Summary
Cilia are organelles involved in motility and sensory transduction, but how these two functions coexist has not been elucidated in depth. Here, the involvement of the ciliary transient receptor potential (TRP) channel TRP11 in mechanoresponses is studied in Chlamydomonas reinhardtii using a TRP11-knockout mutant. The mutant has defects in the conversion of the bending mode of the cilium from forward to reverse when tapped with a glass rod, the detachment of cilia when shear is applied, the increase in ciliary beat frequency upon application of mechanical agitation by vortex mixing, and the initiation of gliding while both cilia are attached in opposite directions to a glass surface. These observations indicate that TRP11 can perceive mechanical stimuli with distinct intensities and durations and induce various types of ciliary responses.
Subject areas: Microbiology, Microbial physiology
Graphical abstract
Highlights
-
•
TRP11 is a TRP channel expressed in Chlamydomonas cilia
-
•
Cilia change motility or detach from cell body upon mechanical stimulation
-
•
TRP11 is involved in various types of mechanoresponses
Microbiology; Microbial physiology
Introduction
The natural environment is full of mechanical stresses such as wind, flow, vibration, acceleration, and osmotic challenge. Living organisms have ways of sensing mechanical stimuli to cope with or make use of them. Examples of mechanosensation include hearing, sense of touch, and gravity sensing in animals, gravitropism in plants, and osmotic response in bacteria.
Mechanoresponses in protists have attracted interest for more than 100 years since becoming readily observable under a microscope.1,2 For example, when swimming microorganisms collide with an obstacle, they show transient backward swimming, without which they might push against the obstacle until their energy runs out. In Paramecium, this avoiding reaction is initiated by a mechanoreceptor potential, which triggers an action potential in cilia.3 The influx of Ca2+ ions caused by the action potential increases the intraciliary Ca2+ concentration and converts the ciliary bending motility from the forward mode to the reverse mode.4 Mechanical stimulation on the anterior end of the cell depolarizes the membrane potential, leading to the avoiding reaction, whereas, on the posterior end, it hyperpolarizes the membrane potential and augments, rather than reverses, ciliary beating.3 The molecular entities of the anterior and posterior mechanoreceptors have not been identified in Paramecium; however, a variety of mechanoresponses have been reported in protists, such as mechanosensitive contraction in Stentor coeruleus and Vorticella convallaria, gravitaxis in Euglena, mechanoshock response in Spermatozopsis similis, and osmoregulation in Trypanosoma cruzi.5,6,7,8,9 Membrane excitability and mechanoreception in protists have previously been reviewed.10,11
Chlamydomonas cells also show a wide range of mechanoresponses. Like Paramecium cells, they display an avoiding reaction when they collide with an obstacle.12 On application of mechanical vibration, Chlamydomonas cells increase their ciliary beat frequency and swimming velocity.13 Chlamydomonas cells also increase the power of their ciliary motility when the viscosity of the medium increases.14 Because the increase in ciliary power does not occur in cells that have been demembranated and reactivated, some membrane components such as ion channels are likely to be involved in this response. Harsher mechanical stimulation by mechanical shear causes detachment of cilia from the cell body.15 The deciliation might be due to activation of severing machinery located at the base of the cilia, or it might simply be a mechanical consequence rather than a physiological process.16 Chlamydomonas cells also display negative gravitaxis and accumulate toward the upper surface of the medium, although the specific gravity of the cell is larger than that of the medium.17 Physical factors and physiological factors may both be involved in this negative gravitaxis.18,19 Besides swimming, Chlamydomonas cells show gliding behavior in which they attach to a solid surface with their cilia and glide along the surface. This gliding movement is generated by retrograde (i.e., toward the base of the cilia) movement of an array of intraflagellar transport (IFT) particles that interact with axonemal microtubules and contain a motor protein, dynein.20,21,22,23 Given that the force generated by dynein is unidirectional, when the two cilia are attached to the surface in opposite directions, the force generated by one cilium should be antagonized by the force generated by the other cilium, resulting in a “tug-of-war” condition.20,24 Therefore, one cilium must cease its force generation to initiate gliding in the direction of the other cilium. The cell then glides in the direction of the leading cilium, which generates force, while the trailing cilium is passively dragged by the force of the leading cilium. The cessation of force generation in the trailing cilium is due to an increase in the intraciliary Ca2+ concentration.24 This rise in Ca2+ concentration has been proposed to be mediated by a mechanosensitive channel because the Ca2+ concentration increases when the trailing cilium is pulled.
Despite the various mechanoresponses in Chlamydomonas, the ion channel responsible for mechanoresponse has been explored only in the avoiding reaction. We previously reported that TRP11, a member of the transient receptor potential (TRP) group of ion channels, is responsible for the avoiding reaction.12 TRP11 is located mainly in the proximal end of cilia and may provide mechanosensitivity to cilia, but it is absent in the cell body.12 TRP channels are multimodal ion channels that are activated by various physical and chemical stimuli. Many TRP channels such as TRPA1, TRPC1, TRPC3, TRPC6, TRPV2, TRPV4, and TRPP2 have been reported to be involved in mechanoresponses.25,26,27,28,29,30,31 Because these TRP channels cannot be activated by membrane stretching when they are purified and incorporated into a lipid bilayer, it is probable that they are activated by the force delivered through tethers attached to them.32,33
In this study, we tested the hypothesis that TRP11 is universally involved in the mechanoresponses of Chlamydomonas reinhardtii using a TRP11-knockout mutant. Our results show that TRP11 mediates many, but not all, mechanoresponses in Chlamydomonas.
Results
TRP11 is involved in contact-induced ciliary waveform conversion
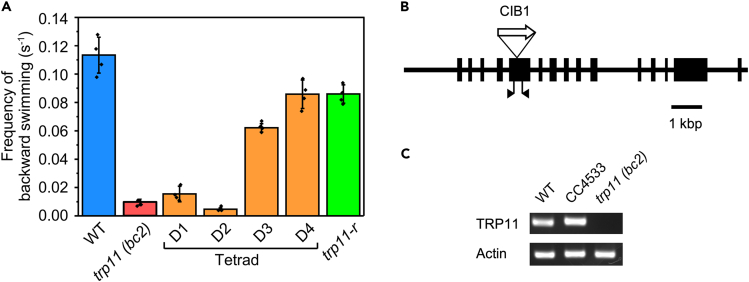
Swimming microorganisms show an avoiding reaction when they collide with a solid surface. When Chlamydomonas cells were placed between a slide and a cover glass separated by approximately 30 μm, they collided frequently with the glass surface and showed avoiding reaction at a rate of around 0.11 times per second (Figure 1A). To examine the involvement of TRP11 in the avoiding reaction, we obtained putative trp11 mutants from the CLiP.34 One of the mutants had an insertion of the transformation plasmid (CIB1) in the fifth exon of the TRP11 gene (Figure 1B). We created strain trp11 (bc2) by backcrossing the mutant twice to wild-type Chlamydomonas cells to remove unrecognized mutations. Absence of TRP11 expression in trp11 (bc2) was confirmed by RT-PCR (Figure 1C).
Figure 1.
Avoiding reaction in TRP11 mutants
(A) The rate of avoiding reaction in wild type (WT) and TRP11 mutants. trp11 (bc2) was created by backcrossing trp11 twice with the WT; D1 to D4 are from a tetrad obtained by crossing trp11 (bc2) with the WT; trp11-r was created by restoring TRP11 in trp11 (bc2) by transformation. Each measurement is based on 70 to 80 cells and the average and standard deviation from 4 measurements are shown.
