reporting bias) | Low risk | Registration in ClinicalTrials.gov after study initiation. |
| Other bias | Low risk | No evidence that other significant sources of bias exist. |
Pappa 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: US |
|
| Participants |
State of disease/disease type: active and inactive CD and UC Inclusion criteria: children and adults (aged 5–21 years) with IBD and 25(OH)D > 20 ng/mL within 8 weeks of enrolment Exclusion criteria: inability to take oral medications, pregnancy, liver or kidney failure, use of antiepileptic medications metabolised through cytochrome P450 Age: mean: 15.1 years (Group 1); 14.5 years (Group 2) Sex (female): 59% (Group 1); 55% (Group 2) Number randomised: 32 (Group 1); 31 (Group 2) Number analysed: 19 (Group 1); 15 (Group 2) |
|
| Interventions |
Group 1: vitamin D2 400 IU/day Group 2: vitamin D2 1000 IU/day (between 1 May and 31 October) and 2000 IU/day (between 1 November and 30 April) |
|
| Outcomes |
Duration of follow‐up: 12 months Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: National Institutes of Health K23, Crohn's and Colitis Foundation of America, Children's Digestive Health and Nutrition Foundation, National Institutes of Health MO1, Harvard Catalyst Grant Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation. |
| Allocation concealment (selection bias) | Low risk | Investigators were blinded to next treatment assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study flow described with balanced withdrawals. |
| Selective reporting (reporting bias) | Low risk | Prospective trial registration but outcome measures not added until after trial completion. |
| Other bias | Low risk | No evidence that other significant sources of bias exist. |
Raftery 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Ireland |
|
| Participants |
State of disease/disease type: inactive CD Inclusion criteria: adults (aged ≥ 18 years) with CD in remission (CDAI < 150, CRP < 5 mg/L) and stable therapy for a minimum of 3 months Exclusion criteria: extensive bowel resection, hypersensitivity to vitamin D, history of hypercalcaemia (corrected serum calcium > 2.66 mmol/L), supplemental vitamin D intake > 1000 IU/day, antibiotic use within 4 weeks prior to enrolment, renal impairment, diabetes mellitus, alcohol dependency, urinary tract infection, pregnancy, on bisphosphonates Age: mean: 36.5 years (Group 1); 36.7 years (Group 2) Sex (male): 7 (Group 1); 6 (Group 2) Number randomised: 13 (Group 1); 14 (Group 2) Number analysed: 13 (Group 1); 14 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 2000 IU/day Group 2: placebo |
|
| Outcomes |
Duration of follow‐up: 3 months Outcomes
|
|
| Notes |
Funding source: Irish Research Council Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central block randomisation in groups of 10. |
| Allocation concealment (selection bias) | Low risk | Randomisation and allocation of assignments were performed centrally by a third party. Assignments were stored in a sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All packaging and tablets were identical. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessors were blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants completed the study. |
| Selective reporting (reporting bias) | Low risk | Registration in ClinicalTrials.gov after study initiation. Listed outcomes were reported. |
| Other bias | Low risk | Balanced baseline characteristics and no other concerns. |
Sassine 2020.
| Study characteristics | ||
| Methods |
Study design: RCT (abstract) Setting: Canada |
|
| Participants |
State of disease/disease type: inactive or mildly active CD Inclusion criteria: children (aged 9–18 years) with newly‐diagnosed CD (≤ 3 months) with PCDAI < 30 Exclusion criteria: not stated Age: 9–18 years Sex: 11 boys; 14 girls Number randomised: 12 (Group 1); 13 (Group 2) Number analysed: not stated |
|
| Interventions |
Group 1: vitamin D3 3000 IU/day (< 40 kg participant) or 4000 IU/day (≥ 40 kg participant) for 4 weeks, then 2000 IU/day for 48 weeks Group 2: vitamin D3 800 IU/day for 52 weeks |
|
| Outcomes |
Duration of follow‐up: 52 weeks Outcomes
|
|
| Notes |
Funding source: not stated Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, although method of randomisation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information to assess allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Interventions were provided in identical soft gel capsules with similar size and colours. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported as double‐blind trial, although method of blinding was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information to assess attrition. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported per methods but no trial registration. |
| Other bias | Low risk | No evidence that other significant sources of bias exist. |
Sharifi 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Iran |
|
| Participants |
State of disease/disease type: inactive UC Inclusion criteria: adults (aged 18–50 years) with UC Exclusion criteria: BMI < 18.5 kg/m2 or > 30 kg/m2; anti‐TNF therapy; use of any form of vitamin D3 supplementation in the 3 months preceding the study; history of hyperparathyroidism, nephrolithiasis, malignancy, renal failure, or hepatic failure; pregnancy; breastfeeding Age: mean: 37.5 years (Group 1); 35.0 years (Group 2) Sex (male): 26 (Group 1); 25 (Group 2) Number randomised: 46 (Group 1); 44 (Group 2) Number analysed: 46 (Group 1); 40 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 300,000 IU intramuscularly Group 2: normal saline intramuscularly |
|
| Outcomes |
Duration of follow‐up: 90 days Outcomes
|
|
| Notes |
Funding source: Tehran University of Medical Sciences and Health Services Conflicts of interest: none Additional information from author after email contact: requested information on clinical relapse but author stated such data not available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation in groups of 4. |
| Allocation concealment (selection bias) | Unclear risk | No details about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient details about blinding. Quote: "Investigators and participants were kept masked to allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were different from personnel who performed intervention. Insufficient details about blinding. Quote: "Investigators and participants were kept masked to allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/90 participants withdrew from the study. Control arm: 4/44 were lost to follow‐up, reasons given for withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Preregistered in the Iranian Registry of Clinical Trials. Some listed outcomes were reported, total 18 primary and secondary outcomes, outcomes in the paper were secondary outcomes as per trial registration. |
| Other bias | Low risk | Balanced baseline characteristics and no other concerns. |
Tan 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: China |
|
| Participants |
State of disease/disease type: active and inactive CD and UC Inclusion criteria: adults (ages ≥ 18 years) with CD or UC, 25(OH)D < 20 ng/mL within 12 months of follow‐up Exclusion criteria: no 25(OH)D data available; endocrine or metabolic diseases that led to secondary osteoporosis, such as severe renal and liver function impairment, primary hyperparathyroidism, type 1 diabetes mellitus, hypogonadism, hyperthyroidism, malignant bone tumours, multiple myeloma, tumour‐associated bone metastasis, connective tissue disease; history of bone fracture or had received treatment for osteoporosis; severe malabsorption or malnutrition Age: mean: 38.9 years (CD); 42.2 years (UC) Sex (male): 33 (CD); 39 (UC) Number randomised: CD: 23 (Group 1); 23 (Group 2); 25 (Group 3); UC: 25 (Group 1); 24 (Group 2); 25 (Group 3) Number analysed: CD: 23 (Group 1); 17 (Group 2); 19 (Group 3); UC: 24 (Group 1); 16 (Group 2); 25 (Group 3) |
