Abstract
Importance:
There is increasing recognition that vascular disease, which can be treated, is a key contributor to dementia risk. However, the contribution of specific markers of vascular disease is unclear and, as a consequence, optimal prevention strategies remain unclear.
Objective:
To disentangle the causal relation of several key vascular traits to dementia risk: (i) white matter hyperintensity (WMH) burden, a highly prevalent imaging marker of covert cerebral small vessel disease (cSVD); (ii) clinical stroke; and (iii) blood pressure (BP), the leading risk factor for cSVD and stroke, for which efficient therapies exist. To account for potential epidemiological biases inherent to late-onset conditions like dementia.
Design, Setting, and Participants:
This study first explored the association of genetically determined WMH, BP levels and stroke risk with AD using summary-level data from large genome-wide association studies (GWASs) in a two-sample Mendelian randomization (MR) framework. Second, leveraging individual-level data from large longitudinal population-based cohorts and biobanks with prospective dementia surveillance, the association of weighted genetic risk scores (wGRSs) for WMH, BP, and stroke with incident all-cause-dementia was explored using Cox-proportional hazard and multi-state models. The data analysis was performed from July 26, 2020, through July 24, 2022.
Exposures:
Genetically determined levels of WMH volume and BP (systolic, diastolic and pulse blood pressures) and genetic liability to stroke.
Main outcomes and measures:
The summary-level MR analyses focused on the outcomes from GWAS of clinically diagnosed AD (n-cases=21,982) and GWAS additionally including self-reported parental history of dementia as a proxy for AD diagnosis (ADmeta, n-cases=53,042). For the longitudinal analyses, individual-level data of 157,698 participants with 10,699 incident all-cause-dementia were studied, exploring AD, vascular or mixed dementia in secondary analyses.
Results:
In the two-sample MR analyses, WMH showed strong evidence for a causal association with increased risk of ADmeta (OR, 1.16; 95%CI:1.05-1.28; P=.003) and AD (OR, 1.28; 95%CI:1.07-1.53; P=.008), after accounting for genetically determined pulse pressure for the latter. Genetically predicted BP traits showed evidence for a protective association with both clinically defined AD and ADmeta, with evidence for confounding by shared genetic instruments. In longitudinal analyses the wGRSs for WMH, but not BP or stroke, showed suggestive association with incident all-cause-dementia (HR, 1.02; 95%CI:1.00-1.04; P=.06). BP and stroke wGRSs were strongly associated with mortality but there was no evidence for selective survival bias during follow-up. In secondary analyses, polygenic scores with more liberal instrument definition showed association of both WMH and stroke with all-cause-dementia, AD, and vascular or mixed dementia; associations of stroke, but not WMH, with dementia outcomes were markedly attenuated after adjusting for interim stroke.
Conclusion:
These findings provide converging evidence that WMH is a leading vascular contributor to dementia risk, which may better capture the brain damage caused by BP (and other etiologies) than BP itself and should be targeted in priority for dementia prevention in the population.
Introduction
As life expectancy rises worldwide, the prevalence of dementia is expected to reach 75 million by 2030.1,2 Devising strategies to prevent or delay its occurrence is therefore a major public health priority. As has been documented over decades, it is now widely recognized by the scientific community that a majority of dementia cases, including Alzheimer’s disease (AD) in the population are, in fact, due to a combination of vascular and neurodegenerative lesions.3-6 A large majority (80%) of patients with clinically diagnosed AD are found to have cerebrovascular lesions on post-mortem examinations.7 Epidemiological and clinical studies have shown that stroke patients have at least a doubling of their risk for incident dementia.8,9 At the population level, covert cerebral small vessel disease (cSVD), detectable on brain imaging in the absence of clinical stroke, is thought to be the main pathological substrate underlying the vascular contribution to cognitive decline and dementia,10 with nearly half of dementia cases exhibiting overlap of AD neuropathology with cSVD.11
White matter hyperintensity burden (WMH) is the most common magnetic resonance imaging (MRI) feature of covert cSVD. Evidence from observational studies has established strong associations of WMH with increased stroke and dementia risk, including AD,12 yet with no proof of causality. Recently, a putative causal relation between WMH and AD has been suggested in a preliminary Mendelian randomization (MR) analysis that used genetic instruments as proxies for WMH volume, thus leveraging the natural randomization of genetic variation at conception to mitigate risks of confounding and reverse causation as seen in observational studies.13 Intriguingly, however, while high blood pressure is by far the strongest risk factor for WMH and easily accessible to efficient therapy, several MR studies14 have reported inverse associations of genetically predicted blood pressure levels15 with AD. These associations were observed both in datasets using standard AD diagnostic criteria (IGAP)16-18 and in studies using self-reported parental history as a proxy for AD diagnosis (UK Biobank).19 “Selective survivorship”20 or age-dependent structural changes (arterial stiffness)21 and neurodegenerative lesions in blood pressure regulated regions resulting in reverse causation have been discussed as possible hypotheses.22,23 These inconsistencies have led to many discussions about the causal role of vascular damage in AD, but an understanding of the contributions of specific vascular pathology is still limited. Indeed, understanding such causal relationships is crucial to prioritize interventions and target populations to prevent cognitive decline and dementia.
Based on the sharing of a large proportion of genetic risk variants between WMH and blood pressure (BP) traits,24 we aim to address from a genetic epidemiologic perspective: 1) what are the putative causal associations of genetically defined different vascular pathologies to AD and all-cause-dementia; 2) what are the potential biases that may contribute to the MR associations.
Methods
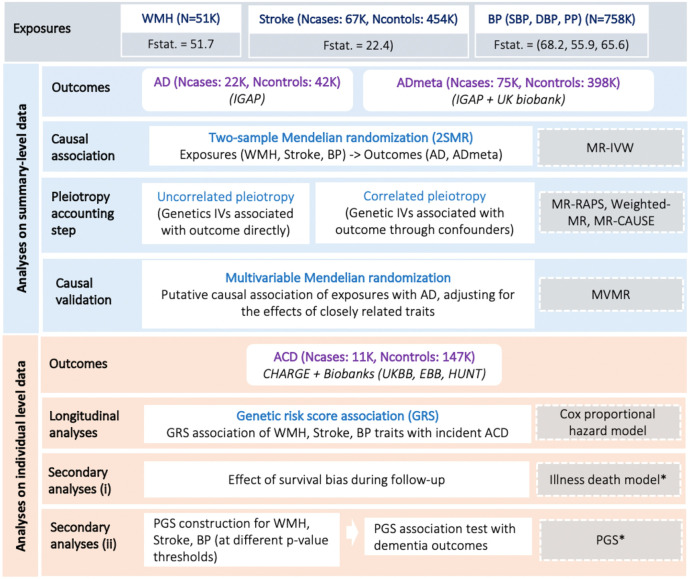
The study design is summarized in Figure 1.
