Abstract
Background
Montelukast is a highly selective and specific cysteinyl leukotriene receptor antagonist used in the treatment of asthma. Whether montelukast as adjuvant therapy can significantly and safely treat adults with cough variant asthma (CVA) remains inconclusive.
Aims
This meta‐analysis systematically evaluated the efficacy and safety of montelukast as an adjuvant treatment for adults with CVA.
Materials and methods
Randomized controlled trials (RCTs) on montelukast combined with inhaled corticosteroids (ICS) and long‐acting β2 agonists (LABAs) to treat CVA in adults, from inception to March 6, 2023, were retrieved from the CNKI, Wanfang, VIP, CBM, PubMed, Embase, Cochrane Library, and Web of Science databases and Clinical Trials website. Review Manager (version 5.4) and Stata (version 15.0) were used to conduct the meta‐analysis.
Results
A total of 15 RCTs were ultimately included in the meta‐analysis. It was established that montelukast as adjuvant therapy raised the total effective rate (RR = 1.20, 95% confidence interval [CI] [1.13, 1.27], P < 0.01) and improved the FEV1% (SMD = 0.91, 95% CI [0.40, 1.41], P < 0.01), PEF% (SMD = 0.63, 95% CI [0.38, 0.88], P < 0.01), FEV1 (SMD = 1.15, 95% CI [0.53, 1.77], P < 0.01), PEF (SMD = 0.64, 95% CI [0.42, 0.86], P < 0.01), and FEV1/FVC% (SMD = 0.76, 95% CI [0.51, 1.01], P < 0.01) and reduced the recurrence rate (RR = 0.28, 95% CI [0.15, 0.53], P < 0.01). The incidence of adverse reactions was higher in the montelukast auxiliary group compared to the control group but with no statistical difference (RR = 1.32, 95% CI [0.89, 1.96], P = 0.17).
Conclusion
Existing evidence indicated that the use of montelukast as an adjuvant therapy had therapeutic efficacy superior to ICS + LABA alone for the treatment of adult patients with CVA. However, further research is needed, especially a combination of high‐quality long‐term prospective studies and carefully designed RCTs.
Keywords: cough variant asthma, meta‐analysis, montelukast, randomized controlled trial
Whether montelukast as adjuvant therapy can significantly and safely treat adults with cough variant asthma (CVA) remains inconclusive. After a comprehensive retrieve, a total of 15 RCTs were ultimately included in the meta‐analysis. Our results demonstrated that montelukast can play a better curative effect in the adjuvant treatment of adults with CVA.

1. INTRODUCTION
Cough variant asthma (CVA), which is primarily characterized by cough, is an atypical form of asthma. The cough is irritating and dry, mild during the day, and severe at night. There is airway hyperresponsiveness, but symptoms or signs such as shortness of breath or wheezing are absent, and treatment with anti‐asthmatic drugs is beneficial in patients with CVA. 1 Epidemiological studies have shown that CVA is the leading cause of chronic cough in China, accounting for about 32–34%. 2 , 3 It is the second most common cause after upper airway cough syndrome in Europe and the United States. 4 Several studies have shown that CVA accounts for about 25–32.6% of chronic cough in adults 2 , 5 and about 30–35.7% of patients with CVA eventually develop typical asthma. 6 The principles for the treatment of CVA are similar to those for asthma. 1 Treatment with inhaled corticosteroids (ICS) or inhaled corticosteroids combined with long‐acting β2 agonists (ICS + LABA) is recommended for more than 8 weeks.
Treatment with ICS + LABA therapy provides rapid and effective cough relief. However, in patients with poor ICS or severe airway inflammation, a combination of leukotriene receptor antagonists may be used. 7 Montelukast is a highly selective and specific cysteinyl leukotriene receptor antagonist (CysLTRA). It can relieve bronchospasms and airway mucosal edema by binding to leukotriene receptors, thereby reducing inflammatory cell infiltration and mucus secretion and promoting disease improvement. 8 , 9
Clinical studies on montelukast as adjuvant therapy versus ICS + LABA in the treatment of adult CVA have been conducted, but the results are controversial. 10 , 11 No meta‐analyses are currently available on this topic. Our study systematically integrated the related randomized controlled trials (RCTs) published at home and abroad on montelukast used as an adjuvant in combination with ICS + LABA for treating adults with CVA.
2. MATERIALS AND METHODS
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) statement 12 and registered in PROSPERO (No. CRD42021289588). Ethical approval and patient consent were not required, as all analyses were based on previously published studies.