|
| Interventions |
Group 1: vitamin D 150,000 IU every 3 months plus elemental calcium 200 mg 3 times daily Group 2: elemental calcium 200 mg 3 times daily Group 3: "vehicle control group" |
|
| Outcomes |
Duration of follow‐up: 12 months Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: Chinese National Scientific Research Special‐Purpose Project in Public Health Professions, Doctoral Fund of the Ministry of Education of China Conflicts of interest: none Additional data received from author after email contact in 2021
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, although method of randomisation was not reported. Quote: "randomly assigned to the arms A, B or C according to the randomization schedule with a ratio of 1:1:1". |
| Allocation concealment (selection bias) | Unclear risk | No information to assess allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals accounted for. |
| Selective reporting (reporting bias) | Low risk | Trial registered in the Chinese Clinical Trial Registry after study initiation. Listed outcomes were reported. |
| Other bias | Low risk | Balanced baseline characteristics and no other concerns. |
Vogelsang 1995.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Austria |
|
| Participants |
State of disease/disease type: active and inactive CD Inclusion criteria: ambulatory people with CD who were unlikely to need hospitalisation or surgery within the next months Exclusion: use of oestrogen, cholestyramine, calcitonin, fluoride Age: median: 39 years (Group 1); 31 years (Group 2) Sex (male): 17 (Group 1); 14 (Group 2) Number randomised: 37 (Group 1); 38 (Group 2) Number analysed: 30 (Group 1); 30 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 1000 IU/day Group 2: no supplementation |
|
| Outcomes |
Duration of follow‐up: 1 year Outcomes
|
|
| Notes |
Funding source: not stated Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Emailed study author to confirm method of generating random numbers. Quote: "Random numbers in sealed envelopes." |
| Allocation concealment (selection bias) | Low risk | Assigned intervention arms through random numbers in sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was relatively balanced across treatment arms. Insufficient information was provided to assess reasons for withdrawals. Group 1: 7/37 withdrawals (1 had very low bone density with lumbar pain). Group 2: 8/38 withdrawals (1 had a low bone density with lumbar pain and 1 had a pathological food fracture). |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported per methods but no trial registration. |
| Other bias | Low risk | No evidence that other significant sources of bias exist. |
Wingate 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Setting: Canada |
|
| Participants |
State of disease/disease type: inactive CD Inclusion criteria: children and adolescents (aged 8–18 years) with quiescent CD Exclusion criteria: on corticosteroids within the prior 6 months, on vitamin D supplementation > 1000 IU/day Age: mean: 14.0 years (Group 1); 14.5 years (Group 2) Sex (female): 46% (Group 1); 44% (Group 2) Number randomised: 40 (Group 1); 43 (Group 2) Number analysed: 34 (Group 1); 35 (Group 2) |
|
| Interventions |
Group 1: vitamin D3 400 IU/day Group 2: vitamin D3 2000 IU/day |
|
| Outcomes |
Duration of follow‐up: 6 months Primary outcome
Secondary outcomes
|
|
| Notes |
Funding source: University of British Columbia Vitamin Research Fund Conflicts of interest: supplements provided by Natural Factors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised at baseline in a 1:1 allocation to either a 400 or 2000IU/d vitamin D3 supplement dose." "Subjects and research staff were blinded to supplement doses, which were coded by lot number. Lot numbers were assigned randomly to a sequential study subject identification number by a statistician." |
| Allocation concealment (selection bias) | Low risk | Central allocation of assignments by coded lot numbers. Quote: "Subjects and research staff were blinded to supplement doses, which were coded by lot number. Lot numbers were assigned randomly to a sequential study subject identification number by a statistician." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and study investigators were reportedly blinded to treatment assignment, unstated how this was achieved. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and study investigators were reportedly blinded to treatment assignment, unstated how this was achieved. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14/83 participants dropped out of the study. Vitamin D3 400 IU/day arm: 5/40 withdrew and 1/40 was lost to follow‐up. Vitamin D3 2000 IU/day arm: 3/43 withdrew, 4/43 were lost to follow‐up, and 1/43 had a protocol error. Withdrawals were balanced across treatment arms. Insufficient data provided to assess reasons for withdrawal within each treatment arm. |
| Selective reporting (reporting bias) | Low risk | Preregistered in ClinicalTrials.gov. Listed outcomes were reported. |
| Other bias | Low risk | Balanced baseline characteristics, no other concerns. |
25(OH)D: 25‐hydroxy vitamin D (calcifediol); BMI: body mass index; CD: Crohn's disease; CDAI: Crohn's Disease Activity Index; CDEIS: Crohn's Disease Endoscopic Index of Severity; CRP: C‐reactive protein; CT: computer tomography; ESR: erythrocyte sedimentation rate; HBI: Harvey‐Bradshaw Index; IBD: inflammatory bowel disease; IBDQ: Inflammatory Bowel Disease Questionnaire; IU: international unit; RCT: randomised controlled trial; SCCAI: Simple Clinical Colitis Activity Index; SD: standard deviation; SIBDQ: Short Inflammatory Bowel Disease Questionnaire; TNF: tumour necrosis factor; UC: ulcerative colitis; UCDAI: Ulcerative Colitis Disease Activity Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| JPRN‐UMIN000025961 | Not an RCT |
| Kojecky 2020 | Ineligible intervention (not IBD treatment) |
| Laing 2020 | Not an RCT |
| Lee 2020 | Ineligible intervention (not IBD treatment) |
| Mullin 2011 | Not an RCT |
| O'Sullivan 2019 | Not an RCT |
| Sharifi 2020 | Ineligible intervention (not IBD treatment) |
| Simek 2016 | Ineligible intervention (not IBD treatment) |
IBD: inflammatory bowel disease; RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
ACTRN12617000836336.
| Methods | RCT |
| Participants | Children (aged 5 to 18 years) with IBD |
| Interventions |
Arm 1 Intervention 1
Intervention 2
Arm 2 Intervention 1
Intervention 2
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Australian New Zealand Clinical Trials Registry Retrospectively registered (first enrolment in 2015; registered in 2017) The author was unwilling to share data until their manuscript has been accepted for publication. |
Berriche‐Yahi 2022.
| Methods | RCT |
| Participants | 262 |
| Interventions | Vitamin D 200,000 IU/month (D200 group) Vitamin D 6000 IU/day (D6 group) |
| Outcomes |
|
| Notes | This study was identified during the update search and will be included in the update of this review. |
CTRI/2017/11/010336.
| Methods | RCT |
| Participants | Adults (aged 18–60 years) with inactive IBD (CDAI < 150 for CD; Mayo UC score ≤ 2 for UC) |
| Interventions | Vitamin D3 60,000 IU weekly for 8 weeks and calcium carbonate 1000 mg/day for 1 year Calcium carbonate 1000 mg/day for 1 year |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Clinical Trials Registry – India (CTRI) Retrospectively registered (first enrolment on 2 January 2017; registered on 11 February 2017) We contacted the study author but received no response. |
EUCTR2007‐006692‐37‐GB.