(B) Intron/exon map of TRP11 showing the position of the CIB1 insertion to create the knockout. The positions of the primers used to confirm the insertion and expression are indicated by triangles.
(C) Expression of TRP11 and actin examined by RT-PCR.
When tpr11 (bc2) cells were placed between a slide and cover glass, the rate of avoiding reaction was reduced by around 90% compared with that of wild type (Figure 1A). The link between the reduction of avoiding reaction and the TRP11 mutation was confirmed by backcross and rescue experiments. When tpr11 (bc2) was backcrossed to the wild type, two progenies (D1 and D2) of the resulting tetrad had a mutation in the TRP11 gene and showed defective avoiding reaction, while the other two progenies from the tetrad (D3 and D4) had an intact TRP11 gene and showed wild-type avoiding reaction (Figure 1A). D1 was designated as strain trp11 (bc3) and used in subsequent experiments. When the trp11 mutation was compensated by transforming tpr11 (bc2) with intact, exogenous TRP11 cDNA, creating strain “trp11-r,” the defect in avoiding reaction was ablated (Figure 1A). These observations show that the defective avoiding reaction was due to TRP11 mutation, which is in agreement with a previous experiment that used RNAi to suppress TRP11 expression.12
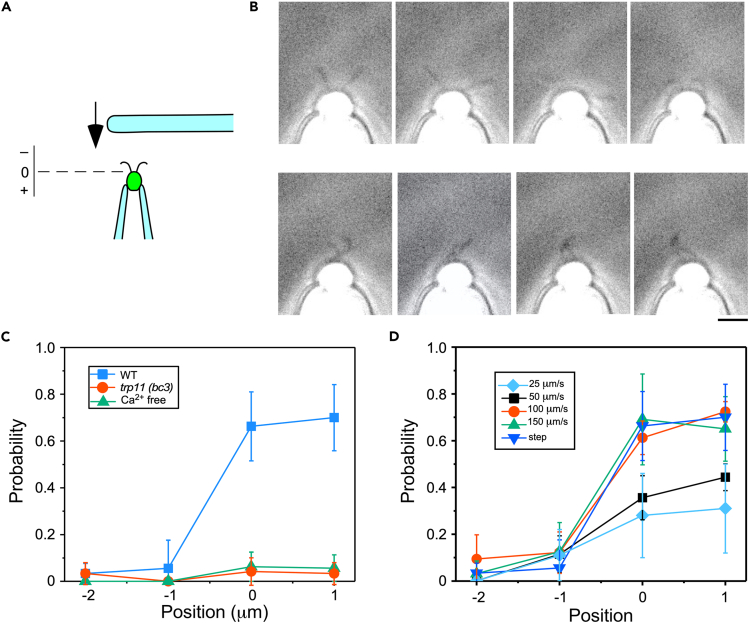
Dense localization of TRP11 in the proximal region of cilia has been shown by immunofluorescence studies; however, it is not known if the localization coincides with mechanosensitivity.12 We examined the localization of mechanosensitivity by catching single Chlamydomonas cells on a glass pipette and tapping the anterior end of each captured cell with a glass rod (Figure 2A). The rod approached the cell at a constant speed, made contact with the anterior end of the cell, also hitting the cilia whose whole length is ∼15 μm, and then retracted quickly. In the absence of stimulation, the cilia showed a bending pattern in the forward mode, beating in a breast-stroke pattern (Figure 2B).35,36 When the anterior end of the cell was tapped at a speed close to the swimming velocity (∼100 μm s−1), the bending pattern changed to the reverse mode, propagating S-shaped waves toward the distal end (Figure 2B, Video S1). This waveform conversion is similar to the one observed in the photoshock response, which results in transient backward swimming.36,37,38 The waveform conversion hardly ever occurred when the glass rod approached a position 1–2 μm from the anterior end of the cell body but did not contact the cell body (Figure 2C). In contrast, when the glass rod was used to push the cell body a distance of 1 μm, a slight flattening of the cell body was observed, but the rate of waveform conversion did not increase. These observations indicate that the mechanosensitivity is localized in the proximal part of the cilia or the anterior end of the cell body.
Figure 2.
Ciliary response to tapping at the anterior end of Chlamydomonas cells
(A) The design of the experiment.
(B) Bending pattern of cilia before (top) and after (bottom) the anterior end of the cell was tapped with a glass rod. The speed of the rod was 100 μm s−1. Bar: 10 μm. The interval between successive frames is 16 ms. See also Video S1.
(C) The probability of conversion of the bending mode from forward to reverse in wild type (blue) and trp11 (bc3) (red). The glass rod approached the cell up to the position indicated by the abscissa. The definition of the position is given in panel A. The response of the wild type in the absence of extracellular Ca2+ is shown in green.
(D) The probability of bending mode conversion when the rod approached up to position 0 at different speeds. Each cell was tapped eight times. The average and standard deviation from four cells are shown.
The video rate is reduced by 1/30. Note that the rod (30 μm in diameter) appears much thicker because the brightness and contrast are adjusted so that the cilia are visible.
The ciliary response to tapping was nearly absent in tpr11 (bc3) cells (Figure 2C). Removal of extracellular Ca2+ also diminished the response in wild-type cells. Therefore, TRP11 is likely responsible for the Ca2+-dependent waveform conversion after mechanical stimulation. Because TRP11 is localized in the proximal end of the cilia,12 our observations suggest that the force resulting from collision activates TRP11 directly. There is another possibility that collision deforms the anterior end of the cell body and activates TRP11 indirectly.
When the cell was tapped at different speeds, the maximal response was observed with tapping at speeds of 100 μm s−1 or higher, whereas the response was less frequent with tapping at 25 μm s−1 or 50 μm s−1 (Figure 2D). These observations imply that the threshold for mechanosensitivity is set approximately at the speed of swimming.
Response to shear requires TRP11
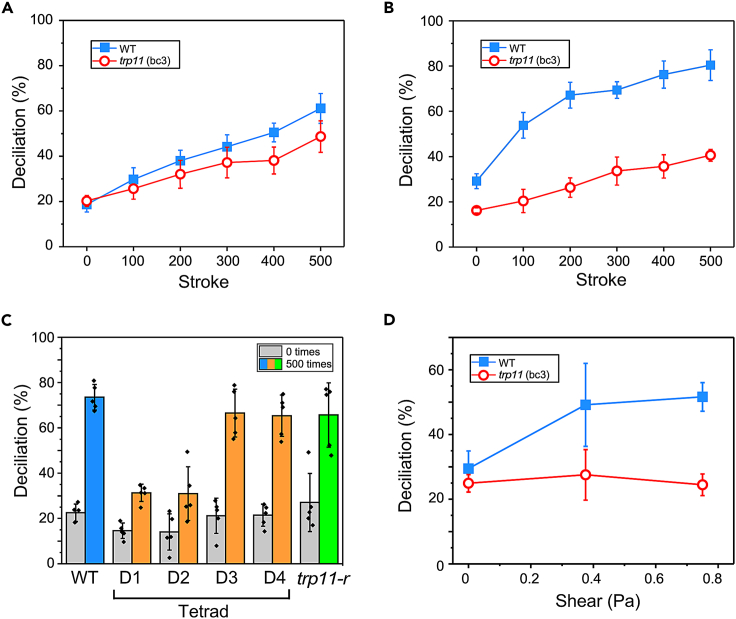
Cilia become detached from the Chlamydomonas cell body when shear is applied by homogenization.15 It has not been established whether this is a physical process or a physiological one. We applied shear to cell suspensions using a Dounce homogenizer. The space between the pestle and the cylindrical glass tube of the homogenizer is around 150 μm (according to the manufacturer), whereas the diameter of Chlamydomonas cells is around 10 μm. In wild-type cells, the application of shear resulted in detachment of cilia from the cell body at the ciliary base, while the cell body remained intact. When the concentration of CaCl2 in the medium was 0.3 mM, the proportion of deciliated cells increased with the number of pestle strokes (Figure 3A). When the CaCl2 concentration was increased to 5 mM, the proportion of deciliated cells increased much more rapidly after the first 100 strokes (Figure 3B), suggesting that shear-induced deciliation is a physiological Ca2+-dependent process.