2.1. Literature search
A comprehensive search of the CNKI, Wanfang, VIP, China Biomedical Literature Database (CBM), PubMed, Embase, Cochrane Library, Web of Science databases, and Clinical Trials (http://www.chictr.org.cn/; https://clinicaltrials.gov) was conducted from database inception to March 6, 2023. The search strategy was as follows: (“cough variant asthma” or “cough variance asthma” or “cough type asthma”) and “montelukast” and (“randomized controlled trial” or “controlled clinical trial” or “random*” or “trial” or “RCT”) (Table S1).
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) patients aged 18 years or older who were diagnosed with CVA, regardless of gender or race; (2) intervention treatments that included montelukast combined with ICS + LABA versus ICS + LABA for at least 8 weeks; and (3) RCTs, without any restrictions on language or publication type.
The primary outcomes were as follows: (1) the total effective rate and (2) lung function indicators: forced expiratory volume in the first second as a percentage of the predicted value (FEV1%), peak expiratory flow expressed as a percentage of the predicted value (PEF%). The secondary outcomes included (1) lung function indicators: forced expiratory volume in the first second (FEV1), peak expiratory flow (PEF), ratio of the forced expiratory volume in the first second to the forced vital capacity expressed as a percentage (FEV1/FVC%); (2) the recurrence rate; and (3) the incidence of adverse reactions.
Repetitive studies, studies with insufficient data available, animal experiments, literature reviews, meta‐analyses, conference abstracts, case reports, and studies without explicit randomization methods were excluded from our analysis.
2.3. Data extraction and quality assessment
Data extraction was conducted by two independent researchers (QX and TTL). 13 , 14 It included the first author, publication year, baseline characteristics, intervention measures, treatment course, and outcomes. In case of disagreement, the third author (ZYS) was consulted to reach an agreement. 15 , 16 , 17
The methodological quality assessment of included trials was independently evaluated by the two authors (QX and TTL) based on the Cochrane Collaboration risk of bias assessment tool (ROB). 18 The evaluation domains were as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Each domain was rated as low, unclear, or high risk of bias.
2.4. Statistical analysis
Dichotomous outcomes are expressed as the relative risk (RR) with a 95% confidence interval (CI), while continuous outcomes are presented by the standardized mean difference (SMD) with 95% CI. Heterogeneity was evaluated using the Chi‐square and I 2 statistics. Only when the P value was >0.1 or I 2 was ≤50% was the fixed‐effects model performed to combine effect sizes. In all other cases, the random‐effects model was adopted. Subgroup analysis was conducted according to cough symptoms and recurrence and cough symptoms and bronchial provocation tests in the studies, which were included in the analysis. Sensitivity analysis was also performed to further identify potential sources of heterogeneity. Data analysis was performed by Review Manager (version 5.4), and Stata (version 15.0) was applied to detect publication bias. A P value of less than 0.05 was considered to indicate a statistically significant difference.
3. RESULTS
3.1. Literature search
A total of 4836 studies were screened. After removing duplicates, 2537 remained for screening of the titles and abstracts, and the full text of 109 studies was read. Eventually, 15 studies, 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 which were all conducted in China, were included according to the eligibility criteria (Figure 1).
FIGURE 1.

PRISMA flow diagram of the study selection process.
3.2. Study characteristics
Fifteen RCTs, 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 which were published from 2014 to 2022, with a total of 1314 participants, were included in the analysis. Montelukast combined with budesonide formoterol was used for treatment in seven studies, 19 , 21 , 23 , 24 , 29 , 32 , 33 and montelukast combined with salmeterol‐ticasone was used in the remaining studies. 20 , 22 , 25 , 26 , 27 , 28 , 30 , 31 The total course of treatment was 8 weeks in 10 of the studies 19 , 20 , 21 , 22 , 26 , 27 , 29 , 30 , 32 , 33 and 12 weeks in the other studies. 23 , 24 , 25 , 28 , 31 The main characteristics of the included studies are summarized in Table 1.
TABLE 1.