| Methods | RCT |
| Participants | Aged ≥ 18 year with active CD, CDAI score 200–450. Diagnosis of IBD and distribution of disease will have been confirmed during the course of their diagnostic investigations including endoscopic and histological parameters compatible with this diagnosis. Participant must be able to fully understand patient information sheet and sign an informed consent form Participants will be on a stable dose of the following medications prior to inclusion: 5‐aminosalicylates (≥ 4 weeks), thiopurines (≥ 8 weeks), no corticosteroids (≥ 4 weeks), no biological agents (≥ 8 weeks) |
| Interventions | Vigantol Oel TM |
| Outcomes |
|
| Notes | This study was terminated early with no results posted or published. |
IRCT20100524004010N22.
| Methods | RCT |
| Participants | Adults (aged 18–80 years) with mild‐to‐moderate UC |
| Interventions | Vitamin D (2 pearls of 1000 IU/day) for 12 weeks Vitamin D (1 pearl of 1000 IU/day and 1 placebo) for 12 weeks |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes | Iranian Registry of Clinical Trials Retrospectively registered (first enrolment in 2017; registered in 2018). We contacted the author but received no response. |
Lin 2023.
| Methods | RCT |
| Participants | 102 |
| Interventions | Vitamin D supplementation to routine treatment Routine treatment alone |
| Outcomes |
|
| Notes | This study was identified during the update search and will be included to the review's update |
NCT00132184.
| Methods | RCT |
| Participants | 110 |
| Interventions | Vitamin D Placebo |
| Outcomes |
|
| Notes | No results posted or published |
NCT00287170.
| Methods | RCT |
| Participants | Adults (aged 18–75 years) with moderate CD (CDAI ≥ 220 and ≤ 400) |
| Interventions | Delayed‐release 6‐mercaptopurine 40 mg/day or calcitriol 5 μg 3 times weekly 6‐mercaptopurine 1–2 mg/kg |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes | The author informed us that the study was prematurely terminated. No results have been posted or published. |
NCT01121796.
| Methods | RCT |
| Participants | Adults (aged ≥ 18 years) with mild‐to‐moderate IBD |
| Interventions | Vitamin D Vitamin D‐enriched milk Placebo (water or milk) |
| Outcomes |
Primary outcome
|
| Notes | Unknown status. We contacted the authors but received no response. |
NCT01369667.
| Methods | RCT |
| Participants | 117 |
| Interventions | Vitamin D Placebo |
| Outcomes |
|
| Notes | Completed but no results posted or published |
NCT01640496.
| Methods | RCT |
| Participants | Adults aged > 18 years with diagnosis of UC confirmed by histology. UC must have been active but mild disease as confirmed by a Mayo Clinic endoscopy score 2–4. Not requiring medication adjustment during the trial. |
| Interventions | Vitamin D |
| Outcomes |
|
| Notes | The study was terminated early. |
NCT01692808.
| Methods | RCT |
| Participants | Children (aged 10–18 years) with CD |
| Interventions | Exclusive enteral nutrition Exclusive enteral nutrition and cholecalciferol 3000 IU/day for 1 month Corticosteroids (1 mg/kg/day), vitamin D3 800 IU/day, calcium 1000 mg/day Corticosteroids (1 mg/kg/day), vitamin D3 4000 IU/day, calcium 1000 mg/day Cholecalciferol 4000 IU/day for children in remission |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Completed in 2014 but no published results |
NCT01846026.
| Methods | RCT |
| Participants | Men and women aged > 18 years, diagnosed with UC (either debut or relapsed chronic UC), moderate or severe, where it is an indication to treat with infliximab. |
| Interventions | Vitamin D |
| Outcomes |
|
| Notes | We contacted the author but received no response. |
NCT02186275.
| Methods | RCT |
| Participants | 25 |
| Interventions | High‐dose vitamin D Low‐dose vitamin D |
| Outcomes |
Primary outcome
Secondary outcomes
Other outcomes
|
| Notes | Completed but no results posted or published |
NCT02208310.
| Methods | RCT |
| Participants | Age ≥ 18 and < 75 years with diagnosis of CD; vitamin D deficiency or insufficiency (serum 25‐(OH)D3 < 30 ng/mL) |
| Interventions | Low‐dose vitamin D High‐dose vitamin D |
| Outcomes |
Composite endpoint
|
| Notes | Study was terminated early. |
Xia 2020.
| Methods | RCT |
| Participants | 120 |
| Interventions | Vitamin D plus mesalamine Mesalamine |
| Outcomes |
|
| Notes | Identified during the update search and to be included in the update of the review. |
25‐(OH)D: 25‐hydroxy vitamin D (calcifediol); CD: Crohn's disease; CDAI: Crohn's Disease Activity Index; CRP: C‐reactive protein; ELISA: enzyme‐linked immunosorbent assay; ESR: erythrocyte sedimentation rate; IBD: inflammatory bowel disease; IBDQ: Inflammatory Bowel Disease Questionnaire; IL: interleukin; PCDAI: Pediatric Crohn's Disease Activity Index; PUCAI: Paediatric Ulcerative Colitis Activity Index; SCCAI: Simple Clinical Colitis Activity Index; TNF: tumour necrosis factor; UC: ulcerative colitis.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800015174.
| Study name | Effects of vitamin D supplementation on clinical prognosis for patients with Crohn's disease |
| Methods | RCT |
| Participants | Adults (aged 18–75 years) with IBD |
| Interventions | Vitamin D 800 IU/day Placebo |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | First enrolment on 1 May 2018 |
| Contact information | Shixue Dai (shixuedai@hotmail.com) Guangdong General Hospital, Guangzhou, Guangdong, China |
| Notes |
CTRI/2021/03/031675.
| Study name | Assessment of malnutrition in patients with ulcerative colitis and effect of supplementation of calcitriol in patients with active disease |
| Methods | RCT |
| Participants | 60 |
| Interventions | Calcitriol plus standard treatment Placebo plus standard treatment |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 11 March 2021 |
| Contact information | ushadutta@gmail.com anuragsachan223@gmail.com |
| Notes |
CTRI/2021/07/035128.
| Study name | Role of vitamin D as an add on therapy in ulcerative colitis patients with anaemia |
| Methods | RCT |
| Participants | 60 |
| Interventions | Vitamin D plus mesalamine plus prednisolone for 3 months Mesalamine plus prednisolone for 3 months |
| Outcomes |
|
| Starting date | 1 August 2021 |
| Contact information | drsaritagoyal@rediffmail.com Komal.dalal99@gmail.com |
| Notes |
EUCTR 2009‐015649‐21‐NO.
| Study name | Immunomodulating and clinical effect of vitamin D on the induction of remission in the patients with moderate to severe ulcerative colitis under the treatment with infliximab |
| Methods | RCT |
| Participants | Adults (aged 18–75 years) with active UC |
| Interventions | Cholecalciferol plus infliximab Placebo plus infliximab |
| Outcomes |
Primary outcomes
Secondary outcome
|
| Starting date | First enrolment on 21 October 2009 |
| Contact information | University Hospital of North Norway |
| Notes | EU Clinical Trials Register Recruitment status currently unknown |
IRCT201011075123N1.
