Abstract
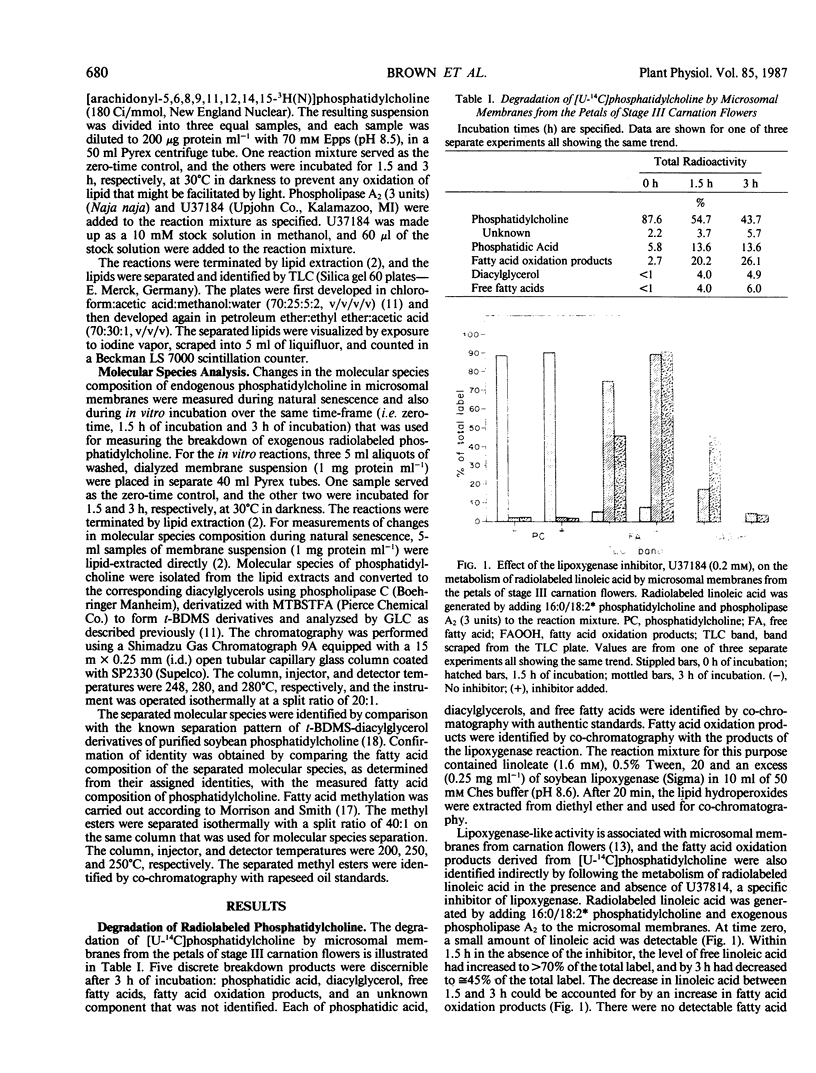
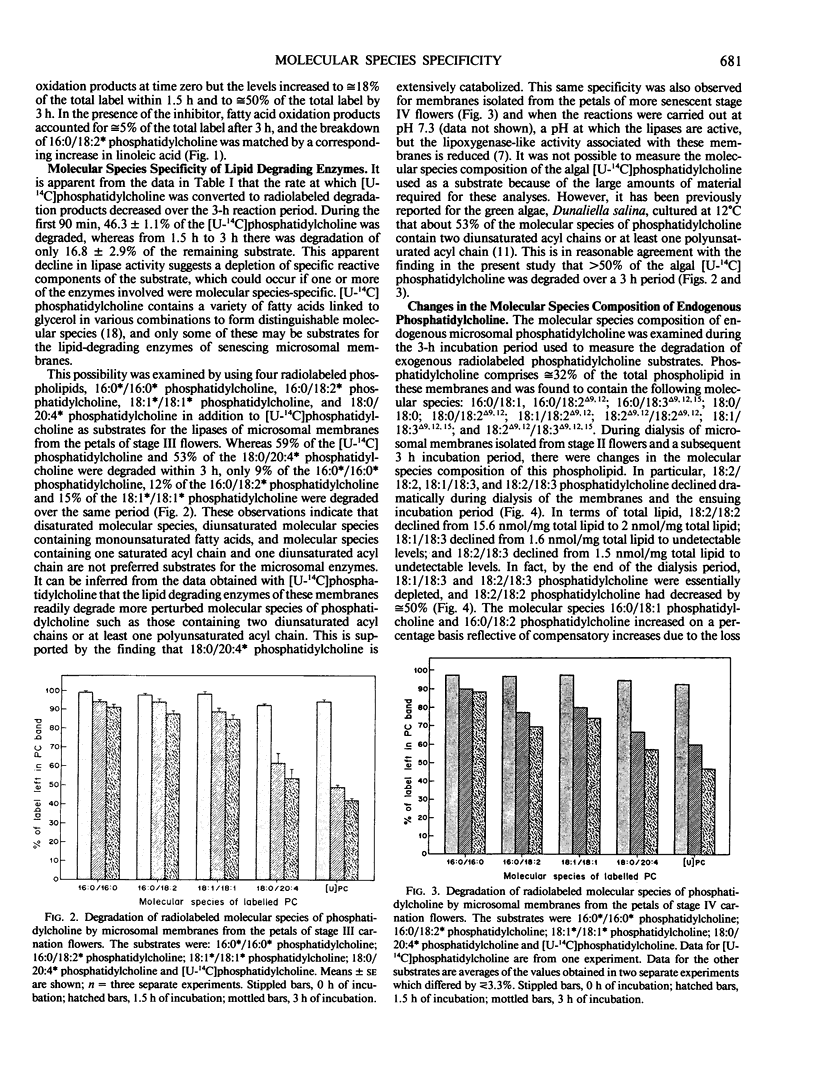
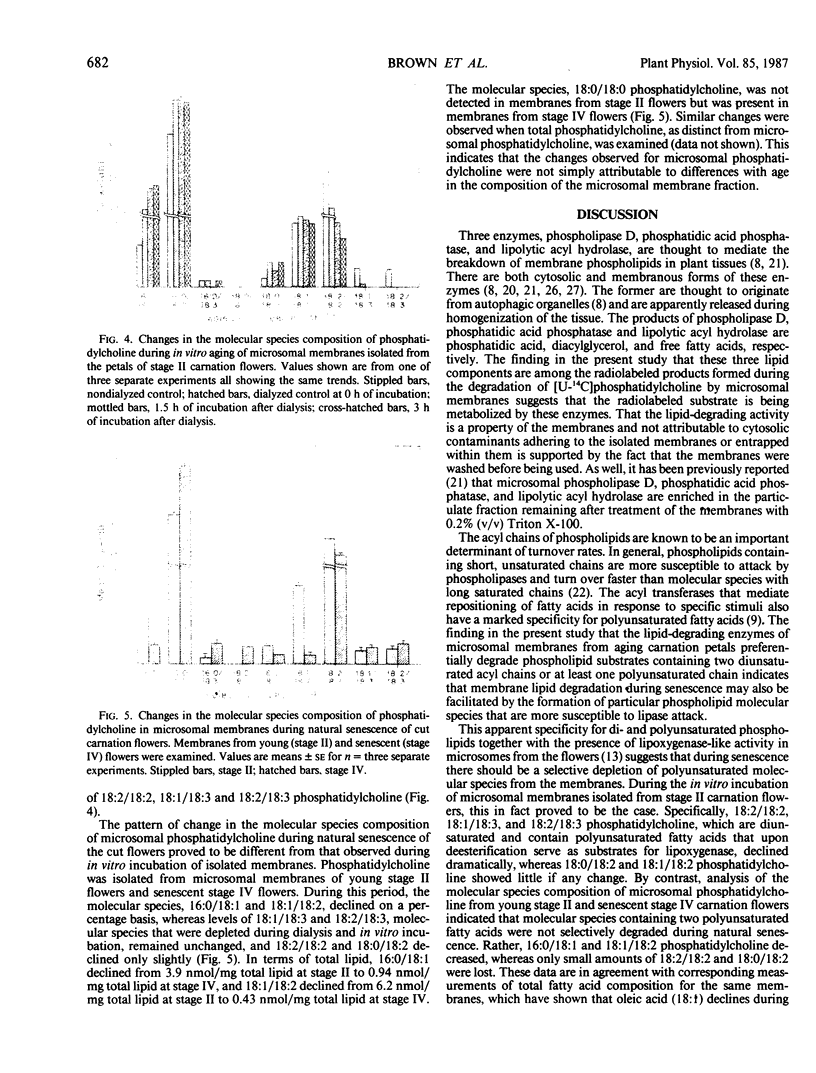
During senescence of cut carnation flowers, there is extensive breakdown of microsomal phospholipid. This is attributable, at least in part, to lipolytic activity associated directly with the microsomal membranes. Evidence indicating that one or more of the lipid-degrading enzymes in these membranes preferentially degrade phospholipid molecular species containing two diunsaturated acyl chains or at least one polyunsaturated acyl chain has been obtained by using radiolabeled phosphatidylcholine substrates. 16:0*/16:0*, 16:0/18:2*, and 18:1*/18:1* phosphatidylcholine were degraded only minimally over a 3 hour period by microsomes isolated from senescing flowers. By contrast, [U-14C]phosphatidylcholine, which comprises various molecular species including those containing polyunsaturated acyl chains, and 18:0/20:4* phosphatidylcholine were extensively degraded. Under identical conditions, but in the absence of added radiolabeled substrate, endogenous 18:2/18:2, 18:1/18:3, and 18:2/18:3 phosphatidylcholine were selectively depleted from the membranes. During natural senescence of the flowers, there was a sharp decline in microsomal 16:0/18:1 and 18:1/18:2 phosphatidylcholine, whereas molecular species containing two diunsaturated acyl chains or at least one polyunsaturated acyl chain remained unchanged or decreased only slightly. The data have been interpreted as indicating that provision of particular molecular species susceptible to lipase attack is a prerequisite to phospholipid catabolism in senescing membranes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beutelmann P., Kende H. Membrane Lipids in Senescing Flower Tissue of Ipomoea tricolor. Plant Physiol. 1977 May;59(5):888–893. doi: 10.1104/pp.59.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov A., Halevy A. H. Microviscosity of plasmalemmas in rose petals as affected by age and environmental factors. Plant Physiol. 