Characteristics of the included studies
| Study ID | Sample size (n) | Gender (male/female) | Age (mean ± standard deviation) | Course of disease (mean ± standard deviation) | Intervention | Course of treatment (w) | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Contr‐ol | Treatment | Contr‐ol | Treatment | Control | Treatment | Control | Treatment | Control | |||
| Chen/2017 | 40 | 40 | 19/21 | 17/23 | 32.9 ± 16.2 | 32.3 ± 16.8 |
3.8 ± 3.5 years |
3.9 ± 4.1 years |
Budesonide formoterol+ montelukast |
Budesonide formoterol | 8 | 1 |
| Feng/2015 | 49 | 49 | 24/25 | 22/27 | 45.7 ± 3.8 | 46.4 ± 3.5 | Not described |
Salmeterol ticasone+ montelukast |
Salmeterol ticasone | 8 | 1, 7 | |
| Gao/2022 | 34 | 34 | 14/20 | 16/18 | 40.24 ± 6.61 | 40.89 ± 6.68 |
6.21 ± 0.55 months |
6.08 ± 0.53 months |
Budesonide formoterol+ montelukast |
Budesonide formoterol | 8 | 1, 4, 5, 8 |
| Guo/2019 | 52 | 52 | 29/23 | 27/25 | 34.78 ± 5.36 | 34.93 ± 5.24 | 15.36 ± 3.49 months | 15.19 ± 3.57
months |
Salmeterol ticasone+ montelukast |
Salmeterol ticasone | 8 | 4, 8 |
| Li/2018 | 47 | 47 | 26/21 | 25/22 | 51.14 ± 11.05 | 50.83 ± 10.95 |
6.48 ± 2.37 years |
6.26 ± 2.28 years |
Budesonide formoterol+ montelukast |
Budesonide formoterol | 12 | 1 |
| Liu/2014 | 49 | 48 | 52/45 | 65.1 ± 3.8 | 7.05 ± 0.75 years | Budesonide formoterol+ montelukast | Budesonide formoterol | 12 | 4, 5, 6 | |||
| Su/2021 | 62 | 62 | 33/29 | 34/28 | 68.41 ± 4.42 | 67.32 ± 4.54 |
5.61 ± 1.13 months |
5.53 ± 1.16 months |
Salmeterol ticasone+ montelukast |
Salmeterol ticasone | 12 | 1, 7, 8 |
| Wang/2016 | 48 | 48 | 22/26 | 21/27 | 35.8 ± 8.6 | 36.3 ± 8.3 |
16.4 ± 5.7 months |
16.1 ± 5.4 months |
Salmeterol ticasone+ montelukast |
Salmeterol ticasone | 8 | 1, 2, 3, 4, 6, 7, 8 |
| Wu/2012 | 36 | 36 | 25/11 | 24/12 | 38.5 ± 11.8 | 37.8 ± 12.2 |
11.8 ± 5.7 months |
12.6 ± 6.1 months |
Salmeterol ticasone+ montelukast |
Salmeterol ticasone | 8 | 1, 2, 3, 8 |
| Ye/2018 | 36 | 39 | 20/16 | 21/18 | 33.1 ± 8.6 | 33.5 ± 7.9 |
17.1 ± 4.2 months |
16.8 ± 3.6 months |
Salmeterol ticasone+ montelukast |
Salmeterol ticasone | 12 | 1, 4, 6, 7, 8 |
| Ye/2019 | 53 | 53 | 33/20 | 30/23 | 38.63 ± 4.61 | 37.93 ± 4.17 |
5.13 ± 0.78 months |
5.20 ± 1.10 months |
Budesonide formoterol+ montelukast |
Budesonide formoterol | 8 | 1, 4, 5, 8 |
| Zhang/2015 | 49 | 49 | 20/29 | 22/27 | 38.3 ± 8.2 | 38.8 ± 7.8 |
15.5 ± 6.6 months |
15.6 ± 6.7 months |
Salmeterol ticasone+ montelukast |
Salmeterol ticasone | 8 | 1, 8 |
| Zhang/2022 | 25 | 25 | 15/10 | 17/8 | 65.85 ± 2.44 | 65.56 ± 2.37 | Not described |
Salmeterol ticasone+ montelukast |
Salmeterol ticasone | 12 | 1, 8 | |
| Zhou/2021 | 30 | 30 | 17/13 | 15/15 | 37.35 ± 9.31 | 38.27 ± 9.65 | 12.82 ± 4.84 weeks | 13.34 ± 4.90 weeks |
Budesonide formoterol+ montelukast |
Budesonide formoterol | 8 | 4, 5, 8 |
| Zhu/2018 | 46 | 46 | 21/25 | 19/27 | 41.36 ± 9.49 | 42.04 ± 9.21 |
3.16 ± 0.80 months |
2.97 ± 0.93 months |
Budesonide formoterol+ montelukast | Budesonide formoterol | 8 | 2, 3, 8 |
3.3. Quality assessment
All included studies were evaluated according to the Cochrane Collaboration ROB tool. Random sequence generation was explicit in all studies, of which one study used random envelopes 19 while the others used a random number table. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 The assessment results were unclear since neither allocation concealment nor the blinding method was mentioned in the overall studies. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 All studies 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 had complete outcome data, but there was no detailed information for evaluating whether they had selective outcome reporting or other sources of risk of bias (Figure 2).
FIGURE 2.

Assessment of risk of bias: (A) risk of bias graph; (B) risk of bias summary.