Figure 3.
Ciliary detachment after application of shear
(A) The proportion of deciliated cells after application of shear to wild type (WT; blue) or trp11 (bc3) (red) by a homogenizer in the presence of 0.3 mM CaCl2. The number of strokes is shown on the abscissa.
(B) As in panel A but with 5 mM CaCl2.
(C) The proportion of deciliated cells in WT, tetrad cells (D1 to D4), and rescued cells (trp11-r) after 0 (gray) or 500 strokes (color) with 5 mM CaCl2.
(D) The percentage of deciliated cells after application of shear for 5 m with a cone-and plate viscometer. The average and standard deviation of five experiments are shown.
When the same shear was applied to tpr11 (bc3) cells, the proportion of deciliated cells was reduced, especially at 5 mM CaCl2, compared with the wild type (Figures 3A and 3B). The proportion of deciliated cells increased slightly with the number of strokes, and the slopes at 0.3 mM and 5 mM CaCl2 were similar (5.28% and 5.34% per 100 strokes at 0.3 and 5 mM, respectively, according to the regression lines). This CaCl2 independence suggests that deciliation in tpr11 (bc3) cells is not a Ca2+-dependent physiological response; rather, deciliation probably occurs as a result of physical tearing. A comparison between wild-type and tpr11 (bc3) cells showed that a large proportion of deciliation in the wild type was mediated by TRP11 in the presence of 5 mM CaCl2. A link between the defective shear response and the TRP11 mutation was confirmed by tetrad and rescue experiments: D1 and D2 showed defective shear-induced deciliation, whereas D3, D4, and trp11-r showed the same deciliation in response to shear as the wild type (Figure 3C).
The shear generated by the homogenizer was estimated to be roughly 0.34 Pa based on the values of the clearance (150 μm), the speed of the pestle (85 mm/1.5 s), and the viscosity coefficient (0.00089 Pa s). When a designated amount of shear was applied with a cone-and-plate viscometer for 5 min in a medium containing 5 mM CaCl2, deciliation was increased at 0.4 Pa or higher in wild-type cells but not in trp11 (bc3) cells (Figure 3D), supporting the idea that shear-induced deciliation requires TRP11.
TRP11 generates the response to agitation
When cell suspensions were subjected to mechanical agitation by vortex mixing, the ciliary beat frequency in wild-type cells increased by around 20 Hz (Figure 4), as reported previously.13 However, no significant increase was observed in tpr11 (bc3) cells. A link between defective motility augmentation and the TRP11 mutation was confirmed by tetrad and rescue experiments: D1 and D2 had defective agitation-induced motility augmentation, whereas D3, D4, and trp11-r showed the same agitation-induced motility augmentation as the wild type (Figure 4).
Figure 4.
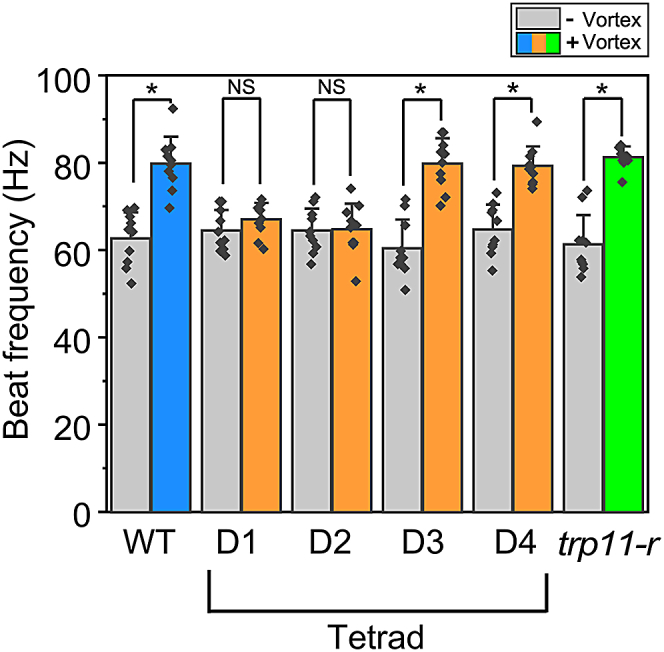
Augmentation of ciliary motility after mechanical agitation
Ciliary beat frequency in wild-type (WT), tetrad (D1 to D4), and rescued cells (trp11-r) before (gray) and after (color) 5 s of vortex mixing. The average and standard deviation are shown. Asterisks indicate significant difference at p < 0.05 (t-test, n = 10).
TRP11 is responsible for the initiation of gliding
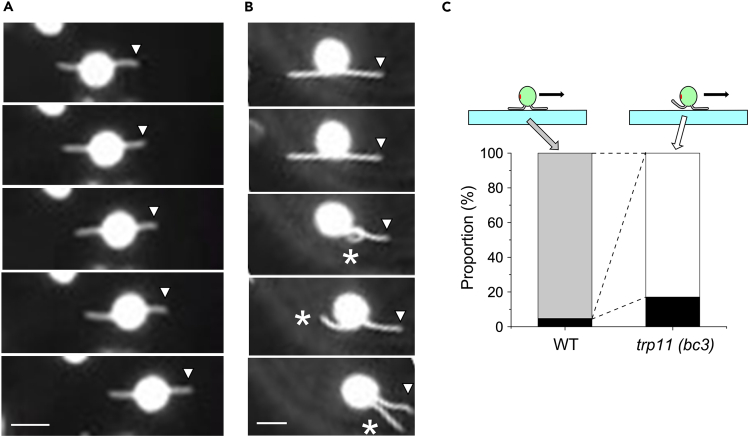
Chlamydomonas cells tend to attach via their two cilia to a smooth surface and then glide along the surface while their cilia remain attached to it. We focused on stationary cells whose cilia were oriented in opposite directions on the surface because the forces generated by the two cilia in these cells are in the tug-of-war condition. When we observed wild-type cells, most cells started gliding in the direction of one of the two cilia after a brief stationary period (Video S2, Figures 5A and 5C). In contrast, tpr11 (bc3) cells did not start gliding while both cilia were attached to the surface. Instead, they started gliding only when one of the two cilia became detached from the surface (Video S3, Figures 5B and 5C). The detached cilium jiggled while gliding but did not beat as in swimming cells. These observations suggest that TRP11 is required for cilia to enter the trailing state, in which they remain attached to the surface but do not exert motive force.
