Abstract
The Aeromonas veronii bv. sobria metallo-β-lactamase gene, imiS, was cloned. The imiS open reading frame extends for 762 bp and encodes a protein of 254 amino acids with a secreted modified protein of 227 amino acids and a predicted pI of 8.1. To confirm the predicted sequence, purified ImiS was digested and the resulting peptides were identified, yielding an identical sequence for ImiS, with 98% identity to CphA. Both possessed the putative active-site sequence Asn-Tyr-His-Thr-Asp at positions 88 to 92, which is unique to the Aeromonas metallo-β-lactamases.
The functional group 3 β-lactamases (2) have assumed increasing clinical significance due to their ability to hydrolyze carbapenems such as imipenem and meropenem, which, apart from a few exceptions, are poorly hydrolyzed by serine β-lactamases. These enzymes are also resistant to all commercially available serine β-lactamase inhibitors. Plasmid-mediated metallo-β-lactamases have now been identified in key pathogens such as Klebsiella pneumoniae, Serratia marcescens, Pseudomonas aeruginosa, and Bacteroides fragilis (1, 10, 25, 26). Furthermore, the metallo-β-lactamase from S. marcescens, IMP1, has been mobilized on an integron-like gene, intI3, and has spread to P. putida, K. pneumoniae, and Alcaligenes spp. (19). These enzymes have also been identified and characterized from emerging pathogens such as Aeromonas spp., Stenotrophomonas maltophilia, and Burkholderia cepacia and have been the subject of recent reviews (14, 15).
While all metallo-β-lactamases possess the ability to hydrolyze carbapenems, their abilities to hydrolyze other β-lactams, such as penicillins and cephalosporins, vary from enzyme to enzyme. Whereas most metallo-β-lactamases have a broad spectrum of activity, enzymes isolated from Aeromonas spp. can readily hydrolyze only carbapenems (17). The sequence of the gene encoding the Aeromonas hydrophila enzyme CphA was determined by Massidda et al. (13) and shows significant difference from those of the metallo-β-lactamases of Bacillus cereus (9) and B. fragilis (20), including conserved amino acids thought to be involved with zinc ion binding (3, 4). Recently, a second A. hydrophila metallo-β-lactamase gene, cphA2, has been sequenced which, not surprisingly, shows strong homology to cphA (15). Hybridization studies using the cphA gene probe illustrated that homologs of the A. hydrophila gene are found in A. veronii, A. caviae, A. jandaei, and other strains of A. hydrophila, illustrating the widespread occurrence of this gene in Aeromonas spp. (16).
A metallo-β-lactamase from A. veronii bv. sobria, ImiS, has been purified and characterized and shown to have a substrate profile very similar to those of both the CphA and A. jandei AsbM1 β-lactamases (5, 24, 27). In addition, the first 40 N-terminal amino acids of ImiS were found to be identical to those of the CphA enzyme. Interestingly, the A. jandei AsbM1 enzyme also has had its N-terminal sequence determined and shows only 26% similarity to CphA over the first 27 amino acids (27). The purpose of this work was to determine the sequences of both the A. veronii bv. sobria metallo-β-lactamase gene, imiS, and its purified product and to compare these specifically with those of the homologous system in A. hydrophila.
A. veronii bv. sobria 163a is a clinical isolate obtained from Hammersmith Hospital, London, United Kingdom. Escherichia coli DH5α (7) was used as the host strain for transformation of the A. veronii bv. sobria gene banks. pSU18 was used as the cloning vector and has been previously described (12). The A. hydrophila metallo-β-lactamase gene, cphA, carried on plasmid pAS20R, was a gift from G. M. Rossolini and has been previously described (13). All of the media and compounds used have been previously described (23).
Induction of bacterial strains with cefoxitin and imipenem and β-lactamase assays were carried out as previously described (22). Imipenem hydrolysis was assayed at 298 nm. One unit of β-lactamase activity is defined as the amount of enzyme required to hydrolyze 1 nmol of substrate/min/mg of protein in the linear phase of the reaction at 37°C.
