Abstract
Malaria elimination interventions in low-transmission settings aim to extinguish hot spots and prevent transmission to nearby areas. In malaria elimination settings, the World Health Organization recommends reactive, focal interventions targeted to the area near malaria cases shortly after they are detected. A key question is whether these interventions reduce transmission to nearby uninfected or asymptomatic individuals who did not receive interventions. Here, we measured direct effects (among intervention recipients) and spillover effects (among non-recipients) of reactive, focal interventions delivered within 500m of confirmed malaria index cases in a cluster-randomized trial in Namibia. The trial delivered malaria chemoprevention (artemether lumefantrine) and vector control (indoor residual spraying with Actellic) separately and in combination using a factorial design. We compared incidence, infection prevalence, and seroprevalence between study arms among intervention recipients (direct effects) and non-recipients (spillover effects) up to 3 km away from index cases. We calculated incremental cost-effectiveness ratios accounting for spillover effects. The combined chemoprevention and vector control intervention produced direct effects and spillover effects. In the primary analysis among non-recipients within 1 km from index cases, the combined intervention reduced malaria incidence by 43% (95% CI 20%, 59%). In secondary analyses among non-recipients 500m-3 km from interventions, the combined intervention reduced infection by 79% (6%, 95%) and seroprevalence 34% (20%, 45%). Accounting for spillover effects increased the cost-effectiveness of the combined intervention by 37%. Our findings provide the first evidence that targeting hot spots with combined chemoprevention and vector control interventions can indirectly benefit non-recipients up to 3 km away.
Introduction
In the past decade, there has been renewed attention towards global malaria eradication, and many countries have set targets for the elimination of local malaria transmission (1). In Southern Africa, eight countries hope to achieve malaria elimination by 2030 as part of the Elimination Eight Initiative (E8). Yet, global progress has plateaued: annual global malaria cases have increased since 2015, and in 2021, there were 59 cases per 1,000 population at risk, an increase from 57 per 1,000 in 2019 (2).
The ideal malaria elimination intervention would not only prevent disease among recipients but would also prevent onward transmission to nearby non-recipients through spillover effects (i.e., “herd effects”, “indirect effects”) (4, 5), like some vaccines do (4, 6–12). Prior studies have reported spillover effects for mass drug administration for trachoma (13, 14), school-based deworming (15), insecticide treated bed nets (16–18), and chemoprevention and vector control for malaria (19, 20).
When an intervention reduces disease among intervention non-recipients, accounting for spillover effects can substantially increase cost-effectiveness (21, 22). Identifying cost-effective interventions is crucial to the elimination and eradication enterprise because elimination efforts are projected to cost significantly more than existing malaria control programs in the medium term (3). Even after elimination, countries must continue to conduct intensive surveillance and outbreak response for imported cases to prevent re-establishment.
In settings approaching malaria elimination, the World Health Organization recommends interventions that are “reactive” – delivered soon after a confirmed malaria case is detected – and “focal” – delivered to higher risk individuals who reside near the case (23). A recent cluster-randomized trial in Namibia found that reactive, focal chemoprevention and vector control substantially reduced malaria incidence (24). Spillover effects of these interventions are plausible: chemoprevention may reduce gametocyte biomass in recipients (25), and vector control can reduce the mosquito population near malaria cases. To shed light on whether focal interventions reduce transmission to nearby uninfected or asymptomatic individuals who did not receive interventions, we separately estimated direct effects among intervention recipients and spillover effects among nearby non-recipients. Our approach can be used to estimate spillover effects of other interventions, such as malaria vaccines.
Results
Interventions
We analyzed data from a previously reported cluster-randomized trial of focal malaria interventions conducted in Zambezi region of Namibia in 2017 (NCT02610400) (24) (Figure 1). The region has low Plasmodium falciparum malaria transmission (26). Using a two-by-two factorial design, the trial randomized 56 clusters to four arms: 1) reactive case detection (RACD), 2) reactive focal mass drug administration (rfMDA) only, 3) reactive vector control (RAVC) + RACD, 4) RAVC + rfMDA. rfMDA included presumptive treatment with artemether-lumefrantrine to individuals in target areas (Table S1). RACD included testing with rapid diagnostic tests and treatment with artemether-lumefrantrine and single-dose primaquine for those who tested positive. RAVC included indoor residual spraying (IRS) with pirimiphosmethyl. The trial delivered interventions in “target areas” within approximately 500 m of confirmed malaria cases detected through passive surveillance.
Figure 1. Map of target areas and spillover zones in the study site.