| Study name | The effect of different levels of vitamin D on the supply of children with inflammatory bowel disease |
| Methods | RCT |
| Participants | People (aged 1–24 years) with IBD |
| Interventions | Vitamin D 3000 IU/day equivalent (administered as 50,000 IU pearls) for 6 weeks Vitamin D 800 IU/day equivalent (administered as 50,000 IU pearls) for 6 weeks |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | Expected start date on 22 August 2016 (actual start date not listed) |
| Contact information | Hamidreza Kianifar (kianifarhr@mums.ac.ir) Mashhad University of Medical Sciences, Mashhad, Iran |
| Notes | Iranian Registry of Clinical Trials Retrospectively registered (expected start date in 2016; registered in 2017) |
NCT02704624.
| Study name | Effects of supplementation of vitamin D in patients with Crohn's disease |
| Methods | RCT |
| Participants | Adults (aged 18–50 years) with moderate‐to‐severe CD in remission and vitamin D deficiency |
| Interventions | Vitamin D3 50,000 IU weekly for 6 months Placebo |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | December 2016 |
| Contact information | Júlio Chebli Federal University of Juiz de Fora, Juiz de Fora, Minas Gerais, Brazil |
| Notes |
NCT03718182.
| Study name | Can vitamin D supplementation in people with Crohn's disease improve symptoms as an adjunct therapy? (D‐CODE) |
| Methods | RCT |
| Participants | Adults aged ≥ 18 years with confirmed diagnosis of CD; identified as having vitamin D deficiency < 50 nmol/L in the Winter screening study; already receiving treatment for CD as per National Institute for Health and Care Excellence (NICE) Guidance or those in remission and not currently receiving treatment but who continue to attend hospital outpatient appointments; have provided written informed consent |
| Interventions | Vitamin D3 3200 IU daily oral capsule for 12 weeks then switch to vitamin D3 800 IU daily oral capsule for 12 weeks Vitamin D3 400 IU daily oral capsule for 24 weeks |
| Outcomes |
|
| Starting date | 17 September 2019 |
| Contact information | Jane Fletcher, University Hospital Birmingham NHS Foundation Trust |
| Notes |
NCT03999580.
| Study name | The vitamin D in pediatric Crohn's disease (ViDiPeC‐2) |
| Methods | RCT |
| Participants | Children (aged 4–18 years) with CD in remission (PCDAI ≤ 10, no clinical symptoms, faecal calprotectin < 250 μg/g stool) |
| Interventions | Vitamin D3 3000 IU/day (< 40 kg participant) or 4000 IU/day (≥ 40 kg participant) for 4 weeks, then 2000 IU/day for 48 weeks Vitamin D3 600 IU/day for 52 weeks |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | August 2019 |
| Contact information | Prevost Jantchou (prevost.jantchou@umontreal.ca) St Justine's Hospital, Montreal, Quebec, Canada |
| Notes | Not yet recruiting |
NCT04134065.
| Study name | The effect of vitamin D in Crohn's disease |
| Methods | RCT |
| Participants | Adults (aged 20–60 years) with CD and risk factors for surgery (smoking, stricturing or fistulizing behaviour, early corticosteroid use, ileal disease, jejunal disease, or young age at diagnosis) |
| Interventions | Liquid vitamin D3 (prescribed with dose adjustment protocol for target of 40–50 ng/mL) for 12 weeks Placebo |
| Outcomes |
Primary outcomes
|
| Starting date | Estimated start date in December 2019 |
| Contact information | Yougsheng Li Shanghai Ninth People's Hospital, Shanghai, China |
| Notes | Not yet recruiting |
NCT04225819.
| Study name | Adjunctive treatment with vitamin D3 in patients with active IBD (ACTIVATED) |
| Methods | RCT |
| Participants | Adults (aged ≥ 18 years) with active IBD (CRP > 8 mg/L or calprotectin > 150 μg/g; HBI > 4 for CD or SCCAI > 2 for UC) and initiating anti‐TNF therapy within 2 weeks of randomisation |
| Interventions | Vitamin D3 10,000 IU/day Placebo |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | Estimated start date in April 2020 |
| Contact information | Ashwin Ananthakrishnan Massachusetts General Hospital, Boston, Massachusetts, USA |
| Notes | Not yet recruiting |
NCT04991324.
| Study name | The 5C‐study (5C) |
| Methods | RCT |
| Participants | 150 |
| Interventions | Vitamin D 24,000 IU per week (corresponding to a dose of approximately 3500 IU/day) Vitamin D 24,000 IU per month (corresponding to a dose of approximately 800 IU/day) |
| Outcomes |
Primary outcome
Secondary outcome
Other outcomes
|
| Starting date | 21 September 2022 |
| Contact information | jp.rothen@unibas.ch petr.hruz@clarunis.ch |
| Notes |
NCT05733117.
| Study name | Oral nano vitamin D supplementation efficacy in inflammatory bowel disease |
| Methods | RCT |
| Participants | 120 |
| Interventions | Vitamin D Vitamin D substitution |
| Outcomes |
|
| Starting date | 25 October 2022 (retrospective registration) |
| Contact information | jan.matous1@fnkv.cz kojecky@bnzlin.cz |
| Notes |
CDAI: Crohn's Disease Activity Index; CRP: C‐reactive protein; HBI: Harvey‐Bradshaw Index; IBD: inflammatory bowel disease; PCDAI: Pediatric Crohn's Disease Activity Index; RCT: randomised controlled trial; SIBDQ: Short Inflammatory Bowel Disease Questionnaire; TNF: tumour necrosis factor; UC: ulcerative colitis.
Differences between protocol and review
The protocol for this review was published in 2015 (Limketkai 2015), and since then the authoring team has changed considerably.
We have updated the methods section to correspond with the most recent Cochrane methodology standards.
We have changed the outcomes and preplanned subgroup and sensitivity analyses based on consensus from the current authors.
We agreed on a classification on what constitutes a supplemental dose, low‐treatment dose, or high‐treatment dose in order to facilitate the synthesis of our data. We have added more information on included populations, interventions, and extracted data. All changes were decided based on clinical criteria and there were no changes based on the findings of the review.
Any preplanned analyses not performed were due to lack of data.
Contributions of authors
CW: led the writing, screened, extracted, resolved conflicts, assessed certainty, approved the final version prior to submission.
MG: secured funding, designed and developed, screened, extracted, resolved conflicts, assessed certainty, contributed to writing and editing, advised on, approved the final version prior to submission, and is a guarantor of the review.
VS: screened, extracted, resolved conflicts, assessed certainty, contributed to writing and editing, advised on, approved the final version prior to submission.
BNL: screened, extracted, resolved conflicts, contributed to writing, advised on, approved the final version prior to submission.
Sources of support
Internal sources
-
University of Central Lancashire, UK
Internal funding for MG and VS comes from their salary for their employment by the University of Central Lancashire.
External sources
-
NIHR grant, UK
This study/project is funded by the NIHR Evidence Synthesis Programme Grant (NIHR132748). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Declarations of interest
CW: none.
MG is an editor for Cochrane Gut Group, but had no role in the editorial process for this review.
VS: none.
BNL: none.