Figure 1: Study workflow.
WMH, White matter hyperintensity burden; BP, Blood pressure (Systolic-SBP, Diastolic-DBP, Pulse pressure-PP); AD, clinically diagnosed late-onset Alzheimer’s disease; ADmeta, combination of parental dementia status and AD; ACD, all-cause-dementia; Fstat, F statistics (genetic instrument strength); IVW, Inverse variance weighted; IVs, Instrumental variables; RAPS, Robust adjusted profile score; MVMR, Multivariable mendelian randomization; PGS, Polygenic profile score; *Association analyses in a subset of CHARGE cohorts (3C, AGES).
Analyses on summary level data
A comprehensive suite of MR-based analyses leveraging summary statistics of large GWAS was used to establish causality of associations between vascular traits (WMH, BP, stroke) and dementia and to test for alternative interpretations of causality. A P value < .017 correcting for 3 independent traits was considered significant.
Selection of genetic instruments for the vascular trait exposures in MR analyses
Independent genetic variants (single-nucleotide polymorphisms [SNPs]) that are genome-wide significant (P < 5x10−8) in large published GWAS and satisfying the instrumental variable definition25,26 were considered as genetic instruments for each of the exposures. These were derived from GWAS of WMH on 50,970 participants, of stroke on 67,162/454,450 cases and controls, and of systolic blood pressure [SBP], diastolic blood pressure [DBP], and pulse pressure [PP] on up to 757,601 participants.15,24,27 (eMethods). The Cragg-Donald F statistic was used to evaluate instrument strength (eMethods).
Dementia phenotypes used as outcomes in MR analyses
For the MR analyses, we used the GWAS summary statistics of clinically diagnosed late-onset AD (N=21,982/41,944 cases/controls)28 and of the combination of clinical AD cases (n-cases=21,982) and proxy AD cases (n-cases=53,402) defined by parental history of dementia in the UK biobank (this combination being hereafter referred to as ADmeta, N=75,024/397844 cases/controls).29 ADmeta is sometimes considered closer to a definition of all-cause-dementia than pure AD.30 In all instances AD diagnosis was based on clinical criteria and family history only, and not on neuropathology criteria (eMethods).
Suite of MR analyses
As a starting point the putative causal effect of a given risk factor (WMH, stroke, SBP, DBP, PP) on AD was estimated using a suite of instrumental variable analyses based on two-sample Mendelian randomization (2SMR). We first accounted for potential pleiotropic effects of genetic instruments (SNPs) directly on the outcome and that is uncorrelated with the exposure (uncorrelated pleiotropy)31 using MR Egger, MR-RAPs, weighted median and mode based methods (eMethods). Second, we used MR-CAUSE to account for possible correlated pleiotropy, whereby genetic instruments are correlated with both exposure and outcome through the unmeasured confounders (eFigures 1-2 and eMethods).32 MR-CAUSE is a Bayesian approach that differentiates the causal model (γ) from the correlated-sharing (q) model based on the extent of the contribution of genetic instruments to the predicted effect. It provides a prediction accuracy (expected log pointwise posterior density, ELPD) for both models and their corresponding difference (ΔELPD=ELPDγ-ELPDq), which, if greater than zero, indicates that the causal model (γ) is a better fit than the correlated-sharing model (q) (eMethods). Third, for exposures for which MR-CAUSE suggested the causal model to be a better fit (ΔELPD > 0) but with significant residual effects in the sharing model (q), we performed multivariable MR conditioning for effects from potential confounders to confirm the putative causal relation using MVMR.33 Finally, for exposures with significant MVMR association (P value < .013, for 4 independent traits) the following sensitivity analyses were conducted: i) using Qhet-MVMR, confounding due to potentially weak instruments was accounted for, and the effect direction was confirmed,34 and ii) causal direction among the multiple exposures was further determined using two-sample bidirectional MR (eMethods).
Analyses on individual level data from longitudinal cohort studies
We additionally conducted analyses on individual level data from prospective cohort studies to study the relation of genetically determined WMH burden, BP, and stroke with incident dementia outcomes in a longitudinal setting, while exploring the impact of potential selective survival bias.35
Association analyses of weighted genetic risk scores for vascular traits with incident dementia
Analyses are based on genotype and phenotype information from 13 longitudinal cohorts (participating in the Cohorts for Heart and Aging Research in Genomic Epidemiology [CHARGE],36 and large biobanks (Trøndelag health study: HUNT, Estonian biobank: EstBB, and UK biobank: UKB). Nearly all cohorts were population-based, except MEMENTO (memory clinic patients with cognitive complaints and no dementia) and Knight ADRC. Together these cohorts included 157,698 unrelated participants of European origin, of whom 10,699 developed all-cause-dementia (which is more inclusive than ADmeta). Dementia diagnosis in these studies is based on standard criteria described in the eMethods along with the study design and participant selection. The mean age at dementia diagnosis across the cohorts is 71.4 years with a median duration of follow-up ranging between 3-25 years. Cox proportional hazard regression models were used to examine the association of genetic risk scores for the different exposures (WMH, stroke, BP traits) studied in the 2SMR analyses with incident all-cause-dementia. For each trait, we constructed an individual weighted genetic risk score (wGRS) using as weights the number of ‘effect’ alleles times the effect estimates (betas) of the respective genetic instruments constructed for a given exposure in the 2SMR analysis.37 The wGRSs were standardized to have mean of 0 and a variance of 1, so that each unit change in the wGRS corresponds to one standard deviation (SD). Analyses were restricted to participants with at least one follow-up visit and no dementia at baseline. The Cox model with delayed entry included birth as time origin and age as the time scale and controlled for sex, education level, principal components of population stratification, and study-specific criteria (eTable 3). Data were censored at the age of dementia diagnosis for cases or age at the last follow-up for controls. Individual cohort-specific estimates were combined using a fixed-effects inverse variance weighted meta-analysis, implemented as an R (meta) package. Additional sensitivity analyses excluding individuals with a history of stroke at inclusion and adjusting for interim incident stroke were conducted (except in Knight ADRC where this information was not available).
