Abstract
Background
Understanding the role of circulating proteins in prostate cancer risk can reveal key biological pathways and identify novel targets for cancer prevention.
Methods
We investigated the association of 2,002 genetically predicted circulating protein levels with risk of prostate cancer overall, and of aggressive and early onset disease, using cis-pQTL Mendelian randomization (MR) and colocalization. Findings for proteins with support from both MR, after correction for multiple-testing, and colocalization were replicated using two independent cancer GWAS, one of European and one of African ancestry. Proteins with evidence of prostate-specific tissue expression were additionally investigated using spatial transcriptomic data in prostate tumor tissue to assess their role in tumor aggressiveness. Finally, we mapped risk proteins to drug and ongoing clinical trials targets.
Results
We identified 20 proteins genetically linked to prostate cancer risk (14 for overall [8 specific], 7 for aggressive [3 specific], and 8 for early onset disease [2 specific]), of which a majority were novel and replicated. Among these were proteins associated with aggressive disease, such as PPA2 [Odds Ratio (OR) per 1 SD increment = 2.13, 95% CI: 1.54–2.93], PYY [OR = 1.87, 95% CI: 1.43–2.44] and PRSS3 [OR = 0.80, 95% CI: 0.73–0.89], and those associated with early onset disease, including EHPB1 [OR = 2.89, 95% CI: 1.99–4.21], POGLUT3 [OR = 0.76, 95% CI: 0.67–0.86] and TPM3 [OR = 0.47, 95% CI: 0.34–0.64]. We confirm an inverse association of MSMB with prostate cancer overall [OR = 0.81, 95% CI: 0.80–0.82], and also find an inverse association with both aggressive [OR = 0.84, 95% CI: 0.82–0.86] and early onset disease [OR = 0.71, 95% CI: 0.68–0.74]. Using spatial transcriptomics data, we identified MSMB as the genome-wide top-most predictive gene to distinguish benign regions from high grade cancer regions that had five-fold lower MSMB expression. Additionally, ten proteins that were associated with prostate cancer risk mapped to existing therapeutic interventions.
Conclusion
Our findings emphasize the importance of proteomics for improving our understanding of prostate cancer etiology and of opportunities for novel therapeutic interventions. Additionally, we demonstrate the added benefit of in-depth functional analyses to triangulate the role of risk proteins in the clinical aggressiveness of prostate tumors. Using these integrated methods, we identify a subset of risk proteins associated with aggressive and early onset disease as priorities for investigation for the future prevention and treatment of prostate cancer.
Keywords: Proteomics, cis-pQTL, Prostate Cancer, plasma, protein
Introduction
Prostate cancer is a heterogeneous disease with a high survival rate for those diagnosed with indolent or low-stage disease, but a less than 50% 5-year survival rate for those diagnosed with aggressive or metastatic cancer.1 The proportion of these clinically aggressive cases is higher among men younger than 55 years (early onset disease), which contributes to premature death among these men.2,3 However, few risk factors for prostate cancer have been established. These include: advanced age, African ancestry, family history of the disease, circulating levels of insulin-like growth factor I and microseminoprotein-beta (MSMB), with little evidence for successful strategies for prevention. 4–7
Recent advances in multiplexed and high throughput platforms as well as the widespread availability of genotypic arrays have identified genetic variants that determine circulating levels of thousands of circulating proteins, known as protein-quantitative trait loci (pQTL). PQTL, in particular those lying in or near a protein’s cognate gene (referred to as cis-pQTL), can be leveraged to identify candidate etiological proteins for cancer risk through Mendelian randomization (MR) analyses, an approach that can limit the impact of reverse causality.8,9 MR can also be complemented with colocalization analyses to further exclude confounding by linkage disequilibrium (LD).10 Candidate etiological proteins for cancer risk identified using these methods can provide a valuable starting point for further analyses using more resource-intense methods, such as spatial transcriptomics, where their functional importance at the tissue level can be directly interrogated to triangulate their role in etiology.11,12
Using an integrated cis-pQTL MR and colocalization pipeline, we analyzed the associations of 2,002 unique proteins with overall, aggressive, and early onset prostate cancer and replicated and mapped those with significant findings to drug targets. Additionally, we investigated the spatial distribution and gene expression profiles of a subset of these proteins in prostate tumor tissue using spatial transcriptomics. In doing so, we demonstrate the value of protein MR and colocalization analyses to identify proteins that may have a causal role in the tumor aggressiveness.
Methods
Overall study design
We extracted cis genetic instruments for circulating protein levels from publicly available datasets, and harmonized these cis-pQTL with the GWAS results from an international prostate cancer consortium, including aggressive and early onset subtypes (Supplementary Figure 1). We subsequently estimated risk associations for protein levels using cis-pQTL MR against each of these three prostate cancer endpoints. All associations passing a multiple testing threshold in MR analyses were then followed up with colocalization analyses. Where data were available, we performed replication analyses in an external prostate cancer GWAS in European and African ancestry (using African ancestry specific cis-pQTLs – see below) populations for proteins with evidence from MR and colocalization analyses. For proteins identified as risk factors for prostate cancer with evidence of specific expression in the prostate tissue, we additionally performed analyses using spatial transcriptomics to gain insights into the spatial distribution and gene expression patterns of these proteins in prostate tumor samples. Finally, we conducted an exploratory analysis restricting to cis-pQTL whose cognate genes are established drug targets.
Identification of cis-pQTL
Genetic instruments for cis-pQTL were extracted from 4 publicly available protein GWAS at p < 5 × 10−8 and clumped at R2=0.01 within their originating panel (instruments presented in Supplementary Table 1).13–16 Cis-instruments were defined in the first instance as those that were genome-wide significant (p< 5 × 10−8) within 1 Mb of the transcription start side of the measured protein encoded gene, or as the sentinel cis-pQTL for the measured protein depending on data availability. We additionally gathered data on cis-pQTL from published GWAS present on the OpenGWAS platform using a relaxed p-value threshold of 5 × 10−5 due to the high biological plausibility of identifying cis-pQTL at or near a protein’s cognate gene.17,18 Specifically, we extracted unreported cis-pQTL from the genomic region 1 megabase up and downstream of the cognate gene for a given protein GWAS (Supplementary Table 1). We subsequently extracted all instruments where no cis-pQTL was present at p < 5 × 10−8 but at least one cis-pQTL was present at p < 5 × 10−05.
All instruments were mapped to Uniprot IDs, and cis-pQTL with weak instrument strength at Fstat < 10 [β2/σ2] were excluded from the study. For cis-pQTL that were not present in the cancer outcome data, SNP proxies were selected at r2max where r2 > 0.8 in 1000 genomes CEU population with the index cis-pQTL. In total, 2,002 unique plasma proteins that fit these criteria were included in analyses.