1978 May;61(5):812–815. doi: 10.1104/pp.61.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov A., Halevy A. H., Shinitzky M. Senescence and the Fluidity of Rose Petal Membranes : RELATIONSHIP TO PHOSPHOLIPID METABOLISM. Plant Physiol. 1982 Feb;69(2):296–299. doi: 10.1104/pp.69.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Herman E. M., Chrispeels M. J. Characteristics and subcellular localization of phospholipase d and phosphatidic Acid phosphatase in mung bean cotyledons. Plant Physiol. 1980 Nov;66(5):1001–1007. doi: 10.1104/pp.66.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R., Kino M., Saito K., Masuda T. Phospholipid acyl chain metabolism during the differentiation of murine leukemia cell lines. On the redistribution of polyunsaturated acyl chains among phospholipids. Biochim Biophys Acta. 1982 Jul 20;712(1):161–168. [PubMed] [Google Scholar]
- Lippiello P. M., Holloway C. T., Garfield S. A., Holloway P. W. The effects of estradiol on stearyl-CoA desaturase activity and microsomal membrane properties in rooster liver. J Biol Chem. 1979 Mar 25;254(6):2004–2009. [PubMed] [Google Scholar]
- Lynch D. V., Thompson G. A. Microsomal Phospholipid Molecular Species Alterations during Low Temperature Acclimation in Dunaliella. Plant Physiol. 1984 Feb;74(2):193–197. doi: 10.1104/pp.74.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Martin C. E., Hiramitsu K., Kitajima Y., Nozawa Y., Skriver L., Thompson G. A. Molecular control of membrane properties during temperature acclimation. Fatty acid desaturase regulation of membrane fluidity in acclimating Tetrahymena cells. Biochemistry. 1976 Nov 30;15(24):5218–5227. doi: 10.1021/bi00669a004. [DOI] [PubMed] [Google Scholar]