For the preparation of DNA probes, large quantities of plasmid pSA20R were prepared and cut with EcoRI to release the cloned A. hydrophila AE036 insert. The DNA fragments were separated by gel electrophoresis, and the fragment carrying cphA (2.0 kb) was recovered, purified by phenol-chloroform extraction, and precipitated as previously described (11). The E. coli recombinants to be blotted were spot inoculated onto nutrient agar, and the plate was incubated at 30°C for 4 h. E. coli(pAS20R) and E. coli(pSU18) were used as positive and negative controls, respectively. The colony blotting and subsequent DNA hybridization were carried out under conditions previously described (11).
DNA sequence determination was performed with the Du Pont Genesis 2000 automated sequencer. Sequences were determined on both strands with a custom primer walking strategy. Compilation of resulting DNA sequences, database searches, and sequence alignments were performed with the LASERGENE suite of programs (DNASTAR, West Ealing, London, United Kingdom).
ImiS was purified as previously described (24). The enzyme was digested with either trypsin or endoproteinase Glu-C. In both cases, digestion was carried out for 16 h at 37°C at an ImiS-to-restriction enzyme ratio of 200:1 (6). Analysis of endoproteinase Glu-C peptides was undertaken only when sequence information could not be obtained from the trypsin-peptide mixture. Aliquots of the peptide mixture were separated by reversed-phase high-pressure liquid chromatography (Hewlett-Packard 1090) with an Aquapor RP-300 column (200 by 2.1 mm) eluting at a flow rate of 200 μl ml−1 with a 1%/min linear increase in acetonitrile (eluent system, water-trifluoroacetic acid-acetonitrile). Detection was by UV at 214 nm. A 50-μl volume of digested ImiS was injected per run, and the eluent was split such that individual peptides could be collected for off-line Edman N-terminal amino acid sequencing (ABI477A Pulse liquid sequencer) and also for direct peptide molecular weight determination. Individual peptides were confirmed by a combination of Edman sequencing (8) and mass spectroscopy.
The 2.0-kb insert from pAS20R was used as a probe for hybridization with various digests of A. veronii bv. sobria 163a chromosomal DNA to identify a restriction enzyme combination suitable for cloning of the imiS gene. The most suitable was EcoRI and BamHI; the A. hydrophila cphA 2.0-kb insert hybridized to a 5.5-kb 163a chromosomal fragment. Both pSU18 and chromosomal DNAs were cut with EcoRI and BamHI. The cut chromosomal DNA was fractionated on a 0.7% agarose gel, and DNA fragments of 3.5 to 7.5 kb were excised and purified. The selected fraction was ligated into pSU18 and subsequently used to transform E. coli DH5α to chloramphenicol resistance. Colonies containing recombinant molecules were blotted and probed with the cphA probe. Four positive signals were found after screening of approximately 4,000 transformants, and the recombinant plasmids were designated pUB5826 to pUB5829. On digestion with EcoRI and BamHI, each recombinant gave an insert of 5.5 kb plus the cloning vector. The restricted recombinants were run on an agarose gel, blotted, and probed with labelled cphA to confirm the identities of the inserts. All were positive when probed.
When E. coli strains carrying the clones were used to check the MICs of various β-lactams by using standard inocula, they showed no increase in resistance over that of the host, E. coli DH5α. However, when a larger inoculum (108 bacteria) was used, the MIC of imipenem increased 8- to 16-fold to a value similar to that of E. coli(pAS20R) (13). Cell lysates of E. coli(pUB5826 to pUB5829) were analyzed for β-lactamase production, both with and without induction with cefoxitin and imipenem. The β-lactamase activities of all of the cell extracts of strains carrying the imiS clones were very similar to the activity displayed by E. coli(pAS20R). The A. veronii bv. sobria metallo-β-lactamase gene was noninducible when expressed in an E. coli background.