Figure 5.
Initiation of gliding
(A and B) (A) Wild-type and (B) trp11 (bc3) cells attached to a glass surface. Images at 2 s intervals are shown. The cell starts gliding in the third frame. The position of the tip of the leading cilium is indicated by arrowheads. Asterisks in (B) denote the cilium detached from the glass surface. Bar: 10 μm. See also Videos S2 and S3.
(C) Proportions of cells gliding with two flagella attached in opposite directions (gray) and with one cilium detached from the glass (white). Cells that did not start gliding during the observation period (10 s) are indicated by black. Data for wild type and trp11 (bc3) are from 41 to 42 cells (9 and 7 experiments), respectively.
TRP11 is not involved in the responses to viscosity and gravity
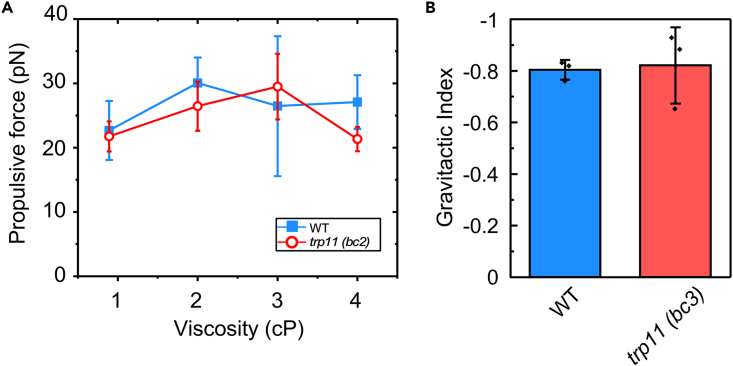
Wild-type Chlamydomonas cells generated greater propulsive force for swimming when the viscosity of the medium increased from 1 cP to 2 or 3 cP (Figure 6A), as reported previously by Minoura and Kamiya.14 We observed a similar increase in propulsive force generated by tpr11 (bc2) cells when the viscosity of the medium was increased. As for gravitaxis, wild-type cells and tpr11 (bc3) cells showed similar capacities for negative gravitaxis (Figure 6B). These observations suggest that TRP11 is not involved in the mechanoresponses to viscosity or gravity.
Figure 6.
Responses to viscosity or gravity
(A) Propulsive force of swimming cells in solutions with different viscosities (n = 4).
(B) Gravitaxis as assessed by accumulation in the upper half of a glass tube (n = 3). The average and standard deviation are shown.
Discussion
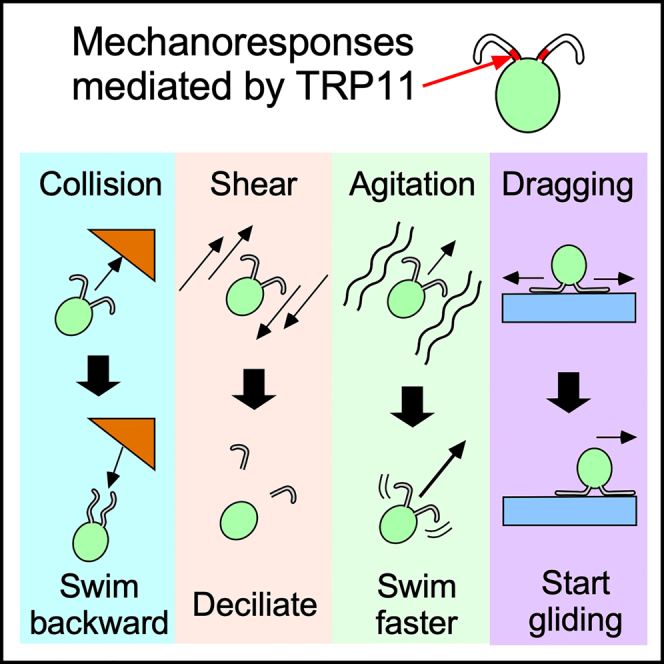
TRP11 mutation caused defects in multiple mechanoresponses, including avoiding reaction, contact-induced ciliary waveform conversion, shear-induced deciliation, agitation-induced motility augmentation, and initiation of gliding. On the other hand, the responses to increased viscosity and gravity were not affected by TRP11 mutation. Therefore, a wide range of mechanoresponses are mediated by TRP11, although some other mechanoresponses are likely brought about by a still unknown mechanoreceptor.
It is not clear how TRP11, as a single protein, can mediate various responses depending on the type of mechanical stimulation. We surmise that the response differences derive from differences in the intensity and duration of the stimuli as well as the ciliary motility state. Waveform conversion to the reverse mode probably occurs when a single instantaneous mechanical stimulus is applied to beating cilia by a collision, whereas an increase in beat frequency occurs when repeated stimuli are applied at moderate intensity by vortex mixing. In contrast, deciliation occurs when intense repeated stimuli are applied with a homogenizer. The generation of gliding force stops when a single instantaneous mechanical stimulus is applied to a quiescent cilium. These differences in the mode of mechanical stimulus probably result in variation in the degree and time course of Ca2+ concentration increase, which determines the type of response, together with the motility state.
Our results suggest that TRP11 is a mechanosensitive channel; however, no mammalian mechanoresponsive TRP channels can be activated by membrane stretching after purification and incorporation into a lipid bilayer.33 Considering that there are two types of mechanosensitive channels, those activated by force from the lipid bilayer and those activated by an attached tether, TRP channels might represent the latter type.32 TRPN (NompC) in Drosophila is likely activated by force transmitted from microtubules.39 TRP11 may also be tethered, as will be discussed below.
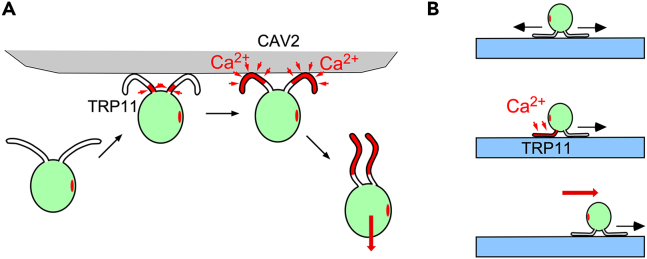
In the avoiding reaction, a mechanoreceptor potential is generated in response to a collision, triggering an action potential in cilia. This scheme has been established in Paramecium and is probably also applicable to Chlamydomonas.3,40 The current study and previous studies indicate that TRP11 produces the mechanoreceptor potential, and CAV2, a voltage-dependent calcium channel, produces the action potential.12,41 It is noteworthy that TRP11 localization and CAV2 localization are mutually exclusive (Figure 7A). TRP11 is concentrated at the proximal end of cilia, where the curvature due to bending motion for swimming is smallest and misactivation by active bending is unlikely to occur.42 The response to tapping by a glass rod in our experiments indicates that the mechanosensitivity is also localized at the proximal end of the cilia, although there remains a possibility that TRP11 is activated indirectly by the deformation of the anterior end of the cell body. On the other hand, CAV2 is present in the middle-to-distal region of the cilia. The absence of robust Ca2+ influx in the proximal region might prevent activation of the Ca2+-dependent cilia-severing machinery at the ciliary base.41,43 The fixed localizations of TRP11 and CAV2 suggest the presence of a transport mechanism and an anchor for tethering the two types of ion channels. Such a tether may be involved in mechanoreception by TRP11.
