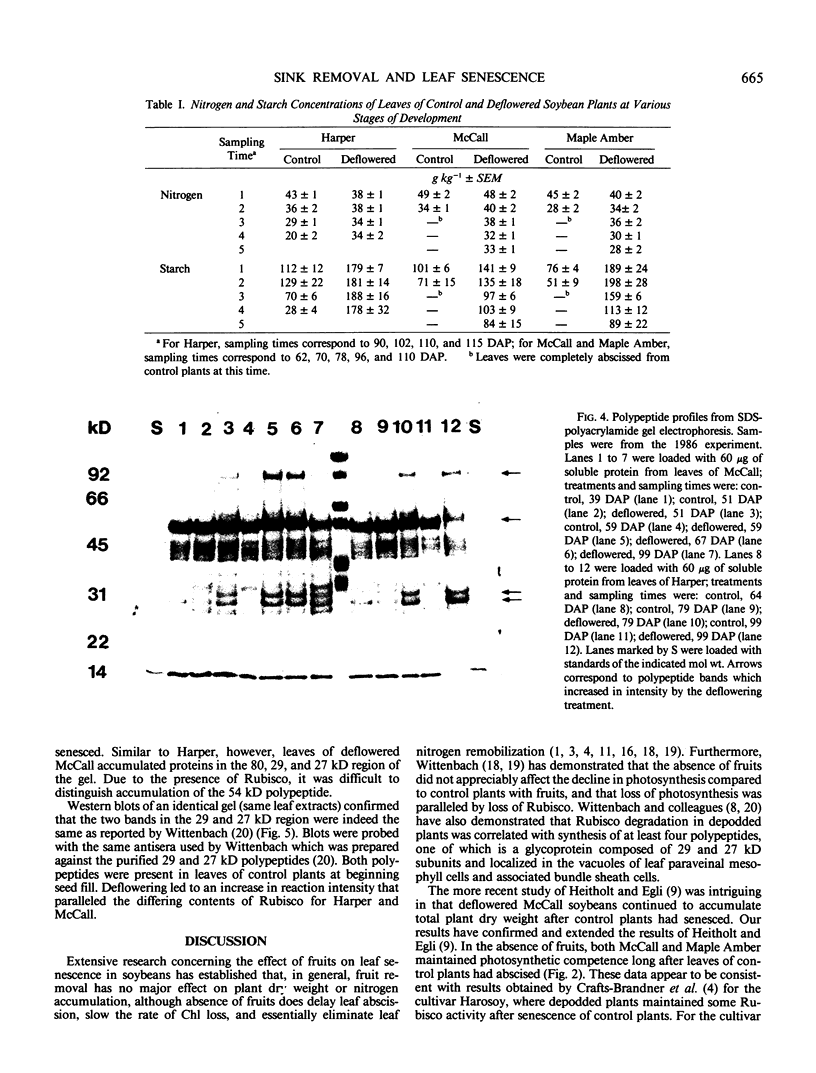
Abstract
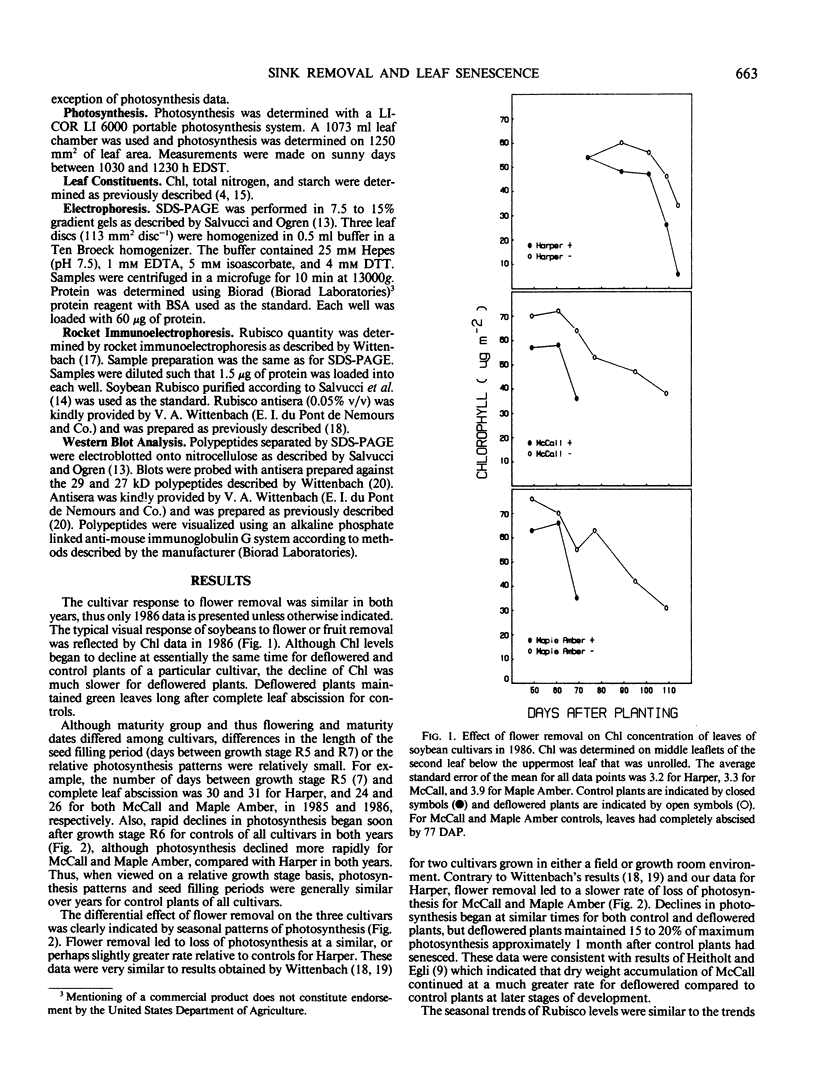
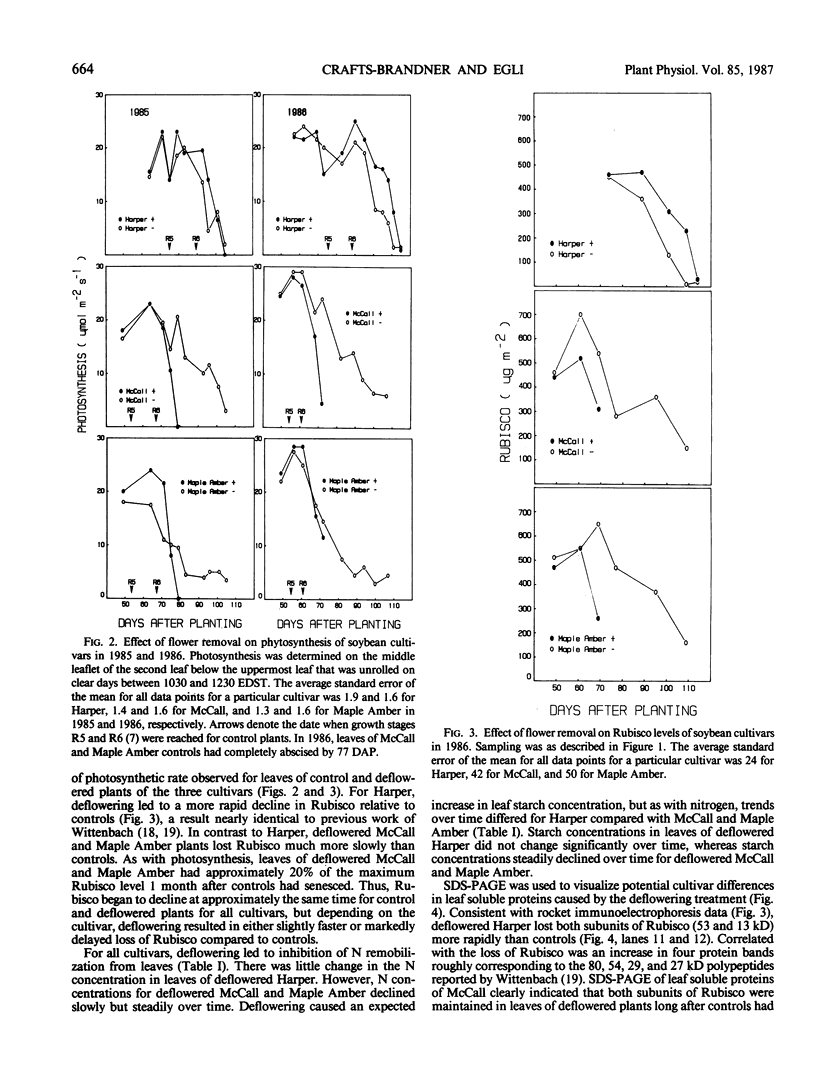
Three cultivars of soybean (Glycine max [L.] Merr. cvs Harper, McCall, and Maple Amber) were grown in the field and kept continuously deflowered throughout the normal seedfill period. For all cultivars, deflowering led to delayed leaf abscission and a slower rate of chlorophyll loss. Compared to control plants, photosynthesis and ribulose 1,5-bis-phosphate carboxylase/oxygenase (Rubisco) level declined slightly faster for deflowered Harper, but for both McCall and Maple Amber, leaves of deflowered plants maintained approximately 20% of maximum photosynthesis and Rubisco level 1 month after control plants had senesced. Deflowering led to decreased leaf N remobilization and increased starch accumulation for all cultivars, but cultivars differed in that for McCall and Maple Amber, N and starch concentrations slowly but steadily declined over time whereas for Harper, N and starch concentrations remained essentially constant over time. SDS-PAGE of leaf proteins indicated that for all cultivars, deflowering led to accumulation of four polypeptides (80, 54, 29, and 27 kilodaltons). Western analysis using antisera prepared against the 29 and 27 kilodalton polypeptides verified that these polypeptides were the glycoproteins previously reported to accumulate in vacuoles of paraveinal mesophyll cells of depodded soybean plants. The results indicated that depending on the cultivar, sink removal can lead to either slightly faster or markedly slower loss of photosynthesis and Rubisco. This difference, however, was not associated with the ability to synthesize leaf storage proteins. For any particular cultivar, declines in chlorophyll, photosynthesis, and