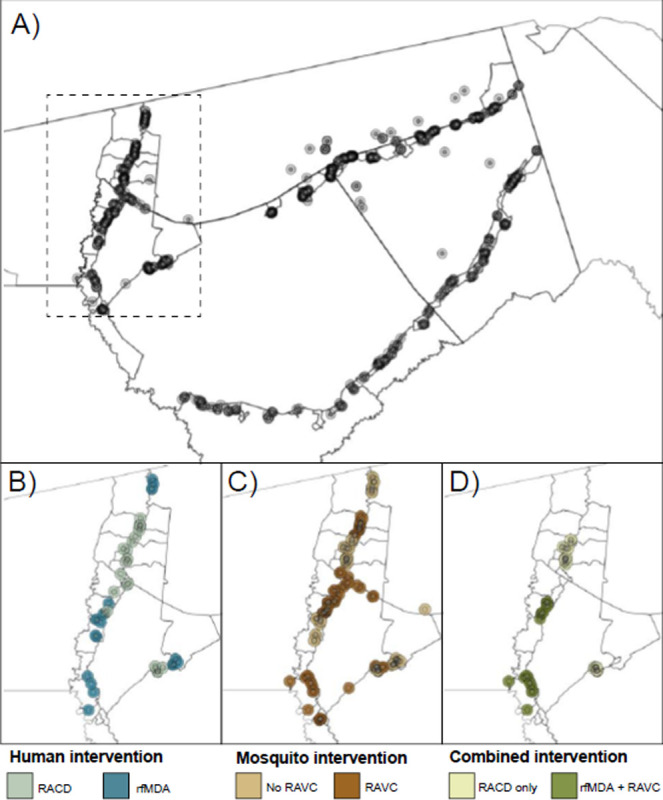
a) All index cases during the study period. The centroid of each circle is the residence location of a treated index case. Inner circles indicate 500m target areas where interventions were delivered. Outer circles indicate the 1km radius around each index case in which spillover effects were estimated in primary analyses. The dashed line indicates insets in panels B-D showing index cases during the follow-up periods with the largest number of treated index cases. For each comparison of study arms, panels b-d depict examples of analytic cohorts from a single follow-up period (i.e., subsample of person-time) in a subset of the study area. b) Inset of study area with index cases in the RACD and rfMDA arms (5-week period: April 25, 2017 – May 30, 2017). C) Inset of study area with index cases in the no RAVC and RAVC arms (6-month period: January 1, 2017 - June 30, 2017). d) Inset of study area with index cases in the RAVC and rfMDA+RAVC arms (6-month period: January 1, 2017 - June 30, 2017).
RACD: reactive case detection
rfMDA: reactive focal mass drug administration
RAVC: reactive focal vector control
Effects on malaria incidence
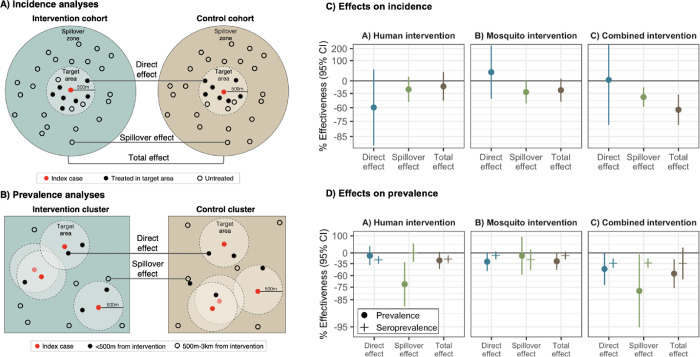
Primary analyses estimated effects on malaria incidence. We estimated three types of effects on the cumulative incidence of locally acquired, confirmed Pf malaria: 1) direct effects among intervention recipients in target areas within 500 m of confirmed malaria index cases, 2) spillover effects among non-recipients within 1 km of index cases, and 3) total effects among all individuals within 1 km of index cases (Figure 2a). We created analytic cohorts including individuals residing within 1 km of each index case to capture the area and time period in which we expected each intervention to reduce infections (direct effect) and secondary transmission to nearby individuals (spillover effect). (Figure 1; Figure S1 see details in Materials and Methods). We measured effects of the chemoprevention intervention comparing arms with rfMDA vs. RACD, the vector control intervention by comparing arms with RAVC vs. no RAVC, and the combined intervention by comparing the rfMDA + RAVC vs. RACD only arms.
Figure 2. Effects of reactive, focal malaria interventions.
a) Definition of effects in incidence analyses. b) Definition of effects in prevalence analyses. c) Effects on incidence estimated with hierarchical TMLE. All incidence outcome models were fit with cohort-level data except for models of spillover effects of rfMDA vs. RACD and rfMDA + RAVC vs. RACD only. Models were adjusted for covariates that were screened separately for each model using a likelihood ratio test. Confidence intervals shown here do not account for potential outcome correlation. For rfMDA and RACD arms, the incidence analysis includes the period from 0–35 days following index case detection for direct effects and 21–56 days for spillover effects. For rfMDA+RAVC and RAVC only arms, the analysis includes the period from 0–6 months following index case detection for direct effects and 17 days to 6 months for spillover effects. Total effects analyses include the person-time for the direct effects and spillover effects analyses. For incidence analyses, direct effects include treated in target zone, spillover effects include intervention non-recipients up to 1km from an index case, and total effects include all individuals (intervention recipients and non-recipients) up to 1km from index case. d) Effects on prevalence estimated with TMLE using individual-level data; standard errors were adjusted for clustering at the enumeration area level. Models were unadjusted because there were fewer than 10 malaria cases per variable. Direct effects include individuals who resided within 500m of any intervention recipients, spillover effects include individuals with no intervention recipients < 500m and any intervention recipients 500m-3km, and total effects include individuals with any intervention recipients <3km during the study. In c) and d), % effectiveness was calculated as the ratio of incidence or prevalence between study arms minus 1 × 100%. The upper bound of the 95% CI for the combined intervention direct effect was truncated from its original value of 381%.