Secondary analyses accounting for interval-censoring, competing risk of death, and dementia subtypes
These in-depth analyses were conducted in two large CHARGE cohorts (3C [Three city], AGES [Age, Gene/Environment Susceptibility - Reykjavik study]) with nearly identical characteristics (sample sizes, age distributions, and dementia ascertainment) (eMethods). We first explored whether survival bias during follow-up might affect our results using illness death models (IDMs)38 accounting for interval-censoring of time-to-onset of dementia and competing risk of death. Second, we examined whether our vascular exposures of interest exhibit an association with different subtypes of incident dementia (all-cause-dementia, AD and vascular and/or mixed dementia, Supplementary Methods) at more liberal instrument selection thresholds using polygenic profile scores (PGSs), created by binning observations by p-value of the exposures in the original GWASs. A P value < .017 correcting for 3 independent traits was considered significant.
Results
Exploring causal relations of WMH, BP, and stroke with AD risk using GWAS summary-level data
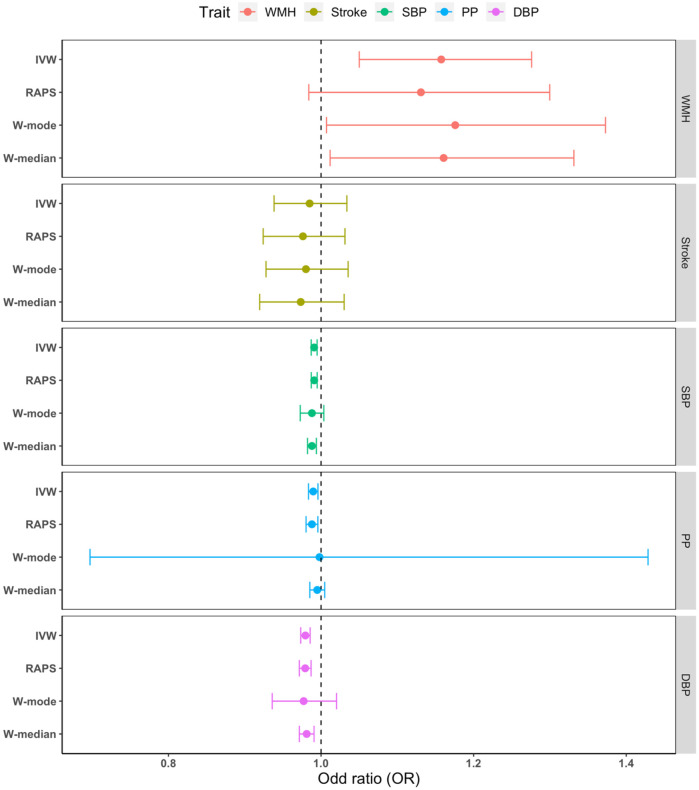
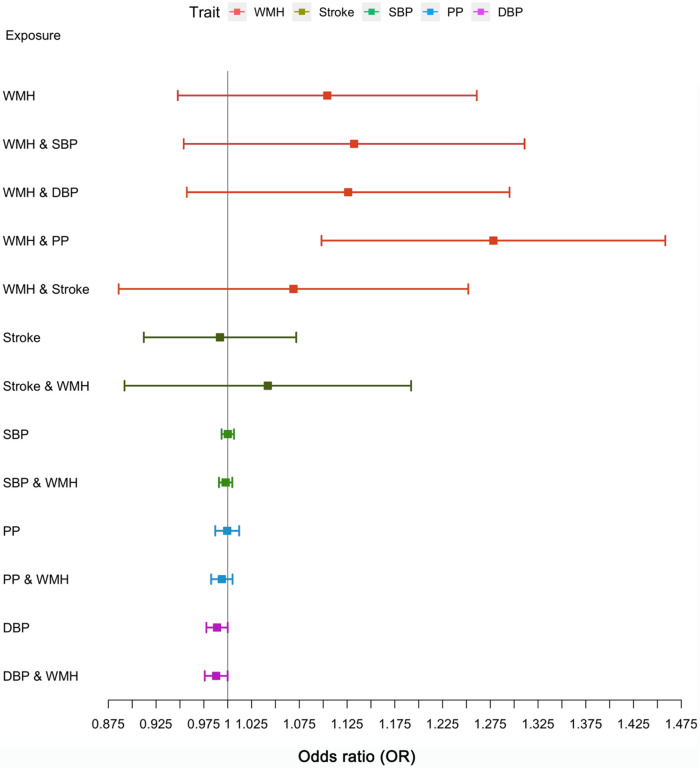
The genetic instruments derived from GWAS of WMH, BP, and stroke strongly predicted the exposures, with a Cragg-Donald F statistic ranging between 22 and 65 (eTables 1-2). Using the inverse variance weighting method we found significant associations (P value < .017) of genetically determined larger WMH burden and lower BP (DBP, SBP, PP) levels with ADmeta (clinically diagnosed AD or parental dementia) risk (Figure 2, eTable 4a) and of lower DBP with clinically diagnosed AD risk (eFigure 3, eTable 5a). The complementary MR tools MR-RAPS, weighted-median and mode enabled us to robustly rule out uncorrelated pleiotropic effects (eTable 4b, 5b). The Bayesian MR-CAUSE method that accounts for correlated pleiotropy (Methods) supported a causal relation of WMH with both AD outcomes (clinical AD and ADmeta), with a posterior distribution of the causal model distinctively different from the sharing model (ΔELPD = 0.91 for AD; 0.50 for ADmeta) (eTable 6, eFigures 4-5). On the contrary, stroke and BP traits suggested a better fit of the sharing model with potential unmeasured confounders (ΔELPD < 0) than a causal model (eTable 6, eFigures 4-5). Notably, for WMH with AD and not for other exposure-outcome combinations, we observed the presence of a significant proportion of genetic instruments being shared with unmeasured confounders (q P value: 8.37x10−9, eFigure 5) along with a better fit of the causal model (ΔELPD > 0) (eTable 6). We, therefore, sought to confirm any putative causal association of WMH with AD in a multivariable analysis setting, adjusting for the effects of closely related traits using MVMR. Greater genetically predicted WMH burden was associated with a 27.8% increase in the probability of AD risk (OR: 1.28, CI:1.07-1.53, P=.008, per unit increase in WMH risk alleles) after accounting for PP effects (Figure 3, eTable 7). This represents a 16.5% increase in disease risk compared to the univariable estimates (OR:1.11, CI:0.95-1.31, P=.19), with a consistent direction of effect. The effect of other exposures (stroke, BP traits) on AD remained non-significant using MVMR after adjusting for the closely related traits (eTable 7). Finally, a bidirectional MR analysis between the exposures showing significant MVMR results (WMH, PP) suggested a causal path of higher genetically predicted levels of PP with larger WMH burden (eTable 8).
Figure 2: Mendelian randomization results of vascular risk factors with ADmeta.
Point estimates and confidence intervals from the inverse-variance weighted (IVW) method are shown. WMH White matter hyperintensity burden. SBP systolic blood pressure, DBP diastolic blood pressure, PP pulse pressure, RAPS Robust adjusted profile score, W-mode Weighted mode, W-median Weighted median.
