Abstract
Two ganciclovir (GCV)-resistant human cytomegalovirus (HCMV) strains recovered from an AIDS patient (strain VR4990) and a heart transplant recipient (strain VR5474) showed a Cys607→Tyr change in the UL97-encoded phosphotransferase. No amino acid substitutions were observed in the viral DNA polymerase. Marker transfer experiments showed marked reduction in GCV phosphorylation and drug susceptibility of the recombinant HCMV strain VR4990rec2-1-1. These results further extend the region of the carboxy-terminal domain of the UL97 phosphotransferase involved in GCV substrate recognition.
Human cytomegalovirus (HCMV) is an important pathogen in immunocompromised patients, and ganciclovir (GCV) is a first-choice drug in the management of HCMV infections. However, GCV resistance has now become a substantial problem (1–4, 6–13, 15, 16, 18, 20–22, 25). A better understanding of the genetic mechanisms involved in the GCV resistance may be useful in designing rapid molecular diagnostic tests for the detection of drug-resistant HCMV strains and in developing new anti-HCMV drugs.
In the UL97 phosphotransferase, mutations proven to confer GCV resistance are clustered in three distinct regions including residues 460, 520, and 590 to 595 (1, 2, 4, 6, 7, 13, 18, 21, 24, 25). Recently, Chou and colleagues reported that a Cys603→Trp change is responsible for GCV resistance (8). Comparative sequencing data indicated that residues 596, 600, and 607 may also play a role in this phenomenon (1, 3, 7). To date, no mutations associated with GCV resistance have been reported in between residues 460, 520, and 590.
Recently, the biochemical properties of HCMV UL97-encoded phosphotransferase have been investigated by expression of the protein in vaccinia and baculovirus systems (14, 19), showing its functional homology with serine/threonine protein kinases and suggesting that the deletion of residues 590 to 593 present in the GCV-resistant strain 759rD100 (24) alters GCV phosphorylation, with only a modest reduction in the protein kinase activity (14, 19).
In this study we demonstrate that a Cys→Tyr substitution in position 607 of UL97 phosphotransferase reduces the GCV kinase activity and confers GCV resistance. Our data suggest that a larger domain of the carboxy-terminal region of the UL97 phosphotransferase (residues 590 to 607) is involved in substrate recognition.
(This work was presented in part at the 6th International Cytomegalovirus Workshop, Orange Beach, Perdido Beach Resort, Ala., 5 to 9 March 1997, and at the 10th International Conference on Antiviral Research, Atlanta, Ga., 6 to 11 April 1997.)
Two HCMV isolates recovered from an AIDS patient (strain VR4990) and a heart transplant recipient (strain VR5747) following prolonged treatment with GCV showed more than eightfold increases in GCV 50% inhibitory doses (ID50s) with respect to ID50s for pretherapy isolates VR4631 and VR5549, respectively, from the same patients (Table 1), as well as with respect to the mean ID50 ± the standard deviation (2.9 ± 2.1) for 10 HCMV isolates from treatment-naive immunocompromised patients (11). Drug susceptibility was measured by an immediate-early antigen plaque reduction assay, as previously reported (12).
TABLE 1.
Ganciclovir susceptibilities of two HCMV clinical isolates and an HCMV recombinant strain with a Cys607→Tyr change in the UL97-encoded phosphotransferasea
| Patient | Status | HCMV strain (nature) | GCV ID50 (μM) | SI50b |
|---|---|---|---|---|
| 1 | AIDS patient | VR4631 (pretherapy) | 2.7 | NA |
| VR4990 (posttherapy) | 25.0 | 9.25 | ||
| 2 | HTRc | VR5549 (pretherapy) | 2.3 | NA |
| VR5747 (posttherapy) | 25.0 | 10.86 | ||
| VR4990rec2-1-1 (recombinant) | 10.0 | 12.5 | ||
| AD169 (wild type) | 0.8 | NA |
With respect to pretherapy HCMV isolates from the same patients and the reference strain AD169.