To estimate direct effects and spillover effects, we used hierarchical targeted maximum likelihood estimation, a doubly-robust, semiparametric method that adjusts for potential confounders using ensemble machine learning (27). This approach is appropriate for cluster-level interventions that may result in statistical dependence between outcomes (see Methods) (28–30). We adjusted for covariates such as baseline malaria incidence and population size to account for differences in baseline characteristics between study arms (Tables S2–3).
In analyses of direct effects among intervention recipients, the chemoprevention intervention reduced malaria incidence among intervention recipients within 500m of index cases, but the confidence interval included the null. There was no evidence of a direct effect for the vector control or combined interventions, but precision was low (Figure 2, Table S4).
In analyses of spillover effects among intervention non-recipients up to 1 km away from interventions, chemoprevention and vector control interventions reduced incidence, but confidence intervals included the null. There was evidence of a spillover effect of the combined intervention, which reduced malaria incidence by 43% (95% CI 21%, 58%) among non-recipients.
We evaluated spillover effect heterogeneity by cluster-level malaria incidence and IRS coverage prior to the trial, surface temperature, rainfall, the enhanced vegetative index, elevation, and cohort-level treatment coverage, and gender. Across interventions, spillover effects were consistently more protective when pre-trial incidence was below the median (Figure S2). For example, the combined intervention reduced incidence by 68% (95% CI 35%, 84%) when baseline incidence was lower, but there was no effect when baseline incidence was above the median. Intervention spillover effects were generally stronger when environmental conditions favored mosquito breeding and survival (higher rainfall, higher enhanced vegetative index, and lower elevation) (Table S5). Spillover effects of the chemoprevention intervention were present for men but were null for women.
We performed several sensitivity analyses. When we repeated spillover effect analyses using 2 and 3 km radii around index cases to capture mosquito dispersal over longer distances (31, 32), results were similar (Figure S3). When using a shorter follow-up period in which intervention effects may have been stronger (see Methods), results were similar for the chemoprevention and vector control interventions; for the combined intervention, the spillover effect estimate was closer to the null, and precision was lower (Figure S4). When we estimated direct effects including the <3% of intervention recipients who resided >500m from index cases, results were nearly the same (Figure S5).
Overlap in analytic cohorts may have resulted in statistical dependence between outcomes (Figure 1; Table S6). When using alternative standard errors accounting for dependence (see Methods), confidence intervals were wider, but there was still evidence of spillover effects and total effects for the combined interventions (Table S4). When we excluded overlapping cohorts from the analysis, results were similar overall (Figure S4).
Effects on malaria prevalence
We also estimated effects on malaria prevalence measured using qPCR in a cross-sectional survey at the end of the malaria season (May to August 2017). In contrast to incidence analyses, which captured any effects within the period immediately after intervention, prevalence analyses captured effects of cumulative interventions near the end of the trial. Prevalence also captures symptomatic and asymptomatic malaria cases that did not necessarily present at health clinics. Analyses of direct effects included individuals who resided within 500 m any intervention recipients; spillover effects included individuals with no intervention recipients < 500 m and at least one recipient within 500 m-3 km; total effects included individuals with at least one intervention recipient within 3 km (Figure 2b). We estimated prevalence ratios using targeted maximum likelihood estimation and adjusted standard errors for enumeration area-level clustering.
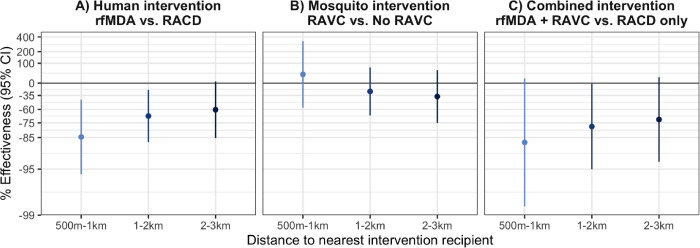
There was evidence of direct effects for all interventions, but confidence intervals included the null (Figure 2, Table S7). There was evidence of spillover effects: among non-recipients near intervention recipients, the chemoprevention intervention reduced prevalence by 72% (95% CI 31%, 88%), and the combined intervention reduced it by 79% (95% CI 6%, 95%). For the chemoprevention and combined interventions, spillover effects decreased in magnitude as distance to the nearest intervention increased (Figure 3). There was also evidence of spillover effects on prevalence of households with multiple malaria cases for the chemoprevention and combined interventions (Table S8).
Figure 3. Spillover effects of reactive, focal malaria interventions on prevalence by distance to nearest intervention recipient.