One clone, pUB5826, was chosen for sequencing. The open reading frame containing imiS extends for 762 nucleotides and encodes a preprotein of 254 amino acids (Fig. 1). Upstream of the imiS ORF lie the ribosome-binding site, a putative −10 promoter box (TATTTT), and a putative −35 promoter box (TTCACA). However, the spacing between the two components of the promoter is far from ideal. Immediately downstream of the termination codon are inverted repeat sequences (GCTGCCGCGGCGGCAGC) representing a possible terminator for transcription of the imiS gene. The imiS ORF sequence shows 94% identity to cphA. The codon preference of imiS strongly favors cytidine (C) and guanosine (G) over uridine (U) and adenosine (A) in the third position. Codon preferences were as follows: NNA, 4.3%; NNU, 10.2%; NNC, 31.2%; NNG, 54.3%. These preferences reflect the high G+C content throughout the ORF (62%), similar to the G+C content of other β-lactamase genes analyzed from the same strain of A. veronii bv. sobria (23). Interestingly, the sequence immediately downstream of imiS shares no homology at all with the downstream sequence of cphA, including the inverted repeat sequences that may represent a terminator for the transcription of the imiS gene.
FIG. 1.
Nucleotide sequence of the imiS ORF and flanking regions. Putative sequences involved in transcription control and the putative ribosome-binding site (RBS) are underlined. The deduced amino acid sequence is also shown.
The results of the tryptic and Glu-C digests were confirmed by Edman sequencing. The complete amino acid sequence of ImiS was derived from a combination of peptide sequence data and peptide molecular mass determination. The molecular mass of the protein determined by liquid chromatography-mass spectrometry of incompletely digested protein was 25,247 Da. This value agrees with the theoretical molecular mass of the protein (25,248 Da). The predicted amino acid sequence shows a perfect match with the protein sequence as determined by Edman sequencing and liquid chromatography-electrospray mass spectrophotometry. The protein sequence confirms the site of the peptide cleavage, between two alanines at positions 27 and 28, and is identical to that for CphA (13).
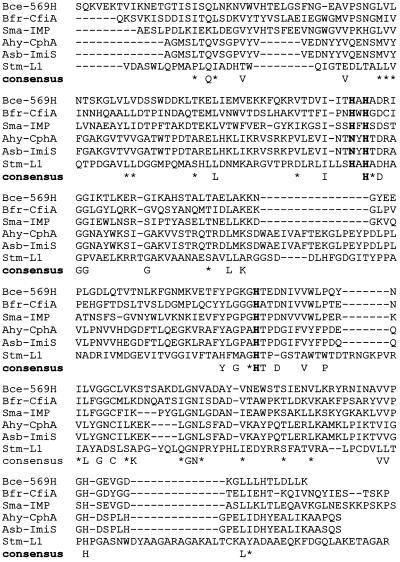
The predicted pI of the secreted gene product is 8.1, similar to the 8.6 reported for CphA (13) but significantly different from that reported for the AsbM1 enzyme (27). The amino acid sequence of ImiS was compared to those of other group 3 β-lactamases. ImiS showed a very high level of identity to CphA (98%), differing at only seven amino acids (88I, 128E, 138L, 143L, 159Q, 201V, and 225S) (Fig. 2). Given that cphA has been shown to hybridize strongly to chromosomal DNAs from several Aeromonas spp. that produce metallo-β-lactamase (16), it is likely that the relevant genes in these strains will show high homologies, a conclusion supported by our findings. Interestingly, the N-terminal protein sequence of the metallo-β-lactamase AsbM1 from A. jandei AER14M shows only 26% similarity to both ImiS and CphA over the first 27 amino acids (27). If this low level of similarity is maintained throughout the AsbM1 protein, it would indicate a major divergence of the metallo-β-lactamases found in Aeromonas spp.
FIG. 2.
Alignment of ImiS with other class B β-lactamases (9, 10, 13, 20, 21). A consensus was noted when five of the six sequences shared the same amino acid residue. An asterisk denotes conserved residues similar in function. Sequences in bold are those thought to be involved in the binding of one of the Zn(II) ions (3, 4). Bce, B. cereus; Bfr, B. fragilis; Sma, S. marcescens; Ahy, A. hydrophila; Asb, A. veronii bv. sobria; Stm, S. maltophilia.