Rubisco were initiated at approximately the same time for control and deflowered plants. Thus, even though cultivars differed in rate of decay of photosynthetic rate and Rubisco level in response to sink removal, the initiation of leaf senescence was not influenced by presence or absence of developing fruits.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crafts-Brandner S. J., Below F. E., Harper J. E., Hageman R. H. Differential Senescence of Maize Hybrids following Ear Removal : I. Whole Plant. Plant Physiol. 1984 Feb;74(2):360–367. doi: 10.1104/pp.74.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner S. J., Below F. E., Harper J. E., Hageman R. H. Effects of Pod Removal on Metabolism and Senescence of Nodulating and Nonnodulating Soybean Isolines: II. Enzymes and Chlorophyll. Plant Physiol. 1984 Jun;75(2):318–322. doi: 10.1104/pp.75.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner S. J., Below F. E., Harper J. E., Hageman R. H. Effects of pod removal on metabolism and senescence of nodulating and nonnodulating soybean isolines: I. Metabolic constituents. Plant Physiol. 1984 Jun;75(2):311–317. doi: 10.1104/pp.75.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner S. J., Poneleit C. G. Effect of Ear Removal on CO(2) Exchange and Activities of Ribulose Bisphosphate Carboxylase/Oxygenase and Phosphoenolpyruvate Carboxylase of Maize Hybrids and Inbred Lines. Plant Physiol. 1987 Jun;84(2):261–265. doi: 10.1104/pp.84.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Wittenbach V. A., Giaquinta R. T. Paraveinal Mesophyll of Soybean Leaves in Relation to Assimilate Transfer and Compartmentation : III. Immunohistochemical Localization of Specific Glycopeptides in the Vacuole after Depodding. Plant Physiol. 1983 Jun;72(2):586–589. doi: 10.1104/pp.72.