Figure 7.
Models of the membrane excitation during avoiding reaction and gliding initiation
(A) In the avoiding reaction, TRP11 produces a receptor potential upon collision. The depolarization activates the voltage-dependent calcium channel CAV2 and triggers an action potential. The influx of calcium ions through CAV2 elevates the intraciliary calcium concentration and converts the bending mode from forward to reverse.
(B) Cilia oriented in opposite directions generate forces in opposing directions, resulting in no net movement (top). When Ca2+ influx occurs through TRP11 in one cilium (middle), the cilium stops generating force and the cell starts gliding (bottom).
Agitation by vortex mixing results in increased ciliary beating and swimming velocity.13 The augmentation of ciliary beating occurs when the Ca2+ concentration increases in a range of 10−8 to 10−7 M.13 Sustained mechanical stimulation by vortex mixing might induce this small increase in Ca2+ concentration through activation of TRP11. An increase in swimming velocity in response to mechanical stimulation has also been reported in Paramecium cells, which respond to mechanical stimulation at their posterior end.3 In Paramecium, the mechanical stimulation hyperpolarizes the membrane potential and increases the cAMP concentration via an adenylate cyclase-coupled potassium channel.44,45 The ciliary beat frequency then rises in a cAMP-dependent manner.46 Mechanoreception at the posterior end is unlikely to occur in Chlamydomonas, however, because the cell body is covered by a stiff cell wall.
Shear force delivered by a homogenizer induces cilia detachment independently of the TRP channel ADF1 and calmodulin, which are required for acid-induced deciliation.15 Our results show that TRP11 is responsible for shear-induced deciliation. Given that deciliation occurs at Ca2+ concentrations higher than 10−6 M, the shear force induced by the homogenizer, which is harsher than vortex mixing, likely produces a larger increase in Ca2+ concentration than the force induced by vortex mixing.16 When we applied shear quantitatively, deciliation occurred with a shear of 0.4 Pa or higher, which is comparable to the shear due to venous blood flow (0–1 Pa). Considering that the shear force in the bed of small and medium-sized streams reaches 7 Pa and 30 Pa, respectively, Chlamydomonas cells probably shed cilia in response to shear in natural streams.47,48 Loss of cilia may be advantageous for survival under conditions of severe mechanical stress because the cell body is protected by the cell wall, whereas the cilia are directly exposed to the environment.
Chlamydomonas cells typically attach to surfaces with their two cilia oriented in opposite directions, but this creates a tug-of-war condition between the two cilia (Figure 7B).23,24 Therefore, one cilium must stop generating force so that gliding can be initiated in the direction of the other cilium. The generation of force halts when an increase in intraciliary Ca2+ concentration induces detachment of the intraflagellar dynein complex from the membrane glycoprotein.24,49 Our finding that trp11 cells did not glide when both cilia were attached to a glass surface suggests that those cells did not end the tug-of-war condition by stopping the force generation in one of the cilia. This is consistent with the idea that TRP11 senses the force of tug-of-war and allows Ca2+ influx once activated. When the Ca2+ level exceeds the threshold in one cilium, the cilium stops generating force (Figure 7B).24,49 However, several reports have shown that Ca2+ is required for the generation, rather than the cessation, of gliding force.21,22,23,50 These studies indicate that a link between IFT particles and FMG1-B, a membrane glycoprotein that adheres to the substrate, is formed in the presence of μM levels of extracellular Ca2+ but is lost at lower Ca2+ concentrations. We speculate that the TRP11-induced cessation of force generation occurs at higher Ca2+ concentrations than those needed for crosslink formation between IFT particles and FMG1-B. In support of this idea, redistribution of FMG1-B along the cilium was shown to occur in a normal culture medium but was inhibited upon addition of excess Ca2+.21
Although gliding is a slow movement, an imbalance of force generated by two cilia may occur abruptly because the number of active IFT arrays in each cilium is not large and the number of IFT particles in each array varies from a few to >40.22,50 Thus, the force generated by each cilium may change stochastically with the number of arrays and dynein molecules.23 Detection of gliding force requires that the detector is connected mechanically to the substrate and the axoneme. It is possible that tethers link TRP11 to the substrate, and TRP11 is activated by the force delivered through the tethers.
When one cilium of a trp11 cell was detached from the substrate while the other was still attached, the detached cilium jiggled and did not beat as that in swimming cells. This observation suggests that immobilization of one cilium suppresses the bending motion of the other cilium. Ciliary beating is possibly inhibited by a mechanism that regulates the activity of the outer dynein arm, and its mutation results in a lack of quiescence while gliding.51
Because viscosity and gravity are physical parameters, it is possible that the responses to them involve mechanosensation. Elevation of the propulsive force in a high-viscosity medium requires an intact cell membrane, but our results indicate that TRP11 is not responsible for this response.14 Likewise, membrane excitability is possibly responsible for gravitaxis, but our results indicate that TRP11 is not related to gravitaxis.18 The responses to viscosity and gravity may be brought about by a TRP channel or mechanosensitive channels other than TRP11. Alternatively, it is possible that these responses are caused by a dynein regulatory factor, Lis1, which increases the ciliary beat frequency under high load conditions.52
Given that several of our results support the idea that TRP11 is attached to a tether, TRP11 may be a mechanosensitive channel activated by force delivered through a tether, although more thorough experiments are needed for a definite conclusion. We speculate that TRP11 is connected to the axoneme and extracellular structure in a manner similar to the Chlamydomonas PKD2 (TRPP2) complex, which binds to the axoneme and the mastigoneme, an extracellular hair-like structure.11 TRP11, similar to PKD2, has a long amino acid sequence between the first and second transmembrane segments. This structure resembles the extracytosolic domain of the TRPP and TRPML channels, which are in the same group of ion channels as algal and unicellular TRP channels.53,54 A candidate for the intracellular tether is the ankyrin repeats, with which TRPN is tethered to microtubules, but TRP11 has no ankyrin motif.55 A long α-helical domain may compose a beam on the intracellular side of the channel, as predicted by AlphaFold (F2Z8J6).56 This and other extracellular and intracellular structures may act as or interact with tethers.
To conclude, TRP11 senses mechanical stimuli with different characteristics and controls cell behavior accordingly. Thus, TRP11 is a mechanotransduction mediator between the sensory and motility functions of cilia. Chlamydomonas cilia are sensitive not only to mechanical stimuli but also to chemical and thermal stimuli. ADF1, also called TRP15, is a ciliary TRP channel that induces deciliation upon application of organic acid, capsaicin, or gingerol.12,57,58 TRP1 is a thermosensitive TRP channel that is possibly present in cilia because the expression is upregulated upon deciliation.12,59 Therefore, the previous studies and the present study show that Chlamydomonas cilia are a multimodal sensor that monitors various environmental conditions.