Cancer outcome data
Genetic associations for overall, aggressive, and early onset prostate cancer were obtained from the Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium (Supplementary material).19 Full study characteristics have been described previously, but briefly, summary statistics for SNP associations with prostate cancer and subtypes were generated from the PRACTICAL consortium using 85,554 overall prostate cancer cases and 91,972 controls (database of Genotypes and Phenotypes [dbGaP] project #31553), 15,167 aggressive PC cases and 58,308 controls, and 6,988 cases of early onset disease and 44,256 controls, all of European ancestry.20,21 Aggressive prostate cancer is defined in PRACTICAL as cases having metastatic disease or Gleason score >=8 or PSA >100 ng/mL or prostate cancer death. Early onset PC cases are defined as those diagnosed before the age of 55 years. Genotype information was imputed for samples using the 2014 release of the 1000 Genomes Project as a reference panel.
Two-sample Mendelian randomization
Cis-pQTL data were harmonized to each cancer outcome by rsID and oriented to the protein-increasing allele. Two-sample Mendelian randomization (MR) was subsequently performed for each cis-pQTL on risk of overall, aggressive, and early onset prostate cancer using the Wald-ratio method (βcancer/βprotein). Resulting associations where the pWald passed a Bonferroni-corrected threshold of significance based on the total number of unique proteins assessed for each of the three prostate cancer outcomes were taken forward in analyses (pWald < 0.05/NProteins analyzed per cancer outcome).22 Multiple independent [r2 < 0.01] cis-pQTL that proxied the same protein and were both associated after correction for multiple testing and that colocalised with the same prostate cancer outcome were combined using the inverse-variance weighted method (IVW). Odds ratio estimates are scaled per standard deviation increment in relative and normalized circulating protein concentrations.
Colocalization
Colocalization was performed to assess the probability that the protein and cancer instruments share a causal variant, fulfilling an important instrumental variable assumption of MR.9,10 Specifically, single and conditional iterative colocalization analysis were performed for all cis-pQTL MR results that passed a Bonferroni correction for multiple testing based on the number of unique proteins in the study (p < 0.05/ N Proteins), using all variants within a 75kb region up- and downstream from the index cis-pQTL to assess confounding by linkage disequilibrium.10,23 To mitigate the chance of false-positive findings, we selected priors of P1: 1 × 10−3, P2: 1 × 10−4, and P12: 1 × 10 −5, which roughly equate to a 0.1% prior belief in colocalization (PP4).24 We defined a threshold PP4 in support of a shared association for a protein and cancer signal at 0.70 to take proteins forward for subsequent analysis and the highest PP4 of any method of colocalization was recorded to assess confidence in the shared association for each SNP assessed.
Replication of robust proteins in European and African ancestry populations
We conducted a replication analysis of cis-pQTL MR associations passing multiple testing correction and that colocalized (referred to as robust proteins) using an external GWAS in a European ancestry population of overall prostate cancer risk. GWAS summary statistics represented a meta-analysis in FinnGen r9 and the UK Biobank (20,907 cases & 289,710 controls).25,26 Additionally, where possible, we performed replication analyses using cis-pQTL identified in an African ancestry protein GWAS in the Atherosclerosis Risk in Communities study (4,657 proteins in 467 African-ancestry participants) and a GWAS of overall prostate cancer among African-ancestry populations obtained from dbGaP (project #31553) containing data from the AAPC GWAS, Ghana Prostate Study, ProHealth Kaiser GWAS, and ELLIPSE OncoArray (10,368 cases and 10,986 controls).21,27 GWAS for aggressive and early onset prostate cancer were unavailable to use as a replicate sample in either ancestry population. We considered a directionally concordant risk estimates and Wald ratio p < 0.05 using external data to indicate replication. No sample overlap was present between samples used to generate protein associations and used to conduct replication analyses.
Drug target pQTL analyses
We restricted our MR results to those cis-pQTL that share a cognate gene that is an established drug target by reference to the DrugBank, Therapeutic Target Database, Pharos consortium, ClinicalTrials.gov or expert curation.28–30 As above, we defined robust associations as Wald p< 0.05/NProteins, where NProteins is the number of unique proteins analyzed for a given cancer outcome that were identified as the cognate gene of a pharmaceutical target and PP4 > 0.7. Additionally, all proteins identified in overall and drug target analyses were queried the Cortellis database (https://www.cortellis.com) to assess the highest current level of clinical development stage.
Statistical analysis was performed in R version 4.1 and all tests of significance were two-sided, where P values <0.05 were considered statistically significant. MR analyses were performed using the TwoSampleMR R package and colocalization analyses were performed using the coloc R package.17,23
Gene expression analysis using spatial transcriptomics
Spatial transcriptomics provides a spatial map of gene expression within the target tissue. This spatial information can be used to investigate the variation in gene expression by healthy tissue and tumor type intratumorally, and as a result, it can provide valuable insights into tumorigenesis and inform causal inference in this molecularly heterogeneous disease.31,32 Spatial transcriptomic analysis was performed for those proteins passing multiple testing correction and that colocalized and also showed high expression in the prostate epithelium.33 Data for spatial transcriptomics were obtained from our previously published dataset derived from radical prostatectomy tissue taken from a patient with multifocal prostate cancer.34 Our analysis focused on eight distinct tissue sections, which collectively comprised 32,156 spots, some of which contained regions of cancer as well as histo-pathologically benign prostate tissue, and some of which did not contain cancer. To ensure data quality, samples with less than 500 Unique Molecular Identifier (UMI) counts were excluded from the analysis. The initial fastq files were processed using the 10x Visium Spaceranger software, enabling the conversion of the files into gene expression data. Subsequently, the data underwent SCTransform normalization and variance reduction procedures. A consensus pathology approach was employed involving two pathologists who independently annotated each spatial transcriptomics spot, with the aim to include those that predominantly contained epithelial cells, which comprised approximately 1–15 cells. Violin plots were generated using Graphpad Prism (version 10).
Iterative random forest network using spatial transcriptomics
We used the iterative random forest (iRF) method to investigate gene interactions.35 With this method, we randomly selected genes and constructed random forests with other genes as branches to identify the most robust gene expression network. The analysis specifically focused on comparing the gene interactions between benign and Gleason grade group 4 histology status. The criteria we used to select credible random forest model was stability > 0.8 and precision > 0.8. The resulting gene network was visualized using Gephi 0.99.
Results
We investigated the associations of 2,002 unique proteins using 4,592 cis-pQTL that harmonized with the GWAS summary statistics for at least one of overall (1,999 proteins; 4,582 cis-pQTL), aggressive (1,986 proteins; 4,543 cis-pQTL), or early onset prostate cancer (1,984 proteins; 4,534 cis-pQTL) (Figure 1). From these analyses we identified 20 proteins that were associated, after correction for multiple testing, with at least one of overall (14 proteins), aggressive (7 proteins), or early-onset (8 proteins) prostate cancer and with support from colocalization analyses (Figure 2, Table 1). Of the 20 proteins associated with any prostate cancer outcome, several showed robust associations in only one outcome, including seven that appeared specific to overall prostate cancer (5NTC, CREBL1, INFA14, ISLR2, MMP7, SERPINA1, TNSFRS10B), three that appeared specific to aggressive disease (C4A, C2, TNFRSF6B), and two that appeared specific to early onset disease (SERPINA3, PYY).