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal M. H., Brun W. A., Brenner M. L. Effects of Sink Removal on Photosynthesis and Senescence in Leaves of Soybean (Glycine max L.) Plants. Plant Physiol. 1978 Mar;61(3):394–397. doi: 10.1104/pp.61.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Portis A. R., Jr, Ogren W. L. Purification of ribulose-1, 5-bisphosphate carboxylase/oxygenase with high specific activity by fast protein liquid chromatography. Anal Biochem. 1986 Feb 15;153(1):97–101. doi: 10.1016/0003-2697(86)90066-7. [DOI] [PubMed] [Google Scholar]
- Swank J. C., Below F. E., Lambert R. J., Hageman R. H. Interaction of carbon and nitrogen metabolism in the productivity of maize. Plant Physiol. 1982 Oct;70(4):1185–1190. doi: 10.1104/pp.70.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Burton J. W., Buck J. A., Brim C. A. Studies on Genetic Male-Sterile Soybeans: I. Distribution of Plant Carbohydrate and Nitrogen during Development. Plant Physiol. 1978 May;61(5):838–841. doi: 10.1104/pp.61.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Effect of pod removal on leaf photosynthesis and soluble protein composition of field-grown soybeans. Plant Physiol. 1983 Sep;73(1):121–124. doi: 10.1104/pp.73.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Effect of pod removal on leaf senescence in soybeans. Plant Physiol. 1982 Nov;70(5):1544–1548. doi: 10.1104/pp.70.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 1983 Sep;73(1):125–129. doi: 10.1104/pp.73.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Ribulose Bisphosphate Carboxylase and Proteolytic Activity in Wheat Leaves from Anthesis through Senescence. Plant Physiol. 1979 Nov;64(5):884–887. doi: 10.1104/pp.64.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]