Abstract
Genital psoriasis affects 3-33% of patients with psoriasis during the course of the disease, usually leading to a severe reduction in the patient’s quality of life. This study aims to retrospectively assess the effectiveness of interleukin (IL)-23 and IL-17 inhibitors in a real-life population affected by moderate-to-severe plaque psoriasis with genital involvement coming from our dermatology department. A total of 86 patients with a diagnosis of moderate-tosevere plaque psoriasis with severe genital involvement were enrolled. Patient characteristics, psoriasis area and severity index (PASI), and static physician global assessment of genitalia (sPGAG) at each visit were recorded. During the treatment, the mean PASI decreased from 12.8 to 0.63 at week 52; a PGA of 0/1 was reached by 97.40% at week 52 and by 100% of patients (37/37) at week 104. No significant differences between IL-23 and IL-17 inhibitors were observed; indeed, the bio-naïve group of patients demonstrated a superior response compared to the group of bioexperienced patients.Our findings confirmed that IL-23 and IL-17 inhibitors are safe and effective therapeutic options for the treatment of genital psoriasis.
Key words: biologics, genital psoriasis, psoriasis treatment
Introduction
Psoriasis is a chronic immune-mediated, inflammatory, systemic disease that affects 2-4% of the general population and has a great impact on the patient’s quality of life. Difficult-to-treat areas include the face/scalp, nails, palms/soles, and genital region.1,2
Genital psoriasis affects 33-63% of patients with psoriasis during the course of the disease, clinically presenting as welldemarcated, erythematous plaques that may not present the typical dry scaling.3 Genital lesions are often associated with itching, burning, or pain and usually lead to significant impairment of psychosocial and sexual function, often resulting in a severe reduction in the patient’s quality of life. This morbidity can be underestimated by physicians.4 It has been demonstrated that genital psoriasis leads to sexual impairment independently of the psoriasis area and severity index (PASI).5 Despite the development of several therapeutic options for plaque psoriasis, genital psoriasis treatment is often challenging, and many patients are undertreated worldwide.6
According to the extension and severity of the disease, psoriasis can be classified as mild or moderate-to-severe. First-line treatments include topical corticosteroids and vitamin D derivates for limited disease. In cases of moderate-to-severe disease, patients are eligible for conventional systemic therapies.7 According to Italian guidelines, in patients with contraindications to conventional therapies or with an inadequate response to them, biological treatments are indicated.8
This study aims to retrospectively assess the effectiveness of interleukin (IL)-23 and IL-17 inhibitors in a real-life population affected by moderate-to-severe plaque psoriasis with genital involvement coming from our dermatology department.
Materials and Methods
We enrolled all patients with a diagnosis of moderate-tosevere plaque psoriasis with genital involvement treated with antiIL-17 and anti-IL-23 inhibitors between January 2020 and October 2022. Institutional review board approval was exempted as all the procedures did not deviate from standard clinical practice. All enrolled patients provided written consent for the retrospective study of the collected data. All patients received biological therapy following the Italian guidelines.
The eventual occurrence of adverse events was recorded at each dermatologic visit. All patients underwent routine blood chemistry tests and screening for HIV, viral hepatitis, and tuberculosis (TB) gold quantiferon before starting the treatment, according to Italian guidelines.8 To be included, the severity of genital psoriasis needed to be scored as 3 or more on the static physician global assessment of genitalia (sPGA-G).
PASI scores were reported at weeks 16, 24, 52, and 104. The percentages of patients achieving a 75%, 90%, and 100% reduction (PASI75, PASI90, and PASI100, respectively) in PASI in comparison with PASI baseline were selected as effectiveness endpoints. Genital psoriasis was evaluated using sPGA-G, a wellestablished, validated subjective tool that assesses the severity of genital psoriasis with a numerical scale ranging from 0 (clear) to 5 (very severe).9
The primary endpoint was the proportion of patients achieving sPGA-G response, defined as a score of 0 (clear) or 1 (minimal) at each timepoint.
Continuous parameters were reported using frequency, mean, and standard deviation (SD) values, while discrete parameters were reported as percentages and counts. The percentages of patients achieving a PGA equal to 0 or 1 (clear or almost clear) and PASI75, PASI90, and PASI100 responses during biological treatment were examined in relation to various parameters: anti- IL-23 treatment vs anti-IL-17 treatment and biologic-naïve vs biologic- experienced patients. The categorical variables were analyzed using the chi-square test. Statistical significance was defined as a probability value of less than 0.05. Stata/SE 17.0 software (StataCorp LLC, College Station, TX, USA) was used for analysis, and Microsoft Excel was used to generate tables.