3.4. Primary outcomes
3.4.1. The total effective rate
A total of 11 RCTs 19 , 20 , 21 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 mentioned the total effective rate. Among them, Gao 21 used lung function improvement as the evaluation criterion, Wang and Li 26 used inconsistent cough symptom scores as evaluation criteria, and Zhang 30 proposed evaluation criteria based on whether cough symptoms were relieved following 2 weeks of treatment. Thus, the effect sizes of the above three studies were not combined. No heterogeneity was seen among the remaining eight studies 19 , 20 , 23 , 25 , 27 , 28 , 29 , 31 that reported the total effective rate (P = 0.30, I 2 = 17%). Meta‐analysis using the fixed‐effects model established that the total effective rate of montelukast adjuvant treatment was higher than that in the control group (RR = 1.20, 95% CI [1.13, 1.27], P < 0.01). Among these eight studies, 19 , 20 , 23 , 25 , 27 , 28 , 29 , 31 there were three different evaluation criteria for the total effective rate. Three studies 20 , 27 , 28 with moderate heterogeneity (I 2 = 54%) used cough symptoms and recurrence as evaluation criteria. The results of this subgroup with the fixed‐effects model apparently confirmed that the total effective rate was higher in the montelukast adjuvant group compared to the control group (RR = 1.17, 95% CI [1.07, 1.28], P < 0.01). After excluding the study by Ye and Feng, 28 the conclusion that montelukast improved the total effective rate remained unchanged (RR = 1.23, 95% CI [1.08, 1.39], P < 0.01), but heterogeneity was decreased (P = 0.80, I 2 = 0%). Three studies 19 , 23 , 29 with no heterogeneity (P = 0.85, I 2 = 0%) were based on cough symptoms and bronchial provocation test results. The fixed‐effects meta‐analysis demonstrated a statistical difference in favor of the montelukast adjuvant group (RR = 1.19, 95% CI [1.09, 1.29], P < 0.01). The other two studies 25 , 31 based on unified cough symptom scores had mild heterogeneity (P = 0.17, I 2 = 46%). Meta‐analysis using the fixed‐effects model showed that the montelukast adjuvant treatment group had a higher total effective rate (RR = 1.27, 95% CI [1.10, 1.45], P < 0.01) (Figure 3A).
FIGURE 3.

Forest plots with montelukast adjuvant group versus the control group: (A) total effective rate; (B) FEV1%; (C) PEF%; (D) FEV1; (E) PEF; (F) FEV1/FVC%; (G) recurrence rate; (H) incidence of adverse reactions.
3.4.2. The lung function indicators: FEV1% and PEF%
FEV1%
A noticeable difference in favor of the montelukast adjuvant group (SMD = 0.91, 95% CI [0.40, 1.41], P < 0.01) was observed using the random‐effects model in three studies 26 , 27 , 33 reporting FEV1% with moderate heterogeneity (P = 0.02, I 2 = 74%) (Figure 3B). Due to the moderate heterogeneity of the results, sensitivity analysis found that the conclusion that montelukast could improve FEV1% after excluding Wang and Li's study 26 remained unchanged, and the difference between groups was statistically significant (SMD = 0.65, 95% CI [0.34, 0.97], P < 0.01). However, the heterogeneity was significantly decreased (P = 0.84, I 2 = 0%).
PEF%
The PEF% index reported in three studies 26 , 27 , 33 without heterogeneity (P = 0.52, I 2 = 0%) was combined by the fixed‐effects model. An obvious difference in favor of the montelukast adjuvant group was demonstrated (SMD = 0.63, 95% CI [0.38, 0.88], P < 0.01) (Figure 3C).
3.5. Secondary outcomes
3.5.1. Lung function indicators: FEV1, PEF, and FEV1/FVC%
FEV1
Seven studies 21 , 22 , 24 , 26 , 28 , 29 , 32 reporting FEV1 showed high heterogeneity (P < 0.01, I 2 = 92%). The random‐effects meta‐analysis demonstrated that the FEV1 was improved by montelukast adjuvant therapy with a statistical difference (SMD = 1.15, 95% CI [0.53, 1.77], P < 0.01) (Figure 3D). Due to the high heterogeneity, sensitivity analysis was conducted by eliminating the studies one by one. After eliminating one study, 22 the conclusion that montelukast improved FEV1 remained unchanged (SMD = 0.77, 95% CI [0.59, 0.95], P < 0.01). However, heterogeneity decreased (P = 0.47, I 2 = 0%).
PEF
Four studies 21 , 24 , 29 , 32 reported the PEF index with no heterogeneity (P = 0.60, I 2 = 0%). The results using the fixed‐effects model demonstrated an obvious difference in favor of the montelukast adjuvant group (SMD = 0.64, 95% CI [0.42, 0.86], P < 0.01) (Figure 3E).