Spillover effects include individuals with no intervention recipients < 500m and any intervention recipients within different distance radii. a) Includes effects of the human intervention by comparing arms with rfMDA vs. those with RACD. b) Includes effects of the mosquito intervention by comparing arms with RAVC vs. those without RAVC. c) Includes effects of the combined intervention by comparing arms with rfMDA vs. RACD and rfMDA + RAVC vs. RACD only. Effects on prevalence estimated with TMLE using individual-level data; standard errors were adjusted for clustering at the enumeration area level. Models were adjusted for covariates that were screened separately for each model using a likelihood ratio test. Models were unadjusted because there were fewer than 10 malaria cases per variable. % Effectiveness was calculated as the ratio of incidence or prevalence between study arms minus 1 × 100%.
Effects on malaria seroprevalence
We also investigated whether there were effects on seroprevalence of early transcribed membrane protein 5 antigen (Etramp5.Ag1), an indicator of recent malaria infection (33) that was measured by Luminex in the cross-sectional survey. The chemoprevention intervention reduced seroprevalence among individuals who resided within 500m of intervention recipients by 25% (95% CI 14%, 34%), and the combined intervention reduced it by 34% (95% CI 10%, 42%) (Figure 2, Table S9). Among intervention non-recipients, the combined intervention reduced seroprevalence by 34% (95% CI 20%, 45%).
Cost-effectiveness
To inform policy decisions, we assessed cost-effectiveness using estimates of direct effects and spillover effects on prevalence. We calculated the incremental cost effectiveness ratio (ICER) by dividing the difference in cost between arms by the difference in prevalent cases averted between arms. We included cases averted for both individuals within 500m of any interventions and those with no intervention recipients < 500 m (direct effect population) and at least one recipient within 500 m-3 km (spillover effect population). Accounting for direct effects and spillover effects, the incremental cost-effectiveness ratios were $156 (95% CI $141, $177), $2,105 (95% CI $1,859, $2,430), and $1,142 (95% CI $944, $1,446) for the chemoprevention, vector control, and combined interventions (Table S10). Compared to the trial’s original incremental cost-effectiveness ratios estimates, accounting for spillover effects increased cost-effectiveness by 3%, 21%, and 37% for the chemoprevention, vector control, and combined interventions (34).
Discussion
Here, we showed that a combined intervention of reactive focal chemoprevention plus IRS reduced malaria infections in intervention recipients as well as non-recipients up to 3 km away. Overall, spillover effects among non-recipients were strongest for the combined intervention, which was designed to reduce the parasite reservoir in both humans and mosquitoes. When accounting for spillover effects, the cost-effectiveness of the combined intervention was 37% higher than the prior estimate (34).
Interventions that produce spillover effects yield greater population health benefits at no additional cost. A prior analysis found that the combined intervention was highly cost-effective, but it did not account for possible spillover effects (34). When accounting for spillover effects, interventions were 3–37% more cost-effective (34). Given that malaria elimination requires substantially larger investments than malaria control (3, 35), evidence about cost savings due to spillover effects is critical to policy decisions about elimination strategies.
We found stronger evidence of spillover effects of the combined chemoprevention and vector control intervention over larger spatial scales than two prior studies of targeted malaria interventions. In Kenya, a trial in a low-transmission area found no change in parasite prevalence within 500m of serologically-defined hot spots that received targeted larviciding, long-lasting insecticide treated nets, IRS, and focal mass drug administration (19). In Zambia, an observational study in a high transmission setting found that IRS targeted to subdistricts with higher malaria incidence and population density reduced parasite prevalence in sprayed and unsprayed households within target areas; it did not measure spillover effects outside of target areas (20). The interventions in our study may have been more likely to produce spillover effects because they were delivered repeatedly in response to subsequent index cases. In this trial, clusters received interventions up to 17 times per cluster; they were repeated annually over three years in the Zambia study and once in the Kenya trial. Further, it is possible that delivering interventions in response to new index cases can more effectively reduce transmission than targeting interventions based on an area’s incidence or seroprevalence.
For the chemoprevention intervention, there was evidence of spillover effects on prevalence and suggestive evidence of spillover effects and direct effects on incidence. Incidence analyses measured effects shortly after interventions, while prevalence analyses measured them at the end of the transmission season. Thus, our findings may indicate that reductions in local transmission accrued as additional rounds of chemoprevention interventions were delivered and population intervention coverage increased. This may especially be the case for the chemoprevention intervention since reductions in infectiousness of malaria cases are typically short-lived following treatment, especially in the absence of concurrent vector control (36). Overall, these findings suggest that reactive, focal chemoprevention can more effectively reduce asymptomatic or subclinical infections among nearby non-intervention recipients than RACD, particularly after repeated rounds.
For the vector control intervention, the primary analysis did not find direct effects, and the spillover effect estimate included the null despite strong biologic plausibility for both types of effects. We used a 6-month follow-up period to capture longer-term effects of IRS, which resulted in spatiotemporal overlap between analytic cohorts (Table S6). This overlap may have induced dependence between outcomes that was not fully accounted for by covariate adjustment, resulting in residual bias (28). Analyses of current infection prevalence were not subject to concerns about cohort overlap and were suggestive of direct effects, but confidence intervals included the null, and there was no evidence of spillover effects. Finally, our pre-specified subgroup analyses suggested that spillover effects of RAVC were driven by baseline transmission levels and environmental conditions: spillover effects on incidence were present in areas with baseline malaria incidence was <14 per 1,000 and when weather conditions favored mosquito breeding and survival (temperature < 31 °C; monthly rainfall < 27 mm).