Figure 1.
Association of genetically predicted protein concentrations with prostate cancer risk presented as a Manhattan plot where position is given by cis-pQTL coordinate (chromosome and base-pair position) labelled with their association with cancer risk and the highest colocalization probability from single or conditional iterative methods (PP4). Points highlighted as filled-in are those with evidence of a shared causal locus (PP4 > 0.7) with point size reflecting PP4 magnitude, which can vary between 0 and 1. Risk associations with MR p > Bonferroni correction threshold were not subject to colocalization analyses. The strongest protein-cancer association per chromosome is labelled and a zoomed-in plot for MSMB (rs10993994) on chromosome 10 is shown in the upper right-hand corner.
Figure 2.
Odds ratios (95% confidence intervals) for genetically predicted protein levels and prostate cancer risk (for proteins with p< Bonferroni threshold based on 0.05/number of proteins analyzed). Odds ratio estimates are scaled per standard deviation increment in genetically predicted relative circulating protein concentrations. Filled circles represent Bonferroni-significant associations and asterisks indicate evidence for colocalization (PP4 > 0.70).
Table 1.
Mendelian randomization and colocalization results for protein-cancer associations that passed Bonferroni correction (0.05/n proteins analyzed) and colocalized at PP4 > 0.70. Results are shown per SNP-cancer association, except for those proteins for which there were multiple SNPs that passed both multiple testing correction and colocalization, and for which the summary estimate using the Inverse Variance Weighted (IVW) method is provided. Odds ratios (95% confidence intervals) are oriented per standard deviation increase in genetically predicted protein level. Maximum PP4 is reported as highest PP4 value from either single or conditional iterative colocalization method assessing the probability of a shared causal locus. Odds ratios (95% confidence intervals) are reported for associations using external UK Biobank/FinnGen and for an African ancestry population where data existed. Drug targets and drug trials are annotated where they existed.
| Prostate Cancer Outcome | Gene | SNP | Proportion Variance Explained (%) | Uniprot ID | Platform | Odds ratio (95% CI) PRACTICAL | P-value (Unadjusted) | Maximum PP4 | Odds ratio (95% CI) UKBB/FinnGen | Odds ratio (95% CI) African Ancestry | Drug Target | Current Drug Trials |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Overall | MSMB | rs 10993994 | 49.60% | P08118 | SomaScan | 0.81 (0.8 –0.82) | 1.32E-165 | 1.00 | 0.83 (0.81–0.85) | 0.85 (0.80–0.91) | Phase I (Prostate Cancer therapy; PCK-3145) | |
| EHBP1 | rs73934251 | 0.53% | Q8NDI1 | SomaScan | 1.89 (1.63 –2.19) | 8.09E-18 | 0.98 | 2.09 (1.66–2.65) | ||||
| TPM3 | rs72696208 | 0.68% | P06753 | SomaScan | 0.64 (0.57 –0.72) | 2.72E-13 | 0.94 | 0.64 (0.53–0.77) | Phenethyl Isothiocyanate | |||
| PRSS3 | rs2005617 | 14.50% | P35030 | SomaScan | 0.91 (0.89 –0.94) | 6.47E-11 | 1.00 | 0.95 (0.91–0.99) | 0.95 (0.87–1.10) | 4-(1,3,2-DIOXABOROLAN-2YLO) | Biological testing | |
| PLG | rs982403 | 0.23% | P00747 | SomaScan | 0.48 (0.38 –0.6) | 2.96E-10 | 1.00 | 0.46 (0.31–0.67) | Tranexamic Acid | 11 Drugs Launched | ||
| rs11751347 | 1.66% | P00747 | SomaScan | 0.8 (0.75 –0.86) | 3.47E-10 | 0.83 | 0.84 (0.77–0.93) | |||||
| rs4252185 | 0.23% | P00747 | SomaScan | 0.6 (0.49 –0.74) | 2.07E-06 | 0.99 | 0.60 (0.44–0.81) | |||||
| IVW | 2.11% | P00747 | SomaScan | 0.75 (0.62–0.92) | 5.80E-03 | |||||||
| MMP7 | rs14983 | 1.56% | P09237 | SomaScan | 0.67 (0.58 –0.76) | 8.84E-10 | 0.98 | 0.62 (0.51–0.74) | 0.83 (0.73–0.95) | Marimastat | 1 in Phase I/II (Colorectal Cancer therapy; IMA-910) | |
| POGLUT3 | rs74911261 | 9.89% | Q7Z4H8 | SomaScan | 0.9 (0.86 –0.93) | 9.99E-09 | 1.00 | 0.85 (0.80–0.91) | 0.97 (0.87–1.10) | |||
| TNSFRS10B | rs2293400 | 4.30% | O14763 | OLINK | 0.87 (0.83 –0.92) | 3.02E-07 | 1.00 | 0.73 (0.68–0.79) | HGS-TR2J; Lexatumumab | 4 in Phase II (Therapy for multiple cancer types) | ||
| SERPINA1 | rs28929474 | 16.10% | P01009 | SomaScan | 1.08 (1.05 –1.11) | 3.01E-07 | 1.00 | 1.10 (1.0–1.10) | 1.06 (0.97–1.15) | Glassia | 1 in Phase II/III (Alpha-1 antitrypsin deficiency; fazirsirar | |
| 5NTC | rs4919682 | 0.50% | P49902 | SomaScan | 0.7 (0.6 –0.81) | 1.43E-06 | 1.00 | 0.64 (0.49–0.84) | 1 in Phase I (Prostate Cancer therapy; FP-253) | |||
| CREBL1 | rs8111 | 1.31% | Q99941 | SomaScan | 1.21 (1.12 –1.31) | 4.92E-06 | 0.99 | 1.23 (1.03–1.48) | ||||