FEV1/FVC%
An evident difference in favor of the montelukast adjuvant group was confirmed in three studies, 24 , 26 , 28 which recorded the FEV1/FVC% index (SMD = 0.76, 95% CI [0.51, 1.01], P < 0.01). The fixed‐effects model was performed because no heterogeneity was seen (P = 0.83, I 2 = 0%) (Figure 3F).
3.5.2. Recurrence rate
Four studies 20 , 25 , 26 , 28 reported the recurrence rate after 6 months of follow‐up. No heterogeneity was observed (P = 0.94, I 2 = 0%). The fixed‐effect model was then conducted and indicated that montelukast adjuvant treatment reduced the recurrence rate (RR = 0.28, 95% CI [0.15, 0.53], P < 0.01) (Figure 3G).
3.5.3. The incidence of adverse reactions
Eleven studies 21 , 22 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 reported the incidence of adverse events, and no apparent heterogeneity was obtained among them (P = 0.97, I 2 = 0%). Although a higher incidence of adverse reactions was seen in the montelukast adjuvant group, the meta‐analysis with the fixed‐effects model showed no statistical difference compared to the control group (RR = 1.32, 95% CI [0.89, 1.96], P = 0.17) (Figure 3H).
3.6. Publication bias
The result of Begg's test showed that the total effective rate of this study had no publication bias (P = 0.079) (Figure 4), which to some extent supported the reliability of the research results.
FIGURE 4.
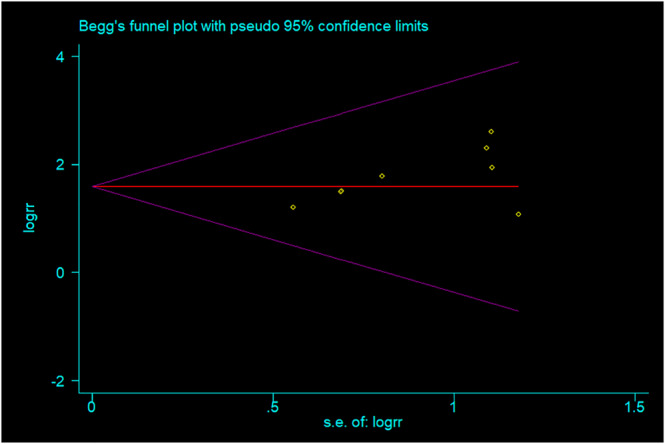
Publication bias funnel chart.
4. DISCUSSION
CVA is the leading cause of chronic cough in China and is the second most common cause of chronic cough in European and American countries. It also accounts for about 25–32.6% of chronic cough in adults. Irritant dry cough is the main clinical symptom of CVA. A positive bronchial provocation test is still essentially the gold standard for diagnosis, and effective anti‐asthma treatment is a necessary diagnostic condition. 34 It is worth noting that uncontrolled and recurrent CVA may lead to the development of classic asthma, 35 which in turn further leads to worsening lung function. Hence, early intervention and standardized CVA treatment are crucial. Domestic and international guidelines 1 , 4 recommend treatment with ICS or ICS + LABA for the management of CVA. Leukotriene receptor antagonists can be used in patients who cannot use hormones or whose hormonal therapy is ineffective.
Currently, airway inflammation, remodeling, and airway hyperresponsiveness are widely recognized to play a critical role in the pathogenesis of CVA. 36 Studies found that although the clinical symptoms of CVA patients were milder compared to those with typical asthma, increases in the degree of eosinophilia in the central airway mucosal tissue biopsy and bronchoalveolar lavage fluid were the same. 37 Cysteinyl leukotrienes (CysLTs), which are closely related to airway inflammation of CVA, are mainly produced by eosinophils. CysLTs mainly include LTC4, LTD4, LTE4, and LTF4, which can lead to bronchial smooth muscle contraction, mucosal edema, and mucus secretion. Montelukast is a cysteinyl leukotriene receptor (CysLTR) antagonist, which can selectively block the CysLT1R to inhibit inflammation of the airway. Leukotrienes are also involved in CVA airway remodeling. Studies suggested that LTC4 and LTD4 are the primary leukotrienes that mediate airway remodeling. Both of them have a high affinity for CysLT1R. 38 Montelukast can inhibit airway remodeling by blocking CysLT1R. Some scholars 39 compared the LTC4 concentration and LTC4/PGE2 ratio in induced sputum of CVA, typical asthma, and eosinophilic bronchitis and suggested that high LTC4 concentrations and high LTC4/PGE2 ratios are the inflammatory basis of airway hyperresponsiveness. Montelukast can inhibit airway hyperresponsiveness by preventing LTC4 from binding to CysLT1R.