The combined intervention appeared to have synergist effects, reducing local transmission to intervention non-recipients via spillover effects in all analyses. This may be because short-lived reductions in host infectiousness following chemotherapy can be sustained over time when coupled with IRS’ long-lasting reductions in mosquito populations. In effect, each intervention reduces the parasite reservoirs in hosts and vectors, and the combination of interventions slows the replenishment of parasite reservoirs (36). This may explain why we found that spillover effects were larger for prevalence of current infection, which captured effects at the end of the season, rather than incidence, which captured short-term effects. Our findings are consistent with two recent studies that found evidence of potential synergistic community-level effects when combining community-wide chemoprevention or seasonal malaria chemoprevention with IRS in high transmission settings (37, 38). Results are also consistent with a modeling study that estimated that the joint effect of chemoprevention and IRS was over 1.5 times higher than effects of intervention alone in low-transmission settings (36). Taken together, our results suggest that the combined intervention may be particularly effective as a reactive intervention or outbreak response in low-transmission settings approaching elimination or possibly following introduction of cases after elimination has been achieved.
Our estimates of direct and spillover effects shed light on the mechanism through which these targeted interventions work in time and space. We found that spillover effect sizes were generally similar to or stronger than direct effect sizes. It is possible that during the time between index case detection and intervention delivery (median 13–14 days) (24), transmission occurred to nearby intervention recipients. Thus, the interventions may not have been rapid enough to reduce malaria among recipients but may have prevented onward transmission to non-recipients further from index cases. In addition, our finding that spillover effects on prevalence were stronger at shorter distances to interventions suggests that the majority of the spillover effect occurred within 1km of index cases.
Our study was subject to several limitations. First, due to rare outcomes, precision was low in some analyses and may have increased the chance of Type II error, especially for direct effects. Second, when constructing analytic cohorts, household relocation after baseline could have resulted in misclassification of households to target areas or spillover zones. Third, incidence analyses could not fully rely on randomization-based inference due to cohort overlap; it is possible that covariate adjustment did not fully account for imbalances between arms. In future studies, using a ring trial design to test focal interventions could improve baseline balance, increase precision, and minimize overlap between target areas (39).
Despite these limitations, the internal consistency between the findings of this secondary analysis and the original trial, which each used different data structures and statistical methods, supports the validity of our findings. Estimates of total effects in this analysis, which pooled across intervention recipients and non-recipients, were consistent overall with those of the original trial, which included all individuals in study clusters (intervention recipients and non-recipients) (24, 40). Additional strengths include pre-specification of spillover analysis methods and use of individual-level, spatially indexed data to measure spillovers.
In conclusion, we found that reactive, focal malaria interventions targeting both human and mosquito parasite reservoirs reduced malaria risk, even among non-intervention recipients up to 3km from index cases. Further, the combined intervention could be particularly useful in responding to imported infections, which pose a persistent threat prior to and following elimination. Our findings suggest that these interventions are an effective strategy for achieving and maintaining malaria elimination.
Materials and Methods
Analysis overview
This study was a secondary analysis of a cluster-randomized trial of focal malaria interventions conducted in Zambezi region of Namibia from January 1 to December 31, 2017 (NCT02610400) (41, 24). Using a two-by-two factorial design, the trial randomly allocated 56 study clusters to study arms: reactive case detection (RACD) only, reactive focal mass drug administration (rfMDA) only, RACD + reactive vector control (RAVC), and rfMDA + RAVC. Here, we separately estimated effects among intervention recipients and non-recipients to estimate direct effects and spillover effects. We estimated the effects of the chemoprevention intervention (rfMDA vs. RACD), the vector control intervention (RAVC vs. no RAVC), or combined interventions (rfMDA + RAVC vs. RACD only), consistent with the original trial (24). The analysis plan for this study was pre-specified at https://osf.io/s8ay4/. Deviations from the pre-analysis plan and details about the study site and trial are in the Supporting Information.
Ethics statement
The trial protocol was approved by the Namibia Ministry of Health and Social Services (17/3/3) and the Institutional Review Boards at the University of California San Francisco (15–17422) and London School of Hygiene & Tropical Medicine (10411). The secondary analysis protocol was approved by the Stanford University Institutional Review Board (60708).
Incidence analyses
The unit of intervention (index cases) and the unit of randomization (clusters) differed, so cluster-level analyses would not have captured fine-scale direct effects and spillover effects. To capture the person-time in which we expected each intervention to influence incident malaria infections, we created analytic cohorts in space and time around each index case that triggered an intervention. The primary analysis used a 1 km radius around each index case because the majority of mosquito movement occurs within < 1 km.