Limitations of the study
Although this study shows the involvement of TRP11 in various mechanoresponses, it is not clear whether TRP11 is a mechanosensitive channel. A mutational study of the ion-conducting pore will be required to show that TRP11 is an ion channel, and an electrophysiological study will be required to show that TRP11 excites the membrane potential.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| Chlamydomonas reinhardtii, wild type | Ueki et al.60 Ide et al.61 | N/A |
| Chlamydomonas reinhardtii, trp11 | CLiP, Chlamydomonas Resource Center, University of Minnesota | LMJ.RY0402.150923 |
| Chlamydomonas reinhardtii, trp11 (bc2) | This study | N/A |
| Chlamydomonas reinhardtii, trp11 D1-D4 | This study | N/A |
| Oligonucleotides | ||
| Forward primer for checking CIB1 insertion in TRP11 TGCTGCACGCATACAAGCTT | This study | N/A |
| Reverse primer for checking CIB1 insertion in TRP11 TCCCAGATGTTCAGCAGCCG | This study | N/A |
| Forward Primer for actin AAGGCCAACCGCGAGAAGAT | This study | N/A |
| Reverse Primer for actin TAATCGGTGAGGTCGCGGC | This study | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Kenjiro Yoshimura (kenjiroy@shibaura-it.ac.jp).
Materials availability
This study did not generate any new or unique reagents.
Experimental model and study participant details
A strain of C. reinhardtii CC124 whose agg1 mutation is removed was used as the wild type.60,61 A TRP11 mutant (LMJ.RY0402.150923), referred to as “trp11,” was obtained from the Chlamydomonas Library Project (CLiP),34 which generates mutants by random insertion of a CIB1 cassette. The trp11 mutant was backcrossed twice to the wild type to obtain “trp11 (bc2).” A tetrad (containing cells D1 to D4) was obtained by crossing trp11 (bc2) with the wild type. D1 and D2 retained the trp11 mutation, whereas D3 and D4 did not, as revealed by PCR using primers encompassing the CIB1 insertion. D1 was designated as “trp11 (bc3),” a TRP11 mutant backcrossed three times to the wild type.
Chlamydomonas cells were grown in Tris-acetate-phosphate (TAP) medium at 25°C under 12 h light-12 h dark conditions.62 Cells were washed three times with an experimental solution containing 1 mM KCl, 0.3 mM CaCl2, 0.2 mM EGTA, and 5 mM Hepes, with pH adjusted to 7.4 with KOH. Where indicated, the concentration of CaCl2 was 5 mM.
Method details
Expression of exogenous TRP11
The open reading frame of TRP11 was obtained by RT-PCR and cloned into pChlamy4 (Invitrogen). The construct was linearized by ScaI and transformed into trp11 (bc2) cells by electroporation, as reported previously.63 Cells were washed with MAX Efficiency Transformation Reagent for Algae (Thermo Fisher Scientific, A24229) and 400 ng of DNA was added. Pulses for transformation was applied with an electroporator (Nepa Gene, NEPA21). Cells were recovered overnight in 40 mM sucrose TAP solution and spreaded on 15 μg/ml zeocin TAP agar plates. Successful transformation was assessed by PCR using primers encompassing the bleomycin resistance gene and TRP11. Expression of intact TRP11 was confirmed by RT-PCR using primers encompassing CIB1.
Mechanical stimulation of Chlamydomonas cells with a glass rod
Single cells were caught on a glass pipette and poked with a glass rod (30 μm in diameter) attached to a Piezo unit (Thorlabs, PB4NB2S). The Piezo unit was driven with a Piezo Driver (NF Corporation, PD-206-150P) controlled with Digidata 1320A and pClamp8 software (Molecular Devices). A glass pipette was filled with the experimental solution and fit to a pipette holder for patch clamping. The holder was attached to an electronic micromanipulator (Scientifica, PS-7000C). Cells were captured on the glass pipette by gentle suction applied through the pipette holder. The glass pipette and glass rod were fabricated using a micropipette puller (Sutter Instrument, P-97) and then heat polished (Narishige, MF-830). Ciliary movement was observed with an inverted microscope equipped with phase contrast optics (Olympus, IX71) and recorded with a CMOS camera (Hamamatsu Photonics, ORCA Flash4.0 v2).
Mechanical stimulation by shear, agitation, increased viscosity, and gravity
Shear was applied to cell suspensions with a Dounce homogenizer (AS ONE, 358034) or a viscometer (Brookfield, LVDV-I Prime fitted with a spindle CPA-40Z). The speed of the pestle was such that a single reciprocal stroke took 3 s. Agitation was applied with a vortex mixer at 3200 r.p.m. for 5 s. The ciliary beat frequency was recorded within 1 min using a fast Fourier transform (FFT)-based method.64 The cell suspension was placed on an inverted microscope fitted with dark field optics and the image was projected on a photomultiplier (Hamamatsu Photonics, H7710-3 with an amplifier C7319). The signal was subjected to FFT using a digital oscilloscope (Tektronix, TBS1052B). The peak on the spectrum was determined by fitting to a Gaussian distribution curve (OriginPro). The viscosity of the medium was increased from 0.89 mPa·s (cP) to 2, 3, or 4 cP by adding Ficoll 400 (Sigma, F9378) to a final concentration of 5.19%, 8.05%, or 10.35% respectively, as calibrated with an Ostwald viscometer at 25°C. The force acting on the cell body, which equals the net force produced by the cilia, was estimated using Stokes’ formula, F = 6πηaυ, where η is the viscosity of the medium, a is the radius of the cell body, and υ is the swimming velocity.14 Gravitaxis was assessed by enclosing cell suspensions (1×106 cells ml−1) in a glass tube and letting the tube stand under red light illumination for 90 min18 The tube was then cut into halves, and the cell concentrations in the upper and lower halves were counted with a cell counter (Olympus, R1). The magnitude of gravitaxis was expressed as the gravitactic index, GI = (nd – nu) / (nd + nu), where nd and nu are the cell density in the lower and upper halves of the glass tube, respectively.
Observation of gliding
Cells were placed on a washed and rinsed glass slide. Gliding was observed with a microscope (Olympus, IX-50) fitted with a dark field condenser. Images were recorded with a CMOS camera (Hamamatsu Photonics, ORCA-Spark).
Quantification and statistical analysis
Data were analyzed using Microsoft Excel (Microsoft) or OriginPro (Origin). Two tailed T-test was used. Details of statistical analysis are described in the figure legends.
Acknowledgments
This study was supported by the Japan Agency for Medical Research and Development (AMED/PRIME) Grant JP18gm5810013 to K.Y., and by the Japan Society for the Promotion of Science KAKENHI Grants JP22K06228 and JP22H05690 to K.Y. and JP21H00420, JP22H05674, JP22H02642, and JP22H01440 to K.W.