| CREB3L4 | rs4845586 | 3.12% | Q8TEY5 | SomaScan | 1.14 (1.08 –1.2) | 7.71E-06 | 0.73 | 1.23 (1.13–1.34) | ||||
| INFA14 | rs662463 | 0.56% | P01570 | SomaScan | 0.74 (0.65 –0.85) | 9.04E-06 | 0.95 | 0.80 (0.64–0.99) | Biological Testing | |||
| ISLR2 | rs751527 | 1.36% | Q6UXK2 | SomaScan | 0.82 (0.75 –0.9) | 1.78E-05 | 0.97 | 0.84 (0.73–0.97) | 0.85 (0.73–0.99) | |||
| Aggressive | MSMB | rs10993994 | 49.60% | P08118 | SomaScan | 0.84 (0.82–0.86) | 1.55E-35 | 1.00 | Phase I (Prostate Cancer therapy; PCK-3145) | |||
| TNFRSF6B | rs6011040 | 0.50% | O95407 | SomaScan | 0.48 (0.37–0.63) | 1.72E-07 | 0.90 | |||||
| PPA2 | rs4699179 | 0.38% | Q9H2U2 | SomaScan | 0.76 (0.67–0.86) | 3.73E-06 | 0.99 | |||||
| C4A | rs2763982 | 22.00% | P0C0L4 | SomaScan | 0.91 (0.88–0.95) | 6.80E-06 | 0.99 | Preclinical | ||||
| TPM3 | rs72696208 | 0.68% | P06753 | SomaScan | 0.61 (0.5–0.76) | 8.56E-06 | 0.96 | Phenethyl Isothiocyanate | ||||
| PRSS3 | rs62555900 | 3.52% | P35030 | SomaScan | 0.8 (0.73–0.89) | 1.13E-05 | 0.99 | 4-(1,3,2-DIOXABOROLAN-2YLO) | Biological testing | |||
| C2 | rs3094662 | 0.75% | P06681 | SomaScan | 0.67 (0.56–0.81) | 2.39E-05 | 0.96 | Phase III (Cancer immunotherapy; 99mTc-ior C5) | ||||
| Early Onset | MSMB | rs10993994 | 49.60% | P08118 | SomaScan | 0.71 (0.68–0.74) | 1.46E-65 | 1.00 | Phase I (Prostate Cancer therapy; PCK-3145) | |||
| PLG | rs982403 | 3.57% | P00747 | SomaScan | 0.63 (0.53–0.73) | 9.38E-09 | 0.72 | 11 Drugs Launched | ||||
| rs11751347 | 1.66% | P00747 | SomaScan | 0.61 (0.52–0.72) | 9.38E-09 | 0.72 | ||||||
| rs4252185 | 0.23% | P00747 | SomaScan | 0.26 (0.14–0.46) | 4.07E-06 | 1.00 | ||||||
| IVW | P00747 | SomaScan | 0.57 (0.36–0.91) | 1.60E-02 | ||||||||
| EHBP1 | rs73934251 | 0.53% | Q8NDI1 | SomaScan | 2.90 (2.0–4.2) | 2.60E-08 | 0.98 | |||||
| TPM3 | rs72696208 | 0.68% | P06753 | SomaScan | 0.47 (0.34–0.64) | 1.92E-06 | 0.95 | Phenethyl Isothiocyanate | 1 Drug discontinued (Neurological Cancer therapy; anisi | |||
| PYY | rs8074783 | 0.00% | P10082 | SomaScan | 1.87 (1.43–2.44) | 4.36E-06 | 0.98 | Preclinical | ||||
| POGLUT3 | rs74911261 | 3.46% | Q7Z4H8 | SomaScan | 0.81 (0.73–0.89) | 6.67E-06 | 1.00 | |||||
| SERPINA3 | rs8023057 | 7.95% | P01011 | SomaScan | 2.08 (1.51–2.88) | 8.04E-06 | 0.99 | Zinc; Acetate, Chloride, Sulfate Preclinical | ||||
| PPA2 | rs4699179 | 0.38% | Q9H2U2 | SomaScan | 2.7 (1.71–4.27) | 1.99E-05 | 0.93 | |||||
The most statistically significant associations per standard deviation increase in protein level with evidence of colocalization were seen for MSMB (a protein that is specifically expressed in the prostate) with a lower risk of all prostate cancer endpoints [OROverall= 0.81, 95% CI: 0.79–0.82, PP4: 100%; ORAggressive = 0.84, 95% CI: 0.82–0.86, PP4: 0.99; OREarly Onset = 0.71, 95% CI: 0.68–0.74, PP4: 1.0, Table 1, Figure 2.]. TPM3 was the only other protein that had a colocalised association with risk of all outcomes [OROverall = 0.64, 95% CI: 0.57–0.73, PP4: 0.94; ORAggressive= 0.61, 95% CI: 0.49–0.76, PP4: 0.96; OREarly Onset = 0.47, 95% CI: 0.34–0.64, PP4: 0.95, Figure 2].
We also reported proteins with a colocalized association for one outcome and little evidence for an association with others after correction for multiple testing, such as IFNA14, ISLR2, MMP7, and TNSFRS10B which were associated exclusively with overall prostate cancer [ORIFNA14= 0.74, 95% CI: 0.70–0.78; ORISLR2= 0.82, 95% CI: 0.75–0.90; ORMMP7= 0.67, 95% CI: 0.58–0.76; ORTNFRSf10B= 0.87, 95% CI: 0.83–0.92, Figure 2]. Similarly, PYY and SERPINA3 associated with an increased risk of early onset prostate cancer only [ORPYY = 1.87, 95% CI: 1.43–2.44; ORSERPINA3 = 2.08, 95% CI: 1.51–2.88, Figure 2] while C2 associated with aggressive prostate cancer only [ORC2 = 0.67, 95% CI: 0.56–0.81, Figure 2].
We additionally identified proteins with evidence for a directionally concordant colocalized association with some but not all prostate cancer outcomes, including TNFRSF6B that had an inverse association with all outcomes but only showed evidence in favor of colocalization for aggressive disease [OROverall = 0.53, 95% CI: 0.46–0.61, PP4: 0.00; ORAggressive = 0.48, 95% CI: 0.0.37–0.63, PP4: 0.90; OREarly Onset = 0.43, 95% CI: 0.29–0.63, PP4: 0.14, Figure 2, Supplementary Table 1]. Likewise PPA2 was associated with an increased risk of both aggressive and early onset disease but lacked support from colocalization analyses for prostate cancer risk overall [OROverall = 1.84, 95% CI: 1.52–2.22, PP4: 0.01; ORAggressive = 2.13, 95% CI: 1.54–2.93, PP4: 0.99; OREarly Onset = 2.70, 95% CI: 1.71–4.27, PP4: 0.93, Figure 2, Supplementary Table 1].