We pre-specified cohort follow-up length based on the period in which we expected each intervention to reduce malaria among intervention recipients (direct effects) and non-recipients (spillover effects). For comparisons of rfMDA and RACD interventions, the direct effect follow-up period was 35 days, the length of intrinsic incubation period for Pf malaria (8). The spillover effect follow-up period was 21 to 56 days; the 3-week lag period allowed for gametocyte clearance in the treated individual, sporozoite development in mosquitos, and development of detectable merozoites in humans. For RAVC interventions, the direct effects follow-up period was 6 months since IRS can remain effective for an entire transmission season (9). The spillover effects follow-up period was from day 17 to 6 months. We conducted a sensitivity analysis with alternative follow-up lengths (rfMDA and RACD direct effects: day 0–21; spillover effects: day 21–42; RAVC direct effects day 0–7; spillover effects day 17–90). Additional details are in the Supporting Information.
Statistical models for incidence
To compare incidence between arms, we used hierarchical targeted maximum likelihood estimation (TMLE), a double robust, semi-parametric approach appropriate for cluster-level exposures (27). TMLE estimates both an outcome regression and a propensity score (the probability of treatment conditional on covariates) and updates the initial parameter estimate using information in the propensity score. Compared to other parametric models for clustered data (e.g., mixed effects models, generalized estimating equations), hierarchical TMLE imposes fewer assumptions and may be more efficient for randomized trials (42). We fit outcome and propensity score models using an ensemble machine learning algorithm (the Superlearner) (43). We adjusted standard errors to account for potential correlation due to overlap between some cohorts using a model of cohort-level influence curves analogous to variance-covariance models used in cross-random effects models (See details in Supporting Information) (44, 45).
Because incidence analyses did not rely on cluster randomization, we adjusted for covariates that were correlated with the outcome (likelihood ratio test p-value < 0.2) (46). Propensity score models adjusted for the following baseline variables: cluster-level indoor residual spray coverage, malaria incidence, median monthly rainfall, median enhanced vegetative index, and median daytime land surface temperature in the season prior to the trial, population size, and median elevation. Outcome models adjusted for individual- and cluster-level covariates. Individual-level covariates included sex, age, calendar month of intervention, distance from an individual’s residence to the residence of the index case that triggered an intervention, number of interventions an individual previously received, number of previously intervention recipients within 0.5, 1, 2, and 3 km of the individual’s residence (from the start of the trial to the start of the cohort’s observation period), and population size within 0.5, 1, 2, and 3 km of the individual’s residence. Cluster-level covariates included those in the propensity score models as well as mean distance to the nearest neighboring household, mean distance to the nearest healthcare facility, and mean time from index case detection to intervention.
Prevalence analyses
To capture effects of cumulative interventions at the end of the malaria transmission season, we estimated effects on prevalence using data from a cross-sectional survey. Direct effects analyses included individuals who resided near any intervention recipients within 500 m of their residence, spillover effects analyses included individuals with no intervention recipients < 500 m and at least one recipient within 500 m-3 km, and total effects included individuals with at least one intervention recipient within 3 km.
Statistical models for prevalence
Prevalence analyses used data from the cross-sectional survey conducted at the end of the malaria transmission season in 2017. We used targeted maximum likelihood estimation (47) with individual-level data with the same learners included in incidence analyses. We accounted for correlation within enumeration area-level clusters using cluster-level influence curve-based standard errors. The covariate set included the same cluster-level covariates included in incidence analyses and the following individual-level covariates: age, sex, occupation, recent travel, household slept under a bed net, slept outdoors in the past two weeks, and the total population, number of intervention recipients, number of intervention recipients in the same study arm, the number of intervention recipients in a different study arm, and the proportion of intervention recipients with the same treatment within 500 m, 1 km, 2 km, and 3 km of sampled individuals. We screened for covariates using the same approach described for incidence analyses.
Cost-effectiveness analysis
We estimated incremental cost effectiveness ratios (ICER) incorporating direct effects and spillover effects. To facilitate comparison with the original cost-effectiveness estimates for the trial (34), we used estimated effects on prevalence measured by qPCR. ICERs estimated using incidence would not be directly comparable between this study and the original trial because we used a cohort-level analysis, but the original trial used a cluster-level analysis. We used previously published estimates of total intervention costs in 2017 in US dollars (34). To obtain the number of prevalent cases averted, we multiplied the difference in prevalence between arms among intervention recipients and non-recipients by the estimated population size included in direct effects and spillover effects analyses. We calculated the ICER by dividing the difference in cost between arms by the difference in prevalent cases averted between arms among individuals who were located within 500m of any intervention recipients and individuals who resided within 500m to 3 km of interventions.
Supplementary Material
Significance Statement.
In settings where malaria transmission is declining and approaching elimination, new malaria cases are clustered in space and time. Prior studies have found that targeting prophylactic antimalarial drugs and vector control in the area around newly detected malaria cases reduced community-wide malaria. Here, we found that when antimalarials and vector control were delivered as a combined strategy in the area near recent cases, malaria incidence was reduced up to 3 kilometers away among individuals who did not receive interventions. Accounting for these benefits to non-recipients increased cost-effectiveness of the intervention. Overall, our findings suggest that combined, targeted malaria interventions can reduce local transmission and support their use for malaria elimination.
