Abstract
Objective:
Wearable technologies for functional brain monitoring in freely behaving subjects can advance our understanding of cognitive processing and adaptive behavior. Existing technologies are lacking in this capability or need procedures that are invasive and/or otherwise impede brain assessments during social behavioral conditions, exercise, and sleep.
Methods:
In response a complete system was developed to combine relative cerebral blood flow (rCBF) measurement, and supplies, and behavior recording for use on conscious, freely behaving mice. An innovative diffuse speckle contrast flowmetry (DSCF) device and associated hardware were miniaturized and optimized for rCBF measurements in small subject applications. The use of this wearable, fiber-free, near-infrared DSCF head-stage/probe allowed no craniotomy, minimally invasive probe implantation, and minimal restraint of the awake animal.
Results and Conclusions:
Significant correlations were found between measurements with the new DSCF design and an optical standard. The system successfully detected rCBF responses to -induced hypercapnia in both anesthetized and freely behaving mice.
Significance:
Collecting rCBF and activity information together during natural behaviors provides realistic physiological results and opens the path to exploring their correlations with pathophysiological conditions.
Keywords: diffuse speckle contrast, wearable sensor, cerebral blood flow, freely moving mice
I. Introduction
Cerebral blood flow (CBF) is a well-established physiological parameter of brain health. Adequate supply of oxygen and metabolic substrates to the brain through CBF supports brain development, maintains normal brain function, achieves successful aging, and navigates acute/chronic medical conditions [1]. Many clinical situations, including stroke, traumatic brain injury, and neonatal asphyxia, expose the brain to insufficient CBF, leading to cerebral ischemic/hypoxic stresses and neurological disorders [2, 3]. On the other hand, cerebral hyperemia (excessive CBF) may raise intracranial pressure or induce hemorrhage, compressing and damaging delicate brain tissue. Moreover, abrupt and dramatic CBF changes are commonly seen in several neurological diseases, such as subarachnoid hemorrhage patients (vasospasm) and premature neonates (underdeveloped cardiovascular system and cerebrovascular autoregulation), presenting life-threatening conditions that require immediate treatment [4, 5]. Thus, it is crucial to monitor CBF variations continuously and longitudinally for early detection and management of cerebrovascular and neurological disorders, and better understanding of underlying pathological mechanisms [6, 7]. However, capable technologies are lacking, especially under free subject movement, hindering realization of abundant clinical benefits as well as impacting neurological animal studies.
Rodents comprise 95% of the animal models used in contemporary biomedical research [8]. Despite this ubiquitous presence, conscious rodents pose technical challenges in taking CBF measurements and are often anesthetized or immobilized [9]. While avoiding motion artifacts, the anesthesia by Isoflurane results in a CBF increase [10, 11]. Anesthesia also impacts neurovascular coupling and cerebral hemodynamics profoundly and interferes with many neurological studies such as psychological/behavioral neuroscience, cognitive function, sleep disorders, and neurological rehabilitation [12, 13]. Moreover, the anesthesia effect can last for several hours, even after the animal regains consciousness. Immobilization techniques inhibit study outcomes by limiting an animal’s social behavior, sleep, exercise, and other regular activities. Avoiding anesthesia and immobilization and continuously monitoring cerebral hemodynamics in conscious, freely behaving animals is imperative to progress. More realistic physiological information can be harnessed for studying pathological conditions and correlations with pathophysiological consequences.
Traditional standard-of-care CBF monitoring modalities such as magnetic resonance imaging (MRI) and transcranial Doppler (TCD) ultrasound are not suitable for continuous monitoring of CBF in freely behaving small animals due to either the large probe size (TCD) or body motion artifacts (MRI) [14, 15]. In response, a variety of wearable technologies have been attempted for continuously monitoring cerebral hemodynamics in awake rodents [16–20]. Wearable microscopes such as 2-photon microscopes, miniature endoscopes, fiber photometers, laser speckle contrast imaging (LSCI), optical intrinsic signal imaging (OISI), and calcium fluorescent probes enable imaging of local field neural activities and mapping of CBF/cerebral oxygenation distributions with high temporal-spatial resolution [18, 21–24], which has already made significant contributions to neuroscience although these microscopes use a widefield illumination with limited imaging depths (generally < 1 mm). Other wearable techniques include ultrasonic probes for brain imaging and implantable microelectrodes for brainwave and oxygen tension measurements [16, 17, 25, 26]. However, most wearable techniques for longitudinal monitoring require invasive craniotomy and/or implantation of probes on the cortex due to limited detection depths. Performing invasive craniotomy and probe implantation requires intricate surgical skill and may disrupt integrity of cerebrovascular network and neural circuitry, impact cerebral hemodynamics, and lead to post-surgery complications [27, 28]. For instance, due to inflammation or physiological changes, the cover glass on the craniotomy window may apply additional pressure disrupting cerebrospinal fluid regulation and intracranial pressure level [28]. Moreover, some of these methods require restraining animal’s head or body during measurements [26, 29, 30], thus impeding brain assessments during social behavioral conditions, exercise, and sleep. All these limitations make it difficult to translate these methods and animal findings to humans.
Near-infrared (NIR) diffuse optical techniques provide a noninvasive approach to probing deep cerebral hemodynamics, including the diffuse correlation spectroscopy (DCS) for blood flow measurements in deep tissues (up to ~15 mm depth) [31, 32]. DCS systems detect the temporal diffuse laser speckle fluctuations resulting from red blood cell (RBC) motions and have recovered CBF in awake rodents [33]. However, their large and expensive long-coherence laser (thousands of dollars) sources and single-photon-counting avalanche photodiode (thousands of dollars) detectors cannot be directly placed on the small animal head. Instead, optical fibers couple the sources and detectors onto the tissue surface, which significantly constrains subject’s movement.
An innovative wearable, fiber-free, dual-wavelength diffuse speckle contrast flow oximetry (DSCFO) technique was recently developed (US Patent #9/861,319, Issued 2020) [34], which provides a simple, low-cost, and compact alternative for continuous and simultaneous monitoring of blood flow and oxygenation variations in tissues up to ~10 mm depth [35–37]. Herein, the work focuses only on a single-wavelength instrumentation as related to acquiring tissue blood flow alone. Components and considerations for measuring oxygenation are not presented in this report, and accordingly the blood flow technique is simply referred to as DSCF (diffuse speckle contrast flowmetry). DSCF utilizes a low-cost small NIR laser diode (~$70, D780–30, US-Lasers) as the source and tiny complementary metal oxide semiconductor (CMOS) camera (~$150, NanEye 2D Black & White, Awaiba) as the 2D detector array. The measured spatial diffuse speckle contrast is then related to tissue blood flow. Thousands of parallel pixels on the inexpensive CMOS sensor significantly improved sampling density and reduced device cost and dimensions. DSCF has been calibrated against established methods (DCS and laser Doppler) in standard tissue-simulating phantoms with known optical properties, anesthetized mice, and human forearms without any motion [35]. In contrast to DCS fiber-optic probes, the connections between the DSCF probe and a compact controlling device are all soft electrical cables/wires (i.e., fiber-free). Despite these benefits, the previous design was not engineered for handling unrestrained subjects with its unstable foam pad probe, unmanaged electrical wire bundle, inappropriate material for a resident presence, and absence of animal behavior monitoring support.
