Abstract
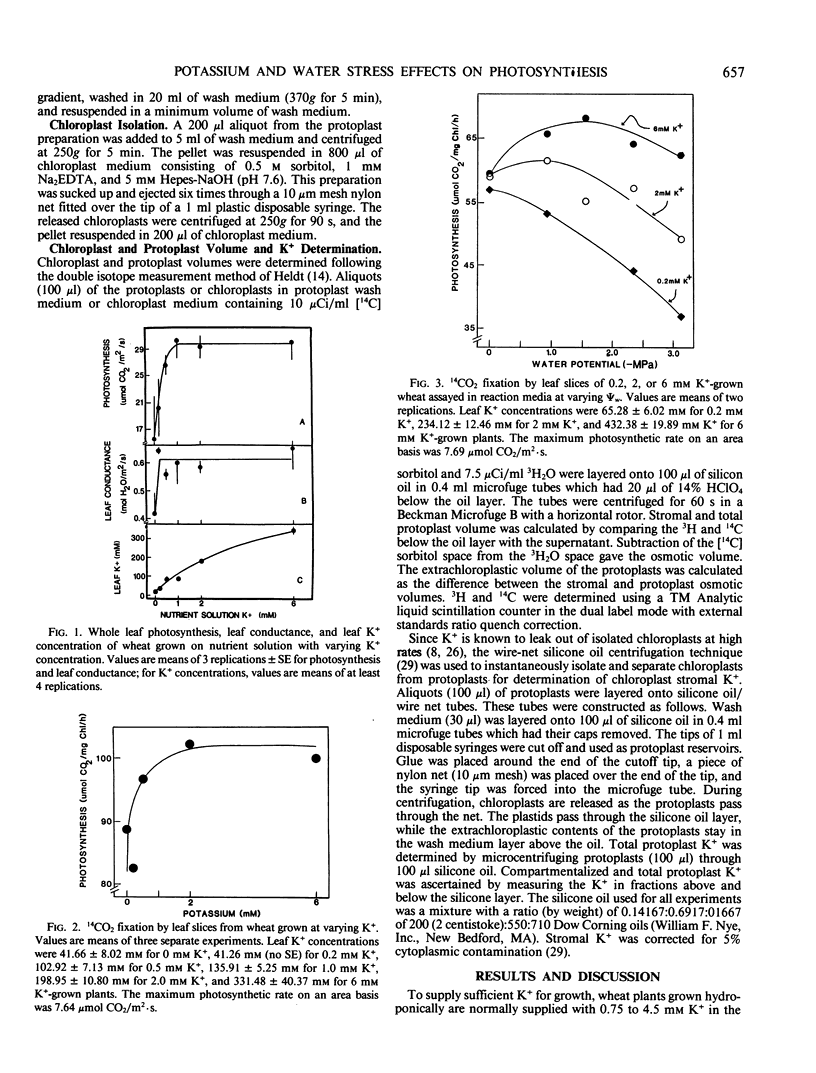
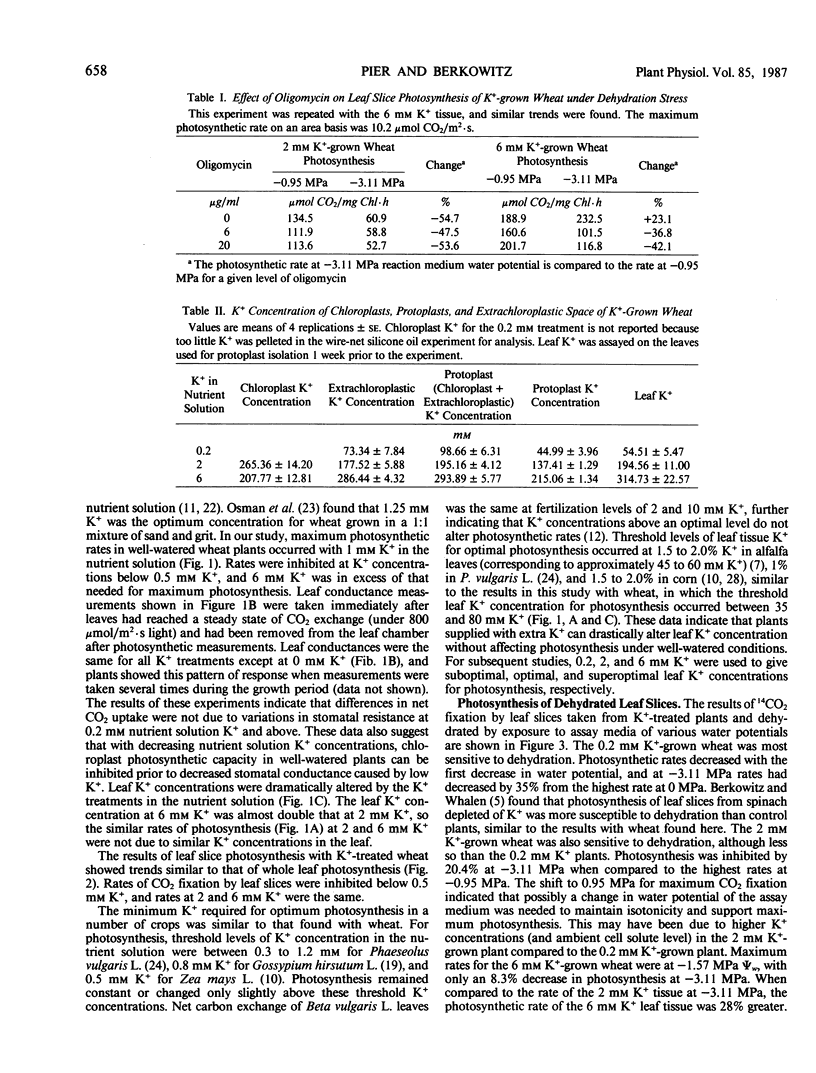
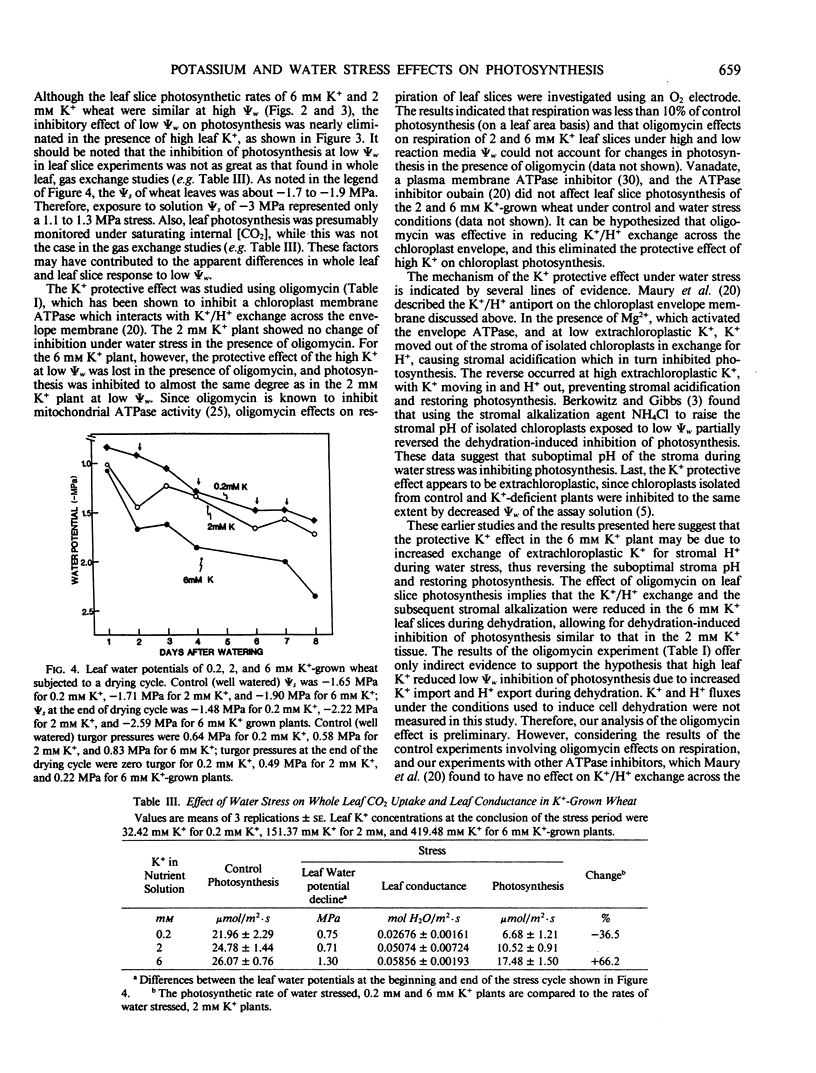
Wheat irrigated with nutrient solutions containing 0, 0.2, 0.5, 1, 2, or 6 millimolar K+ had maximum photosynthetic rates at 1 to 2 millimolar K+ concentrations. Rates in the 6 millimolar K+-grown plants were not higher than the 2 millimolar K+-grown wheat, and rates were inhibited below 0.5 millimolar K+. Photosynthesis was measured by both attached whole leaf CO2 uptake and by 14CO2 fixation of leaf slices in solution. Exposure of leaf slices from 0.2, 2, and 6 millimolar K+-grown wheat to various assay media water potentials showed that photosynthesis of the 0.2 millimolar K+-grown wheat decreased from control (high water potential) rates by 35%, that of the 2 millimolar K+-grown wheat by 20.4%, and that of the 6 millimolar K+-grown wheat by only 8.3% at −3.11 megapascals. Also, photosynthesis of the 6 millimolar K+-grown wheat was enhanced by 28% over that of the 2 millimolar K+ wheat at the most severe water stress (−3.11 megapascals), indicating that the excess leaf K+ in the 6 millimolar K+-grown wheat partially reversed dehydration effects on photosynthesis. Oligomycin eliminated the protective effects of high K+ on photosynthesis in dehydrated leaf slices. These results suggest that the protective effect of high K+ under water stress may involve the exchange of K+ in the cytoplasm for stroma H+, thus altering stromal pH and restoring photosynthesis. The protective effect of high K+ was also observed in attached whole leaf photosynthesis