Abstract
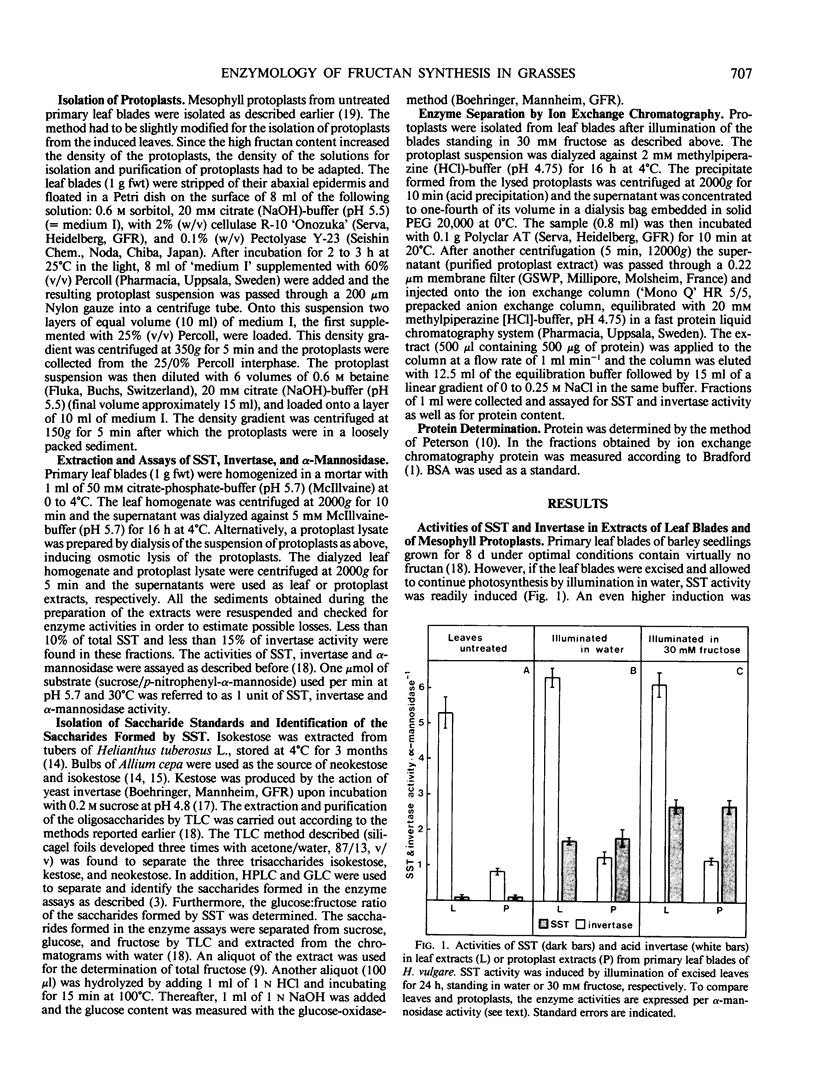
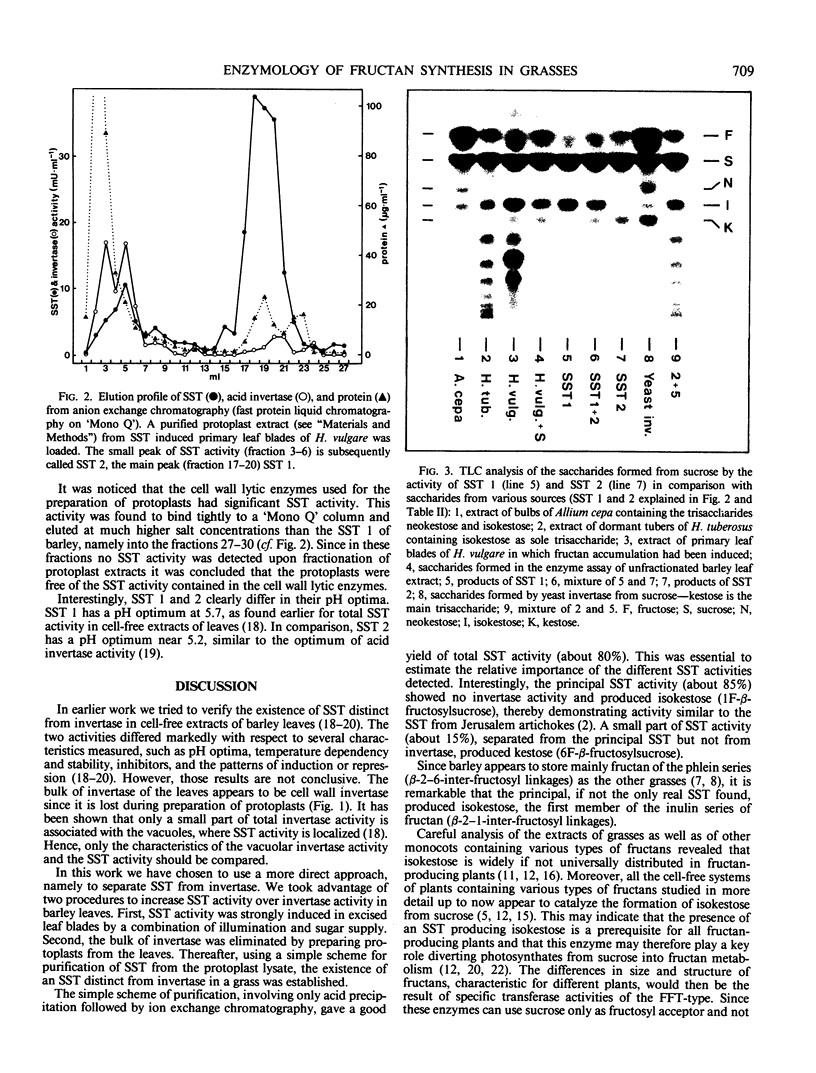
Fructan synthesis was induced in excised primary leaf blades of Hordeum vulgare L. cv Gerbel by illumination in 30 millimolar fructose. This treatment induced a 26-fold increase of sucrose-sucrose-fructosyltransferase (SST, EC 2.4.1.99) activity within 24 hours. Acid invertase (EC 3.2.1.26) activity remained about constant. By preparing protoplasts from induced leaves, approximately 80% of the invertase activity was removed with the cell walls while SST was retained. The protoplast homogenate was used to partially purify and characterize SST. Acid precipitation (pH 4.75) and anion exchange chromatography (fast protein liquid chromatography on Mono `Q') resulted in a recovery of about 80% of total SST activity. The principal activity (SST 1), accounting for 85% of the activity recovered, was purified about 200-fold. It was essentially free of invertase activity and catalyzed the synthesis of a trisaccharide which co-chromatographed with isokestose (1F-β-fructosylsucrose). The remaining 15% of SST activity (SST 2) was purified about 35-fold. It retained substantial invertase activity and catalyzed the synthesis of only one trisaccharide which co-chromatographed with kestose (6F-β-fructosylsucrose). It is concluded that barley leaves which store mainly fructan of the phlein type (β-2-6 polyfructosylsucrose), nevertheless contain sucrose-sucrose 1F-β-d-fructosyltransferase as the key enzyme of fructan synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Housley T. L., Daughtry C. S. Fructan Content and Fructosyltransferase Activity during Wheat Seed Growth. Plant Physiol. 1987 Jan;83(1):4–7. doi: 10.1104/pp.83.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Straathof A. J., Kieboom A. P., van Bekkum H. Invertase-catalysed fructosyl transfer in concentrated solutions of sucrose. Carbohydr Res. 1986 Jan 15;146(1):154–159. doi: 10.1016/0008-6215(86)85033-9. [DOI] [PubMed] [Google Scholar]
- Wagner W., Wiemken A., Matile P. Regulation of Fructan Metabolism in Leaves of Barley (Hordeum vulgare L. cv Gerbel). Plant Physiol. 1986 Jun;81(2):444–447. doi: 10.1104/pp.81.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]