Both Aeromonas metalloenzymes CphA and ImiS and a third enzyme from A. hydrophila, encoded by cphA2, show an Asn-Tyr-His-Thr-Asp sequence at residues 88 to 92, confirming that the Aeromonas metallo-β-lactamases possess a significantly different residue, namely, Asn88, in place of a histidine thought to be involved in zinc coordination and the formation of the active site of these enzymes. In contrast to other metallo-β-lactamases, the kinetic profiles of both the A. hydrophila CphA and A. veronii bv. sobria ImiS metallo-β-lactamases demonstrate that while they can readily hydrolyze carbapenems, they have poor activity against most other β-lactams (18, 24). The metallo-β-lactamases of B. cereus, B. fragilis, and S. maltophilia possess the amino acid motif His-X-His-X-Asp close to the start of the proteins (9, 20, 21). This motif, among other residues, is thought to be responsible for coordinating the two zinc ions found in the active site of the other group 3 enzymes (3, 4). The Aeromonas metallo-β-lactamases CphA, CphA2, and ImiS have the related sequence Asn-Tyr-His-Thr-Asp at the equivalent position. The amino acid residues thought to be needed to bind the second zinc ion in the enzymes from B. cereus and B. fragilis, namely, Asp90, Cys168, and His210 (4), are all conserved in the Aeromonas group 3 enzymes. Thus, the possibility arises that CphA, CphA2, and ImiS may complex just a single zinc ion and that this consequently results in the narrow hydrolytic spectra displayed by these enzymes. The structure of ImiS is currently being determined to resolve, among other points, this particular question.
Nucleotide sequence accession number.
The nucleotide sequence of imiS has been assigned EMBL accession no. Y01415.
Acknowledgments
This work was funded by the Wellcome Trust (grant 038025/2/93/2/1.5).
REFERENCES
- 1.Bandoh K, Watanabe K, Muto Y, Tanaka Y, Kato N, Ueno K. Conjugal transfer of imipenem resistance in Bacterodies fragilis. J Antibiot. 1992;45:542–547. doi: 10.7164/antibiotics.45.542. [DOI] [PubMed] [Google Scholar]
- 2.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structures. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Concha N O, Rasmussen B A, Bush K, Hertzberg O. Crystal structure of the wide spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure. 1996;4:823–836. doi: 10.1016/s0969-2126(96)00089-5. [DOI] [PubMed] [Google Scholar]
- 4.Crowder M W, Wang Z, Franklin S L, Zovinka E P, Benkovic S J. Characterization of the metal-binding sites of the β-lactamase from Bacteroides fragilis. Biochemistry. 1996;35:12126–12132. doi: 10.1021/bi960976h. [DOI] [PubMed] [Google Scholar]
- 5.Felici A, Amicosante G, Oratore A, Strom R, Ledent P, Joris B, Fanuel L, Frere J-M. An overview of the kinetic parameters of class B β-lactamases. Biochem J. 1993;291:151–155. doi: 10.1042/bj2910151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flannery A V, Beynon R J, Bond J S. Proteolysis of proteins for sequence analysis and peptide mapping. In: Beynon R J, Bond J S, editors. Proteolytic enzymes—a practical approach. Oxford, England: IRL Press; 1989. p. 150. [Google Scholar]
- 7.Hanahan D. Studies on the transformation of Escherichia coli with plasmids. J Mol Biol. 1983;45:37–67. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 8.Hewick R M, Hunkapiller M W, Hood L E, Dreyer W J. A gas-liquid solid phase peptide sequenator. J Biol Chem. 1981;256:7990–7997. [PubMed] [Google Scholar]