New
References
References to studies included in this review
Ahamed 2019 {published data only}
- Ahamed ZR, Dutta U, Sharma V, Prasad KK, Popli P, Kalsi D, et al. Oral nano vitamin D supplementation reduces disease activity in ulcerative colitis: a double-blind randomized parallel group placebo-controlled trial. Journal of Clinical Gastroenterology 2019;53(10):e409-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Arihiro 2019 {published data only}
- Arihiro S, Nakashima A, Matsuoka M, Suto S, Uchiyama K, Kato T, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza and upper respiratory infection in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2019;25(6):1088-95. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bafutto 2020 {published data only}
- Bafutto M, Oliveira EC, Filho JR. Use of vitamin D with anti-tumor necrosis factor therapy for Crohn's disease. Gastroenterology Research 2020;13(3):101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bendix 2020 {published data only}
- Bendix M, Dige A, Jorgensen SP, Dahlerup JF, Bibby BM, Deleuran B, et al. Decrease in mucosal IL-17A, IFN-y and IL10 expressions in active Crohn's disease patients treated with high-dose vitamin alone or combined with infliximab. Nutrients 2020;12(12):3699. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boothe 2011 {published data only}
- Boothe D, Lakehomer H, Jacob V, Scherl E, Bosworth B. High dose vitamin D3 improves clinical activity in Crohn's disease. American Journal of Gastroenterology 2011;106:S458. [Google Scholar]
Dadaei 2015 {published data only}
- Dadaei T, Safapoor MH, Aghdaei H, Balaii H, Pourhoseingholi MA, Naderi N, et al. Effect of vitamin D3 supplementation on TNF-α serum level and disease activity index in Iranian IBD patients. Gastroenterology and Hepatology from Bed to Bench 2015;8(1):49-55. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- IRCT2013122915686N1. Association between vitamin D deficiency in patients with inflammatory bowel disease and disease activity, TNF alpha level and quality of life. www.irct.ir/trial/14884 (first received 29 December 2013).
Dash 2019 {published data only}
- Dash KR, Mishra D, Kumar Barik R, Sahu SK, Pradhan S, Parida PK, et al. Effect of vitamin d supplementation in patients of ulcerative colitis (UC) and its correlation with disease severity and health related quality of life (HRQOL). Journal of Gastroenterology and Hepatology 2019;34:209. [Google Scholar]
de Bruyn 2020 {published data only}
- Bruyn JR, Bossuyt P, Ferrante M, West RL, Dijkstra G, Witteman BJ, et al. High-dose vitamin D does not prevent postoperative recurrence of Crohn's disease in a randomized placebo-controlled trial. Clinical Gastroenterology & Hepatology 2020;19(8):1573-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
El Amrousy 2021 {published and unpublished data}
- El Amrousy D, El Ashry H, Hodeib H, Hassan S. Vitamin D in children with inflammatory bowel disease: a randomized controlled clinical trial. Journal of Clinical Gastroenterology 2021;55(9):815-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jing 2019 {published data only}
- Jing Y, Shi Y, Zhang J, Zhao X. Effect of exogenous vitamin D on intestinal mucosal barrier and inflammatory factors in patients. Chinese Journal of Gastroenterology 2019;24(8):493-6. [Google Scholar]
Jorgensen 2010 {published data only}
- Bendix M, Greisen S, Dige A, Hvas CL, Bak N, Jorgensen SP, et al. Vitamin D increases programmed death receptor-1 expression in Crohn's disease. Oncotarget 2017;8(15):24177-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendix-Struve M, Bartels LE, Agnholt J, Dige A, Jørgensen SP, Dahlerup JF. Vitamin D3 treatment of Crohn's disease patients increases stimulated T cell IL-6 production and proliferation. Alimentary Pharmacology and Therapeutics 2010;32:1364-72. [DOI] [PubMed] [Google Scholar]
- Jorgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, et al. Clinical trial: vitamin D3 treatment in Crohn's disease – a randomized double-blind placebo-controlled study. Alimentary Pharmacology and Therapeutics 2010;32(3):377-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karimi 2020 {published and unpublished data}
- Karimi S, Tabataba-Vakili S, Ebrahimi-Daryani N, Yari Z, Karimi A, Hedayati M, et al. Inflammatory biomarkers response to two dosages of vitamin D supplementation in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Clinical Nutrition ESPEN 2020;36:76-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mathur 2017 {published data only (unpublished sought but not used)}
- Mathur J, Naing S, Mills P, Limsui D. A randomized clinical trial of vitamin D3 (cholecalciferol) in ulcerative colitis patients with hypovitaminosis D3. PeerJ 2017;5:e3654. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01877577. Supplementation of vitamin D3 in patients with inflammatory bowel diseases and hypovitaminosis D. clinicaltrials.gov/ct2/show/NCT01877577 (first received 13 June 2013).
Narula 2017 {published data only}
- NCT02615288. High dose vitamin D3 in Crohn's disease. clinicaltrials.gov/ct2/show/NCT02615288 (first received 26 November 2015).
- Narula N, Cooray M, Anglin R, Muqtadir Z, Narula A, Marshall JK. Impact of high-dose vitamin D3 supplementation in patients with Crohn's disease in remission: a pilot randomized double-blind controlled study. Digestive Diseases and Sciences 2017;62(2):448-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pappa 2012 {published data only}
- Pappa HM, Mitchell PD, Jiang H, Kassiff S, Filip-Dhima R, DiFabio D, et al. Treatment of vitamin D insufficiency in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing three regimens. Journal of Clinical Endocrinology and Metabolism 2012;97(6):2134-42. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pappa 2014 {published data only}
- Pappa HM, Jiang H, Filip-Dhima R, Kassiff S, Gordon C. Comparison of two oral vitamin d supplementation doses in maintaining optimal vitamin D level in children and adolescents with inflammatory bowel disease. Journal of Bone and Mineral Research 2012;27:S340. [Google Scholar]
- Pappa HM, Mitchell PD, Jiang H, Kassiff S, Filip-Dhima R, DiFabio D, et al. Maintenance of optimal vitamin D status in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing two regimens. Journal of Clinical Endocrinology and Metabolism 2014;99(9):3408-17. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raftery 2015 {published data only}
- Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn's disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterology Journal 2015;3(3):294-302. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sassine 2020 {published data only}
- Sassine S, Tchogna SA, Deslandres C, Mailhot G, Alos N, Touzot F, et al. A pilot double-blind randomized controlled trial on vitamin D3 in children with newly diagnosed Crohn's disease. Gastroenterology 2020;158:S1200. [Google Scholar]
Sharifi 2016 {published data only (unpublished sought but not used)}
- Hosseinzadeh-Attar MJ, Sharifi A, Nedjat S, Mohamadkhani A, Vahedi H. The effect of vitamin D on serum asymmetric dimethylarginine in patients with mild to moderate ulcerative colitis. International Journal for Vitamin and Nutrition Research 2020;90(1-2):17-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sharifi A, Hosseinzadeh-Attar MJ, Vahedi H, Nedjat S. A randomized controlled trial on the effect of vitamin D3 on inflammation and cathelicidin gene expression in ulcerative colitis patients. Saudi Journal of Gastroenterology 2016;22(4):316-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi A, Vahedi H, Nedjat S, Mohamadkhani A, Hosseinzadeh Attar MJ. Vitamin D decreases Beck Depression Inventory score in patients with mild to moderate ulcerative colitis: a double-blind randomized placebo-controlled trial. Journal of Dietary Supplements 2019;16(5):541-9. [DOI] [PubMed] [Google Scholar]
- Sharifi A, Vahedi H, Nedjat S, Rafiei H, Hosseinzadeh-Attar MJ. Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo-controlled trial. APMIS 2019;127(10):681-7. [DOI] [PubMed] [Google Scholar]
Tan 2018 {published and unpublished data}
- Tan B, Li P, LV H, Yang H, Li Y, Li J, et al. Treatment of vitamin D deficiency in Chinese inflammatory bowel disease patients: a prospective, randomized, open-label, pilot study. Journal of Digestive Diseases 2018;19(4):215-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vogelsang 1995 {published data only}
- Vogelsang H, Ferenci P, Resch H, Kiss A, Gangl A. Prevention of bone mineral loss in patients with Crohn's disease by long-term oral vitamin D supplementation. European Journal of Gastroenterology and Hepatology 1995;7(7):609-14. [PMID: ] [PubMed] [Google Scholar]
Wingate 2014 {published data only}
- Wingate KE, Jacobson K, Issenman R, Carroll M, Barker C, Israel D, et al. 25-Hydroxyvitamin D concentrations in children with Crohn's disease supplemented with either 2000 or 400 IU daily for 6 months: a randomized controlled study. Journal of Pediatrics 2014;164(4):860-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
JPRN‐UMIN000025961 {published data only}
- JPRN-UMIN000025961. The study of effect of alfacalcidol treatment on Crohn's disease. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000029845 (first received 1 March 2017).