The present study addressed these issues on several fronts. First, a wearable, miniaturized, fiber-free optical sensor was created to enable safe and repeated monitoring of CBF variations in freely behaving mice. This leveraged and improved upon the low-cost DSCF platform. The DSCF platform was then expanded to include a multifunctional test cage. A wire commutator (attached to the cage top) acted as a rotary electrical connector to provide low torque, 360-degree smooth and unlimited rotation for electrical signal/data transmission. The wire commutator was added between the DSCF probe and device as an electrical swivel to avoid wire twisting during animal’s behavior test. A piezoelectric pad (cage floor) and nearby video camera supported continuous recording of animal activities, which are vital for assessing behavioral outcomes. To further extend the utility of the system, the test cage included a sensor and gas supply for applying inhalation and anesthesia. The complete system was validated against a comparable DCS system in anesthetized mice during inhalation. Full capability was then demonstrated using inhalation on mice in an anesthetized then freely behaving state. Collectively, this augmented system produced a highly impactful and affordable combination for noninvasive and continuous use in conscious freely behaving subjects, such as rodents and human infants.
II. Methods
A. Fabrication of wearable, fiber-free head-stage/probe optimized for continuous CBF monitoring on anesthetized and unrestrained rodents
The typical probe-tissue interface was first decoupled into wearable head-stage (base) and probe elements (Fig. 1). Both items were designed using SOLIDWORKS (Dassault Systems) and fabricated by 3D printing (X-Max, QiDi Tech). The pieces were made from smooth, nontransparent, safe, and biodegradable polylactic acid material, commonly used in medical implants [38]. The material did not introduce abrasions or tissue interactions and appeared resistant to scratching and chewing. The head-stage held a DSCF probe (Fig. 1a-1f) or a hybrid DCS/DSCF probe (Fig. 1g-1j) during measurements. The components were then optimized for CBF monitoring through intact mouse skull.
Fig. 1.
Wearable fiber-free DSCF (a-f) and hybrid DCS/DSCF (g-j) probes with head-stages. (a) The wearable DSCF head-stage and probe. Dimensions when combined were 15 × 8 × 12 mm3. The probe was embedded and stabilized into the head-stage with locking screws. “S” is the DSCF laser diode. “D” is the NanEye camera. (b) Isolated head-stage side view. (c) Isolated DSCF probe side view, with camera and laser diode distanced 6 mm center-to-center. (d) Bottom view of combined head-stage and DSCF probe. (e) Fixation of a DSCF head-stage on intact mouse skull with zoomed view at top right. (f) An anesthetized mouse wearing DSCF head-stage/probe before a measurement. (g) Hybrid DCS/DSCF head-stage and probe. “S” is the optical fiber connected to the DCS laser. “D1” and “D2” are NanEye camera and optical fiber connected to DCS APD detector, respectively. (h) and (i) Side view and bottom view of hybrid head-stage/probe. (j) An anesthetized mouse wearing hybrid head-stage/probe.
The printable nature and material flexibility of the tiny head-stage promoted accommodating variations in the curvature of the skull (Fig. 1b, 1h) for tight attachment. Prong structures (Fig. 1d) at the base further assisted in proper fitting as well as enlarging the contact area between the skull and head-stage, strengthening the effect of any applied adherents (e.g., superglue). Contours were matched to the probe with openings for passing light to/from the tissue. Minimal surgical procedure and noninvasive NIR skull penetration resulted in head-stage installation and measurement procedures having minimal effect on the subject. In the animal studies to follow, head-stage installation was carried out with superglue and dental cement (Fig. 1f, 1j).
The DSCF head-stage/probe (Fig. 1a-1f) established a measurement paradigm consistent with our previous device [35]. During measurements, the probe was firmly attached to the head stage through two pairs of locking screws (Fig. 1a). Seating was provided for a single NIR laser diode (D780–30, Ø5.6 mm, 30 mW, 780 nm, US-Lasers) and an ultra-small, low-power, high-sensitive CMOS camera (NanEye 2D Black & White; dimensions: 1 mm × 1 mm, 250 × 250 pixel array, power: 4 mW, spectral response: 80% at 780 nm, Awaiba) (Fig. 1a). The laser diode provided point-source illumination for deep tissue penetration while diffuse spatial speckle contrast was extracted from NanEye camera images. The NanEye camera (sensor chip dimensions: 0.75 mm × 0.75 mm, F number: 4) had a dynamic range of 58 dB with 3 µm × 3 µm pixel dimensions. A tiny optical lens (minimal focus length: 3 mm) was placed over the camera sensor chip. The working distance between the integrated camera lens and tissue surfaces were ~ 3 mm to ensure appropriate optical focus and generated images of 4 mm × 4 mm. For protection, the camera was wrapped with transparent film and inserted into a transparent plastic tube. The laser diode body remained exposed allowing air circulation to spread generated heat. The source-detector (S-D) distance between the laser diode and camera sensor center was set at 6 mm (Fig. 1c), limited by the available space on the small mouse head. Note that NIR light penetration depth is approximately one half of the S-D distance [35, 39]. Thus, the S-D separation of 6 mm enabled a maximal penetration depth of ~3 mm, which is sufficient to detect the mouse brain [40]. Our previous publications have verified the sufficiency of laser diode coherence, the NanEye camera feasibility, and using the arrangement for measuring CBF response in mice [35, 37]. The S-D selection is within the optimal instrument range (< 10 mm) [41, 42].
The complete DSCF head-stage/probe assembly weighed only 3.6 g and provided steady optoelectronics housing and a stable skull surface interface. To account for the independent nature of the assembly, a commercial wire commutator (12 wires, 2A, Taidacent) was added to act as an electrical swivel between the DSCF probe and device. This was made possible by using only fiber-free optoelectronic parts (laser diode and NanEye camera) with electrical wire connections in the DSCF probe.
Collectively, this versatile arrangement facilitated continuous quantification of in vivo CBF through intact skull on freely moving subjects (Fig. 1e-1f). Recordings from a separate, standard commercial video camera confirmed no impedance on the animal’s natural behavior during experiments (Supplementary Movie 1).
To conduct a concurrent DCS and DSCF measurement in anesthetized mice, we created another wearable probe with head-stage of similar design (Fig. 1g-1j). This hybrid DCS/DSCF probe consisted of a multi-mode source fiber (S, diameter: 200 µm, Thorlabs) coupled to a long-coherence laser (30 mW, 785 nm, CrystaLaser) for both DCS and DSCF, a DCS single-mode detector fiber (D2, diameter: 5.6 µm, Thorlabs), and a DSCF NanEye camera (D1). The laser remained on continuously (from start of measurement) when used. Diffused light emitted from the tissue was simultaneously collected by the NanEye camera for DSCF measurement and the single-mode fiber for DCS measurement. The DCS detector fiber conveyed the light to an APD module (SPCM-AQ4C, PerkinElmer) which then passed photon counts to an autocorrelation board (Correlator.com). The S-D distance between S and both D1/D2 (Fig. 1i) was 6 mm to match the DSCF probe design (Fig. 1c). The hybrid probe was inserted into its head-stage and fixed with a single screw. As this hybrid probe was only used in stationary mice and included fragile optical fibers, there was no wire commutator integration.
The setup for DSCF measurements satisfied the Nyquist sampling criterion of where is the minimum expected speckle size and the NanEye camera pixel size. The minimum expected speckle size can impact spatial sampling and is related to the optics of the system and light source by [35, 36, 43]:
| (1) |
where is the wavelength of light, M is the magnification, and is the F number. In our design, M = 0.25 at the working distance of ~ 3 mm and . With and the pixel size from the NanEye specifications, , we thus determined that 9.5 μm > 6 μm.