Fifteen studies were eventually included in our analysis. All these studies were based on the use of ICS + LABA combined with montelukast for adjuvant treatment of CVA in adults for at least 8 weeks. Meta‐analysis suggested that montelukast adjuvant therapy increased the total response rate, improved lung function indicators (FEV1%, PEF%, FEV1, PEF, and FEV1/FVC%), and reduced the recurrence rate. Although the incidence of adverse reactions in the montelukast adjuvant group was higher, they were alleviated spontaneously or after symptomatic treatment. There was moderate heterogeneity when analyzing the total effective rate based on cough symptoms combined with recurrence. When Ye and Feng's study was excluded, heterogeneity was significantly decreased. Studying the original text of Ye and Feng 28 found that the patients were 19–57 years old, the course of the disease was 3–23 months, and the montelukast treatment cycle reached 12 weeks. Due to the moderate heterogeneity in FEV1%, sensitivity analysis was conducted, which indicated that the study by Wang and Li 26 may have been the cause of the heterogeneity. In their study, patients were aged 20–55 and had a disease course of 2–25 months. All the patients received 10 mg of montelukast before bedtime for 8 weeks. In the process of analyzing FEV1 attributed to the high heterogeneity, a sensitivity analysis was also carried out, which found that heterogeneity decreased after removing the research by Guo. 22 Analysis of the original study found that patients in the study by Guo 22 were 19–60 years old and had a disease duration of 2–24 months. Montelukast was given at 10 mg once a day for an unlimited medication time, and the treatment course was 8 weeks. Considering the actual situation, all the above had the potential to produce heterogeneity.
A meta‐analysis confirmed that montelukast combined with budesonide significantly increased the total effective rate; improved FEV1, FEV1%, and PEF; and reduced the recurrence rate in the treatment of children with CVA compared to budesonide alone. 40 A study by Feng et al. 41 found that a treatment regimen of montelukast given with a combination of salmeterol‐ticasone had a higher total response rate and apparent efficiency, shorter cough duration, and lower recurrence rate without limitations on the age of CVA patients compared to when salmeterol‐ticasone was given alone. The above studies were consistent with our results. In addition, our study limited the inclusion of adults with CVA, and the treatment of montelukast combined with ICS + LABA was used for at least 8 weeks. The results confirmed that the standardized treatment of montelukast was effective for adult CVA patients. Furthermore, reliable indicators like PEF% and FEV1/FVC%, which also reflect lung function, were added to the outcome and showed improvement, further verifying the effectiveness of montelukast in improving lung function.
Our study had several strengths. First, this is the first study, to the best of our knowledge, that conducted a systematic review and meta‐analysis of montelukast as an adjunctive treatment for adults with cough variant asthma. Second, data screening, extraction, and quality evaluation were all individually completed by two researchers to ensure the reliability of the data. Additionally, subgroup analysis was also carried out to further investigate the source of heterogeneity. Our study also had some limitations. First, blinding was not mentioned in all included studies, which in turn might have led to implementation bias. Second, although the dose of montelukast in all included studies was 10 mg, the frequency and duration of use were not consistent across all the studies. Third, the small number of included studies and small sample size might have impacted the reliability of the results of this systematic review. Therefore, future studies should clarify the implementation of blinding methods when conducting relevant studies, standardize the application of montelukast according to guidelines, and conduct large‐scale multicenter RCTs to improve the reliability of the research results.
Existing evidence indicated that the use of montelukast as an adjuvant therapy had therapeutic efficacy superior to that of ICS + LABA alone for the treatment of adult patients with CVA. However, based on the above limitations, further research is needed, especially a combination of high‐quality long‐term prospective studies and carefully designed RCTs.
AUTHOR CONTRIBUTIONS
Kehu Yang and Zhiming Zhang designed the study. Qian Xu and Tingting Lu were in charge of literature search and data analysis. Qian Xu and Tingting Lu were responsible for the initial manuscript. Zhongyang Song, Peng Zhu, Yana Wu, and Lumei Zhang contributed to the collection of data. All authors commit to be responsible for all aspects of the work.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
ETHICS STATEMENT
All analyses in this study were based on previously published results and did not require ethical approval or patient consent.
Supporting information
Table S1. Search Strategy in PubMed
ACKNOWLEDGMENT
We would like to thank MogoEdit (https://www.mogoedit.com) for its English editing during the preparation of this manuscript.