Acknowledgements
The original trial was supported by Novartis Foundation (A122666), the Bill & Melinda Gates Foundation (OPP1160129), and the Horchow Family Fund (5300375400). Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers K01AI141616 (PI: Benjamin-Chung) and the National Heart, Lung, And Blood Institute of the National Institutes of Health under award number T32HL151323 (Nguyen) and Novartis Foundation Supplement to A122666 (PIs: Gosling, Hsiang). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Jade Benjamin-Chung and Michelle Hsiang are Chan Zuckerberg Biohub Investigators. We also acknowledge the Stanford Research Computing Center for computational resources at the Sherlock high-performance cluster.
Footnotes
Competing Interest Statement: The authors have no competing interests to declare.
Data and Code Availability
Data from the original trial is available at https://clinepidb.org/ce/app/workspace/analyses/DS_f559aee789. Replication scripts are available at https://github.com/jadebc/malaria-focal-spillover-public.
References
- 1.Feachem R. G. A., et al. , Malaria eradication within a generation: ambitious, achievable, and necessary. The Lancet 394, 1056–1112 (2019). [DOI] [PubMed] [Google Scholar]
- 2., “World malaria report 2022” (World Health Organization; ) (May 8, 2023). [Google Scholar]
- 3.Sabot O., et al. , Costs and financial feasibility of malaria elimination. The Lancet 376, 1604–1615 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halloran E., Longini I. M. Jr., Struchiner C. J, Design and Analysis of Vaccine Studies (Springer, 2010). [Google Scholar]
- 5.Benjamin-Chung J., et al. , Spillover effects in epidemiology: parameters, study designs and methodological considerations. Int J Epidemiol 47, 332–347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin-Chung J., et al. , Spillover effects on health outcomes in low- and middle-income countries: a systematic review. Int J Epidemiol 46, 1251–1276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim T. H., Johnstone J., Loeb M., Vaccine herd effect. Scandinavian Journal of Infectious Diseases 43, 683–689 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard S. L., et al. , Estimating the herd immunity effect of rotavirus vaccine. Vaccine 33, 3795–3800 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Tsaban G., Ben-Shimol S., Indirect (herd) protection, following pneumococcal conjugated vaccines introduction: A systematic review of the literature. Vaccine 35, 2882–2891 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Borrow R., et al. , The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Review of Vaccines 16, 313–328 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Mertz D., et al. , Herd Effect From Influenza Vaccination in Non-Healthcare Settings: A Systematic Review of Randomized Controlled and Observational Studies. Open Forum Infect Dis 2, 1916 (2015). [Google Scholar]
- 12.Drolet M., et al. , Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. The Lancet 394, 497–509 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien K. S., et al. , Azithromycin distribution and childhood mortality in compliance-related subgroups in Niger: complier average causal effect and spillovers in a cluster-randomized, placebo-controlled trial. International Journal of Epidemiology 51, 1775–1784 (2022). [DOI] [PubMed] [Google Scholar]
- 14.House J. I., et al. , Assessment of herd protection against trachoma due to repeated mass antibiotic distributions: a cluster-randomised trial. The Lancet 373, 1111–1118 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Miguel E., Kremer M., Worms: Identifying Impacts on Education and Health in the Presence of Treatment Externalities. Econometrica 72, 159–217 (2004). [Google Scholar]
- 16.Binka F. N., Indome F., Smith T., Impact of spatial distribution of permethrin-impregnated bed nets on child mortality in rural northern Ghana. Am J Trop Med Hyg 59, 80–85 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Hawley W. A., et al. , Community-Wide Effects of Permethrin-Treated Bed Nets on Child Mortality and Malaria Morbidity in Western Kenya. Am J Trop Med Hyg 68, 121–127 (2003). [PubMed] [Google Scholar]
- 18.Jarvis C. I., et al. , Spatial Effects of Permethrin-Impregnated Bed Nets on Child Mortality: 26 Years on, a Spatial Reanalysis of a Cluster Randomized Trial. The American Journal of Tropical Medicine and Hygiene 101, 1434–1441 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bousema T., et al. , The Impact of Hotspot-Targeted Interventions on Malaria Transmission in Rachuonyo South District in the Western Kenyan Highlands: A Cluster-Randomized Controlled Trial. PLOS Medicine 13, e1001993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hast M. A., et al. , The Impact of 3 Years of Targeted Indoor Residual Spraying With Pirimiphos-Methyl on Malaria Parasite Prevalence in a High-Transmission Area of Northern Zambia. American Journal of Epidemiology 188, 2120–2130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J. J., The Role of Cost-Effectiveness in U.S. Vaccination Policy. New England Journal of Medicine 365, 1760–1761 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Ma S., Lavelle T. A., Ollendorf D. A., Lin P.-J., Herd Immunity Effects in Cost-Effectiveness Analyses among Low- and Middle-Income Countries. Appl Health Econ Health Policy 20, 395–404 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23., “WHO Guidelines for malaria” (World Health Organization, 2023).