- 9.Hussain M, Carlino A, Madonna M J, Lampen J O. Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569/H in Escherichia coli. J Bacteriol. 1985;164:223–229. doi: 10.1128/jb.164.1.223-229.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito H, Arakawa Y, Ohsuka S, Wacharotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39:824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 12.Martinez E, Bartolome B, de la Cruz F. pACYC184-derived cloning vectors containing the multiple cloning site and lacZ-alpha reporter gene of pUC8/9 and pUC18/19 plasmids. Gene. 1988;68:159–162. doi: 10.1016/0378-1119(88)90608-7. [DOI] [PubMed] [Google Scholar]
- 13.Massidda O, Rossolini G M, Satta G. The Aeromonas hydrophila cphA gene: molecular heterogeneity among class B metallo-β-lactamases. J Bacteriol. 1991;173:4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne D J. Metallo-β-lactamases. J Med Microbiol. 1993;39:93–99. doi: 10.1099/00222615-39-2-93. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossolini G M, Zanchi A, Chiesurin A, Amicosante G, Satta G, Guglielmetti P. Distribution of cphA or related carbapenemase-encoding genes and production of carbapenemase activity in members of the genus Aeromonas. Antimicrob Agents Chemother. 1995;39:346–349. doi: 10.1128/aac.39.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossolini G M, Walsh T R, Amicosante G. The Aeromonas metallo-β-lactamases: genetics, enzymology, and contribution to drug resistance. Microb Drug Resist. 1996;2:245–252. doi: 10.1089/mdr.1996.2.245. [DOI] [PubMed] [Google Scholar]
- 18.Segatore B, Massidda O, Satta G, Setacci D, Aminocosante G. High specificity of cphA-encoded metallo-β-lactamase from Aeromonas hydrophila AE036 for carbapenems and its contribution to β-lactam resistance. Antimicrob Agents Chemother. 1993;37:1324–1328. doi: 10.1128/aac.37.6.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senda K, Arakawa Y, Nakashima K, Ito H, Ichiyama S, Shimokata K, Kato N, Ohta M. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob Agents Chemother. 1996;40:349–353. doi: 10.1128/aac.40.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson J S, Malamy M H. Sequencing the gene for an imipenem-cefoxitin-hydrolyzing enzyme (CfiA) from Bacteroides fragilis TAL2480 reveals strong similarity between CfiA and Bacillus cereus β-lactamase II. J Bacteriol. 1990;172:2584–2593. doi: 10.1128/jb.172.5.2584-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh T R, Hall L, Assinder S J, Nichols W W, Cartwright S J, MacGowan A P, Bennett P M. Sequence analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochim Biophys Acta. 1994;1218:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 22.Walsh T R, Payne D J, MacGowan A P, Bennett P M. A clinical isolate of Aeromonas sobria with three chromosomal mediated inducible β-lactamases: a cephalosporinase, a penicillinase and a third enzyme, displaying carbapenemase activity. J Antimicrob Chemother. 1995;35:271–279. doi: 10.1093/jac/35.2.271. [DOI] [PubMed] [Google Scholar]
- 23.Walsh T R, Hall L, MacGowan A P, Bennett P M. Sequence analysis of two chromosomally mediated inducible β-lactamases from a clinical isolate of Aeromonas sobria, strain 163a, one a group 2d penicillinase, the other a group 1 AmpC cephalosporinase. J Antimicrob Chemother. 1995;36:41–47. doi: 10.1093/jac/36.1.41. [DOI] [PubMed] [Google Scholar]
- 24.Walsh T R, Emery D C, Gamblin S J, MacGowan A P, Bennett P M. Enzyme kinetics and biochemical analysis of ImiS, a metallo-β-lactamase from Aeromonas sobria. J Antimicrob Chemother. 1996;37:423–431. doi: 10.1093/jac/37.3.423. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi H, Nukaga M, Sawai T. Sequence of the Klebsiella pneumoniae RDK4 metallo-β-lactamase. Accession no. D29636. 1994. EMBO Database. [Google Scholar]
- 27.Yang Y, Bush K. Biochemical characterisation of the carbapenem-hydrolysing β-lactamase AsbM1 from Aeromonas sobria AER14M: a member of a novel subgroup of metallo-β-lactamases. FEMS Microbiol Lett. 1996;137:193–200. doi: 10.1111/j.1574-6968.1996.tb08105.x. [DOI] [PubMed] [Google Scholar]