Xu Q, Lu T, Song Z, et al. Efficacy and safety of montelukast adjuvant therapy in adults with cough variant asthma: A systematic review and meta‐analysis. Clin Respir J. 2023;17(10):986‐997. doi: 10.1111/crj.13629
Qian Xu and Tingting Lu contributed equally to this work.
Contributor Information
Kehu Yang, Email: kehuyangebm2006@126.com.
Zhiming Zhang, Email: zhangzhimingys@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Asthma Group of Chinese . Throacic society (2020) guidelines for bronchial asthma prevent and management. Chin J Tuberc Respir Dis. 2020;43:1023‐1048. [DOI] [PubMed] [Google Scholar]
- 2. Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest. 2013;143(3):613‐620. doi: 10.1378/chest.12-0441 [DOI] [PubMed] [Google Scholar]
- 3. Ding H, Xu X, Wen S, et al. Changing etiological frequency of chronic cough in a tertiary hospital in Shanghai, China. J Thorac Dis. 2019;11(8):3482‐3489. doi: 10.21037/jtd.2019.07.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Côté A, Russell RJ, Boulet LP, et al. CHEST expert cough panel. Managing chronic cough due to asthma and NAEB in adults and adolescents: CHEST guideline and expert panel report. Chest. 2020;158(1):68‐96. doi: 10.1016/j.chest.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 5. Corrao WM. Pearls and pitfalls in the diagnosis of cough variant asthma. Allergy Asthma Proc. 2018;39(6):466‐467. doi: 10.2500/aap.2018.39.4168 [DOI] [PubMed] [Google Scholar]
- 6. Miao Q, Wei PC, Fan MR, Zhang YP. Clinical study on treatment of cough variant asthma by Chinese medicine. Chin J Integr Med. 2013;19(7):539‐545. doi: 10.1007/s11655-013-1508-5 [DOI] [PubMed] [Google Scholar]
- 7. Asthma Group of Chinese Throacic Society . Guidelines for the diagnosis and treatment of cough (2015). Chin J Tuberc Respir Dis. 2016;39:323‐354. [Google Scholar]
- 8. Al‐Hamdani FY. Comparative clinical evaluation of ketotifen and montelukast sodium in asthmatic Iraqi patients. Saudi Pharm J. 2010;18(4):245‐249. doi: 10.1016/j.jsps.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu Z, Meng L, Xie Y, Guo W. lncRNA PCGEM1 strengthens anti‐inflammatory and lung protective effects of montelukast sodium in children with cough‐variant asthma. Braz J Med Biol Res. 2020;53(7):e9271. doi: 10.1590/1414-431x20209271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X. Observation on effect of salmeterol/fluticasone combined with montelukast sodium on adult cough variant asthma. China Prac Med. 2020;15:19‐21. [Google Scholar]
- 11. Zeng L, Chen X, Li C, et al. Effect of budesonide/formoterol powder inhalation combined with montelukast sodium in the treatment of cough variant asthma in adults. J Bethune Med Sci. 2017;15:603‐604. [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan B, Ge L, Xun YQ, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta‐analysis. Int J Behav Nutr Phys Act. 2018;15(1):72. doi: 10.1186/s12966-018-0703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li XX, Wei LL, Shang WR, et al. Trace and evaluation systems for health services quality in rural and remote areas: a systematic review. J Public Health. 2018;26(2):127‐135. doi: 10.1007/s10389-017-0858-4 [DOI] [Google Scholar]
- 15. Fu Y, Zha S, Lü N, et al. Carrier frequencies of hearing loss variants in newborns of China: a meta‐analysis. J Evid Based Med. 2019;12(1):40‐50. doi: 10.1111/jebm.12305 [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Cao L, Zhang Z, et al. Reporting and methodological quality of COVID‐19 systematic reviews needs to be improved: an evidence mapping. J Clin Epidemiol. 2021;135:17‐28. doi: 10.1016/j.jclinepi.2021.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang KH, Li XX, Bai ZG. Research Methods of Evidence‐Based Social Science: Systematic Review and Meta‐Analysis. Lanzhou University Press; 2018:66‐67. [Google Scholar]
- 18. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen BY, Jiang RZ, He SM, et al. Clinical observation of budesonide formoterol powder inhalation combined with montelukast sodium in the treatment of cough variant asthma. China Prac Med. 2017;12:115‐117. [Google Scholar]
- 20. Feng RF, Lin ZT. The effect of salmeterol and ticasone powder combined with montelukast sodium on the curative effect and recurrence rate of cough variant asthma. Mod Diagn Treat. 2015;26:3430‐3431. [Google Scholar]