Author contributions
Conceptualization, K.Y.; Methodology, N.W. and K.Y.; Investigation, D.O., M.Y., K.S., N.I., M.T., A.I., K.W., and K.Y.; Writing – Original Draft, K.Y.; Writing – Review and Editing, K.Y.; Supervision, K.W. and K.Y.; Project Administration, K.Y.; Funding Acquisition, K.W. and K.Y.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: September 15, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107926.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This study does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Jennings H.S. Carnegie Institution of Washington; 1904. Contributions to the Study of the Behavior of Lower Organisms. [Google Scholar]
- 2.Jennings H.S. The behavior of Paramecium. Additional features and general relations. J. Comp. Neurol. Psychol. 1904;14:441–510. [Google Scholar]
- 3.Naitoh Y., Eckert R. Ionic mechanisms controlling behavioral responses of paramecium to mechanical stimulation. Science. 1969;164:963–965. doi: 10.1126/science.164.3882.963. [DOI] [PubMed] [Google Scholar]
- 4.Naito Y., Kaneko H. Reactivated Triton-extracted models of Paramecium: modification of ciliary movement by calcium ions. Science. 1972;176:523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- 5.Newman E. Contraction in Stentor coeruleus: a cinematic analysis. Science. 1972;177:447–449. doi: 10.1126/science.177.4047.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katoh K., Naitoh Y. A mechanosensory mechanism for evoking a cellular contraction in Vorticella. J. Exp. Biol. 1992;168:253–267. doi: 10.1242/jeb.168.1.253. [DOI] [Google Scholar]
- 7.Kreimer G., Witman G.B. Novel touch-induced, Ca2+-dependent phobic response in a flagellate green alga. Cell Motil Cytoskeleton. 1994;29:97–109. doi: 10.1002/cm.970290202. [DOI] [PubMed] [Google Scholar]
- 8.Häder D.-P., Lebert M., Richter P., Ntefidou M. Gravitaxis and graviperception in flagellates. Adv. Space Res. 2003;31:2181–2186. doi: 10.1016/s0273-1177(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 9.Dave N., Cetiner U., Arroyo D., Fonbuena J., Tiwari M., Barrera P., Lander N., Anishkin A., Sukharev S., Jimenez V. A novel mechanosensitive channel controls osmoregulation, differentiation, and infectivity in Trypanosoma cruzi. Elife. 2021;10:e67449. doi: 10.7554/eLife.67449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan K.Y., Jékely G. Origins of eukaryotic excitability. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021;376:20190758. doi: 10.1098/rstb.2019.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu P., Liu Y., Zhou J. Ciliary mechanosensation - roles of polycystins and mastigonemes. J. Cell Sci. 2023;136:jcs260565. doi: 10.1242/jcs.260565. [DOI] [PubMed] [Google Scholar]
- 12.Fujiu K., Nakayama Y., Iida H., Sokabe M., Yoshimura K. Mechanoreception in motile flagella of Chlamydomonas. Nat. Cell Biol. 2011;13:630–632. doi: 10.1038/ncb2214. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi K.I., Ide T., Kamiya R. Calcium-dependent flagellar motility activation in Chlamydomonas reinhardtii in response to mechanical agitation. Cell Motil Cytoskeleton. 2009;66:736–742. doi: 10.1002/cm.20402. [DOI] [PubMed] [Google Scholar]
- 14.Minoura I., Kamiya R. Strikingly different propulsive forces generated by different dynein-deficient mutants in viscous media. Cell Motil Cytoskeleton. 1995;31:130–139. doi: 10.1002/cm.970310205. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q., Gao K., Zheng S., Zhu X., Liang Y., Pan J. Calmodulin regulates a TRP channel (ADF1) and phospholipase C (PLC) to mediate elevation of cytosolic calcium during acidic stress that induces deflagellation in Chlamydomonas. FASEB J. 2018;32:3689–3699. doi: 10.1096/fj.201701396RR. [DOI] [PubMed] [Google Scholar]
- 16.Sanders M.A., Salisbury J.L. Centrin-mediated microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J. Cell Biol. 1989;108:1751–1760. doi: 10.1083/jcb.108.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts A.M. Geotaxis in motile micro-organisms. J. Exp. Biol. 1970;53:687–699. doi: 10.1242/jeb.53.3.687. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura K., Matsuo Y., Kamiya R. Gravitaxis in Chlamydomonas reinhardtii studied with novel mutants. Plant Cell Physiol. 2003;44:1112–1118. doi: 10.1093/pcp/pcg134. [DOI] [PubMed] [Google Scholar]
- 19.Kage A., Omori T., Kikuchi K., Ishikawa T. The shape effect of flagella is more important than bottom-heaviness on passive gravitactic orientation in Chlamydomonas reinhardtii. J. Exp. Biol. 2020;223:jeb205989. doi: 10.1242/jeb.205989. [DOI] [PubMed] [Google Scholar]
- 20.Bloodgood R.A. Flagella-dependent gliding motility in Chlamydomonas. Protoplasma. 1981;106:183–192. doi: 10.1007/BF01275550. [DOI] [Google Scholar]
- 21.Bloodgood R.A., Salomonsky N.L. Calcium influx regulates antibody-induced glycoprotein movements within the Chlamydomonas flagellar membrane. J. Cell Sci. 1990;96:27–33. doi: 10.1242/jcs.96.1.27. [DOI] [PubMed] [Google Scholar]
- 22.Shih S.M., Engel B.D., Kocabas F., Bilyard T., Gennerich A., Marshall W.F., Yildiz A. Intraflagellar transport drives flagellar surface motility. Elife. 2013;2:e00744. doi: 10.7554/eLife.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloodgood R.A. In: Chlamydomonas Sourcebook, 3rd edition, Vol. III Cell Motility and Behavior. Dutcher Susan., editor. Elsevier; 2023. The Chlamydomonas flagellar membrane and its dynamic properties. Chapter 10. [Google Scholar]
- 24.Collingridge P., Brownlee C., Wheeler G.L. Compartmentalized calcium signaling in cilia regulates intraflagellar transport. Curr. Biol. 2013;23:2311–2318. doi: 10.1016/j.cub.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 25.Muraki K., Iwata Y., Katanosaka Y., Ito T., Ohya S., Shigekawa M., Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ. Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 26.Nauli S.M., Alenghat F.J., Luo Y., Williams E., Vassilev P., Li X., Elia A.E.H., Lu W., Brown E.M., Quinn S.J., et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 27.Corey D.P., García-Añoveros J., Holt J.R., Kwan K.Y., Lin S.-Y., Vollrath M.A., Amalfitano A., Cheung E.L.-M., Derfler B.H., Duggan A., et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 28.Loukin S., Zhou X., Su Z., Saimi Y., Kung C. Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J. Biol. Chem. 2010;285:27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrison S.R., Dietrich A., Stucky C.L. TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J. Neurophysiol. 2012;107:913–922. doi: 10.1152/jn.00658.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quick K., Zhao J., Eijkelkamp N., Linley J.E., Rugiero F., Cox J.J., Raouf R., Gringhuis M., Sexton J.E., Abramowitz J., et al. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol. 2012;2:120068. doi: 10.1098/rsob.120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katanosaka Y., Iwasaki K., Ujihara Y., Takatsu S., Nishitsuji K., Kanagawa M., Sudo A., Toda T., Katanosaka K., Mohri S., Naruse K. TRPV2 is critical for the maintenance of cardiac structure and function in mice. Nat. Commun. 2014;5:3932. doi: 10.1038/ncomms4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teng J., Loukin S., Anishkin A., Kung C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflügers Arch. 2015;467:27–37. doi: 10.1007/s00424-014-1530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolaev Y.A., Cox C.D., Ridone P., Rohde P.R., Cordero-Morales J.F., Vásquez V., Laver D.R., Martinac B. Mammalian TRP ion channels are insensitive to membrane stretch. J. Cell Sci. 2019;132:jcs238360. doi: 10.1242/jcs.238360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Zhang R., Patena W., Gang S.S., Blum S.R., Ivanova N., Yue R., Robertson J.M., Lefebvre P.A., Fitz-Gibbon S.T., et al. An indexed, mapped mutant library enables reverse genetics studies of biological processes in Chlamydomonas reinhardtii. Plant Cell. 2016;28:367–387. doi: 10.1105/tpc.15.