Replication of robust proteins in European and African ancestry populations
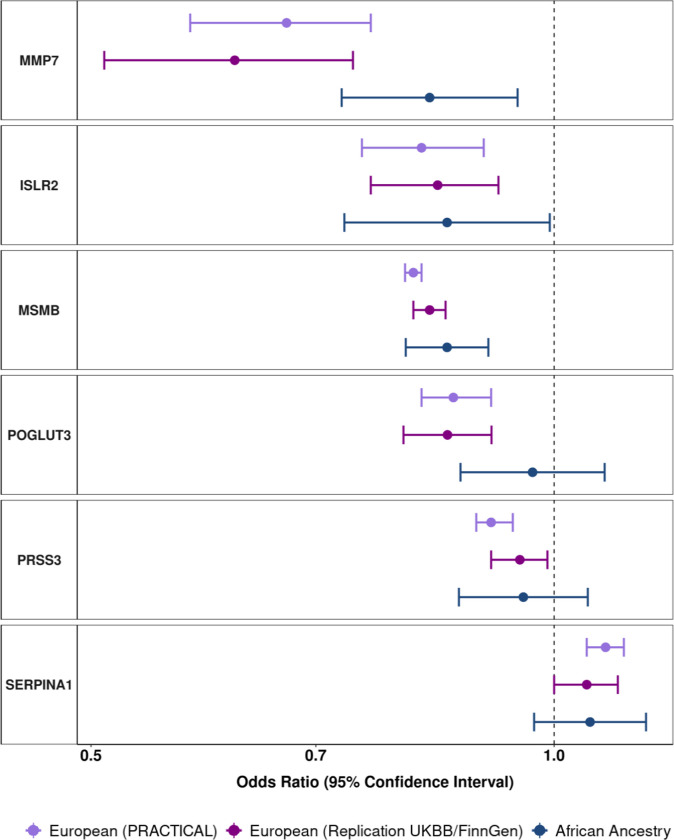
We replicated the association for all 14 proteins that were robustly associated with overall prostate cancer (5NTC, CREBL1, CREB3L4, EHBP1, INFA14, ISLR2, MMP7, MSMB, PRSS3, PLG, POGLUT3, SERPINA1, TNSFRF10B, TPM3) using an independent meta-analysis of European ancestry participants in the UK Biobank and FinnGen cohorts (Table 1). Among these, the most statistically significant association was for MSMB [OROverall = 0.83, 95% CI: 0.81 to 0.85, Table 1] and the largest effect size was for PLG [OROverall = 0.46, 95% CI: 0.07 to 0.84, Table 1]. We additionally identified African ancestry-specific cis-pQTL for six of the 14 proteins (ISLR2, MMP7, MSMB, POGLUT3, PRSS3, SERPINA1; Table 1). Of these, three proteins associations with risk of prostate cancer overall were replicated in men of African ancestry: MSMB [OROverall = 0.85, 95% CI: 0.80 to 0.91], MMP7 [OROverall = 0.83, 95% CI: 0.73 to 0.95], and ISLR2 [OROverall = 0.85, 95% CI: 0.73 to 0.99] (Figure 3, Table 1).
Figure 3.
Odds ratios (95% confidence intervals) for genetically predicted protein levels and overall prostate cancer risk for proteins with p< Bonferroni threshold based on 0.05/number of proteins analyzed in main analyses, and with data available to perform replication in an African ancestry and European ancestry population. Odds ratio estimates are scaled per standard deviation increment in genetically predicted circulating protein concentrations.
Drug target analysis
Out of the 2,002 unique proteins investigated, we identified 525 proteins that could be successfully mapped at the gene level to the target of a therapeutic intervention. Of these, ten (TPM3, PRSS3, PLG, MMP7, SERPINA1, SERPINA3, TNFRSF10B, C4A, HDGF, and LAYN) were associated with risk of at least one prostate cancer outcome after correction for investigating 525 drug target proteins and had evidence of colocalization (Supplementary Table 1). For example, PLG and C4A mapped to the clot dissolving class of fibrinolytics, TPM3 mapped to phenethyl isothiocyanate, and MMP7 mapped to matrix metalloproteinase inhibitor, marimastat.
Spatial transcriptomic analysis
Of the 20 proteins that were associated with at least once prostate cancer outcome, we performed a targeted follow-up analysis for the two proteins with known high expression in the prostate tumor epithelium, MSMB and CREB3L4, using organ-wide spatial transcriptomic data on tissue obtained by radical prostatectomy from a patient with multifocal prostate cancer.34 In analyzing epithelial-rich spots, we observed marked differences of MSMB expression between benign cells, where MSMB was highly abundant, and Gleason grade group 4 (GG4) cells, where MSMB was very low or absent in a majority of cells (log MSMBbenign [median, interquartile range]: 2.73 [2.00–3.12] vs. log MSMBGG4: 0.48 [0.00–1.20], Figure 4.). A similarly, albeit more modestly, lower MSMB expression was observed in GG1 and GG2 cells (log MSMBGG1: 1.62 [0.97–1.88] and log MSMBGG2: 0.95 [0.69–1.32] compared to benign cells (Figure 4.). A lower expression of CREB3L4 was noted in GG2 and GG4 cells compared to benign cells (log CREB3L4benign: 0.60 [0.30–0.85] vs. log CREB3L4GG2: 0.31 [0.00–0.48] vs. log CREB3L4GG4: 0.48 [0.00–0.85], Supplementary Figure 2). Additional genome-wide random forest analyses identified MSMB expression as the most important gene in terms of distinguishing between benign and GG4 cells (Figure 5).
Figure 4.
A) MSMB association with overall, early onset, and aggressive prostate cancer risk with replication in the FinnGen and UK Biobank populations and in an African ancestry population. Odds ratio (95% confidence interval) estimates are scaled per standard deviation increment in genetically predicted circulating MSMB concentrations B) Spatial visualization showing MSMB gene expression (top) and histology and tissue status (bottom) from organ-wide spatial transcriptomic data in two tumor sections (GG: Gleason grade group: GG1, Gleason score of 6 or lower; GG2, Gleason score of 3+4 = 7; GG4, Gleason score of 8). C) Violin plots representing gene expression in each spatial transcriptomics spot according to histological status. Statistical differences are indicated: **** p < 0.0001 (Kruskal–Wallis; post-test: Dunn’s test).
Figure 5.
Gene network from iterative random forests of the difference in gene expression between benign and GG4 prostate histology (Gleason Score = 8). Arrows indicate direction of influence and shape of the network. MSMB is colored to demonstrate its central role in the network.
Discussion
In this analysis, we conducted the largest study to date investigating the genetic associations of up to 2,002 unique proteins with the likely etiological risk of overall, early onset, and aggressive prostate cancer in up to 177,526 men using a MR and colocalization pipeline. In total, we found evidence supporting associations between 20 proteins and prostate cancer risk: 14 proteins for overall prostate cancer risk, seven for aggressive prostate cancer and eight for early onset prostate cancer. Among those 14 proteins that associated with prostate cancer risk overall, 14 were replicated in an external European ancestry population and three (out of six with available data) were replicated in an African ancestry population. A further half of the 20 proteins identified were also found to be the site of action for established drug targets with potential therapeutic implications. Finally, using spatial transcriptomics, we demonstrated a central role for the gene of our most robustly associated prostate cancer protein, MSMB, in distinguishing benign from undifferentiated, high-grade prostate cancer cells.
MSMB
MSMB is a secretory protein and member of the human immunoglobulin family that is released largely by luminal epithelial cells in the prostate epithelium and has a documented role in overall prostate cancer risk for both observational and genetic epidemiology.6,7,36 In this study, we expand upon these previous findings by demonstrating that a 20% lower risk of overall prostate cancer is associated with higher levels of genetically predicted MSMB in two independent European ancestry cancer GWAS, and confirm for the first time its protective role in both aggressive and early onset disease etiology. We further successfully replicated this association with prostate cancer risk overall in an African-ancestry population, an ancestry group with an established higher risk of developing the disease. Subsequently, in reporting that MSMB gene expression is significantly depleted among high grade tumor when compared to expression on benign cells, we reiterate, through an independent line of evidence, that this gene may be particularly relevant to tumorigenesis and risk for aggressive disease.