Results and Discussion
We analyzed a total of 86 patients affected by genital psoriasis who were treated with biological therapy at the dermatologic outpatient clinic of Humanitas Research Hospital. All the patients reached at least 24 weeks of follow-up (n=86), while 77 patients reached 52 weeks of follow-up and 37 completed 104 weeks of treatment.
In particular, 35 patients were treated with IL-17 inhibitors (26 patients with ixekizumab, 5 patients with secukinumab, 2 patients with bimekizumab, and 2 patients with brodalumab), and 51 patients were treated with IL-23 inhibitors (33 patients with risankizumab, 14 with guselkumab, and 4 with tildrakizumab).
The baseline demographic characteristics of the patients are summarized in Table 1. We analyzed 30 female and 56 male patients, with a median age of 49.81 years (SD=15.16), a median body mass index of 26.7 kg/m2 (SD=3.16), and a median disease duration of 14.15 years (SD=10.30). A total of 17 patients had a concomitant diagnosis of psoriatic arthritis (17.44%) and 31 patients reported one or more comorbidities (36.04%). One patient had a positive TB quantiferon test before starting the treatment. 68 patients were naïve to biologic therapy, while 18 had been previously treated with another biologic at least; in particular, anti- TNFα drugs were previously administered to 6 patients (6.98%), ustekinumab to 5 patients (5.81%), anti-IL-17 to 8 patients (9.30%), and other anti-IL-23 inhibitors (including risankizumab and tildrakizumab) to 2 patients (11.63%).
During the treatment, the mean PASI (mPASI) of our population decreased from 12.8 (6.58) at baseline to 1.82 (2.05) at week 16, and 0.94 (1.21) at week 24. Regarding the 77 patients who reached one year of follow-up, their mPASI was 0.63 (0.94) at week 52, while at week 104 (1.06) the mPASI of our 37 patients was 0.42 (Figure 1).
Concerning PASI reduction, during the treatment, PASI75 was reached by 70.93% (61/86) of patients treated at week 16, 89.53% (77/86) at week 24, 93.51% (72/77) at week 52, and 94.59% (34/37) at week 104.
One-half of patients (43/86) reached PASI90 at week 16, 65.12% (86) at week 24, 79.22% at week 52 (61/77), and 94.59% (35/37) at week 104.
Finally, PASI100 was achieved by 33.72% of patients (29/86) at week 16, 47.67% at week 24 (41/86), 55.84% (43/77) at week 52, and 70.27% (26/37) at week 104.
Regarding the effectiveness of biologics on genital psoriasis, a PGA of 0/1was reached by 81.40% (70/86) of patients treated at week 16, 88.37% (76/86) at week 24, 97.40% at week 52, and 100% of patients (37/37) at week 104.
Data concerning PASI75, PASI90, PASI100, and PGA equal to 0 or 1 of our cohort of patients are summarized in Figure 1.
Treatment effectiveness was also evaluated based on different parameters. We compared the treatment responses of patients treated with anti-IL-23 (n=51) and anti-IL-17 (n=35) at weeks 16, 24, and 52.
PASI75 was achieved by 37/51 patients (72.55%) at week 16, 43/51 (84.31%) at week 24 and 41/45 (91.11%) at week 52 in the group treated with anti-IL-23, while 24/35 patients (68.57%) at week 16, 34/35 (97.14%) at week 24, and 32/32 (100%) at week 52 among patients treated with anti-IL-17.
The first group demonstrated PASI90 in 25/51 (49.02%) at week 16, 29/51 (56.86%) at week 24, and 36/45 (80%) at week 52; the same result was achieved in 18/35 patients (51.43%) at week 16, 27/35 (77.14%) at week 24, and 26/32 (81.25%) at week 52 among anti-IL-17 patients.
Table 1.
Demographic characteristics of the 86 patients with genital psoriasis.
| Characteristics | N (%) |
|---|---|
| Number of patients | 86 |
| Male | 50/86 (58.14) |
| Age (years) | 49.81 (SD 15.16) |
| BMI | 26.7 (SD 3.16) |
| Obese (BMI≥30) | 12/86 (13.95) |
| Disease duration (years) | 14.15 (SD 10.30) |
| PsA | 15/86 (17.44) |
| Comorbidity | 31/86 (36.04) |
| Hypertension | 14/86 (16.27) |
| Diabetes | 5/86 (5.81) |
| Bio-experienced | 18/86 (20.93) |
| Anti-IL-17 | 35/86 (40.70) |
| Bimekizumab | 2/86 (2.33) |
| Brodalumab | 2/86 (2.33) |
| Ixekizumab | 26/86 (30.23) |
| Secukinumab | 5/86 (5.81) |
| Anti-IL-23 | 51/86 (59.30) |
| Risankizumab | 33/86 (38.37) |
| Guselkumab | 14/86 (16.28) |
| Tildrakizumab | 4/86 (4.65) |
SD, standard deviation; BMI, body mass index; PsA, psoriatic arthritis; IL, interleukin.