SI50, 50% sensitivity index, representing the ratio between GCV ID50s for VR4990 and VR5747 and the ID50s for pretherapy HCMV isolates from the same patients. Similarly, the SI50 of VR4990rec2-1-1 with respect to AD169 is shown. NA, not applicable.
HTR, heart transplant recipient.
Sequence analysis of the conserved domains of UL97 and UL54 (codons 305 to 706 and 333 to 1004, respectively) was performed on sequential (GCV-sensitive and GCV-resistant) isolates from the two patients, as described previously (2, 4). Restriction fragment length polymorphism of multiple genome regions (20) as well as comparison of nucleotide changes in both UL97 and UL54 suggested that the two patients were infected by genetically unrelated HCMV strains, thus excluding an epidemiological connection between the two cases (data not shown). The GCV-resistant isolates VR4990 and VR5747 showed a TGT→TAT change in UL97 codon 607, leading to a Cys607→Tyr substitution which was not present in GCV-sensitive isolates from either patient. In addition, this mutation was never previously reported for GCV-sensitive HCMV field strains (data not shown). No additional amino acid alterations were predicted based on the sequences of the UL97 genes of VR4990 and VR5747 (Table 2). In contrast, the amino acid sequences of the UL54 gene products of these two strains showed differences between the two isolates which are frequently detected in drug-sensitive HCMV strains (Table 2).
TABLE 2.
UL54 and UL97 sequence analysis of two GCV-resistant HCMV isolates from two immunocompromised patients
| Patient | Status | HCMV isolate | Amino acid change(s) with respect to AD169 in:
|
|
|---|---|---|---|---|
| UL54 | UL97 | |||
| 1 | AIDS patient | VR4990 | Ser655→Leu, Asn685→Ser, Ala885→Thr, Asn898→Asp | Cys607→Tyra |
| 2 | HTRb | VR5747 | Ala885→Thr | Cys607→Tyra |
An amino acid change never observed in GCV-sensitive HCMV field strains.
HTR, heart transplant recipient.
Marker transfer experiments were performed as reported previously (2, 4) by cotransfection of full-length infectious AD169 DNA and a 1,629-bp fragment (nucleotides 915 to 2544) amplified by PCR with primer pair UL97 1 and 6 from the VR4990 UL97 gene (2). Recombinant virus screening was performed by plating viral progeny on MRC-5 cell monolayers in the presence of 50 μM GCV (2). Plaques growing under GCV were picked up, expanded in the absence of drug, and then plaque purified two more times. Finally, recombinant strain VR4990rec2-1-1 was submitted to sequence analysis, GCV susceptibility testing, and GCV phosphorylation. Sequencing showed the presence of the TGT→TAT change in codon 607 of the UL97 gene. In addition, comparison of silent nucleotide variations between AD169 and VR4990 in the fragment amplified by primer pair UL97 1 and 6 demonstrated that the VR4990 UL97 fragment spanning codons 429 to 634 was inserted in the AD169 UL97 gene during the homologous recombination process, thus showing that the change was not generated de novo during the selection of the viral progeny with GCV.
VR4990rec2-1-1 showed a GCV-resistant phenotype in the immediate-early antigen plaque reduction assay, with a GCV ID50 of 10.0 μM, which corresponded to a 12.5-fold increase in GCV resistance with respect to that of parent strain AD169 (Table 1).
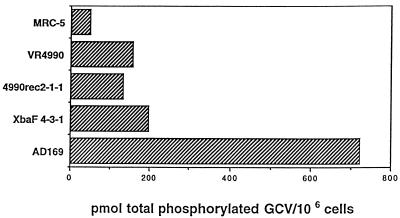
GCV phosphorylation was evaluated with the recombinant strain VR4990rec2-1-1 and the two parent strains, VR4990 (GCV resistant) and AD169 (GCV sensitive), as previously described (2, 4, 22, 24). As controls, GCV-resistant reference strain XbaF4-3-1, derived by transfer of UL97 from 759rD100 back into AD169 (23, 24), and mock-infected MRC-5 cells were included in the experiment. Both VR4990 and VR4990rec2-1-1 showed greatly impaired GCV phosphorylation, which appeared reduced to levels comparable to those of XbaF4-3-1 (Fig. 1). Thus, the Cys607→Tyr change in the AD169 genetic background conferred GCV resistance to and impaired GCV phosphorylation of the recombinant VR4990rec2-1-1 strain.