B. Development of compact DSCF device control hardware and a real-time monitoring interface
Redesigning our recent DSCF device reduced the laser current driving printed circuit board (PCB) size by almost half without increasing cost or loss of essential functionality [35]. The improved, compact DSCF controller assembly ② (Fig. 2a-2c) was formed by stacking the new custom, miniaturized current driver PCB ③ with a microcontroller (Arduino Uno, SparkFun) ④ and a camera electronic interface board (NanoUSB 2.2, Awaiba) ⑤. A laser diode ⑧ affixed to the wearable DSCF probe ⑥ (Fig. 1a) was electrically connected to and powered by the driving circuit (dimensions: 44 mm × 79 mm) which includes a light power stabilization module. The current driving PCB was connected to the Arduino allowing simple programmatic operation. The NanEye camera ⑦, also within the probe, passed through the small camera control board ⑤ (dimensions: 80 mm × 50 mm). The entire controller assembly (i.e., current driver, Arduino, and camera boards) was housed within an electrically insulated container and powered and directed by laptop ①. DSCF controller and laptop communications went over two USB cables.
Fig. 2.
Optimized DSCF device for rCBF measurements on unrestrained rodents. (a) LabVIEW and C# GUIs ① and DSCF device ②. (b) DSCF device components. Miniaturized controller assembly: compacted current-driving PCB ③, Arduino board ④, and camera electronic board ⑤. Tissue interfacing: wearable DSCF head-stage/probe ⑥, NanEye camera ⑦, and laser diode ⑧. (c) Component interconnectivity schematic. (d) A mouse skull with installed DSCF head-stage/probe. The laser diode and camera were at 6 mm S-D distance for skull penetration. Top right: typical banana-shape paths for photons traveling from the laser diode (S) to the camera (D) through upper head layers and brain. Bottom right: raw speckle images captured by the NanEye camera for rCBF measurement.
The stabilization of the laser output light intensity has been discussed in our previous DSCF study [35, 37]. Briefly, a built-in photodiode in the laser diode package continuously detects the light intensity generated by the laser diode. The microcontroller reads this feedback current and adjusts the driving current of the laser diode to stabilize light intensity output. The microcontroller controls the analog output of the digital-to-analog converter via serial peripheral interface, which enables the fine tuning of the current. The operating current of the laser diode was 60 mA in this application while the laser control module could drive current up to 150 mA.
DSCF operation was split into a LabVIEW™ (National Instruments) control panel and custom C# graphical user interface (GUI). The C# GUI acted as primary control interface with real-time manipulations and displays. A LINX module and application logic in LabVIEW mediated interaction between the PC and Arduino Uno. Synchronization of the camera with the laser control/stabilization module was accomplished via transmission control protocol/internet protocol communication (i.e., C# to LabVIEW) through the laptop loopback address. Raw images (Fig. 2d), light intensity, and calculated relative change in CBF (rCBF) were observable in real time. All raw images were stored to local disk for further off-line data analysis.
C. Experimental setup and protocols
All animal experimental procedures and protocols (#2016-2508) on mice were approved by the University of Kentucky Institutional Animal Care and Use Committee (IACUC). Twelve adult mice (C57BL/6, male, 10–12 weeks) were included in this study (five mice for concurrent DCS/DSCF measurements, seven mice for DSCF freely behaving measurements).
Experimental setup.
For conducting measurements in anesthetized and freely behaving mice, we designed and customized a test cage (Fig. 3). This test cage consisted of a video camera (HF S100, Canon), a piezoelectric pad (Signal Solution, LLC, Lexington, KY), a low-torque commutator, and DSCF control assembly. The DSCF control assembly was connected to either the DSCF probe via flexible electrical wires through the commutator or directly to the hybrid DCS/DSCF probe. A user laptop controlled the device via USB cables. The integration of a piezoelectric pad and a video camera allowed for continuous recording of conscious animal activities during behavioral tests. Pressure variations from body interaction with the platform floor were captured by the piezoelectric pad as voltage output. This included pressure from locomotion and, thorax motion (breathing) when the animal was in a low activity state. The Canon video camera was mounted on a tripod near the test area. A commercial sensor (SprintIR, Meter) was used to continuously monitor the concentration in the cage.
Fig. 3.
Full experimental setup for continuous monitoring of rCBF and functional activity in freely behaving rodents. Visible items include the wearable, fiber-free DSCF probe, condensed DSCF hardware with real-time GUI, behavior monitoring elements (piezoelectric pad and Canon video camera), and anesthesia and apparatus.
The outfitted isoflurane and CO2/O2 delivery system facilitated anesthesia and cerebral hypercapnia stimuli. A multi-channel flow regulator connected to the isoflurane vaporizer, through which and premixed gas could be switched from each other to induce transient hypercapnia. concentrations were maintained by monitoring/adjusting levels based on a sensor, with sampling rate of 1 Hz and detection range up to 20%, connected to the end of the gas delivery tube. This sensor was laptop controlled via USB and collected data using proprietary software. The inhalation is an established method for creating hypercapnia [44–46]. The DSCF system, sensor commercial software, and video camera were synchronized by starting simultaneously.
DSCF and hybrid DCS/DSCF head-stage and probe installation.
For head-stage installation, anesthesia was induced with 5% isoflurane and maintained at 1.25% in at a flow rate of 1 L/min. We gave the isoflurane at the minimal level since it is known to elevate rCBF [11]. The mouse head was secured on a stereotaxic (Kopf) frame with 2 ear bars, a bite bar, and a nose cone. An ointment (Rugby) was applied topically to the eye to protect the cornea. The head hair was removed with a clipper and cleaned with hair cream. The mouse scalp was sterilized with Betadine followed by 70% ethanol and then a piece of scalp was surgically removed to expose the skull with an area of approximately 8 mm × 12 mm. After the cranium was cleaned thoroughly with cotton gauze and air-dried, the fascia overlying the skull was removed, and a few drops of 100% ethanol were applied on the surface which will absorb moisture for the bone and evaporate, to achieve a dry surface. Then a head-stage (Fig. 1b, 1h) was permanently bonded onto the center of the intact skull with superglue (LOCTITE) and dental acrylic cement (Stoelting). After the dental cement solidified, the DSCF (or hybrid) probe was inserted into the head-stage cavity and secured with screw(s) for the rCBF measurement (Fig. 1f, 1j).
Methods to evoke rCBF responses.
Firstly, we use low concentration ( in the current study), a well-known vascular dilator, to elevate rCBF increase [47]. Secondly, voluntary physiological activities, such as grooming, walking, and an adhesive tape removal test, a well-established protocol for detecting neurological deficit [48, 49], were used to examine induced rCBF variations from those self-orientated activities and coordinated front limb-paw motion. The amplitude of rCBF elevation has been shown to indicate neurological activities and cerebral neurovascular coupling function [48, 50].
Concurrent DCS and DSCF measurements in anesthetized mice.
DCS is an established diffuse NIR technique for acquiring rCBF changes in small animals. To validate the feasibility of the wearable DSCF system for monitoring rCBF variation, we conducted concurrent DSCF and DCS measurements in anesthetized mice during inhalation (n = 5; Fig. 4a and Supplementary Material Fig. 1). After installation of the hybrid DCS/DSCF head-stage and probe (Fig. 1g), rCBF was recorded continuously under isoflurane in 3 sessions: 1. Baseline, 2. inhalation, 3. Recovery. DCS and DSCF recorded 2 minutes for the baseline, and then switched to a premixed gas of with 1.25% isoflurane using a Matheson Mixer Rotameter for 5 minutes to induce rCBF increase. DCS and DSCF were then recorded for 2 minutes as recovery data. For the DCS/DSCF system setup, the exposure time of DSCF was set as 5 ms where previous studies found a corresponding minimum was reached for speckle contrast noises [51]. The sampling rate of both DSCF and DCS was set as 2 Hz.
Fig. 4.
Experimental protocols for continuous monitoring of rCBF in mice with the miniaturized, wearable DSCF system. (a) Concurrent DCS/DSCF measurements of rCBF in anesthetized mice (n = 5). (b) Continuous monitoring of rCBF in freely behaving mice (n = 7).