Although the mechanism of action for MSMB in prostate cancer is not clear, MSMB has been shown to have a regulatory effect on cell growth, which may be lost during tumorigenesis while a MSMB-derived polypeptide was shown to induce prostate cell death.36,37 Additionally, in a rodent model and in vitro, higher MSMB activity was found to suppress prostate tumor growth while a knockout of MSMB promoter/enhancer regions was characterized by tumor progression and metastases.38,39 Given the integration of several compelling lines of evidence presented in this paper with existing literature, further research is warranted to understand the precise functional role of MSMB in prostate cancer tumorigenesis, identify environmental and lifestyle determinants, and explore potential clinical utility.
Transcription Factors CREBL34 and CREBL1
We identified novel proteins associated with overall, early onset, and aggressive prostate cancer etiology. These include the endoplasmic reticulum (ER) originating transcription factors CREB3L4 and CREBL1 (also known as ATF6B), which we find are associated with an increased risk of prostate cancer overall and that are expressed in the prostate epithelium.33 These transcription factors form part of a transcriptional factor network that regulates the function of the endoplasmic reticulum (ER) and the activity of the unfolded protein response (UPR). There is an established role for the UPR and heat-shock proteins in maintaining AR stability and supporting AR-dependent tumorigenesis.40,41 CREB3L4 has been shown to directly interact with AR in LNCaP cells to increase cellular proliferation and is abundantly expressed in prostate tumor tissue.42,43 Furthermore, evidence suggests that disruptions in CREB3L4 contribute to ER stress downstream initiation of the unfolded protein response. 43,44 In a previous study of differential gene expression in prostate tissue, CREB3L4 was identified as a member of a co-expression gene cluster enriched for a previously described metabolic pathway (hsa05215) in prostate cancer that may regulate apoptosis and cell proliferation.45 Interestingly, we found that CREB3L4 expression is lower in GG2 and GG4 cells as opposed to benign cells.
Previous studies have linked AR activation with members of the ATF6 family in LNCaP and PC3 cells, however these experiments have mostly focused on ATF6A.46,47 For example, a recent in vivo study showed that prostate cancer cells with ATF6A overexpression resisted cellular death by ferroptosis.46 In parallel, a previous MR study reported a lowered risk of prostate cancer overall with genetically elevated circulating ATF6A levels from trans-pQTL.48 Given the promising role of its paralog, and the increased risk we report here, targeted follow up of CREBL1 may prove valuable in characterizing the broader role of ER stress proteins and AR-dependent tumorigenesis.
EHBP1
We observed a more than two-fold increased risk of early-onset prostate cancer associated with higher EHBP1, an adaptor protein with a key role in vesicular trafficking and actin reorganization.49 Variants in the EHBP1 intron have previously been associated with aggressive prostate cancer in a genetic association study the protein and is more highly expressed in prostate tumor tissue and may have a role in determining the invasiveness of PTEN-positive prostate cancer cells according to GWAS and expression data and in a cellular study.50–52 While mechanisms that may link EHBP1 to prostate cancer risk are not yet fully described, it has role as an effector molecule for Rab8 family members that modulate polarized membrane transport via actin reorganization and may have a role in the mechanism of action for atorvastatin.50
Other early onset and aggressive disease proteins
We found several proteins that were associated with early onset and aggressive disease, including PYY, PRSS3, PPA2, C2, C4A, and SERPINA3. For example, PYY is a metabolic hormone involved in appetite regulation, and while there has been some hypothesized relationship between obesity and aggressive prostate cancer risk in the past, recent findings suggest that obesity does not serve as a risk factor for disease itself, but may affect likelihood of diagnosis.53,54 Additionally, one study found that mesotrypsin, a protease encoded by the PRSS3 gene, was essential for prostate cancer metastasis in vitro and mouse models, however the role of this protein has not been widely studied in humans.55
We also note that proteins associated with early onset disease were generally greater in magnitude when compared to their associations with overall or aggressive disease. Two of these proteins, the complement proteins C2 and C4A, sit on chromosome six, which contains a particularly dense genetic region including the MHC complex and is consequently particularly difficult to interpret. However, given the importance of addressing early onset and aggressive disease, future studies are needed to further investigate and replicate the associations with early onset disease to uncover potential subtype specific mechanisms of disease onset and progression.
Drug target proteins
We identified 10 proteins that were associated with both the risk of prostate cancer and that were the site of action for a known drug. These included TNFRSF10B, which is a receptor for the cytotoxic TRAIL ligand, and is essential for CASP8 and ER stress induced apoptosis.56 Further, TNFRSF10B expression is lower in higher grade prostate tumors and a recent study of PARP inhibitors in prostate cancer cell lines suggested that TNFRSF10B may provide a mechanism by which the cancer drug Olaparib induces apoptosis.57 The apparent protective association we observe with prostate cancer risk is in line with the results from multiple phase I/II trials of TNFRSF10B agonists that support their use for the treatment of multiple cancer endpoints, though not yet including prostate cancer. 57–59
We also identified an inverse association of PLG, a serine protease targeted by transexamic acids and several classes of thrombolytics, with prostate cancer risk overall and with early onset disease. Transexamic acids are primarily prescribed to control excessive bleeding while thrombolytics are primarily used to dissolve blot clots and act via plasmin and fibrin pathways.60 One molecular study found that PLG is generated by the cancer-mediated proteolysis of plasminogen which is released by human prostate carcinoma cells.61 PLG in turn has been shown in many lab studies to inhibit angiogenesis which when unregulated can lead to the rapid formation of tumors.62,63 Currently, several studies investigated combination therapy including plasminogen activation or inhibition for treatment of several cancer types, though not specifically for prostate cancer, in phase I/II trials.64,65
SERPINA1 maps to fazisiran, the treatment for alpha-1 antitrypsin deficiency and that is in phase II/III of drug trials. Previous findings have indicated that alpha-1 antitrypsin levels are often elevated in many carcinomas, including prostate.66 However, no agents targeting SERPINA1 have been investigated in cancer trials thus far. MMP7 belongs to a class of matrix metalloproteinases that participate in wound healing, bone growth, and matrix remodeling. There are multiple lines of evidence that this protein is involved in many cancers, and agents targeting metalloproteases, such as marimastat, are currently being investigated in clinical trials at various phases.67 In prostate cancer, marimastat showed some efficacy in early trials, however has not yet progressed further.68
While we highlight proteins that may share the same target site for established drug targets that may have implications for therapeutic use, the suitability of these to act as preventative or remedial agents requires careful considerations including the site specificity, potential downstream effects, routes of administration and effectively capturing the population at risk.69
Strengths and weaknesses
This study offers several strengths including being the largest currently available GWAS of prostate cancer outcomes and the use of both aggressive and early onset endpoints with cis-pQTL covering up to 2,002 proteins. One previous MR study investigated the role of the circulating proteome in prostate cancer risk but did not stratify analyses by cis or trans- pQTL, and did not perform colocalization analyses, making it more challenging to infer causal relationships between individual proteins and cancer risk.48 Additionally, by integrating gene expression data measured using spatial transcriptomics, the current paper introduces a novel translational approach to highlight biological enablers of prostate cancer. To our knowledge, this study provides the first demonstration that MR using cis instruments of plasma protein levels can be used to identify a risk protein that has both specific expression in the cell of cancer origin and is related to tumor aggressiveness – important features to consider when identifying candidate targets for therapeutic prevention.