FIG. 1.
Anabolism of GCV by HCMV strains. MRC-5 cells were mock infected or infected with UL97 recombinant HCMV strain VR4990rec2-1-1 or its parental strains: clinical isolate VR4990 (GCV resistant) and reference strain AD169 (GCV sensitive). For comparison, GCV anabolism in cells infected with reference GCV-resistant HCMV strain XbaF4-3-1, derived by transfer of UL97 from 759rD100 back into AD169 (22, 23), was evaluated.
As already mentioned, HCMV drug resistance is becoming a major problem in the clinical management of HCMV infections in immunocompromised patients (1–4, 6–13, 15, 16, 18, 20–22, 25). Since the discovery of UL97’s function in GCV phosphorylation (5, 17, 24), some progress has been made in the understanding of the molecular mechanism(s) of GCV resistance and several mutations in this gene responsible for GCV resistance have been reported. However, several problems remain unsolved. Why are some mutations more frequent than others? What is the impact of each mutation on viral biology, mainly with respect to the still unknown natural function of UL97 phosphotransferase? Which are the boundaries of the UL97 region(s) involved in GCV binding and processing? To date, only single nucleotide substitutions or small deletions (1–4, 6, 7, 13, 18, 25) in the UL97 gene product conferring impaired GCV phosphorylation have been described, whereas attempts to generate mutants with larger alterations in UL97 have been unsuccessful (14, 19). He et al. have shown that mutations in the carboxy-terminal region of the UL97 gene product only marginally reduce autophosphorylation, whereas these mutations in clinical strains clearly reduce GCV phosphorylation. However, the mutation of the crucial catalytic lysine in position 355 suppresses any UL97 phosphorylation activity, suggesting that it may be lethal in vivo (14). Thus, one can argue that mutations detected in GCV-resistant clinical strains may hamper drug processing without interfering in the natural functions of the enzyme.
Residues 460, 594, and 595 are most frequently mutated (1–4, 6, 7, 18, 21, 25). However, Leu595 may be substituted in several ways and even be deleted without apparent major alterations in viral growth rate, virus yield, or in vivo pathogenicity (2, 4, 6, 7, 21, 25). In contrast, substitutions in positions 460, 520, and 594 present a more conserved pattern (Met460→Val or →Ile, His520→Asn, and Ala594→Val). Interestingly, all the reported mutations in positions 460, 520, and 590 to 607 seem to confer similar degrees of GCV resistance (GCV ID50, ∼20 to 25 μM), giving a 6- to 15-fold increase in ID50s with respect to the mean values for HCMV isolates from untreated patients (2, 6, 7, 12, 13, 18, 20, 21, 25), while higher levels of GCV resistance (12- to >40-fold increases in ID50s with respect to those for controls) are associated with additional mutations in the DNA polymerase gene (8, 21, 23). Three Cys residues are present at positions 592, 603, and 607, and mutations in each of them have been associated with GCV resistance (1, 3, 7, 8, 24). Thus, it seems reasonable to hypothesize that they may play a functional role in substrate recognition. Furthermore, it must be noted that the UL97 gene product has as many as 28 Cys residues located in the catalytic half of the enzyme. The Cys607→Tyr change reported here further extends the domain of the carboxy-terminal region of the UL97 phosphotransferase involved in GCV anabolism. Additional studies are needed to define the impact of each amino acid change on the natural function of the UL97-encoded phosphotransferase.