- McArthur J. A., Marsho T. V., Newman D. W. Lipid Transformations in Plastids of Bean Leaves and Pepper Fruits. Plant Physiol. 1964 Jul;39(4):551–554. doi: 10.1104/pp.39.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- Myher J. J., Kuksis A. Resolution of diacylglycerol moieties of natural glycerophospholipids by gas-liquid chromatography on polar capillary columns. Can J Biochem. 1982 Jun;60(6):638–650. doi: 10.1139/o82-079. [DOI] [PubMed] [Google Scholar]
- Nandini-Kishore S. G., Kitajima Y., Thompson G. A., Jr Membrane fluidizing effects of the general anesthetic methoxyflurane elicit an acclimation response in Tetrahymena. Biochim Biophys Acta. 1977 Nov 15;471(1):157–161. doi: 10.1016/0005-2736(77)90403-5. [DOI] [PubMed] [Google Scholar]
- Paliyath G., Thompson J. E. Calcium- and calmodulin-regulated breakdown of phospholipid by microsomal membranes from bean cotyledons. Plant Physiol. 1987 Jan;83(1):63–68. doi: 10.1104/pp.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Chapman D. The dynamics of membrane structure. CRC Crit Rev Biochem. 1980;8(1):1–117. doi: 10.3109/10409238009105466. [DOI] [PubMed] [Google Scholar]
- Suttle J. C., Kende H. Ethylene Action and Loss of Membrane Integrity during Petal Senescence in Tradescantia. Plant Physiol. 1980 Jun;65(6):1067–1072. doi: 10.1104/pp.65.6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. E., Mayak S., Shinitzky M., Halevy A. H. Acceleration of membrane senescence in cut carnation flowers by treatment with ethylene. Plant Physiol. 1982 Apr;69(4):859–863. doi: 10.1104/pp.69.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Freezing Injury and Phospholipid Degradation in Vivo in Woody Plant Cells: III. Effects of Freezing on Activity of Membrane-bound Phospholipase D in Microsome-enriched Membranes. Plant Physiol. 1979 Aug;64(2):252–256. doi: 10.1104/pp.64.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Freezing injury and phospholipid degradation in vivo in woody plant cells: I. Subcellular localization of phospholipase d in living bark tissues of the black locust tree (robinia pseudoacacia L.). Plant Physiol. 1979 Aug;64(2):241–246. doi: 10.1104/pp.64.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]