Continuous monitoring of rCBF in freely behaving mice.
To demonstrate rCBF monitoring in freely behaving mice using the complete DSCF system, we induced hypercapnia during anesthetized and awake periods on mice (n = 7; Fig. 4b). The mouse was put in the test cage (Fig. 3) and anesthetized using 1.25% isoflurane via a mouse nose cone. The animal’s activities in the cage were continuously recorded by the Canon video camera and piezoelectric pad. After 2 minutes collecting baseline DSCF data, the gas mixture with 1–2% isoflurane was then administered using a Matheson Mixer Rotameter for 10 minutes to induce rCBF variations. After which both and isoflurane were stopped and 100% flowed in. After the mouse regained consciousness and started freely moving, its physiological behaviors were observed and evaluated for ~ 20 minutes. The animal was then subjected to the adhesive tape removal test. A piece of adhesive tape was applied to the hairless area of a forepaw and the mouse was allowed up to 5 minutes to remove it by hand rubbing or biting. After removing the tape (or 5 minutes), a gas mixture was applied again to fill the cage for 10 minutes. After stopping the inhalation, DSCF measurement continued for another 5 minutes to record rCBF recovery. For the DSCF system setup, the exposure time of the camera was set as 5 ms and the sampling rate was 2 Hz.
D. Data processing
DSCF data.
rCBF was extracted from the spatially distributed diffuse speckle information, arising from point-source light as opposed to the wide-field illumination in traditional LSCI, in the images acquired by the tiny NanEye camera [35]. After injection into the mouse head, photons travel through the skull and (primarily) brain tissues before re-emission and focusing onto the NanEye camera sensor. Collected coherent light is integrated over milliseconds time order (~ 5 ms) wherein continuous fluctuations of speckle caused by movement of RBCs blur the contrast in the speckle pattern. We thus relate the speckle pattern contrast to cerebral RBC motions as follows.
Calculation of spatial laser speckle contrast is quantified within a window of pixels which identifies the region representing a local speckle pattern. In agreement with our previous DSCF studies as well as conventional LSCI, a window of 7 × 7 pixels was used for the calculation [35, 36, 43]. The ratio of standard deviation to mean over the 49 pixels defines the basic speckle contrast calculation (i.e., ). Preprocessing of the raw images is described in our previous publications, but essentially consists of correcting for shot and dark noise contributions by use of the modified calculation [35, 36, 52]:
| (2) |
where I is light intensity of a single pixel and is the intensity of dark current. The incorporated shot noise follows Poisson statistics:.
A single detector is logically defined by combining a 3 × 3 grid of windows. The 9 values of are averaged to represent a single contrast value for the detector, thereby increasing the signal-to-noise ratio (SNR). This effective detector has an S-D distance attributed to its center pixel location and a detection area of ~0.18 mm2. A blood flow index (BFI) was then extracted through a nonlinear relationship between and BFI under semi-infinite geometry [36, 53]. The maximal sampling rate of NanEye camera is 50 Hz. A sampling rate of 2 Hz was used in this study for time-course averaging to further improve the SNR, which is consistent with our previous studies [37]. The relative change in BFI (rCBF) was calculated by normalizing BFI data to the baseline value before physiological changes.
DCS data.
rCBF was extracted from the temporal intensity autocorrelation function measurements output by the autocorrelator board. This required use of the correlation diffusion equation (CDE). The CDE describes the diffuse propagation of the temporal electric field autocorrelation function through biological tissues such as the brain with NIR light [54, 55]. An analytic solution to the CDE for semi-infinite media was used as an approximation to the small mouse head areas which exhibited little curvature. Furthermore, as we measured light intensity rather than the electric field directly, we also employed the Siegert relation to relate the analytic intensity to electric field autocorrelation functions. By fitting the measured to theoretical intensity autocorrelations, a BFI was extracted which quantitatively reflected blood flow changes [56]. The normalized form, rCBF, was reported for comparisons with those obtained by DSCF methods.
Animal behavior.
Animal activities were determined from analyzing the piezoelectric voltage signals and manual inspection of recorded Canon videos [57–59]. For piezoelectric analysis, root mean square (RMS) of the cage floor pressure signals directly related to activity level were computed over 1-hour intervals.
Motion artifacts.
MATLAB® (MathWorks, MA) analysis of raw NanEye camera images with structural similarity (SSIM) index identified the presence of any motion artifacts [60]. The ssim function in MATLAB calculates the structural similarity index for the targeted image using a reference image. A value closer to 1 indicates higher similarity. For each measurement in the freely behaving mice, the first image captured by the NanEye camera was selected as the reference image.
Statistical analysis.
For the concurrent DCS and DSCF measurements, their correlation over continuous time points was evaluated by the linear mixed regression model on DCS measurement against DSCF measurement with both fixed and random intercepts and slopes. The corresponding R2 was calculated based on the standardized generalized variance method [61] using the “r2beta” function in R package “r2glmm”. The analyses were performed by using R version 4.0.3.
III. Results
A. Validation of cerebral hemodynamics monitoring in anesthetized mice
Fig. 5a shows average dynamic changes in rCBF during the inhalation over five subjects, measured by DSCF (785 nm) and DCS (785 nm) concurrently. Time-course results are presented as the mean values ± standard error (error bars). Thirty data points were averaged and plotted with corresponding error bars to improve readability. Similar trends in rCBF were observed in both DSCF and DCS measurements with DCS rCBF showing a stronger magnitude of response. Elevation of rCBF was seen by DCS (26.3 ± 7.8%) and DSCF (10.5 ± 2.7%) during inhalation. Significant correlations were observed between the two measurements (Slope = 2.20, p = 0.004; R2 = 0.84; Fig. 5b), supporting the reliable acquisition of important rCBF information using our novel system. From post-mortem histological analyses, no brain tissue injuries were observed in all subjects. Moreover, all animals behave normally after experiments.
Fig. 5.
Concurrent DCS/DSCF measurement of hemodynamic changes in anesthetized mice (n = 5) during inhalation. (a) Average time-course changes of rCBF before, during, and after inhalation measured by DCS/DSCF. Error bars represent standard error over the 5 mice. (b) Correlation between DCS and DSCF measurements of rCBF from the 5 mice.
B. Continuous cerebral hemodynamics monitoring in freely behaving mice
Representative results.
Fig. 6 shows representative responses of rCBF and animal activities in one mouse measured by DSCF and piezoelectric film sensors. The rCBF responses (red curve, Fig. 6b) to inhalations during anesthesia and awake periods were examined for the representative mouse as measured by a wearable DSCF head-stage/probe (Fig. 1a). An expected rCBF elevation (9%) was induced by inhalation while anesthetized. Immediately post-anesthesia, a significant rCBF decrease coincided with isoflurane wash off and withdrawal due to their respective hyperemia and hypercapnia effects subsiding. Small surges in rCBF became evident when the animal was behaving freely (grooming, walking, and tape removal; Fig. 6c and 6d; Supplementary Material Video 1). A larger rCBF increase (35%) was found from inhalation during free movement (gradually filling sealed cage with . A slow decrease is observable during withdrawal (100%) at the recovery stage. Near the procedure end, exposure to 5% isoflurane, for replacing the DSCF probe with a protective dummy block, showed a corresponding rCBF jump illustrating hyperemia response. These rCBF changes were consistent with clinical observations at the recovery phase after anesthesia and match the impacts of isoflurane, hypercapnia , and activity-induced cortical excitations [62, 63]. Animal motion captured by the piezoelectric pad (black curve, Fig. 6a) and activity levels (red curve, Fig. 6a) extracted from RMS of piezoelectric voltage output responded noticeably with the mouse waking and moving freely [57–59]. Raw NanEye images (bottom left of Fig. 6d) presented comparable patterns of the skull with SSIM index of 0.93, suggesting no motion artifacts affected DSCF measurements.