Kojecky 2020 {published data only}
- Kojecky V, Matous J, Kianicka B, Dite P, Zadorova Z, Kubovy J, et al. Vitamin D levels in IBD: a randomised trial of weight-based versus fixed dose vitamin D supplementation. Scandinavian Journal of Gastroenterology 2020;55(6):671-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Laing 2020 {published data only}
- Laing B, Cavadino A, Ellett S, Ferguson LR. Effects of an omega-3 and vitamin D supplement on fatty acids and vitamin D serum levels in double-blinded, randomized, controlled trials in healthy and Crohn's disease populations. Nutrients 2020;12(4):E1139. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2020 {published data only}
- Lee R, Maltz RM, Crandall WV, Plogsted SW, Shaikhkhalil AK, Bowden SA, et al. Single high-dose vitamin D3 supplementation in pediatric patients with inflammatory bowel disease and hypovitaminosis D. Journal of Pediatric Gastroenterology and Nutrition 2020;70(4):e77-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mullin 2011 {published data only}
- Mullin GE. Comment on: clinical trial: vitamin D3 treatment in Crohn's disease: a randomized double-blind placebo-controlled study. Nutrition in Clinical Practice 2011;26(2):204-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Sullivan 2019 {published data only}
- O'Sullivan F, Raftery T, Weele M, Geffen J, McNamara D, O'Morain C, et al. Sunshine is an important determinant of vitamin D status even among high-dose supplement users: secondary analysis of a randomized controlled trial in Crohn's disease patients. Photochemistry and Photobiology 2019;95(4):1060-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharifi 2020 {published data only}
- Sharifi A, Vahedi H, Honarvar MR, Amiriani T, Nikniaz Z, Rad EY, et al. Vitamin D decreases CD40L gene expression in ulcerative colitis patients: a randomized, double-blinded, placebo-controlled trial. Turkish Journal of Gastroenterology 2020;31(2):99-104. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simek 2016 {published data only}
- Simek RZ, Prince J, Syed S, Sauer CG, Martineau B, Hofmekler T, et al. Pilot study evaluating efficacy of 2 regimens for hypovitaminosis D repletion in pediatric inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition 2016;62(2):252-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12617000836336 {published data only}
- ACTRN12617000836336. Vitamin D supplementation in paediatric inflammatory bowel disease: a randomised controlled trial to determine effect of vitamin D status and supplementation method on serum 25-hydroxyvitamin D levels. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373054 (first received 7 June 2017).
Berriche‐Yahi 2022 {published data only}
- Berriche-Yahi N, Tahar A, Asselah H, Ayoub S, Hantala D, Koceir EA. Effects of oral vitamin D3 supplementation in Crohn's disease patients: modulation of clinical active/remission phases by pro-inflammatory cytokines profile and oxidative stress. Annales de Biologie Clinique 2022;80(1):29-46. [DOI] [PubMed] [Google Scholar]
CTRI/2017/11/010336 {published data only}
- CTRI/2017/11/010336. A study to assess the efficacy of vitamin D supplementation in preventing disease relapse in patients with inflammatory bowel disease. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=17504 (first received 2 November 2017).
EUCTR2007‐006692‐37‐GB {published data only}
- EUCTR2007-006692-37-GB. The immunomodulatory role of vitamin D in inflammatory bowel disease (IBDVit): IBDVit1 – trial of adjuvant vitamin D with corticosteroids in active Crohn's disease; IBDVit2 – trial of adjuvant vitamin D with infliximab in active Crohn's disease; IBDVit3 – trial of adjuvant vitamin D with standard maintenance therapy in preventing relapse of inflammatory bowel disease. clinicaltrialsregister.eu/ctr-search/trial/2007-006692-37/GB (date first entered 25 January 2008).
- EUCTR2008-001466-93-GB. Trial of adjuvant vitamin D with Infliximab in active Crohn's disease (IBDVit2). clinicaltrialsregister.eu/ctr-search/trial/2008-001466-93/results (first received 28 March 2008).
- EUCTR2008-001467-10-GB. Trial of adjuvant vitamin D with standard maintenance therapy in preventing relapse of inflammatory bowel disease. clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2008-001467-10 (first received 28 March 2008).
IRCT20100524004010N22 {published data only}
- IRCT20100524004010N22. Study of the effect of vitamin D supplementation on serum vitamin D concentration, quality of life, disease activity index, and some of inflammatory and oxidative factors in patients with ulcerative colitis with vitamin D deficiency. en.irct.ir/trial/4194 (first received 1 February 2018).
Lin 2023 {published data only}
- Lin X, Wu X, Zhang Y, Shao X, Wu H, Zhou L. Effect of vitamin D supplementation on clinical course and T helper 17/ T-regulatory balance in peripheral blood of patients with Crohn's disease. Turkish Journal of Gastroenterology 2023;34(5):463-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00132184 {published data only}
- NCT00132184. Vitamin D treatment for Crohn's disease. clinicaltrials.gov/ct2/show/NCT00132184 (first received 19 August 2005).
NCT00287170 {published data only}
- NCT00287170. Efficacy study of targeted, local delivery of drugs to treat Crohn's disease. clinicaltrials.gov/ct2/show/NCT00287170 (first received 6 February 2006).