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rüffer U., Nultsch W. Comparison of the beating of cis- and trans-flagella of Chlamydomonas cells held on micropipettes. Cell Motil Cytoskeleton. 1987;7:87–93. doi: 10.1002/cm.970070111. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura K., Shingyoji C., Takahashi K. Conversion of beating mode in Chlamydomonas flagella induced by electric stimulation. Cell Motil Cytoskeleton. 1997;36:236–245. doi: 10.1002/(SICI)1097-0169(1997)36:3<236::AID-CM4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Ringo D.L. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J. Cell Biol. 1967;33:543–571. doi: 10.1083/jcb.33.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt J.A., Eckert R. Calcium couples flagellar reversal to photostimulation in Chlamydomonas reinhardtii. Nature. 1976;262:713–715. doi: 10.1038/262713a0. [DOI] [PubMed] [Google Scholar]
- 39.Cheng L.E., Song W., Looger L.L., Jan L.Y., Jan Y.N. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lodh S., Yano J., Valentine M.S., Van Houten J.L. Voltage-gated calcium channels of Paramecium cilia. J. Exp. Biol. 2016;219:3028–3038. doi: 10.1242/jeb.141234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujiu K., Nakayama Y., Yanagisawa A., Sokabe M., Yoshimura K. Chlamydomonas CAV2 encodes a voltage-dependent calcium channel required for the flagellar waveform conversion. Curr. Biol. 2009;19:133–139. doi: 10.1111/jeu.12800. [DOI] [PubMed] [Google Scholar]
- 42.Brokaw C.J., Luck D.J. Bending patterns of Chlamydomonas flagella I. Wild-type bending patterns. Cell Motil. 1983;3:131–150. doi: 10.1002/cm.970030204. [DOI] [PubMed] [Google Scholar]
- 43.Quarmby L. Ciliary ion channels: location, location, location. Curr. Biol. 2009;19:R158–R160. doi: 10.1016/j.cub.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 44.Schultz J.E., Klumpp S., Benz R., Schürhoff-Goeters W.J., Schmid A. Regulation of adenylyl cyclase from Paramecium by an intrinsic potassium conductance. Science. 1992;255:600–603. doi: 10.1126/science.1371017. [DOI] [PubMed] [Google Scholar]
- 45.Kawano M., Tominaga T., Ishida M., Hori M. Roles of adenylate cyclases in ciliary responses of Paramecium to mechanical stimulation. J. Eukaryot. Microbiol. 2020;67:532–540. doi: 10.1111/jeu.12800. [DOI] [PubMed] [Google Scholar]
- 46.Bonini N.M., Nelson D.L. Differential regulation of Paramecium ciliary motility by cAMP and cGMP. J. Cell Biol. 1988;106:1615–1623. doi: 10.1083/jcb.106.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lancaster J., Hildrew A.G. Characterizing in-stream flow refugia. Can. J. Fish. Aquat. Sci. 1993;50:1663–1675. doi: 10.1139/f93-187. [DOI] [Google Scholar]
- 48.Statzner B., Müller R. Standard hemispheres as indicators of flow characteristics in lotic benthos research. Freshw. Biol. 1989;21:445–459. doi: 10.1111/j.1365-2427.1989.tb01377.x. [DOI] [Google Scholar]
- 49.Fort C., Collingridge P., Brownlee C., Wheeler G. Ca2+ elevations disrupt interactions between intraflagellar transport and the flagella membrane in Chlamydomonas. J. Cell Sci. 2021;134:jcs253492. doi: 10.1242/jcs.253492. [DOI] [PubMed] [Google Scholar]
- 50.Kozminski K.G., Johnson K.A., Forscher P., Rosenbaum J.L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell B.F., Grulich L.E., Mader M.M. Flagellar quiescence in Chlamydomonas: Characterization and defective quiescence in cells carrying sup-pf-1 and sup-pf-2 outer dynein arm mutations. Cell Motil Cytoskeleton. 2004;57:186–196. doi: 10.1002/cm.10166. [DOI] [PubMed] [Google Scholar]
- 52.Rompolas P., Patel-King R.S., King S.M. Association of Lis1 with outer arm dynein is modulated in response to alterations in flagellar motility. Mol. Biol. Cell. 2012;23:3554–3565. doi: 10.1091/mbc.E12-04-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arias-Darraz L., Cabezas D., Colenso C.K., Alegría-Arcos M., Bravo-Moraga F., Varas-Concha I., Almonacid D.E., Madrid R., Brauchi S. A transient receptor potential ion channel in Chlamydomonas shares key features with sensory transduction-associated TRP channels in mammals. Plant Cell. 2015;27:177–188. doi: 10.1105/tpc.114.131862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cabezas-Bratesco D., Mcgee F.A., Colenso C.K., Zavala K., Granata D., Carnevale V., Opazo J.C., Brauchi S.E. Sequence and structural conservation reveal fingerprint residues in TRP channels. Elife. 2022;11:e73645. doi: 10.7554/eLife.73645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W., Cheng L.E., Kittelmann M., Li J., Petkovic M., Cheng T., Jin P., Guo Z., Göpfert M.C., Jan L.Y., Jan Y.N. Ankyrin repeats convey force to gate the NOMPC mechanotransduction channel. Cell. 2015;162:1391–1403. doi: 10.1016/j.cell.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hilton L.K., Meili F., Buckoll P.D., Rodriguez-Pike J.C., Choutka C.P., Kirschner J.A., Warner F., Lethan M., Garces F.A., Qi J., Quarmby L.M. A forward genetic screen and whole genome sequencing identify deflagellation defective mutants in Chlamydomonas, including assignment of ADF1 as a TRP channel. G3 (Bethesda) 2016;6:3409–3418. doi: 10.1534/g3.116.034264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wada M., Kaizuka I., Yoshimura K. Responses to transient receptor potential (TRP) channel agonists in Chlamydomonas reinhardtii. Biol. Open. 2020;9:bio053140. doi: 10.1242/bio.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGoldrick L.L., Singh A.K., Demirkhanyan L., Lin T.Y., Casner R.G., Zakharian E., Sobolevsky A.I. Structure of the thermo-sensitive TRP channel TRP1 from the alga Chlamydomonas reinhardtii. Nat. Commun. 2019;10:4180. doi: 10.1038/s41467-019-12121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueki N., Ide T., Mochiji S., Kobayashi Y., Tokutsu R., Ohnishi N., Yamaguchi K., Shigenobu S., Tanaka K., Minagawa J., et al. Eyespot-dependent determination of the phototactic sign in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 2016;113:5299–5304. doi: 10.1073/pnas.1525538113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ide T., Mochiji S., Ueki N., Yamaguchi K., Shigenobu S., Hirono M., Wakabayashi K.I. Identification of the agg1 mutation responsible for negative phototaxis in a "wild-type" strain of Chlamydomonas reinhardtii. Biochem. Biophys. Rep. 2016;7:379–385. doi: 10.1016/j.bbrep.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorman D.S., Levine R.P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamano T., Iguchi H., Fukuzawa H. Rapid transformation of Chlamydomonas reinhardtii without cell-wall removal. J. Biosci. Bioeng. 2013;115:691–694. doi: 10.1016/j.jbiosc.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 64.Kamiya R. Analysis of cell vibration for assessing axonemal motility in Chlamydomonas. Methods. 2000;22:383–387. doi: 10.1006/meth.2000.1090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The video rate is reduced by 1/30. Note that the rod (30 μm in diameter) appears much thicker because the brightness and contrast are adjusted so that the cilia are visible.
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This study does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.