Fig. 6.
Continuous rCBF and behavior monitoring in a representative mouse under anesthesia and awake conditions. (a) Animal activity level (red curve) from the RMS of piezoelectric cage floor signals (black curve). (b) rCBF (red curve) and concentration (black curve) were continuously monitored by a wearable DSCF head-stage/probe (S-D distance: 6 mm) and sensor on the cage floor, respectively. “% ISO” denotes the percentage and periods of isoflurane application. (c) Corresponding rCBF responses (red curve) during grooming, occasional climbing, walking, and tape removal with representative trends using a moving average (blue curve). (d) Raw NanEye camera images and external Canon video recordings in the grooming, climbing, walking, and tape removal periods.
Group results.
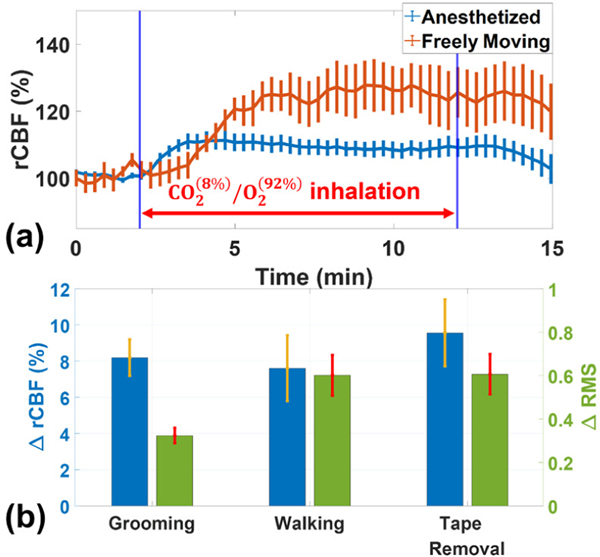
Fig. 7a shows the average rCBF variations before, during, and after inhalation over 7 mice in both anesthetized and freely moving status. Time-course results are presented as the mean values ± standard error (error bars). Thirty data points were averaged and plotted with corresponding error bars to improve readability. Normalization of rCBF was to the stable signal period prior to inhalation, separately for anesthesia and awake responses, due to potential baseline shifts. Increases of 11.3 ± 2.4% in rCBF were found in anesthetized mice while increases of 27.8 ± 6.8% in rCBF were seen in freely behaving mice during inhalation. From post-mortem histological analyses, no brain tissue injuries were observed in all subjects. All animals behave normally after measurements.
Fig. 7.
Continuous rCBF and behavior monitoring in a representative mouse under anesthesia and awake conditions. (a) Time-course rCBF variations during inhalation in anesthetized mice and freely moving mice. Error bars represent standard error across the 7 mice. (b) Variations of rCBF (ΔrCBF) and animal activities (ΔRMS) during freely behaving periods (grooming, walking, and tape removal). The values of ΔrCBF and ΔRMS are assigned “0” at the baselines before the freely behaving periods.
Additional variations of rCBF (ΔrCBF) and animal activities (ΔRMS) during freely behaving periods (grooming, walking, and tape removal) were also observed (Fig. 7b). The values of ΔrCBF and ΔRMS were assigned “0” at the baselines before each of the freely behaving periods. In grooming periods, group ΔrCBF increased 8.2 ± 1.0% while ΔRMS increased 0.32 ± 0.03; in walking periods, ΔrCBF increased 7.6 ± 1.8% while ΔRMS increased 0.60 ± 0.09; in tape removal periods, ΔrCBF increased 9.6 ± 1.8% while ΔRMS increased 0.61 ± 0.09. The results indicated that rCBF increased with the increase of RMS. Tape removal induced the highest rCBF response, followed by walking and grooming. There was no significant correlation between the ΔrCBF and ΔRMS. The mean (with standard error) of SSIM indices across the seven mice was 0.93 ± 0.01, a value closer to 1, indicating high similarity of NanEye images (i.e., no motion artifacts).
Collectively, these data demonstrated the ability of the integrated DSCF system to detect cerebral hemodynamics and animal activities in both anesthetized and freely behaving mice. No overt adverse events occurred during the experiments.
IV. Discussion
Abnormal rCBF stems from varied disease/injury originations and transient circumstances. The monitoring thereof can offer deep insights into treatments and preventions, ideally in a transparent fashion to the subject. As small animals remain the primary current target of many research designs it is also important to establish low overhead in translating findings. In the spirit of these considerations, a succession of system improvements was presented, collectively tested, and found to exhibit a highly useful combination of features towards these ends.
Versatile, fiber-free head-stage/probe optimizes rCBF measurement on unrestrained mouse head.
A custom lightweight, 3D-printed head-stage and probe provides a solid starting point for rCBF measurements on freely moving subjects such as mice. The adaptable, low-cost 3D printing technology enables forming head-stage/probe combinations tailored to different purposes (e.g., DSCF and hybrid DCS/DSCF). The specific designs for the heads of mice each required only a single contour measurement for matching the head surface in all cases herein. Preparation in installing these head-stage/probes on mice involves minimal surgical procedures and animal manipulation. Unlike the most used craniotomy window for probe installation [64–66], the DSCF head-stage was fixed on the intact skull using superglue and dental cement. Other materials and head surface and/or probe geometries are readily possible. The decoupled head-stage from a removable probe also encourages interchanging options (i.e., probes with varied SD arrangements) in future studies. The head-stage can hold a dummy block as a placeholder for the probe when measurements are not in progress. This allows the head-stage to remain over long periods without skull exposure, while ensuring animals’ natural behavior and static measurement location. Testing on a few mice with the DSCF head-stage attached to exposed skull, in the same manner as the current study, have been used up to 14 months after initial measurement, providing a great potential to conduct animal behavioral tests in long term.
The DSCF probe comprised of only optoelectronic components transmits electric signals through a wire commutator back to locally mounted control hardware. This fiber-free setup enables free movement of the studied subject. For the DSCF probe used on mice, the influence of motion artifacts is determined to be negligible as there are no obvious shifts in raw NanEye image details by visual inspection and structural similarity analysis. The high SSIM indices (0.93 ± 0.01) for measurements during free movement indicate that the images within each measurement are highly similar to the reference image (i.e., negligible motion artifacts) [67]. Fig. 6 also exhibits no notable relation between RMS (mouse movement) and rCBF trends.
Validation of novel compact DSCF.
Hypercapnia challenge is a widely accepted method in clinics to examine cardio and cerebrovascular responsive functions or induce a panic attack to examine the subject’s anxiety level [44, 45]. In this study, we have demonstrated the ability of DSCF for continuous monitoring of rCBF responses to hypercapnia in anesthetized (Fig. 5) and freely behaving mice (Fig. 6). Concurrent DSCF and DCS measurements on anesthetized mice show an evident response to hypercapnia induced by inhalation (Fig. 5a). Correlation between the two systems is found to be significant (Slope = 2.20, p = 0.004, R2 = 0.84, Fig. 5b). This maintained correlation between DSCF and DCS, after miniaturization and free movement optimization of the DSCF platform, shows this study is a positive continuation of our previous efforts [35]. From these results, it is concluded that the novel DSCF system gives similar results to an equivalent DCS system.