NCT01121796 {published data only}
- NCT01121796. Influence of vitamin D on inflammatory bowel disease remission. clinicaltrials.gov/ct2/show/NCT01121796 (first received 12 May 2010).
NCT01369667 {published data only}
- NCT01369667. Vitamin D supplementation in adult Crohn's disease (VITD-CD). clinicaltrials.gov/ct2/show/NCT01369667 (first received 9 June 2011).
NCT01640496 {published data only}
- NCT01640496. Vitamin D treatment in ulcerative colitis. clinicaltrials.gov/ct2/show/NCT01640496 (first received 13 July 2012).
NCT01692808 {published data only}
- NCT01692808. Bioavailability of vitamin D in children and adolescents with Crohn's disease. clinicaltrials.gov/ct2/show/NCT01692808 (first received 25 September 2012).
NCT01846026 {published data only}
- NCT01846026. Ulcerative colitis and vitamin D supplementation. clinicaltrials.gov/ct2/show/NCT01846026 (first received 2 June 2015).
NCT02186275 {published data only}
- NCT02186275. The vitamin D in pediatric Crohn's disease (ViDiPeC). clinicaltrials.gov/ct2/show/NCT02186275 (first received 10 July 2014).
NCT02208310 {published data only}
- NCT02208310. Trial of high dose vitamin D in patient's with Crohn's disease (RODIN-CD). clinicaltrials.gov/ct2/show/NCT02208310 (first received 6 October 2017).
Xia 2020 {published data only}
- Xia Y, Gao H. Clinical study on effect of vitamin D3 combined with mesalazine in reparation of intestinal mucosal oxidative stress injury in patients with ulcerative colitis. Chinese Journal of Gastroenterology 2020;25(7):409-12. [Google Scholar]
References to ongoing studies
ChiCTR1800015174 {published data only}
- ChiCTR1800015174. Effects of vitamin D supplementation on clinical prognosis for patients with Crohn's disease. https://www.chictr.org.cn/showproj.html?proj=19597 (date of last refreshed on 12 March 2018).
CTRI/2021/03/031675 {published data only}
- CTRI/2021/03/031675. Assessment of malnutrition in patients with ulcerative colitis and effect of supplementation of calcitriol in patients with active disease. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2021/03/031675 (first received 3 March 2021).
CTRI/2021/07/035128 {published data only}
- CTRI/2021/07/035128. Role of vitamin D as an add on therapy in ulcerative colitis patients with anaemia. ctri.nic.in/Clinicaltrials/showallp.php?mid1=57677&EncHid=&userName=CTRI/2021/07/035128 (first received 23 July 2021).
EUCTR 2009‐015649‐21‐NO {published data only}
- EUCTR 2009-015649-21-NO. Immunmodulating and clinical effect of vitamin D on the induction of remission in the patients with moderate to severe ulcerative colitis under the treatment with Infliximab. www.clinicaltrialsregister.eu/ctr-search/search?query=2009-015649-21 (first received 19 March 2020).
IRCT201011075123N1 {published data only}
- IRCT201011075123N1. The effect of different levels of vitamin D on the supply of children with inflammatory bowel disease. en.irct.ir/trial/5450 (first received 15 July 2017).
NCT02704624 {published data only}
- NCT02704624. Effects of supplementation of vitamin D in patients with Crohn's disease. clinicaltrials.gov/ct2/show/NCT02704624 (first received 10 March 2016).
NCT03718182 {published data only}
- Fletcher J, Bedson E, Brown M, Hewison M, Swift A, Cooper SC. Protocol for an open-label feasibility study for a randomised controlled trial of vitamin D supplementation in Crohn's Disease patients with vitamin D deficiency: D-CODE Feasibility study. Pilot and Feasibility Studies 2021;7(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03718182. Can vitamin D supplementation in people with Crohn's disease improve symptoms as an adjunct therapy? (D-CODE). clinicaltrials.gov/ct2/show/NCT03718182 (first received 24 October 2018).
NCT03999580 {published data only}
- NCT03999580. The vitamin D in pediatric Crohn's disease (ViDiPeC-2). clinicaltrials.gov/ct2/show/NCT03999580 (first received 26 June 2019).
NCT04134065 {published data only}
- NCT04134065. The effect of vitamin D in Crohn's disease. clinicaltrials.gov/ct2/show/NCT04134065 (first received 21 October 2019).
NCT04225819 {published data only}
- NCT04225819. Adjunctive treatment with vitamin D3 in patients with active IBD (ACTIVATED). clinicaltrials.gov/ct2/show/NCT04225819 (first received 13 January 2020).
NCT04991324 {published data only}
- NCT04991324. Cholecalciferol comedication in IBD – the 5C-study (5C). clinicaltrials.gov/ct2/show/NCT04991324 (first received 5 August 2021).
NCT05733117 {published data only}
- NCT05733117. Oral nano vitamin D supplementation efficacy in inflammatory bowel disease. clinicaltrials.gov/ct2/show/NCT05733117 (first received 17 February 2023).
Additional references
Aali 2021
- Aali G, Shokraneh F. No limitations to language, date, publication type, and publication status in search step of systematic reviews. Journal of Clinical Epidemiology 2021;133:165-7. [DOI: 10.1016/j.jclinepi.2021.02.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Alatab 2019
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;5(1):17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ananthakrishnan 2012
- Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology 2012;142(3):482-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ananthakrishnan 2013
- Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng SC, Savova G, et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn's disease. Inflammatory Bowel Diseases 2013;19(9):1921-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 2010
- Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, et al. Estimation of total usual calcium and vitamin D intakes in the United States. Journal of Nutrition 2010;140(4):817-22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blanck 2013
- Blanck S, Aberra F. Vitamin D deficiency is associated with ulcerative colitis disease activity. Digestive Diseases and Sciences 2013;58(6):1698-702. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2007
- Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. Journal of Immunology 2007;179(3):1634-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, 2022. Available at www.covidence.org.