of in situ water-stressed wheat grown on 0.2, 2, and 6 millimolar K+. Under water stress, rates of the 6 millimolar K+-grown wheat were enhanced by 66.2% and 113.9% over that of 2 millimolar K+-grown wheat in two separate experiments. Internal CO2 concentration of the 6 millimolar K+-grown wheat was lower than that of the 0.2 and 2 millimolar K+-grown wheat. These results suggest that the high K+ effects on chloroplast photosynthesis seen in leaf slices also occur at the whole plant level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. A., Gibbs M. Reduced osmotic potential effects on photosynthesis : identification of stromal acidification as a mediating factor. Plant Physiol. 1983 Apr;71(4):905–911. doi: 10.1104/pp.71.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. A., Gibbs M. Reduced osmotic potential inhibition of photosynthesis : site-specific effects of osmotically induced stromal acidification. Plant Physiol. 1983 Aug;72(4):1100–1109. doi: 10.1104/pp.72.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. A., Whalen C. Leaf k interaction with water stress inhibition of nonstomatal-controlled photosynthesis. Plant Physiol. 1985 Sep;79(1):189–193. doi: 10.1104/pp.79.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Gimmler H. Properties of the Isolated Intact Chloroplast at Cytoplasmic K Concentrations : I. Light-Induced Cation Uptake into Intact Chloroplasts is Driven by an Electrical Potential Difference. Plant Physiol. 1983 Sep;73(1):169–174. doi: 10.1104/pp.73.