- 24.Hsiang M. S., et al. , The effectiveness of reactive focal mass drug administration (rfMDA) and reactive focal vector control (RAVC) to reduce malaria transmission: a cluster-randomised controlled open label two-by-two factorial design trial from the low-endemic setting of Namibia. 395, 1361–1373 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makanga M., A review of the effects of artemether-lumefantrine on gametocyte carriage and disease transmission. Malar J 13, 291 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chanda E., et al. , An investigation of the Plasmodium falciparum malaria epidemic in Kavango and Zambezi regions of Namibia in 2016. Transactions of The Royal Society of Tropical Medicine and Hygiene 112, 546–554 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Zheng W., van der Laan M. J., “Cross-Validated Targeted Minimum-Loss-Based Estimation” in Targeted Learning: Causal Inference for Observational and Experimental Data, Springer Series in Statistics., van der Laan M. J, Rose S, Eds. (Springer, 2011), pp. 459–474. [Google Scholar]
- 28.Sobel M. E., What Do Randomized Studies of Housing Mobility Demonstrate? Journal of the American Statistical Association 101, 1398–1407 (2006). [Google Scholar]
- 29.Rubin D. B., Comment on: ‘Randomisation analysis of experimental data in the fisher randomisation test’ by D. Basu. Journal of the American Statistical Association 75, 591–593 (1980). [Google Scholar]
- 30.Manski C. F., Economic Analysis of Social Interactions. Journal of Economic Perspectives 14, 115–136 (2000). [Google Scholar]
- 31.Costantini C., et al. , Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a West African Sudan savanna village. Medical and Veterinary Entomology 10, 203–219 (1996). [DOI] [PubMed] [Google Scholar]
- 32.Huestis D. L., et al. , Windborne long-distance migration of malaria mosquitoes in the Sahel. Nature 574, 404–408 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Hoogen L. L., et al. , Selection of Antibody Responses Associated With Plasmodium falciparum Infections in the Context of Malaria Elimination. Frontiers in Immunology 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ntuku H., et al. , Cost and cost effectiveness of reactive case detection (RACD), reactive focal mass drug administration (rfMDA) and reactive focal vector control (RAVC) to reduce malaria in the low endemic setting of Namibia: an analysis alongside a 2×2 factorial design cluster randomised controlled trial. BMJ Open 12, e049050 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shretta R., Avanceña A. L. V., Hatefi A., The economics of malaria control and elimination: a systematic review. Malaria Journal 15, 593 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elliott R. C., Smith D. L., Echodu D. C., Synergy and timing: a concurrent mass medical campaign predicted to augment indoor residual spraying for malaria. Malaria Journal 18, 160 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Echodu D. C., et al. , Impact of population based indoor residual spraying with and without mass drug administration with dihydroartemisinin-piperaquine on malaria prevalence in a high transmission setting: a quasi-experimental controlled before-and-after trial in northeastern Uganda. BMC Infect Dis 23, 72 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagman J., et al. , Combining next-generation indoor residual spraying and drug-based malaria control strategies: observational evidence of a combined effect in Mali. Malaria Journal 19, 293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butzin-Dozier Z., Athni T. S., Benjamin-Chung J., A review of the ring trial design for evaluating ring interventions for infectious diseases. Epidemiologic Reviews In Press; (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L., et al. , Serological evaluation of the effectiveness of reactive focal mass drug administration and reactive vector control to reduce malaria transmission in Zambezi Region, Namibia: Results from a secondary analysis of a cluster randomised trial. eClinicalMedicine 44, 101272 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medzihradsky O. F., et al. , Study protocol for a cluster randomised controlled factorial design trial to assess the effectiveness and feasibility of reactive focal mass drug administration and vector control to reduce malaria transmission in the low endemic setting of Namibia. BMJ Open 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balzer L. B., Zheng W., van der Laan M. J., Petersen M. L., A new approach to hierarchical data analysis: Targeted maximum likelihood estimation for the causal effect of a cluster-level exposure. Stat Methods Med Res 28, 1761–1780 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Laan M. J., Polley E. C., Hubbard A. E., Super learner. Stat Appl Genet Mol Biol 6, Article 25 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Goldstein H., Multilevel Covariance Component Models. Biometrika 74, 430–431 (1987). [Google Scholar]
- 45.Raudenbush S. W., A Crossed Random Effects Model for Unbalanced Data With Applications in Cross-Sectional and Longitudinal Research. Journal of Educational Statistics 18, 321–349 (1993). [Google Scholar]
- 46.Pocock S. J., Assmann S. E., Enos L. E., Kasten L. E., Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Statistics in Medicine 21, 2917–2930 (2002). [DOI] [PubMed] [Google Scholar]
- 47.van der Laan M. J., Rose S., Targeted Learning: Causal Inference for Observational and Experimental Data (Springer, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the original trial is available at https://clinepidb.org/ce/app/workspace/analyses/DS_f559aee789. Replication scripts are available at https://github.com/jadebc/malaria-focal-spillover-public.