Figure 3: Multivariable Mendelian randomization (MVMR) along with the univariable MR for AD as the outcome.
WMH White matter hyperintensity burden. SBP systolic blood pressure, DBP diastolic blood pressure, PP pulse pressure.
Association of genetic risk scores for WMH, BP, and stroke with incident dementia using individual-level data
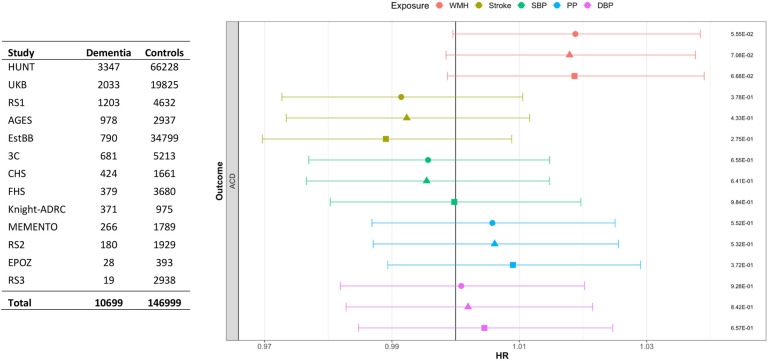
In a meta-analysis of thirteen longitudinal cohort studies, we observed a borderline significant association of increasing genetically predicted WMH burden with increased risk of incident all-cause-dementia (HR:1.02, CI:1.00-1.04, P=.06, per SD increase in WMH wGRS) (Figure 4, eTable 9). After adjusting for education and interim stroke, this association remained substantially unchanged (Figure 4). There was no significant heterogeneity across cohorts (I2: 7%, p=0.38; eFigure 6). Genetically determined BP traits and genetic liability to stroke failed to show significant associations with incident all-cause-dementia, with negative point estimates for stroke and SBP. Notably, all exposures showed at least a nominally significant association with increased risk for mortality, the most significant association being observed for SBP (eTable 9-10).
Figure 4: Forest plot showing the meta-analysis results of risk factor wGRSs (per standard deviation increase) with incident ACD.
Circle represents the model not adjusted for education level. Triangle represents the model adjusted for education. Square represents the model after further adjustment for interim stroke (prevalent stroke excluded). Association p-values are shown on the far right. ACD: all-cause-dementia. HUNT: Trøndelag health study, UKB: UK Biobank, RS: Rotterdam study, AGES: Age, Gene/Environment Susceptibility - Reykjavik study, EstBB: Estonian Biobank, 3C: Three-city, CHS: Cardiovascular Health Study, FHS: Framingham Heart Study, Knight-ADRC: Alzheimer’s Disease Research Centre, EPOZ: Epidemiological Prevention Study of Zoetermeer.
In secondary analyses, using illness-death models (IDM) in two older population-based cohorts (3C, AGES, Methods), we ruled out potential biases related to competing risk of death during follow-up in the context of interval censoring. Indeed, per SD increase in genetically predicted WMH and BP levels, and genetic liability to stroke risk were not associated with incident all-cause-dementia in the IDM model, and the effect estimates were generally similar to those from the Cox model (eTable 11) in both cohorts.
When examining the PGSs, where associations are binned according to significance of genetic instruments with less stringent instrument-significance thresholds (eMethods), we found that PGSs for WMH and stroke were significantly associated (P < .017) with increased all-cause-dementia risk in both cohorts (eTable 12-13, eFigures 7-8). In sensitivity analyses excluding prevalent stroke and adjusting for interim stroke, the WMH PGSs associations with dementia outcomes remained unchanged, while the stroke PGSs associations with dementia risk were markedly attenuated in both cohorts (eTable 13, eFigure 8). Meta-analyses of effect estimates from individual PGSs bins in 3C and AGES showed a significant association of WMH and stroke PGSs with increased risk of all-cause-dementia, AD, and (for stroke) vascular and/or mixed dementia (eTable 14). Finally, most of the PGSs for BP traits failed to show significant associations with dementia risk; the significant protective association observed between SBP/DBP PGSs and AD in the AGES cohort was attenuated in sensitivity analyses excluding prevalent stroke and adjusting for interim stroke (eTable 15).
Discussion
In this study capitalizing on a comprehensive MR workflow, large GWASs and numerous longitudinal cohort studies and biobanks, we report a putative causal association of genetically predicted WMH burden with increased risk of both clinically diagnosed AD28 and ADmeta (AD and parental history of dementia).29 The former association was strengthened after accounting for PP using multivariable MR. In contrast we observed protective effects of BP traits with ADmeta (all BP traits) and AD (DBP) risk using two-sample MR,16-19 and provide evidence that these may at least partly be driven by the sharing of BP genetic instruments with unmeasured confounders. Genetic liability to stroke was not associated with AD or ADmeta. Next, using 13 large longitudinal cohorts and biobanks gathering 157,698 participants with prospective dementia surveillance (N=10,699 incident dementia cases), we report a similar, borderline significant association trend of WMH with increased risk of incident all-cause-dementia, while genetic instruments for BP traits and stroke showed no association. In a subset of population-based cohorts, polygenic scores (encompassing risk variants at less stringent significance thresholds than MR instruments) for WMH burden and stroke were associated with increased risk of incident all-cause-dementia, AD, and vascular and/or mixed dementia. While the association between stroke PGSs and all-cause-dementia attenuated markedly after adjusting for interim stroke, the WMH PGSs association with all-cause-dementia remained significant.
Overall, out of all the vascular phenotypes considered (WMH, BP traits, stroke), genetic instruments for WMH appear to show the most robust associations with dementia risk, including ADmeta, AD, and all-cause-dementia. These MR findings suggest causal mechanisms and highlighting WMH as an important causal pathway to target for the prevention of dementia (Figure 5). Our results support association of extensive WMH burden with dementia and AD risks described in observational studies,39-43 providing additional strong evidence for a possible causal relation. This reinforces our earlier preliminary observations of a putative causal association of WMH with ADmeta,24 and expands it to a larger ADmeta GWAS resource,29 and, importantly also to a smaller and more conservative definition of only clinically diagnosed AD.28 The fact that the latter association was more prominent after accounting for PP effects in a multivariable model, with a 16.5% increase in the disease risk compared to the univariable estimates, is intriguing. PP is a marker of the pulsatile component of BP and is correlated with measures of arterial stiffness,44,45 which was suggested to promote both white matter pathology and amyloid deposition.46,47 Interestingly, recent evidence for a causal relation of arterial stiffness with larger WMH burden was found to be reinforced after accounting for PP,48 in line with the present findings. Underlying mechanisms are speculative, but could potentially involve oxidative stress, implicated in BP-associated vascular damage49 and also an early and prominent feature of aging and neurodegeneration.50 Elevated PP may dysregulate brain endothelial cells and increase cellular production of oxidative and inflammatory molecules, which may in turn increased amyloid-β secretion by cerebral endothelial cells and induce blood-brain barrier breakdown.51-53 Interestingly, although WMH is known to be associated with increased risk of stroke,12,13 and stroke with a substantial increase in dementia incidence,54-56 associations of WMH PGSs with risk of dementia were unchanged after accounting for baseline and interim stroke, suggesting an independent effect of WMH on dementia risk.