Figure 1.
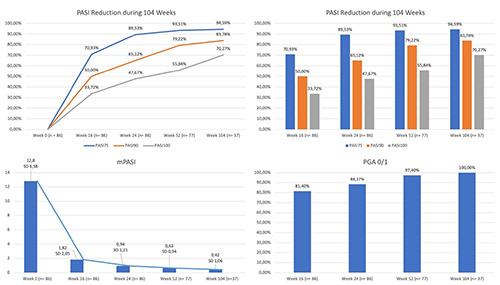
Mean psoriasis area and severity index reduction, percentages of patients achieving psoriasis area and severity index 100/90/75 compared with baseline and physician global assessment 0/1 during 104 weeks of treatment. PASI, psoriasis area and severity index; mPASI, mean psoriasis area and severity index; PGA, physician global assessment; SD, standard deviation.
Figure 2.

Physician global assessment 0/1 and percentages of patients achieving psoriasis area and severity index 100/90/75 compared with baseline in bio-naïve vs bio-experienced. PASI, psoriasis area and severity index; PGA, physician global assessment.
PASI100 was reached in the anti-IL-23 group by 16/51 patients (31.37%) at week 16, 29/51 (56.86%) at week 24, and 24/45 (53.33%) at week 52, compared to 13/35 patients (37.14%) at week 16, 17/35 (48.57%) at week 24, and 19/32 (59.38%) at week 52 in the group treated with IL-17 inhibitors.
At week 16, anti-IL-23 patients reached a PGA of 0/1 in 43/51 cases (84.31%), 46/51 (90.20%) at week 24, and 45/45 (100%) at week 52; similarly, patients treated with IL-17 inhibitors achieved the primary endpoint in 27/35 cases (77.14%) at week 16, 30/35 (85.71%) at week 24, and 30/32 (93.75%) at week 52.
Regarding all effectiveness endpoints, no significant differences between the two groups were observed.
Concerning the previous exposition to biologic therapies, the bio-naïve group of patients demonstrated superior response compared to the group of bio-experienced patients (Figure 2); in particular, we observed PASI90 at week 16 in 56.82% (50/68) of bionaïve patients vs 16.67% (3/18) of bio-experienced patients (p=0.001); at week 52, PGA 0/1, PASI75 and PASI90 were also superior in the first group (100% vs 87.50%, p=0.005; 100% vs 75%, p<0.001; 88.52% vs 50%, p=0.001) (Figure 2).
Regarding the safety of biologics, none of our 86 patients had to discontinue the treatment because of adverse events and no severe adverse events were reported. No relapses of TB were observed during the entire study period.
Conclusions
The treatment of genital psoriasis is challenging, and the involvement of this area can have a significant impact on the quality of life of the patients. Many patients remain undertreated because they are often excluded from clinical trials due to lower PASI and body surface area scores at baseline.6
Given the reduced skin thickness and occlusion of the genital area, the application of topical therapies is often ineffective and poorly tolerated by patients. Moreover, patients with genital or other difficult-to-treat localizations of psoriasis tend to be more refractory to systemic therapies and show an increased risk of disease relapse.7
For these reasons, recent studies have highlighted the importance of biological therapies in cases of genital psoriasis that are severe or non-responsive to traditional therapy.
In two phase-III clinical trials, ixekizumab demonstrated superiority vs placebo for the treatment of moderate-to-severe genital psoriasis, with an improvement of the disease and symptoms related to sexual activity.10,11
In a randomized clinical trial in 2021, the IL-17 inhibitors secukinumab and ixekizumab demonstrated efficacy in terms of reduction of genital psoriasis symptoms and impact on sexual activity.12
A real-life experience with 237 patients affected by psoriasis in a multicentric study revealed a good response in difficult-totreat areas.13
In a cohort of patients affected by moderate-to-severe plaque psoriasis treated with risankizumab, at week 52, comparable percentages of patients achieved PASI100, regardless of the involvement of difficult-to-treat areas, including the genital region.14
In our study, we did not observe any significant difference in response to therapy between IL-23 and IL-17 inhibitors, with comparable PASI75/90/100 and PGA 0/1 at week 16, 24, and 52.