Acknowledgments
We thank Linda D’Arrigo for revision of the English.
This work was partially supported by the Ministero della Sanità, Istituto Superiore di Sanità, IX Progetto Nazionale AIDS 1996, grant 9404-13, and by Ricerca Corrente, IRCCS Policlinico S. Matteo, grant 820RCR96/03.
REFERENCES
- 1.Baldanti F, Sarasini A, Silini E, Barbi M, Lazzarin A, Biron K K, Gerna G. Four dually resistant human cytomegalovirus strains from AIDS patients: single mutations in UL97 and UL54 open reading frames are responsible for ganciclovir- and foscarnet-specific resistance, respectively. Scand J Infect Dis Suppl. 1995;99:103–104. [PubMed] [Google Scholar]
- 2.Baldanti F, Silini E, Sarasini A, Talarico C L, Stanat S C, Biron K K, Furione M, Bono F, Palù G, Gerna G. A three-nucleotide deletion in the UL97 open reading frame is responsible for the ganciclovir resistance of a human cytomegalovirus clinical isolate. J Virol. 1995;69:796–800. doi: 10.1128/jvi.69.2.796-800.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldanti, F., M. Boeckh, S. Chou, C. Crumpacker, S. Danner, W. L. Drew, D. Emanuel, A. Erice, W. D. Hardy, and S. Spector. 1996. Drug resistance in cytomegalovirus: current knowledge and implications for patient management. J. Acquired Immune Defic. Syndr. Hum. Retrovirol. 12(Suppl. 1):S1–S22. [PubMed]
- 4.Baldanti F, Underwood M R, Stanat S C, Biron K K, Chou S, Sarasini A, Silini E, Gerna G. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J Virol. 1996;70:1390–1395. doi: 10.1128/jvi.70.3.1390-1395.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chee M S, Lawrence G L, Barrell B G. Alpha-, beta- and gamma herpesviruses encode a putative phosphotranspherase. J Gen Virol. 1989;70:1151–1160. doi: 10.1099/0022-1317-70-5-1151. [DOI] [PubMed] [Google Scholar]
- 6.Chou S, Erice A, Colin Jordan M, Vercellotti G M, Michels K R, Talarico C L, Stanat S C, Biron K K. Analysis of the UL97 phosphotransferase coding sequence in clinical isolates and identification of mutations conferring ganciclovir resistance. J Infect Dis. 1995;171:576–583. doi: 10.1093/infdis/171.3.576. [DOI] [PubMed] [Google Scholar]
- 7.Chou S, Guentzel S, Michels K R, Miner R C, Drew W L. Frequency of UL97 phosphotransferase mutations related to ganciclovir resistance in clinical cytomegalovirus isolates. J Infect Dis. 1995;172:239–242. doi: 10.1093/infdis/172.1.239. [DOI] [PubMed] [Google Scholar]
- 8.Chou S, Marousek G, Guentzel S, Follansbee S E, Poscher M E, Lalezari J P, Miner R C, Drew W L. Evolution of mutations conferring multidrug resistance during prophylaxis and therapy for cytomegalovirus disease. J Infect Dis. 1997;176:786–789. doi: 10.1086/517302. [DOI] [PubMed] [Google Scholar]
- 9.Drew W L, Miner R C, Busch D F, Follansbee S E, Gullet J, Mehalko S G, Gordon S M, Owen W F, Matthews T R, Bushless W C, DeArmond B. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infections. J Infect Dis. 1991;163:716–719. doi: 10.1093/infdis/163.4.716. [DOI] [PubMed] [Google Scholar]
- 10.Erice A, Chou S, Biron K K, Stanat S C, Balfour H H, Jordan M C. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N Engl J Med. 1989;320:289–293. doi: 10.1056/NEJM198902023200505. [DOI] [PubMed] [Google Scholar]