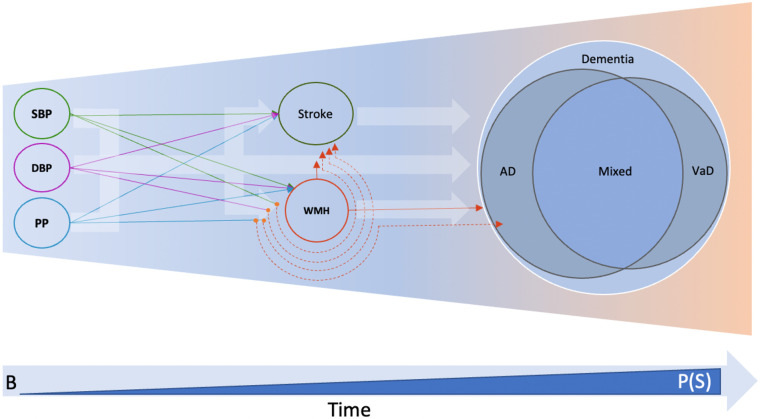
Figure 5: Central role of WMH with dementia outcomes.
VaD: Vascular dementia, B: baseline, P(S): probability of survival bias. Thick background arrows indicate inferences made from observational studies, and solid coloured lines indicate inferences from univariable Mendelian randomization studies. Orange dots and dashed lines represent the multivariable MR setting.
High blood pressure is the strongest known risk factor for WMH and other MRI-markers of cSVD, with MR studies suggesting a causal relation of increasing genetically determined BP with WMH volume, even in persons without clinically defined hypertension.24 Moreover, evidence from randomized controlled trials shows that WMH volume progression is slowed down by BP-lowering treatments in hypertensive individuals,57-61 especially by intensive vs. standard BP-lowering.61 Given the aforementioned associations of genetically determined WMH with dementia and AD it therefore appears “counterintuitive” to observe an inverse association of genetically determined high BP with lower risk of ADmeta and AD in our two-sample MR analysis. It is however in line with earlier MR studies deriving instruments from earlier, smaller BP GWAS or using genetic proxies for the effect of BP-lowering drugs.16-19,62 Our sensitivity analyses suggest that this unexpected directionality of association might at least partly be explained by pleiotropic effects from unmeasured confounders (MR-CAUSE), prompting caution with applying methods designed only to remove or downweigh pleiotropic variants (e.g., weighted median based MR methods).
Moreover, while we saw suggestive evidence for selective survival during follow-up, given the late age of onset of dementia (85 years on average63), the strong association of high BP and stroke with premature death in our longitudinal data and extensive evidence from other studies,64-67 it is possible that the apparent protective effect of high BP on dementia risk could potentially reflect selective survival bias before entry into the study. Indeed, if the selection is a function of the exposure or the outcome, it could result in collider (index event) bias,68 leading to an association in the absence of a causal effect.69-71 While we did not see any evidence for selective survival during follow-up using the illness-death model in our longitudinal cohorts, we cannot formally rule out selective survival before study entry. Although non-significant, the association of genetically determined PP and DBP with incident all-cause-dementia had point estimates above 1 in the longitudinal cohort studies, which are likely less exposed to selective survival than the AD case-control GWAS used for the 2SMR analyses, although they also tend to overrepresent healthy individuals at inclusion (as for any voluntary participation).72-74 Beyond these possible biases our results highlight the complexity of the relation between BP and dementia risk, with weak epidemiological evidence varying by age.75-77 Recent meta-analyses of clinical trials show evidence that antihypertensive medication reduces risk of secondary outcomes combining dementia with cognitive impairment,78,79 but not dementia alone. In a meta-analysis of 6 population-based cohorts, antihypertensive medication was associated with a reduced risk of incident dementia and AD in individuals with clinically defined high BP at baseline.78 However in another recent meta-analysis of 7 population-based cohorts, dementia risk appeared lower for older individuals with higher SBP levels, with more distinctly U-shaped associations for participants older than 75 years, which were not explained by SBP-associated changes in mortality risk.80
In contrast with BP measurements, which show high intra-individual variability,81 WMH volume is also a more stable marker, reflecting white matter damage secondary to changes in structure or function of cerebral small vessels. Assuming that WMH at least partly mediates the association of BP with dementia in the population, WMH may better capture the brain damage caused by BP than BP itself. WMH and covert cSVD more broadly likely also reflects the impact of other parameters on white matter integrity, including other risk factors for cSVD such as cerebral amyloid angiopathy, or factors influencing the resilience of the brain white matter to vascular insults. Given the high prevalence of WMH in the general population in the absence of clinical stroke,75-77 our results highlight WMH as a major causal pathway to consider for the prevention of dementia (Figure 5).
Strengths and Limitations
Strengths of our study include the diversity and complementarity of the analytical approaches used, ranging from various state-of-the-art MR analyses, based on powerful published GWAS, to longitudinal analyses in large population-based cohort studies with prospective dementia ascertainment. We also acknowledge limitations. First, the GWASs used for the MR analyses have likely predominantly recruited patients with clinically diagnosed AD, thus leading to an underrepresentation of patients with mixed dementia, who likely represent the majority of AD cases in the population. Second, in our longitudinal analyses, the number of incident dementia cases remained modest, with some differences in the methods for dementia ascertainment, thus limiting power to detect associations. Third, we considered only one MRI-marker for cSVD, WMH, for which there is most evidence for an association with dementia risk and the strongest genetic instruments. With increasing availability of larger GWAS for other MRI-cSVD markers (cerebral microbleeds, lacunes, perivascular spaces) future studies concomitantly assessing the impact of various genetically determined MRI-cSVD markers are warranted.82-85 Finally, validation of our findings in populations of non-European ancestry, as larger datasets become available, will be crucial.