Discrepancies in rCBF magnitudes and apparent trends between DSCF and DCS during validation (Fig. 5) are attributed to several details. The DCS optoelectronics includes a single optical fiber connected to a high temporal sampling, super sensitive, and expensive APD (thousands of dollars). The APD feeds into the autocorrelator board to generate a full temporal intensity decorrelation curve for extracting flow information. By contrast, the cost-effective 2D NanEye camera spatial sampling sensor ($150) selects a single exposure to maximize spatial speckle contrast sensitivity and minimize noise [37, 51]. The spatial averaging by DSCF increases the SNR but reduces the detection sensitivity and might cause underestimation in rCBF. The high sensitivity of DCS using a fiber-optic probe with a small detection area (diameter of the single-mode fiber = 5.6 μm) makes it susceptible to motion artifacts. Additionally, DCS is also more sensitive to the location of its detector (diameter: 5.6 μm), which is close to the reported average diameter of mouse microvessels (3.5 – 4.0 μm) [68]. Therefore, variations may be introduced in the results due to influences of underlying location-specific physiology.
Consequently, the differences between the two systems results in a lower peak response by DSCF than as measured by DCS (see Fig. 5a). This effect also subtly occurs in the gradual decrease that follows, resulting in a less pronounced decreasing trend as compared with DCS. Combining data from the 5 mice accentuates the consistency across the group, but also works against visual trend inspection. In any case, DSCF by design compromises hitting this DCS benchmark to graduate other important features. Use of multiple exposures or other techniques can improve DSCF accuracy but increase system and operational complexity [69]. Nevertheless, the sacrifice to rCBF recovery sensitivity by DSCF as compared to DCS are offset in many scenarios by reducing cost and motion artifact as well as improving spatial sampling, miniaturization, wearability, and portability.
Integrated DSCF system enables analysis of both rCBF and behavior in moving subjects.
Investigation reveals DSCF sensitivity to hypercapnia clearly and consistently during anesthetized and awake periods (Fig. 7). The group average rCBF changes in conscious and anesthetized rodents during inhalations are consistent with previous findings using LDF, LSCI, positron emission tomography, and magnetic resonance imaging [14, 70, 71]. Variations in rCBF coincide with the simultaneously measured RMS and video indicators of animal behavior. The activities exhibited (Fig. 6c and Fig. 7b) are representative of those typically encountered in conscious mice. Furthermore, rCBF elevations induced by the animal behaviors agree with previously published research on freely moving rodents [72, 73]. The ΔrCBF and ΔRMS variations were not statistically correlated. Different sampling rates between the two measurements (2 Hz for DSCF and 120 Hz for RMS) may affect the correlation. Moreover, there might be a delay between CBF responses and physical activities (i.e., grooming, walking, and tape removal). More subjects with further technology improvements are needed to confirm whether a significant correlation exists between the ΔrCBF and ΔRMS.
These results reflect the system’s capability to monitor rCBF without regard to mouse consciousness and motion. Acquiring these awake state responses fills a previously lacking capability and is critical for translatable results and clinical use. The path has been cleared to exploring correlations between behavior and rCBF response which are crucial to understanding many pathological conditions.
Study limitations.
The head-stage/probe, when installed directly on the mouse scalp, may cause the scalp shifting with respect to the skull, thus resulting in motion artifacts during behavior tests. However, given our previous studies with different scales of DSCF/DSCFO probes affixed on human forearms and human infant heads by elastic bandages, DSCF/DSCFO probes can be worn by a controlled subject without the need of any invasive procedure [37].
For the current study, behavior and hypercapnia effects on rCBF are not completely decoupled (Fig. 6). Differences in -induced rCBF response magnitudes between anesthetized (11.3 ± 2.4%) and free movement (27.8 ± 6.8%) states are attributable to several influences. During anesthesia, the increase in rCBF induced by inhalation is less than that during free movement because Isoflurane raises the baseline of rCBF (Fig. 6b). While conscious, free movements also raise rCBF during but the events occur at random times and durations. Thus, direct comparison on the response magnitude of each state is not straightforward. Nevertheless, comparisons of rCBF to pre-baseline values in both cases shows that still succeeds in evoking a sufficient increase for our analysis. Both responses may also depend on subject medicinal response, activity exertion, sleep and hunger status, among others. Furthermore, introduction of the gas mixture is carried out through connected nose cone during anesthesia or by gradual cage filling for conscious mice. The latter method may result in remnants of the gas mixture within the cage and a potentially protracted hypercapnia effect. Resolving these issues could include techniques such as image analysis of recorded videos (e.g., Canon camera videos) to identify behaviors and group corresponding rCBF data, more complete control of the animal conditions, and alternative introduction mechanisms.
Future perspectives.
There are several avenues for sublimation of the device and application extension. More subjects may be recruited to instill greater confidence concerning DSCF sensitivity and constraints. Only relative changes in CBF (i.e., rCBF) are reported in the current study. Absolute measurements of CBF are important for accurately acquiring blood flow information by differentiating the contribution of static scatterers (i.e., skull) from that of moving scatterers (i.e., RBCs) to the speckle patterns and longitudinal monitoring of cerebral hemodynamics at discrete periods [74]. Use of multiple exposures enables extraction of an absolute BFI [69], which can be calibrated against other gold standards to obtain an absolute CBF.
The working distance and spatial resolution are expandable, without significant modification, to extents suitable for larger subjects. Additional laser diodes would increase S-D pairs for partial volume (skull/surface layer) compensation [56]. Increasing working distance, region of interest, and S-D pairs also facilitates 2D rCBF mapping (using 2D NanEye), differential analysis, and 3D tomography.
We have already extended the DSCF to DSCFO for both blood flow and oxygenation measurements in large tissue volumes including human forearms [35, 37]. Very recently, we have successfully completed preliminary testing with a wearable DSCFO sensor to monitor both CBF and cerebral oxygenation in newborn piglets and preterm neonates. The head-stage attachment mechanism employed custom 3D-printed head straps instead of superglue and dental cement. We are also implementing battery power and wireless connectivity features. These results will be reported in future papers. In the future, we will further miniaturize DSCFO probes for use in small rodent heads.
V. Conclusions
We developed a complete and fully integrated system to combine rCBF measurement, and supplies, and behavior recording for use on conscious, freely behaving rodents. The system can conduct tests involving anesthesia, inhalation, and natural movements while providing real-time feedback. We foresee utility in many neurological studies with this integrated system for continuous noninvasive monitoring of animals in research laboratories and newborn infants in clinics.
Supplementary Material
Acknowledgments
This work was partially supported by National Institutes of Health (NIH) #R01 EB028792 (GY), #R01 HD101508 (GY), #R21 HD091118 (GY), #R41 NS122722 (GY), and #R56 NS117587 (GY); American Heart Association (AHA) #16GRNT30820006 (GY) and #14SDG20480186 (GY); National Science Foundation (NSF) #1539068 (GY); and University of Kentucky (UK) Halcomb Fellowship in Medicine and Engineering (XL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, AHA, NSF or UK.
Contributor Information
Xuhui Liu, Department of Biomedical Engineering, University of Kentucky, Lexington, KY, USA.
Daniel A. Irwin, Department of Biomedical Engineering, University of Kentucky, Lexington, KY, USA
Chong Huang, Department of Biomedical Engineering, University of Kentucky, Lexington, KY, USA.
Yutong Gu, Department of Biomedical Engineering, University of Kentucky, Lexington, KY, USA.
Li Chen, Biostatistics and Bioinformatics Shared Resource Facility, Markey Cancer Center, University of Kentucky, Lexington, KY, USA..
Kevin D. Donohue, Department of Electrical and Computer Engineering, University of Kentucky, Lexington, KY, USA.
Lei Chen, Department of Physiology, Spinal Cord and Brain Injury Research Center, University of Kentucky, Lexington, KY, USA.
Guoqiang Yu, Department of Biomedical Engineering, University of Kentucky, Lexington, KY, USA.