While we have analyzed a wide array of proteins, we have not investigated the entire human plasma proteome (n ~ 20,000 protein-coding genes). As more protein GWAS data become available, it will become possible to use genetic methods to investigate more proteins. However, some blood proteins are unlikely to have a cis-pQTL due to the degree of evolutionary constraint for a protein’s cognate gene. Additional limitations include the more modest GWAS sample sizes for aggressive and early onset prostate cancer, which have lower power to discover novel protein associations. Finally, while we were able to perform additional analyses to replicate some, but not all, of our robust proteins in populations of African ancestry, we note as a limitation that GWAS sample sizes in this group are not yet sufficient to perform well-powered discovery analyses. Especially given the increased risk for prostate cancer among populations of African ancestry, it is essential that future studies identify risk proteins in more diverse populations and allow for the discovery of ancestry-specific markers of risk.
Conclusion
This paper provides a catalogue of 20 proteins with evidence of etiological significance for prostate cancer. These proteins present an opportunity to direct further molecular and epidemiological investigations aimed at exploring the specific roles that the proteome plays in tumorigenesis and ultimately may inform future research into therapeutic prevention. In particular, converging evidence from population genetic and tumor sequencing analyses implicates MSMB as having an important protective role in prostate tumorigenesis, both in European and African-ancestry men, which is particularly marked for aggressive and early onset disease.
Supplementary Material
Funding:
This work was supported by Cancer Research UK (grant no. C8221/A29017). TAD is supported by a Cancer Research UK studentship grant number (C8221/A30904). KSB is supported by Cancer Research UK (grant nos. C8221/A29017 and C16077/A29186) and UKRI grant no. 10063259. ELW is supported by the Intramural Research Program of the National Institutes of Health (NIH) RMM is a National Institute for Health Research Senior Investigator (NIHR202411). RMM is supported by a Cancer Research UK 25 (C18281/A29019) program grant (the Integrative Cancer Epidemiology Program). RMM is also supported by the NIHR Bristol Biomedical Research Centre which is funded by the NIHR and is a partnership between University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. RMM is affiliated with the Medical Research Council Integrative Epidemiology Unit at the University of Bristol which is supported by the Medical Research Council (MC_UU_00011/1, MC_UU_00011/3, MC_UU_00011/6, and MC_UU_00011/4) and the University of Bristol. Sandy Figiel is funded by the The Hanson Trust.
Footnotes
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer / World Health Organization. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the National Institutes of Health. Department of Health and Social Care disclaimer: The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Anders Malarstig, Åsa Hedman, and Marios Dimitriou are employees of Pfizer Inc.
References
- 1.Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 68, 7–30 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Salinas C. A., Tsodikov A., Ishak-Howard M. & Cooney K. A. Prostate Cancer in Young Men: An Important Clinical Entity. Nat Rev Urol 11, 317–323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shih H.-J., Fang S.-C., An L. & Shao Y.-H. J. Early-onset prostate cancer is associated with increased risks of disease progression and cancer-specific mortality. Prostate 81, 118–126 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Bergengren O. et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors—A Systematic Review. European Urology 84, 191–206 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watts E. L. et al. Circulating insulin-like growth factors and risks of overall, aggressive and early-onset prostate cancer: a collaborative analysis of 20 prospective studies and Mendelian randomization analysis. International Journal of Epidemiology 52, 71–86 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith Byrne K. et al. The role of plasma microseminoprotein-beta in prostate cancer: an observational nested case-control and Mendelian randomization study in the European prospective investigation into cancer and nutrition. Ann Oncol 30, 983–989 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haiman C. A. et al. Levels of Beta-Microseminoprotein in Blood and Risk of Prostate Cancer in Multiple Populations. J Natl Cancer Inst 105, 237–243 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swerdlow D. I. et al. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int J Epidemiol 45, 1600–1616 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess S., Foley C. N. & Zuber V. Inferring Causal Relationships Between Risk Factors and Outcomes from Genome-Wide Association Study Data. Annual Review of Genomics and Human Genetics 19, 303–327 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giambartolomei C. et al. Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics. PLoS Genet 10, e1004383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawlor D. A., Tilling K. & Davey Smith G. Triangulation in aetiological epidemiology. Int. J. Epidemiol. dyw314 (2017) doi: 10.1093/ije/dyw314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams C. G., Lee H. J., Asatsuma T., Vento-Tormo R. & Haque A. An introduction to spatial transcriptomics for biomedical research. Genome Medicine 14, 68 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferkingstad E. et al. Large-scale integration of the plasma proteome with genetics and disease. Nat Genet 53, 1712–1721 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Zheng J. et al. Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat Genet 52, 1122–1131 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folkersen L. et al. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat Metab 2, 1135–1148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X. et al. Genetically predicted levels of circulating cytokines and prostate cancer risk: A Mendelian randomization study. Int. J. Cancer 147, 2469–2478 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Hemani G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 7, e34408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fauman E. B. & Hyde C. An optimal variant to gene distance window derived from an empirical definition of cis and trans protein QTLs. BMC Bioinformatics 23, 169 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.PRACTICAL. http://practical.icr.ac.uk/. [Google Scholar]
- 20.Schumacher F. R. et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet 50, 928–936 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conti D. V. et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat Genet 53, 65–75 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S., Foley C. N., Allara E., Staley J. R. & Howson J. M. M. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun 11, 376 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace C. A more accurate method for colocalisation analysis allowing for multiple causal variants. PLOS Genetics 17, e1009440 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet 16, e1008720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurki M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewitt J., Walters M., Padmanabhan S. & Dawson J. Cohort profile of the UK Biobank: diagnosis and characteristics of cerebrovascular disease. BMJ Open 6, e009161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J. et al. Plasma proteome analyses in individuals of European and African ancestry identify cis-pQTLs and models for proteome-wide association studies. Nat Genet 54, 593–602 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wishart D. S. et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46, D1074–D1082 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheils T. K. et al. TCRD and Pharos 2021: mining the human proteome for disease biology. Nucleic Acids Res 49, D1334–D1346 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y. et al. Therapeutic target database update 2022: facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Research 50, D1398–D1407 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erickson A. et al. A Systematic Review of