- 11.Gerna G, Sarasini A, Percivalle E, Zavattoni M, Baldanti F, Revello M G. Rapid screening of resistance to ganciclovir and foscarnet of human cytomegalovirus primary isolates from culture-positive blood samples. J Clin Microbiol. 1995;33:738–741. doi: 10.1128/jcm.33.3.738-741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerna G, Baldanti F, Zavattoni M, Sarasini A, Percivalle E, Revello M G. Monitoring of ganciclovir sensitivity of human cytomegalovirus strains coinfecting blood of an AIDS patient by an immediate-early antigen plaque assay. Antivir Res. 1992;19:333–345. doi: 10.1016/0166-3542(92)90014-v. [DOI] [PubMed] [Google Scholar]
- 13.Hanson M N, Preheim L C, Chou S, Talarico C L, Biron K K, Erice A. Novel mutation in the UL97 gene of a clinical cytomegalovirus strain conferring resistance to ganciclovir. Antimicrob Agents Chemother. 1995;39:1204–1205. doi: 10.1128/aac.39.5.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Z, He Y-S, Kim Y, Chu L, Ohmstede C, Biron K K, Coen D. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J Virol. 1997;71:405–411. doi: 10.1128/jvi.71.1.405-411.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson M A, Drew W L, Feinberg J, O’Donnell J J, Withmore P V, Miner R D, Parenti D. Foscarnet therapy for ganciclovir-resistant cytomegalovirus retinitis in patients with AIDS. J Infect Dis. 1991;163:1348–1351. doi: 10.1093/infdis/163.6.1348. [DOI] [PubMed] [Google Scholar]
- 16.Knox K K, Drobyski W R, Carrigan D R. Cytomegalovirus isolate resistant to ganciclovir and foscarnet from a marrow transplant patient. Lancet. 1991;2:1292–1293. doi: 10.1016/0140-6736(91)92965-5. [DOI] [PubMed] [Google Scholar]
- 17.Littler E, Stuart A D, Chee M S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- 18.Lurain N S, Spafford L E, Thompson K D. Mutation in the UL97 open reading frame of human cytomegalovirus strains resistant to ganciclovir. J Virol. 1994;68:4427–4431. doi: 10.1128/jvi.68.7.4427-4431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel D, Pavic I, Zimmermann A, Haupt E, Wunderlich K, Heuschmid M, Mertens T. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J Virol. 1996;70:6340–6346. doi: 10.1128/jvi.70.9.6340-6346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarasini A, Baldanti F, Furione M, Percivalle E, Brerra R, Barbi M, Gerna G. Double resistance to ganciclovir and foscarnet of four human cytomegalovirus strains recovered from AIDS patients. J Med Virol. 1995;47:237–244. doi: 10.1002/jmv.1890470309. [DOI] [PubMed] [Google Scholar]
- 21.Smith I L, Cherrington J M, Jiles R E, Fuller M D, Freeman W R, Spector S A. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J Infect Dis. 1997;176:69–77. doi: 10.1086/514041. [DOI] [PubMed] [Google Scholar]
- 22.Stanat S C, Reardon J E, Erice A, Jordan M C, Drew W L, Biron K K. Ganciclovir-resistant cytomegalovirus clinical isolates: mode of resistance to ganciclovir. Antimicrob Agents Chemother. 1991;35:2191–2197. doi: 10.1128/aac.35.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan V, Biron K K, Talarico C, Stanat S C, Davis M, Pozzi L M, Coen D M. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob Agents Chemother. 1993;37:19–25. doi: 10.1128/aac.37.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan V, Talarico C L, Stanat S C, Davis M, Coen D M, Biron K K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;358:162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- 25.Wolf D G, Smith I L, Lee D J, Freeman W R, Flores-Aguillar M, Spector S A. Mutations in human cytomegalovirus UL97 gene confer clinical resistance to ganciclovir and can be detected directly in patient plasma. J Clin Invest. 1995;95:257–263. doi: 10.1172/JCI117648. [DOI] [PMC free article] [PubMed] [Google Scholar]