REFERENCES
- [1].Brew N, Walker D, and Wong FY, “Cerebral vascular regulation and brain injury in preterm infants,” American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, vol. 306, no. 11, pp. R773–R786, 2014. [DOI] [PubMed] [Google Scholar]
- [2].Chai CL, Tu Y-K, and Huang S-J, “Can cerebral hypoperfusion after sympathetic storm be used to diagnose brain death? A retrospective survey in traumatic brain injury patients,” Journal of Trauma and Acute Care Surgery, vol. 64, no. 3, pp. 688–697, 2008. [DOI] [PubMed] [Google Scholar]
- [3].Attwell D et al. , “Glial and neuronal control of brain blood flow,” Nature, vol. 468, no. 7321, pp. 232–243, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reynolds RA et al. , “Hyperoxemia and cerebral vasospasm in aneurysmal subarachnoid hemorrhage,” Neurocritical Care, vol. 35, no. 1, pp. 30–38, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rhee CJ et al. , “Neonatal cerebrovascular autoregulation,” Pediatric research, vol. 84, no. 5, pp. 602–610, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mesquita RC et al. , “Direct measurement of tissue blood flow and metabolism with diffuse optics,” Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, vol. 369, no. 1955, pp. 4390–4406, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Durduran T et al. , “Optical measurement of cerebral hemodynamics and oxygen metabolism in neonates with congenital heart defects,” Journal of biomedical optics, vol. 15, no. 3, p. 037004, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ellenbroek B and Youn J, “Rodent models in neuroscience research: is it a rat race?,” Dis Model Mech, vol. 9, no. 10, pp. 1079–1087, Oct 1 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Iida H et al. , “Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation,” Anesthesiology, vol. 89, no. 4, pp. 954–60, Oct 1998. [DOI] [PubMed] [Google Scholar]
- [10].Drummond JC et al. , “A comparison of the direct cerebral vasodilating potencies of halothane and isoflurane in the New Zealand white rabbit,” (in eng), Anesthesiology, vol. 65, no. 5, pp. 462–7, Nov 1986. [DOI] [PubMed] [Google Scholar]
- [11].Matta Basil F. et al. , “Direct Cerebral Vasodilatory Effects of Sevoflurane and Isoflurane “ Anesthesiology, vol. 91, no. 3, pp. 677-677, 1999. [DOI] [PubMed] [Google Scholar]
- [12].Eger EI, “IsofluraneA Review,” Anesthesiology: The Journal of the American Society of Anesthesiologists, vol. 55, no. 5, pp. 559–576, 1981. [DOI] [PubMed] [Google Scholar]
- [13].McPherson RW et al. , “Cerebral blood flow in primates is increased by isoflurane over time and is decreased by nitric oxide synthase inhibition,” Anesthesiology: The Journal of the American Society of Anesthesiologists, vol. 80, no. 6, pp. 1320–1327, 1994. [DOI] [PubMed] [Google Scholar]
- [14].Sicard K et al. , “Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies,” Journal of Cerebral Blood Flow & Metabolism, vol. 23, no. 4, pp. 472–481, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fantini S et al. , “Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods,” (in eng), Neurophotonics, vol. 3, no. 3, p. 031411, Jul 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Holschneider D et al. , “Functional brain mapping in freely moving rats during treadmill walking,” Journal of Cerebral Blood Flow & Metabolism, vol. 23, no. 8, pp. 925–932, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cao R et al. , “Functional and oxygen-metabolic photoacoustic microscopy of the awake mouse brain,” Neuroimage, vol. 150, pp. 77–87, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zong W et al. , “Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice,” Nature methods, vol. 14, no. 7, pp. 713–719, 2017. [DOI] [PubMed] [Google Scholar]
- [19].Senarathna J et al. , “A miniature multi-contrast microscope for functional imaging in freely behaving animals,” Nature communications, vol. 10, no. 1, pp. 1–13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miao P et al. , “Micro-device combining electrophysiology and optical imaging for functional brain monitoring in freely moving animals,” in Optogenetics and Optical Manipulation, 2017, vol. 10052, pp. 19–25: SPIE. [Google Scholar]
- [21].Helmchen F et al. , “A Miniature Head-Mounted Two-Photon Microscope: High-Resolution Brain Imaging in Freely Moving Animals,” Neuron, vol. 31, no. 6, pp. 903–912, 2001/09/27/2001. [DOI] [PubMed] [Google Scholar]
- [22].Ghosh KK et al. , “Miniaturized integration of a fluorescence microscope,” Nature methods, vol. 8, no. 10, p. 871, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hayashi Y et al. , “Spatio-temporal control of neural activity in vivo using fluorescence microendoscopy,” Eur J Neurosci, vol. 36, no. 6, pp. 2722–32, Sep 2012. [DOI] [PubMed] [Google Scholar]
- [24].Kobayashi T et al. , ““Optical communication with brain cells by means of an implanted duplex micro-device with optogenetics and Ca(2+) fluoroimaging”,” Sci Rep, vol. 6, p. 21247, Feb 16 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Limnuson K et al. , “A User-Configurable Headstage for Multimodality Neuromonitoring in Freely Moving Rats,” Front Neurosci, vol. 10, p. 382, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dombeck DA et al. , “Imaging large-scale neural activity with cellular resolution in awake, mobile mice,” Neuron, vol. 56, no. 1, pp. 43–57, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goldey GJ et al. , “Removable cranial windows for long-term imaging in awake mice,” Nature protocols, vol. 9, no. 11, p. 2515, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xu H-T et al. , “Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex,” Nature neuroscience, vol. 10, no. 5, pp. 549–551, 2007. [DOI] [PubMed] [Google Scholar]
- [29].Guo ZV et al. , “Procedures for behavioral experiments in head-fixed mice,” PloS one, vol. 9, no. 2, p. e88678, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dombeck DA, Graziano MS, and Tank DW, “Functional clustering of neurons in motor cortex determined by cellular resolution imaging in awake behaving mice,” Journal of Neuroscience, vol. 29, no. 44, pp. 13751–13760, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shang Y, Gurley K, and Yu G, “Diffuse Correlation Spectroscopy (DCS) for Assessment of Tissue Blood Flow in Skeletal Muscle: Recent Progress,” Anat Physiol, vol. 3, no. 2, p. 128, Dec 1 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shang Y “Portable optical tissue flow oximeter based on diffuse correlation spectroscopy,” Opt Lett, vol. 34, no. 22, pp. 3556–8, Nov 15 2009. [DOI] [PubMed] [Google Scholar]
- [33].Brothers RO “Cerebrovascular reactivity measured in awake mice using diffuse correlation spectroscopy,” Neurophotonics, vol. 8, no. 1, p. 015007, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yu G, Huang C, and Hastings JT, “Compact low-cost fiberless diffuse speckle contrast flow-oximeter,” ed: Google Patents, 2020. [Google Scholar]