Conclusions
In summary, our findings provide converging evidence that WMH is a leading vascular contributor to dementia risk and should be targeted in priority for the prevention of cognitive decline and dementia in the population. They also support WMH as a relevant surrogate marker for clinical trials testing interventions to prevent dementia by better controlling vascular risk.61,86 Our data also prompt caution when interpreting MR studies where the outcome of interest is a late-onset disease such as dementia, especially if the instrument for the exposure of interest shows a strong association with survival. They highlight the importance of combining several complementary epidemiological approaches to compensate for respective limitations and of combining different studies, cohorts and biobanks rather than drawing definitive conclusions on single datasets to minimize the impact of study-specific shortcomings and biases.
Supplementary Material
Key points.
Question:
Do instrumental variable analyses leveraging genetic information provide evidence for a causal association of various vascular traits with Alzheimer's disease (AD) and all-cause-dementia? How do these associations compare for white matter hyperintensity (WMH) burden, a highly prevalent marker of covert cerebral small vessel disease (cSVD), stroke, and blood pressure traits, the strongest known risk factor for cSVD and stroke?
Findings:
Using Mendelian randomization (MR) leveraging large, published genome-wide association studies, this study showed a putative causal association of larger WMH burden with increased AD risk after accounting for pulse pressure effects, and some evidence for association of lower BP with AD risk with possible confounding by shared genetic instruments. Longitudinal analyses on individual-level data also supported association of genetically determined WMH with incident all-cause-dementia and AD, independently of interim stroke.
Meaning:
This study using complementary genetic epidemiology approaches, identified increasing WMH burden to be associated with dementia and AD risk, suggesting the association as specific for cSVD and independent of BP and stroke.
References
- 1.Prince M, Ali G-C, Guerchet M, Prina AM, Albanese E, Wu Y-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer's Research & Therapy. 2016-December-01 2016;8(1)doi: 10.1186/s13195-016-0188-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia How to move forward? VIEWS & REVIEWS. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. NIH Public Access; 2011. p. 723–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141–1145. doi: 10.1016/S0140-6736(96)90608-X [DOI] [PubMed] [Google Scholar]
- 6.Nichols E, Merrick R, Hay SI, et al. The prevalence, correlation, and co-occurrence of neuropathology in old age: harmonisation of 12 measures across six community-based autopsy studies of dementia. Lancet Healthy Longev. Mar 2023;4(3):e115–e125. doi: 10.1016/S2666-7568(23)00019-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. A JOURNAL OF NEUROLOGY. doi: 10.1093/brain/awt188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savva GM, Stephan BCM. Epidemiological studies of the effect of stroke on incident dementia: A systematic review. Stroke; 2010. [DOI] [PubMed] [Google Scholar]
- 9.Levine DA, Galecki AT, Langa KM, et al. Trajectory of Cognitive Decline After Incident Stroke. JAMA. 2015;314(1):41–51. doi: 10.1001/jama.2015.6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol; 2013. p. 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iadecola C. The Pathobiology of Vascular Dementia. NIH Public Access; 2013. p. 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debette S, Schilling S, Duperron M-G, Larsson SC, Markus HS. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury. JAMA Neurology. 2019-January-01 2019;76(1):81. doi: 10.1001/jamaneurol.2018.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sargurupremraj M, Suzuki H, Jian X, et al. Cerebral small vessel disease genomics and its implications across the lifespan. Nature Communications. 2020-December-01 2020;11(1)doi: 10.1038/s41467-020-19111-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess S, Swanson SA, Labrecque JA. Are Mendelian randomization investigations immune from bias due to reverse causation? European Journal of Epidemiology. 2021-March-01 2021;36(3):253–257. doi: 10.1007/s10654-021-00726-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evangelou E, Warren HR, Mosen-Ansorena D, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nature Genetics. 2018-October-01 2018;50(10):1412–1425. doi: 10.1038/s41588-018-0205-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Østergaard SD, Mukherjee S, Sharp SJ, et al. Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study. PLOS Medicine. 2015;12(6):e1001841–e1001841. doi: 10.1371/journal.pmed.1001841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsson SC, Traylor M, Malik R, Dichgans M, Burgess S, Markus HS. Modifiable pathways in Alzheimer's disease: Mendelian randomisation analysis. BMJ (Clinical research ed). 2017;359:j5375–j5375. doi: 10.1136/bmj.j5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews SJ, Fulton-Howard B, O'Reilly P, et al. Causal Associations Between Modifiable Risk Factors and the Alzheimer's Phenome. Annals of Neurology. 2021-January-01 2021;89(1):54–65. doi: 10.1002/ana.25918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sproviero W, Winchester L, Newby D, et al. High Blood Pressure and Risk of Dementia: A Two-Sample Mendelian Randomization Study in the UK Biobank. Biological Psychiatry. 2021-April-01 2021;89(8):817–824. doi: 10.1016/j.biopsych.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 20.Smit RAJ, Trompet S, Dekkers OM, Jukema JW, le Cessie S. Survival Bias in Mendelian Randomization Studies. Epidemiology. 2019;30(6):813–816. doi: 10.1097/EDE.0000000000001072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franklin SS, Gustin W, Wong ND, et al. Hemodynamic Patterns of Age-Related Changes in Blood Pressure. Circulation. 1997;96(1):308–315. doi: 10.1161/01.CIR.96.1.308 [DOI] [PubMed] [Google Scholar]
- 22.Qiu C, Von Strauss E, Winblad B, Fratiglioni L. Decline in blood pressure over time and risk of dementia: A longitudinal study from the Kungsholmen project. Stroke. 2004;35(8):1810–1815. doi: 10.1161/01.STR.0000133128.42462.ef [DOI] [PubMed] [Google Scholar]
- 23.Gregson J, Qizilbash N, Iwagami M, et al. Blood pressure and risk of dementia and its subtypes: a historical cohort study with long-term follow-up in 2.6 million people. European Journal of Neurology. 2019;26(12):1479–1486. doi: 10.1111/ene.14030 [DOI] [PubMed] [Google Scholar]