- 21. Gao CW. Evaluation of the efficacy of budesonide and formoterol powder inhalation combined with montelukast sodium in the treatment of cough variant asthma. Friends Health. 2022;5:17‐18. [Google Scholar]
- 22. Guo Q. Effects of salmeterol fluticasone combined with montelukast on inflammatory factor levels and pulmonary functions in patients with cough variant asthma. Acta Med Sin. 2019;32:47‐50. [Google Scholar]
- 23. Li GY. Clinical study of leukotriene receptor antagonist combined with budesonide formoterol powder inhalation in the treatment of cough variant asthma. Strait Pharmaceut J. 2018;30:188‐189. [Google Scholar]
- 24. Liu K, Dai AG, Jiang YL. The effect of montelukast sodium on inflammatory factor helper T cell 17, regulatory T cell in elderly patients with cough variant asthma. J Commun Med. 2014;12:42‐44. [Google Scholar]
- 25. Su X, Chen F, Wang YB, et al. Montelukast sodium combined with salmeterol and fluticasone in the treatment of older patients with cough variant asthma. Int J Geriatr. 2021;42:228‐230. [Google Scholar]
- 26. Wang CH, Li CH. Clinical observation of therapeutic effects of seretide plus montelukast on patinets with cough variant asthma. Chin Hosp Pharm J. 2016;36:50‐53. [Google Scholar]
- 27. Wu QH. The effects of montelukast combine with seretide in the treatment of patients with cough variant asthma. J Front Med. 2012;28:83‐84. [Google Scholar]
- 28. Ye ZL, Feng XP. Analysis of the recurrence rate and safety of seretide combined with montelukast in patients with cough variant asthma. Modern Med Imageol. 2018;27:1723‐1724. [Google Scholar]
- 29. Ye JP, Dai JG, Zhao WY, et al. Effect of budesonide and formoterol fumarate powder for inhalation combined with montelukast on lung function and hypersensitive C‐reactive protein in patients of cough variant asthma. Clin Educ Gen Pract. 2019;17:326‐328. [Google Scholar]
- 30. Zhang FE. Clinical study of montelukast combined with seretide in the treatment of cough variant asthma. China Pract Med. 2015;10:142‐143. [Google Scholar]
- 31. Zhang ZY. Clinical effect of montelukast sodium combined with salmeterol fluticasone in the elderly patients with cough variant asthma. Reflex Ther Rehab Med. 2022;3:141‐143. [Google Scholar]
- 32. Zhou J, Chi CT. Efficacy of budesonide formoterol powder inhalation combined with montelukast sodium in the treatment of cough variant asthma and its effect on lung function. Chin Med J (Engl). 2021;56:1200‐1203. [Google Scholar]
- 33. Zhu SQ, Li XH, Han WL. Observation on efficacy of montelukast sodium tablets combined with budesonide fumotrol powder inhalation in treatment of cough variant asthma. Eval Anal Drug‐Use Hospitals China. 2018;18:1225‐1227. [Google Scholar]
- 34. Qiu ZM. Advance in the management of cough variant asthma. J Gannan Med Univ. 2021;41:797‐800+807. [Google Scholar]
- 35. Chen SY, Fang ZK, Fang S, et al. Comparison of functional parameters of small airways between patients with typical asthma and cough‐variant asthma. J South Med Univ. 2017;37:330‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao L, Yan Y, Bao H, et al. Modern research progress of cough variant asthma. CJTCMP. 2019;34:4171‐4174. [Google Scholar]
- 37. Niimi A, Amitani R, Suzuki K, Tanaka E, Murayama T, Kuze F. Eosinophilic inflammation in cough variant asthma. Eur Respir J. 1998;11(5):1064‐1069. doi: 10.1183/09031936.98.11051064 [DOI] [PubMed] [Google Scholar]
- 38. Bankova LG, Lai J, Yoshimoto E, et al. Leukotriene E4 elicits respiratory epithelial cell mucin release through the G‐protein‐coupled receptor, GPR99. Proc Natl Acad Sci U S A. 2016;113(22):6242‐6247. doi: 10.1073/pnas.1605957113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terasaki G, Paauw DS. Evaluation and treatment of chronic cough. Med Clin North Am. 2014;98(3):391‐403. doi: 10.1016/j.mcna.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 40. Wei Y, Li DS, Liu JJ, Zhang J, Zhao HE. Therapeutic effect and safety of montelukast sodium combined with budesonide in children with cough variant asthma:a meta analysis. Chinese Journal of Contemporary Pediatrics. 2016;18(11):1100‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Feng WT, Yi H, Yuan HY. Efficacay and safety of salmeterol/fluticasone combined with montelukast versus salmeterol/fluticasone in the treatment of cough variant asthma:a meta‐analysis. China Pharmacy. 2018;29:699‐703. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Strategy in PubMed
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