- [35].Huang C et al. , “A Wearable Fiberless Optical Sensor for Continuous Monitoring of Cerebral Blood Flow in Mice,” IEEE J Sel Top Quantum Electron, vol. 25, no. 1, Jan-Feb 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Huang C et al. , “Low-cost compact diffuse speckle contrast flowmeter using small laser diode and bare charge-coupled-device,” Journal of Biomedical Optics, vol. 21, no. 8, pp. 080501–080501, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu X et al. , “Simultaneous measurements of tissue blood flow and oxygenation using a wearable fiber-free optical sensor,” Journal of Biomedical Optics, vol. 26, no. 1, p. 012705, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Da Silva D et al. , “Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems,” vol. 340, pp. 9–14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huang C et al. , “Alignment of sources and detectors on breast surface for noncontact diffuse correlation tomography of breast tumors,” Applied Optics, vol. 54, no. 29, pp. 8808–8816, 2015/10/10 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shang Y et al. , “Diffuse optical monitoring of repeated cerebral ischemia in mice,” vol. 19, no. 21, pp. 20301–20315, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tang Y and Yao J, “3D Monte Carlo simulation of light distribution in mouse brain in quantitative photoacoustic computed tomography,” Quant Imaging Med Surg, vol. 11, no. 3, pp. 1046–1059, Mar 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Salehpour F et al. , “Penetration Profiles of Visible and Near-Infrared Lasers and Light-Emitting Diode Light Through the Head Tissues in Animal and Human Species: A Review of Literature,” Photobiomodul Photomed Laser Surg, vol. 37, no. 10, pp. 581–595, Oct 2019. [DOI] [PubMed] [Google Scholar]
- [43].Boas DA and Dunn AK, “Laser speckle contrast imaging in biomedical optics,” J Biomed Opt, vol. 15, no. 1, p. 011109, Jan-Feb 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Martino PF et al. , “Cardiorespiratory Response to Moderate Hypercapnia in Female College Students Expressing Behaviorally Inhibited Temperament,” (in eng), Front Neurosci, vol. 14, p. 588813, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu P, De Vis JB, and Lu H, “Cerebrovascular reactivity (CVR) MRI with challenge: A technical review,” (in eng), Neuroimage, vol. 187, pp. 104–115, Feb 15 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Brian Johnny E., “Carbon Dioxide and the Cerebral Circulation “ Anesthesiology, vol. 88, no. 5, pp. 1365–1386, 1998. [DOI] [PubMed] [Google Scholar]
- [47].Jones M, Berwick J, and Mayhew J, “Changes in blood flow, oxygenation, and volume following extended stimulation of rodent barrel cortex,” Neuroimage, vol. 15, no. 3, pp. 474–487, 2002. [DOI] [PubMed] [Google Scholar]
- [48].Bouet V et al. , “The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice,” Nature protocols, vol. 4, no. 10, pp. 1560–1564, 2009. [DOI] [PubMed] [Google Scholar]
- [49].Komotar RJ et al. , “Neurologic assessment of somatosensory dysfunction following an experimental rodent model of cerebral ischemia,” Nature protocols, vol. 2, no. 10, pp. 2345–2347, 2007. [DOI] [PubMed] [Google Scholar]
- [50].Bouët V et al. , “Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse,” Experimental neurology, vol. 203, no. 2, pp. 555–567, 2007. [DOI] [PubMed] [Google Scholar]
- [51].Yuan S et al. , “Determination of optimal exposure time for imaging of blood flow changes with laser speckle contrast imaging,” Applied optics, vol. 44, no. 10, pp. 1823–1830, 2005. [DOI] [PubMed] [Google Scholar]
- [52].Huang C et al. , “Speckle contrast diffuse correlation tomography of complex turbid medium flow,” Med Phys, vol. 42, no. 7, pp. 4000–6, Jul 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Huang C et al. , “Noncontact 3-dimensional Speckle Contrast Diffuse Correlation Tomography of Tissue Blood Flow Distribution,” IEEE Transactions on Medical Imaging, vol. 36, no. 10, pp. 2068–2076, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Boas DA and Yodh AG, “Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation,” J Opt Soc Am A Opt Image Sci Vis, vol. 14, no. 1, pp. 192–215, JAN 1997. [Google Scholar]
- [55].Shang Y, Li T, and Yu G, “Clinical applications of near-infrared diffuse correlation spectroscopy and tomography for tissue blood flow monitoring and imaging,” Physiological measurement, vol. 38, no. 4, p. R1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Huang C et al. , “Noninvasive noncontact speckle contrast diffuse correlation tomography of cerebral blood flow in rats,” Neuroimage, vol. 198, pp. 160–169, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Donohue KD et al. , “Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice,” Biomedical engineering online, vol. 7, no. 1, p. 14, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mang GM et al. , “Evaluation of a piezoelectric system as an alternative to electroencephalogram/electromyogram recordings in mouse sleep studies,” Sleep, vol. 37, no. 8, pp. 1383–92, Aug 1 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Harrison JL et al. , “Resolvins AT-D1 and E1 differentially impact functional outcome, post-traumatic sleep, and microglial activation following diffuse brain injury in the mouse,” Brain Behav Immun, vol. 47, pp. 131–40, Jul 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang Z et al. , “Image quality assessment: from error visibility to structural similarity,” vol. 13, no. 4, pp. 600–612, 2004. [DOI] [PubMed] [Google Scholar]
- [61].Jaeger BC et al. , “An R 2 statistic for fixed effects in the generalized linear mixed model,” Journal of Applied Statistics, vol. 44, no. 6, pp. 1086–1105, 2017. [Google Scholar]
- [62].Henriksen L, “Brain luxury perfusion during cardiopulmonary bypass in humans. A study of the cerebral blood flow response to changes in , , and blood pressure,” Journal of Cerebral Blood Flow & Metabolism, vol. 6, no. 3, pp. 366–378, 1986. [DOI] [PubMed] [Google Scholar]
- [63].Takuwa H et al. , “Hemodynamic changes during somatosensory stimulation in awake and isoflurane-anesthetized mice measured by laser-Doppler flowmetry,” Brain research, vol. 1472, pp. 107–112, 2012. [DOI] [PubMed] [Google Scholar]
- [64].Liu R et al. , “Extendable, miniaturized multi-modal optical imaging system: cortical hemodynamic observation in freely moving animals,” Opt Express, vol. 21, no. 2, pp. 1911–24, Jan 28 2013. [DOI] [PubMed] [Google Scholar]
- [65].Guo H et al. , “Detachable head-mounted photoacoustic microscope in freely moving mice,” Opt Lett, vol. 46, no. 24, pp. 6055–6058, Dec 15 2021. [DOI] [PubMed] [Google Scholar]
- [66].Rynes ML et al. , “Miniaturized head-mounted microscope for whole-cortex mesoscale imaging in freely behaving mice,” Nat Methods, vol. 18, no. 4, pp. 417–425, Apr 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Istepanian R et al. , “Subjective and objective quality assessment in wireless teleultrasonography imaging,” in 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2008, pp. 5346–5349: IEEE. [DOI] [PubMed] [Google Scholar]
- [68].Tsai PS et al. , “Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels,” J Neurosci, vol. 29, no. 46, pp. 14553–70, Nov 18 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Liu J et al. , “Establishing the quantitative relationship between diffuse speckle contrast analysis signals with absolute blood flow,” vol. 9, no. 10, pp. 4792–4806, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Takuwa H et al. , “Development of a simultaneous optical/PET imaging system for awake mice,” Physics in Medicine & Biology, vol. 61, no. 17, p. 6430, 2016. [DOI] [PubMed] [Google Scholar]
- [71].Nishino A et al. , “Vasodilation mechanism of cerebral microvessels induced by neural activation under high baseline cerebral blood flow level results from hypercapnia in awake mice,” Microcirculation, vol. 22, no. 8, pp. 744–752, 2015. [DOI] [PubMed] [Google Scholar]
- [72].Tiran E et al. , “Transcranial functional ultrasound imaging in freely moving awake mice and anesthetized young rats without contrast agent,” Ultrasound in medicine & biology, vol. 43, no. 8, pp. 1679–1689, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Miao P et al. , “Chronic wide-field imaging of brain hemodynamics in behaving animals,” Biomedical optics express, vol. 8, no. 1, pp. 436–445, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chammas M and Pain F, “Synthetic exposure with a CMOS camera for multiple exposure speckle imaging of blood flow,” Sci Rep, vol. 12, no. 1, p. 4708, Mar 18 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.