Egger 1997
- Egger M, Smith GD, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feuerstein 2020
- Feuerstein J, Isaacs K, Schneider Y, Siddique S, Falck-Ytter Y, Singh S, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020;158:1450-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feuerstein 2021
- Feuerstein JD, Ho EY, Shmidt E, Singh H, Falck-Ytter Y, Sultan S, et al, American Gastroenterological Association Institute Clinical Guidelines Committee. AGA clinical practice guidelines on the medical management of moderate to severe luminal and perianal fistulizing Crohn's disease. Gastroenterology 2021;160:2496-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 2019
- Fletcher J, Cooper SC, Ghosh S, Hewison M. The role of vitamin D in inflammatory bowel disease: mechanism to management. Nutrients 2019;11(5):1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frigstad 2017
- Frigstad SO, Høivik M, Jahnsen J, Dahl SR, Cvancarova M, Grimstad T, et al. Vitamin D deficiency in inflammatory bowel disease: prevalence and predictors in a Norwegian outpatient population. Scandinavian Journal of Gastroenterology 2017;52(1):100-6. [DOI: 10.1080/00365521.2016.1233577] [DOI] [PubMed] [Google Scholar]
Froicu 2003
- Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Molecular Endocrinology 2003;17(12):2386-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Froicu 2006
- Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology 2006;117(3):310-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gjuladin‐Hellon 2019a
- Gjuladin-Hellon T, Iheozor-Ejiofor, Gordon M et al. Azathioprine and 6-mercaptopurine for maintenance of surgically-induced remission in Crohn's disease. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD010233. [DOI: 10.1002/14651858.CD010233.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gjuladin‐Hellon 2019b
- Gjuladin‐Hellon T, Gordon M, Iheozor‐Ejiofor Z, Akobeng AK. Oral 5-aminosalicylic acid for maintenance of surgically-induced remission in Crohn's disease. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD008414. [DOI: 10.1002/14651858.CD008414.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2021a
- Gordon M, Grafton-Clarke C, Akobeng A, Macdonald J, Chande N, Hanauer S, et al. Pancreatitis associated with azathioprine and 6-mercaptopurine use in Crohn's disease: a systematic review. Frontline Gastroenterology 2021;12:423-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2021b
- Gordon M, Lakunina S, Sinopoulou V, Akobeng A. Minimum sample size estimates for trials in inflammatory bowel disease: a systematic review of a support resource. World Journal of Gastroenterology 2021;27:7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2022. Available at gradepro.org.
Guo 2021
- Guo Y, Zhang T, Wang Y, Liu R, Chang M, Wang X. Effects of oral vitamin D supplementation on inflammatory bowel disease: a systematic review and meta-analysis. Food & Function 2021;12(17):7588-606. [DOI] [PubMed] [Google Scholar]
Guzman‐Prado 2020
- Guzman-Prado Y, Samson O, Segal JP, Limdi JK, Hayee BH. Vitamin D therapy in adults with inflammatory bowel disease: a systematic review and meta-analysis. Inflammatory Bowel Diseases 2020;26(12):1819-30. [DOI] [PubMed] [Google Scholar]
Głąbska 2021
- Głąbska D, Kołota A, Lachowicz K, Skolmowska D, Stachoń M, Guzek D. Vitamin D supplementation and mental health in inflammatory bowel diseases and irritable bowel syndrome patients: a systematic review. Nutrients 2021;13(10):3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2021b
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Iheozor‐Ejiofor 2019
- Iheozor-Ejiofor Z, Gordon M, Clegg A, Freeman SC, Gjuladin-Hellon T, MacDonald JK, et al. Interventions for maintenance of surgically induced remission in Crohn's disease: a network meta-analysis. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013210. [DOI: 10.1002/14651858.CD013210.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iheozor‐Ejiofor 2021
- Iheozor-Ejiofor Z, Lakunina S, Gordon M, Akintelure D, Sinopoulou V, Akobeng A. Sample-size estimation is not reported in 24% of randomised controlled trials of inflammatory bowel disease: a systematic review. UEG Journal 2021;9:47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeffery 2009
- Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. Journal of Immunology 2009;183(9):5458-67. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabbani 2016
- Kabbani TA, Koutroubakis IE, Schoen RE, Ramos-Rivers C, Shah N, Swoger J, et al. Association of vitamin D level with clinical status in inflammatory bowel disease: a 5-year longitudinal study. American Journal of Gastroenterology 2016;111(5):712-9. [DOI: 10.1038/ajg.2016.53] [DOI] [PubMed] [Google Scholar]
Kaplan 2015
- Kaplan GG. The global burden of IBD: from 2015 to 2025. Nature Reviews: Gastroenterology and Hepatology 2015;12(12):720-7. [DOI: 10.1038/nrgastro.2015.150] [DOI] [PubMed] [Google Scholar]
Lamb 2019
- Lamb C, Kennedy N, Raine T, Hendy P, Smith P, Limdi J, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68(Suppl 1):1-106. [DOI: 10.1136/gutjnl-2019-318484] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laverny 2010
- Laverny G, Penna G, Vetrano S, Correale C, Nebuloni M, Danese S, et al. Efficacy of a potent and safe vitamin D receptor agonist for the treatment of inflammatory bowel disease. Immunology Letters 2010;131(1):49-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
Limketkai 2014
- Limketkai BN, Bayless TM, Brant SR, Hutfless SM. Lower regional and temporal ultraviolet exposure is associated with increased rates and severity of inflammatory bowel disease hospitalisation. Alimentary Pharmacology & Therapeutics 2014;40(5):508-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Manolagas 1986
- Manolagas SC, Provvedini DM, Murray EJ, Tsoukas CD, Deftos LJ. The antiproliferative effect of calcitriol on human peripheral blood mononuclear cells. Journal of Clinical Endocrinology and Metabolism 1986;63(2):394-400. [PMID: ] [DOI] [PubMed] [Google Scholar]
Merlino 2004
- Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis and Rheumatism 2004;50(1):72-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Molodecky 2012
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Munger 2006
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296(23):2832-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ng 2017
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390(10114):2769-78. [DOI: 10.1016/S0140-6736(17)32448-0] [DOI] [PubMed] [Google Scholar]
NHS 2020
- NHS. Vitamin D. www.nhs.uk/conditions/vitamins-and-minerals/vitamin-d (accessed 13 September 2023).
Pappa 2008
- Pappa HM, Bern E, Kamin D, Grand RJ. Vitamin D status in gastrointestinal and liver disease. Current Opinion in Gastroenterology 2008;24(2):176-83. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PRISMA 2020
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372(7):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rigterink 2019
- Rigterink T, Appleton L, Day AS. Vitamin D therapy in children with inflammatory bowel disease: a systematic review. World Journal of Clinical Pediatrics 2019;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 2011
- Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutrition 2011;14(5):938-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Sun 2023
- Sun YH, Tian DD, Zhou JM, Ye Q. Association between vitamin D level and pediatric inflammatory bowel disease: a systematic review and meta-analysis. Frontiers in Pediatrics 2023;11:1155004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torres 2020
- Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert J, Raine T, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. Journal of Crohn's and Colitis 2020;14:4-22. [DOI] [PubMed] [Google Scholar]
Tsoukas 1984
- Tsoukas CD, Provvedini DM, Manolagas SC. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science 1984;224(4656):1438-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ulitsky 2011
- Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. Journal of Parenteral and Enteral Nutrition 2011;35(3):308-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Valvano 2022
- Valvano M, Magistroni M, Cesaro N, Carlino G, Monaco S, Fabiani S, et al. Effectiveness of vitamin D supplementation on disease course in inflammatory bowel disease patients: systematic review with meta-analysis. Inflammatory Bowel Diseases 2022;2022:izac253. [DOI: 10.1093/ibd/izac253] [DOI] [PubMed] [Google Scholar]
Webb 2006
- Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a health vitamin D status. Photochemistry and Photobiology 2006;82(6):1697-703. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Limketkai 2015
- Limketkai BN, Kavuru R, Parian A, Al Kazzi ES, Hutfless SM. Vitamin D for the treatment of inflammatory bowel disease. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD011806. [DOI: 10.1002/14651858.CD011806] [DOI] [PMC free article] [PubMed] [Google Scholar]