- 24.Sargurupremraj M, Suzuki H, Jian X, et al. Cerebral small vessel disease genomics and its implications across the lifespan. Nat Commun. Dec 8 2020;11(1):6285. doi: 10.1038/s41467-020-19111-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. International Journal of Epidemiology. 2015-April-01 2015;44(2):512–525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swerdlow DI, Kuchenbaecker KB, Shah S, et al. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. International Journal of Epidemiology. 2016-October-01 2016;45(5):1600–1616. doi: 10.1093/ije/dyw088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malik R, Chauhan G, Traylor M, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nature Genetics. 2018-April-01 2018;50(4):524–537. doi: 10.1038/s41588-018-0058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunkle BW, Grenier-Boley B, Sims R, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nature Genetics. 2019;51(3):414–430. doi: 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartzentruber J, Cooper S, Liu JZ, et al. Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer’s disease risk genes. Nature Genetics. 2021-March-01 2021;53(3):392–402. doi: 10.1038/s41588-020-00776-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fongang B, Sargurupremraj M, Jian X, et al. Genetic insights of all-cause and vascular dementia through genome-wide association studies. Alzheimer's & Dementia. 2022-December-01 2022;18(S3)doi: 10.1002/alz.067165 [DOI] [Google Scholar]
- 31.Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. Feb 2003;32(1):1–22. doi: 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 32.Morrison J, Knoblauch N, Marcus JH, Stephens M, He X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nature Genetics. 2020-July-01 2020;52(7):740–747. doi: 10.1038/s41588-020-0631-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. International Journal of Epidemiology. 2019-June-01 2019;48(3):713–727. doi: 10.1093/ije/dyy262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanderson E, Spiller W, Bowden J. Testing and Correcting for Weak and Pleiotropic Instruments in Two-Sample Multivariable Mendelian Randomisation. Cold Spring Harbor Laboratory; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson SA. A Practical Guide to Selection Bias in Instrumental Variable Analyses. Lippincott Williams and Wilkins; 2019. p. 345–349. [DOI] [PubMed] [Google Scholar]
- 36.Psaty BM, O'Donnell CJ, Gudnason V, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet. Feb 2009;2(1):73–80. doi: 10.1161/CIRCGENETICS.108.829747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chouraki V, Reitz C, Maury F, et al. Evaluation of a Genetic Risk Score to Improve Risk Prediction for Alzheimer's Disease. J Alzheimers Dis. Jun 18 2016;53(3):921–32. doi: 10.3233/JAD-150749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Touraine C, Gerds TA, Joly P. SmoothHazard: An R Package for Fitting Regression Models to Interval-Censored Observations of Illness-Death Models. Journal of Statistical Software. July/13 2017;79(7):1 – 22. doi: 10.18637/jss.v079.i0730220889 [DOI] [Google Scholar]
- 39.Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurology. 2019;76(1):81–94. doi: 10.1001/jamaneurol.2018.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo W, Shi J. White matter hyperintensities volume and cognition: A meta-analysis. Front Aging Neurosci. 2022;14:949763. doi: 10.3389/fnagi.2022.949763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roseborough AD, Saad L, Goodman M, Cipriano LE, Hachinski VC, Whitehead SN. White matter hyperintensities and longitudinal cognitive decline in cognitively normal populations and across diagnostic categories: A meta-analysis, systematic review, and recommendations for future study harmonization. Alzheimers Dement. Jan 2023;19(1):194–207. doi: 10.1002/alz.12642 [DOI] [PubMed] [Google Scholar]
- 42.Hu HY, Ou YN, Shen XN, et al. White matter hyperintensities and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 36 prospective studies. Neurosci Biobehav Rev. Jan 2021;120:16–27. doi: 10.1016/j.neubiorev.2020.11.007 [DOI] [PubMed] [Google Scholar]
- 43.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010-July-26 2010;341(jul26 1):c3666–c3666. doi: 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niiranen TJ, Kalesan B, Mitchell GF, Vasan RS. Relative Contributions of Pulse Pressure and Arterial Stiffness to Cardiovascular Disease. Hypertension. Mar 2019;73(3):712–717. doi: 10.1161/HYPERTENSIONAHA.118.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safar ME, Blacher J, Jankowski P. Arterial stiffness, pulse pressure, and cardiovascular disease-is it possible to break the vicious circle? Atherosclerosis. Oct 2011;218(2):263–71. doi: 10.1016/j.atherosclerosis.2011.04.039 [DOI] [PubMed] [Google Scholar]
- 46.Hughes TM, Kuller LH, Barinas-Mitchell EJ, et al. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology. Nov 5 2013;81(19):1711–8. doi: 10.1212/01.wnl.0000435301.64776.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes TM, Kuller LH, Barinas-Mitchell EJ, et al. Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol. May 2014;71(5):562–8. doi: 10.1001/jamaneurol.2014.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Francis CM, Futschik ME, Huang J, et al. GENOME-WIDE ASSOCIATIONS OF AORTIC DISTENSIBILITY SUGGEST CAUSAL RELATIONSHIPS WITH AORTIC ANEURYSMS AND BRAIN WHITE MATTER HYPERINTENSITIES. medRxiv. 2021-January-01 00:00:00 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodrigo R, González J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertension Research. 2011-April-01 2011;34(4):431–440. doi: 10.1038/hr.2010.264 [DOI] [PubMed] [Google Scholar]
- 50.Kim GH, Kim JE, Rhie SJ, Yoon S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp Neurobiol. Dec 2015;24(4):325–40. doi: 10.5607/en.2015.24.4.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tong Y, Zhou W, Fung V, et al. Oxidative stress potentiates BACE1 gene expression and Abeta generation. J Neural Transm (Vienna). Mar 2005;112(3):455–69. doi: 10.1007/s00702-004-0255-3 [DOI] [PubMed] [Google Scholar]
- 52.Levin RA, Carnegie MH, Celermajer DS. Pulse Pressure: An Emerging Therapeutic Target for Dementia. Frontiers in Neuroscience. 2020-June-24 2020;14 doi: 10.3389/fnins.2020.00669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mceniery CM, Wallace S, Mackenzie IS, et al. Endothelial Function Is Associated With Pulse Pressure, Pulse Wave Velocity, and Augmentation Index in Healthy Humans. Hypertension. 2006-October-01 2006;48(4):602–608. doi: 10.1161/01.hyp.0000239206.64270.5f [DOI] [PubMed] [Google Scholar]
- 54.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. Nov 2009;8(11):1006–18. doi: 10.1016/S1474-4422(09)70236-4 [DOI] [PubMed] [Google Scholar]