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Conti T. R. Relation of increased potassium nutrition to photosynthesis and translocation of carbon. Plant Physiol. 1983 Jan;71(1):141–144. doi: 10.1104/pp.71.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R. L., Leech R. M. The stimulation of CO2-supported O2 evolution in intact spinach chloroplasts by ammonium ion. Arch Biochem Biophys. 1978 Sep;190(1):221–226. doi: 10.1016/0003-9861(78)90271-0. [DOI] [PubMed] [Google Scholar]
- Hutmacher R. B., Krieg D. R. Photosynthetic rate control in cotton : stomatal and nonstomatal factors. Plant Physiol. 1983 Nov;73(3):658–661. doi: 10.1104/pp.73.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Longstreth D. J., Nobel P. S. Nutrient Influences on Leaf Photosynthesis: EFFECTS OF NITROGEN, PHOSPHORUS, AND POTASSIUM FOR GOSSYPIUM HIRSUTUM L. Plant Physiol. 1980 Mar;65(3):541–543. doi: 10.1104/pp.65.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury W. J., Huber S. C., Moreland D. E. Effects of Magnesium on Intact Chloroplasts : II. CATION SPECIFICITY AND INVOLVEMENT OF THE ENVELOPE ATPase IN (SODIUM) POTASSIUM/PROTON EXCHANGE ACROSS THE ENVELOPE. Plant Physiol. 1981 Dec;68(6):1257–1263. doi: 10.1104/pp.68.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. ATPases associated with electron transport. Methods Enzymol. 1979;55:297–303. doi: 10.1016/0076-6879(79)55034-4. [DOI] [PubMed] [Google Scholar]
- Tu S. I., Sliwinski B. J. Mechanistic investigation on the temperature dependence and inhibition of corn root plasma membrane ATPase. Arch Biochem Biophys. 1985 Sep;241(2):348–355. doi: 10.1016/0003-9861(85)90556-9. [DOI] [PubMed] [Google Scholar]