Due to the lack of work demonstrating the specific efficacy of anti-IL-23 on genital psoriasis, our data could support their use in this difficult-to-treat area.
Comparing the groups between the two cohorts of bio-naïve and bio-experienced patients, the first group improved more consistently in both PASI reduction and PGA 0/1 score; this data is consistent with what was previously reported in the literature, and it may be explained by the fact that refractory or multi-refractory patients may develop resistance mechanisms such as antibodies directed against the biological drug, making it less effective.15
The main limitations of our work are the monocentric setting and retrospective analysis. Large prospective and multicentric studies are needed to eventually confirm this preliminary report.
Comments
Our article confirms the tendency of biologic drugs to work better in bio-naïve patients than in bio-experienced ones, highlighting the importance of careful drug selection at the beginning of patient management.
Most importantly, our case series adds to the evidence of the treatment efficacy of both anti-IL-23 and anti-IL-17 biological drugs, with no significant differences between the two classes, in the management of patients with genital psoriasis, which often results in non-responders to conventional therapies.
References
- 1.Boehncke WH, Schön MP. Psoriasis. Lancet 2015;386:983-94. [DOI] [PubMed] [Google Scholar]
- 2.Narcisi A, Valenti M, Cortese A, et al. Anti-IL17 and anti-IL23 biologic drugs for scalp psoriasis: a single-center retrospective comparative study. Dermatol Ther 2022;35:e15228. [DOI] [PubMed] [Google Scholar]
- 3.Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat 2018;29:754-60. [DOI] [PubMed] [Google Scholar]
- 4.Kelly A, Ryan C. Genital psoriasis: impact on quality of life and treatment options. Am J Clin Dermatol 2019;20:639-46. [DOI] [PubMed] [Google Scholar]
- 5.Duarte GV, Calmon H, Radel G, de Fátima Paim de Oliveira M. Psoriasis and sexual dysfunction: links, risks, and management challenges. Psoriasis (Auckl) 2018;8:93-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol 2019;58:649-58. [DOI] [PubMed] [Google Scholar]
- 7.Hong JJ, Mosca ML, Hadeler EK, et al. Genital and inverse/intertriginous psoriasis: an updated review of therapies and recommendations for practical management. Dermatol Ther (Heidelb) 2021;11:833-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gisondi P, Fargnoli MC, Amerio P, et al. Italian adaptation of EuroGuiDerm guideline on the systemic treatment of chronic plaque psoriasis. Ital J Dermatol Venerol 2022;157:1-78. [DOI] [PubMed] [Google Scholar]
- 9.Merola JF, Bleakman AP, Gottlieb AB, et al. The static physician's global assessment of genitalia: a clinical outcome measure for the severity of genital psoriasis. J Drugs Dermatol 2017;16:793-9. [PubMed] [Google Scholar]
- 10.Guenther L, Potts Bleakman A, Weisman J, et al. Ixekizumab results in persistent clinical improvement in moderate-tosevere genital psoriasis during a 52 week, randomized, placebo- controlled, phase 3 clinical trial. Acta Derm Venereol 2020;100:adv00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yosipovitch G, Foley P, Ryan C, et al. Ixekizumab improved patient-reported genital psoriasis symptoms and impact of symptoms on sexual activity vs placebo in a randomized, double- blind study. J Sex Med 2018;15:1645-52. [DOI] [PubMed] [Google Scholar]
- 12.Almutairi N, Eassa BI. A randomized controlled ixekizumab vs secukinumab trial to study the impact on sexual activity in adult patients with genital psoriasis. Expert Opin Biol Ther 2021;21:297-8. [DOI] [PubMed] [Google Scholar]
- 13.Narcisi A, Valenti M, Gargiulo L, et al. Real-life effectiveness of tildrakizumab in chronic plaque psoriasis: a 52-week multicentre retrospective study-IL PSO (Italian landscape psoriasis). J Eur Acad Dermatol Venereol 2023;37:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gargiulo L, Ibba L, Pavia G, et al. Real-life effectiveness and safety of risankizumab in 131 patients affected by moderateto- severe plaque psoriasis: a 52-week retrospective study. Dermatol Ther (Heidelb) 2022;12:2309-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu L, Armstrong AW. Anti-drug antibodies in psoriasis: a critical evaluation of clinical significance and impact on treatment response. Expert Rev Clin Immunol 2013;9:949-58. [DOI] [PubMed] [Google Scholar]