- 55.Pendlebury ST, Rothwell PM, Oxford Vascular S. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. Mar 2019;18(3):248–258. doi: 10.1016/S1474-4422(18)30442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koton S, Pike JR, Johansen M, et al. Association of Ischemic Stroke Incidence, Severity, and Recurrence With Dementia in the Atherosclerosis Risk in Communities Cohort Study. JAMA Neurology. 2022-March-01 2022;79(3):271. doi: 10.1001/jamaneurol.2021.5080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kjeldsen SE, Narkiewicz K, Burnier M, Oparil S. Intensive blood pressure lowering prevents mild cognitive impairment and possible dementia and slows development of white matter lesions in brain: the SPRINT Memory and Cognition IN Decreased Hypertension (SPRINT MIND) study. Blood Pressure. 2018-September-03 2018;27(5):247–248. doi: 10.1080/08037051.2018.1507621 [DOI] [PubMed] [Google Scholar]
- 58.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. Sep 13 2005;112(11):1644–50. doi: 10.1161/CIRCULATIONAHA.104.501163 [DOI] [PubMed] [Google Scholar]
- 59.de Havenon A, Majersik JJ, Tirschwell DL, McNally JS, Stoddard G, Rost NS. Blood pressure, glycemic control, and white matter hyperintensity progression in type 2 diabetics. Neurology. Mar 12 2019;92(11):e1168–e1175. doi: 10.1212/WNL.0000000000007093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White WB, Wakefield DB, Moscufo N, et al. Effects of Intensive Versus Standard Ambulatory Blood Pressure Control on Cerebrovascular Outcomes in Older People (INFINITY). Circulation. Nov 12 2019;140(20):1626–1635. doi: 10.1161/CIRCULATIONAHA.119.041603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wardlaw JM, Debette S, Jokinen H, et al. ESO Guideline on covert cerebral small vessel disease. European Stroke Journal. 2021-June-01 2021;6(2):CXI–CLXII. doi: 10.1177/23969873211012132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker VM, Kehoe PG, Martin RM, Davies NM. Repurposing antihypertensive drugs for the prevention of Alzheimer’s disease: a Mendelian randomization study. International Journal of Epidemiology. 2020;49(4):1132–1140. doi: 10.1093/ije/dyz155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Satizabal C, Beiser AS, Seshadri S. Incidence of Dementia over Three Decades in the Framingham Heart Study. N Engl J Med. Jul 7 2016;375(1):93–4. doi: 10.1056/NEJMc1604823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perkovic V, Huxley R, Wu Y, Prabhakaran D, Macmahon S. The Burden of Blood Pressure-Related Disease. Hypertension. 2007-December-01 2007;50(6):991–997. doi: 10.1161/hypertensionaha.107.095497 [DOI] [PubMed] [Google Scholar]
- 65.Miura K, Daviglus ML, Dyer AR, et al. Relationship of Blood Pressure to 25-Year Mortality Due to Coronary Heart Disease, Cardiovascular Diseases, and All Causes in Young Adult Men. Archives of Internal Medicine. 2001-June-25 2001;161(12):1501. doi: 10.1001/archinte.161.12.1501 [DOI] [PubMed] [Google Scholar]
- 66.Forouzanfar MH, Liu P, Roth GA, et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA. 2017-January-10 2017;317(2):165. doi: 10.1001/jama.2016.19043 [DOI] [PubMed] [Google Scholar]
- 67.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. Dec 2007;6(12):1106–14. doi: 10.1016/S1474-4422(07)70291-0 [DOI] [PubMed] [Google Scholar]
- 68.Dudbridge F, Allen RJ, Sheehan NA, et al. Adjustment for index event bias in genome-wide association studies of subsequent events. Nature Communications. 2019-December-01 2019;10(1)doi: 10.1038/s41467-019-09381-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boef AG, le Cessie S, Dekkers OM. Mendelian randomization studies in the elderly. Epidemiology. Mar 2015;26(2):e15–6. doi: 10.1097/EDE.0000000000000243 [DOI] [PubMed] [Google Scholar]
- 70.Munafò MR, Tilling K, Taylor AE, Evans DM, Smith GD. Collider scope: when selection bias can substantially influence observed associations. International Journal of Epidemiology. 2018:226–235. doi: 10.1093/ije/dyx206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cole SR, Platt RW, Schisterman EF, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. Apr 2010;39(2):417–20. doi: 10.1093/ije/dyp334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Health. Aug 2004;58(8):635–41. doi: 10.1136/jech.2003.008466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manolio TA, Weis BK, Cowie CC, et al. New models for large prospective studies: is there a better way? Am J Epidemiol. May 1 2012;175(9):859–66. doi: 10.1093/aje/kwr453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol. Nov 1 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. Jan-Feb 2000;21(1):49–55. doi: 10.1016/s0197-4580(00)00096-8 [DOI] [PubMed] [Google Scholar]
- 76.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Vol. 4. 2005:487–487. http://neurology.thelancet.com [DOI] [PubMed] [Google Scholar]
- 77.Tzourio C, Laurent S, Debette S. Is Hypertension Associated With an Accelerated Aging of the Brain? Hypertension. 2014-May-01 2014;63(5):894–903. doi: 10.1161/hypertensionaha.113.00147 [DOI] [PubMed] [Google Scholar]
- 78.Ding J, Davis-Plourde KL, Sedaghat S, et al. Antihypertensive medications and risk for incident dementia and Alzheimer's disease: a meta-analysis of individual participant data from prospective cohort studies. The Lancet Neurology. 2020-January-01 2020;19(1):61–70. doi: 10.1016/s1474-4422(19)30393-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hughes D, Judge C, Murphy R, et al. Association of Blood Pressure Lowering with Incident Dementia or Cognitive Impairment: A Systematic Review and Meta-analysis. American Medical Association; 2020. p. 1934–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Dalen JW, Brayne C, Crane PK, et al. Association of Systolic Blood Pressure With Dementia Risk and the Role of Age, U-Shaped Associations, and Mortality. JAMA Internal Medicine. 2022-February-01 2022;182(2):142. doi: 10.1001/jamainternmed.2021.7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. Mar 2013;10(3):143–55. doi: 10.1038/nrcardio.2013.1 [DOI] [PubMed] [Google Scholar]
- 82.Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Altheimer's disease: do white matter hyperintensities matter? Dialogues Clin Neurosci. 2009;11(2):181–90. doi: 10.31887/DCNS.2009.11.2/ambrickman [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willey JZ, Scarmeas N, Provenzano FA, Luchsinger JA, Mayeux R, Brickman AM. White matter hyperintensity volume and impaired mobility among older adults. J Neurol. Mar 2013;260(3):884–90. doi: 10.1007/s00415-012-6731-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: A systematic review and meta-analysis. Neurosci Biobehav Rev. Jul 2018;90:164–173. doi: 10.1016/j.neubiorev.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Phuah C-L, Chen Y, Strain JF, et al. Association of Data-Driven White Matter Hyperintensity Spatial Signatures With Distinct Cerebral Small Vessel Disease Etiologies. Neurology. 2022-December-06 2022;99(23):e2535–e2547. doi: 10.1212/wnl.0000000000201186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown R, Low A, Markus HS. Rate of, and risk factors for, white matter hyperintensity growth: a systematic review and meta-analysis with implications for clinical trial design. J Neurol Neurosurg Psychiatry. Dec 2021;92(12):1271–1277. doi: 10.1136/jnnp-2021-326569 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.