Prostate Cancer Heterogeneity: Understanding the Clonal Ancestry of Multifocal Disease. European Urology Oncology 4, 358–369 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Hunter M. V., Moncada R., Weiss J. M., Yanai I. & White R. M. Spatially resolved transcriptomics reveals the architecture of the tumor-microenvironment interface. Nat Commun 12, 6278 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.THE TABULA SAPIENS CONSORTIUM. The Tabula Sapiens: A multiple-organ, single-cell transcriptomic atlas of humans. Science 376, eabl4896 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson A. et al. Spatially resolved clonal copy number alterations in benign and malignant tissue. Nature 608, 360–367 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basu S., Kumbier K., Brown J. B. & Yu B. Iterative random forests to discover predictive and stable high-order interactions. Proceedings of the National Academy of Sciences 115, 1943–1948 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitaker H. C., Warren A. Y., Eeles R., Kote-Jarai Z. & Neal D. E. The potential value of microseminoprotein-beta as a prostate cancer biomarker and therapeutic target. Prostate 70, 333–340 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Annabi B. et al. Contribution of the 37-kDa laminin receptor precursor in the anti-metastatic PSP94-derived peptide PCK3145 cell surface binding. Biochem Biophys Res Commun 346, 358–366 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Annabi B. et al. A PSP94-derived peptide PCK3145 inhibits MMP-9 secretion and triggers CD44 cell surface shedding: implication in tumor metastasis. Clin Exp Metastasis 22, 429–439 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Lamy S. et al. A prostate secretory protein94-derived synthetic peptide PCK3145 inhibits VEGF signalling in endothelial cells: implication in tumor angiogenesis. Int J Cancer 118, 2350–2358 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Jin Y. & Saatcioglu F. Targeting the Unfolded Protein Response in Hormone-Regulated Cancers. Trends in Cancer 6, 160–171 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Centenera M. M., Fitzpatrick A. K., Tilley W. D. & Butler L. M. Hsp90: Still a viable target in prostate cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1835, 211–218 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Labrie C. et al. Androgen-regulated transcription factor AIbZIP in prostate cancer. The Journal of Steroid Biochemistry and Molecular Biology 108, 237–244 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Kim T.-H., Park J.-M., Kim M.-Y. & Ahn Y.-H. The role of CREB3L4 in the proliferation of prostate cancer cells. Sci Rep 7, 45300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pu Q. et al. The Novel Transcription Factor CREB3L4 Contributes to the Progression of Human Breast Carcinoma. J Mammary Gland Biol Neoplasia 25, 37–50 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Pidò S., Ceddia G. & Masseroli M. Computational analysis of fused co-expression networks for the identification of candidate cancer gene biomarkers. NPJ Syst Biol Appl 7, 17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao R. et al. ATF6α promotes prostate cancer progression by enhancing PLA2G4A-mediated arachidonic acid metabolism and protecting tumor cells against ferroptosis. Prostate 82, 617–629 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou H. et al. The functional implication of ATF6α in castration-resistant prostate cancer cells. The FASEB Journal 37, e22758 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Wu L. et al. Analysis of over 140,000 European descendants identifies genetically-predicted blood protein biomarkers associated with prostate cancer risk. Cancer Res 79, 4592–4598 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rai A., Bleimling N., Vetter I. R. & Goody R. S. The mechanism of activation of the actin binding protein EHBP1 by Rab8 family members. Nat Commun 11, 4187 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghalali A., Wiklund F., Zheng H., Stenius U. & Högberg J. Atorvastatin prevents ATP-driven invasiveness via P2X7 and EHBP1 signaling in PTEN-expressing prostate cancer cells. Carcinogenesis 35, 1547–1555 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Gudmundsson J. et al. Common sequence variants on 2p15 and Xp11.22 confer susceptibility to prostate cancer. Nat Genet 40, 281–283 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mamidi T. K. K., Wu J. & Hicks C. Integrating germline and somatic variation information using genomic data for the discovery of biomarkers in prostate cancer. BMC Cancer 19, 229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hurwitz L. M., Dogbe N., Barry K. H., Koutros S. & Berndt S. I. Obesity and prostate cin ancer screening, incidence, and mortality in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. JNCI: Journal of the National Cancer Institute djad113 (2023) doi: 10.1093/jnci/djad113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez-Cornago A., Dunneram Y., Watts E. L., Key T. J. & Travis R. C. Adiposity and risk of prostate cancer death: a prospective analysis in UK Biobank and meta-analysis of published studies. 2021.10.05.21264556 10.1101/2021.10.05.21264556v1 (2021) doi:. [DOI] [PMC free article] [PubMed]
- 55.Hockla A. et al. PRSS3/Mesotrypsin is a therapeutic target for metastatic prostate cancer. Molecular cancer research : MCR 10, 1555 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hagenlocher C. et al. ER stress-induced cell death proceeds independently of the TRAIL-R2 signaling axis in pancreatic β cells. Cell Death Discov. 8, 1–9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hernandez-Cueto A. et al. Death receptor 5 expression is inversely correlated with prostate cancer progression. Molecular Medicine Reports 10, 2279–2286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subbiah V. et al. Preclinical Characterization and Phase I Trial Results of INBRX-109, A Third-Generation, Recombinant, Humanized, Death Receptor 5 Agonist Antibody, in Chondrosarcoma. Clin Cancer Res 29, 2988–3003 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forero-Torres A. et al. TBCRC 019: An open label, randomized, phase II trial of nanoparticle albumin-bound paclitaxel (nab-PAC or Abraxane®) with or without the antideath receptor 5 (DR5) monoclonal antibody tigatuzumab in patients with metastatic triple negative breast cancer. Clin Cancer Res 21, 2722–2729 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reed M. R. & Woolley L. T. Uses of tranexamic acid. Continuing Education in Anaesthesia Critical Care & Pain 15, 32–37 (2015). [Google Scholar]
- 61.Didiasova M., Wujak L., Wygrecka M. & Zakrzewicz D. From Plasminogen to Plasmin: Role of Plasminogen Receptors in Human Cancer. Int J Mol Sci 15, 21229–21252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Capello M., Ferri-Borgogno S., Cappello P. & Novelli F. α-Enolase: a promising therapeutic and diagnostic tumor target. FEBS J 278, 1064–1074 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Kwaan H. C. & McMahon B. The role of plasminogen-plasmin system in cancer. Cancer Treat Res 148, 43–66 (2009). [DOI] [PubMed] [Google Scholar]
- 64.University of Aarhus. Perioperative Treatment With Tranexamic Acid in Melanoma; Prognostic and Treatment-related Impact of the Plasminogen-plasmin Pathway. https://clinicaltrials.gov/study/NCT05899465 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Northwestern University. Phase I/II Trial of In Vivo Angiostatin Generation With Tissue Plasminogen Activator (tPA) and Captopril in Patients With Progressive, Metastatic Cancer. https://clinicaltrials.gov/study/NCT00086723 (2012). [Google Scholar]
- 66.El-Akawi Z. J., Abu-awad A. M. & Khouri N. A. Alpha-1 Antitrypsin Blood Levels as Indicator for the Efficacy of Cancer Treatment. World J Oncol 4, 83–86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao H.-Y., Da C.-M., Liao B. & Zhang H.-H. Roles of matrix metalloproteinase-7 (MMP-7) in cancer. Clinical Biochemistry 92, 9–18 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Rosenbaum E. et al. Marimastat in the treatment of patients with biochemically relapsed prostate cancer: a prospective randomized, double-blind, phase I/II trial. Clin Cancer Res 11, 4437–4443 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Bull S. C. & Doig A. J. Properties of Protein Drug Target Classes. PLoS One 10, e0117955 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.