Abstract
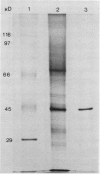
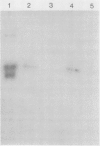
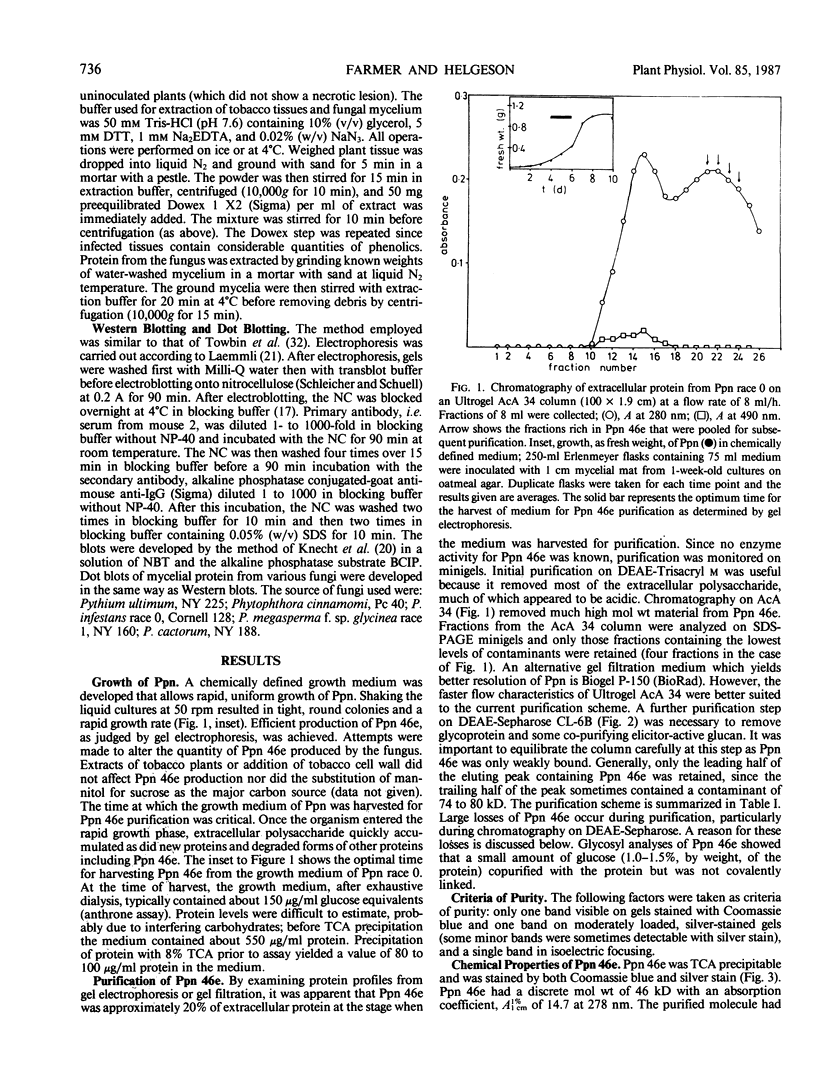
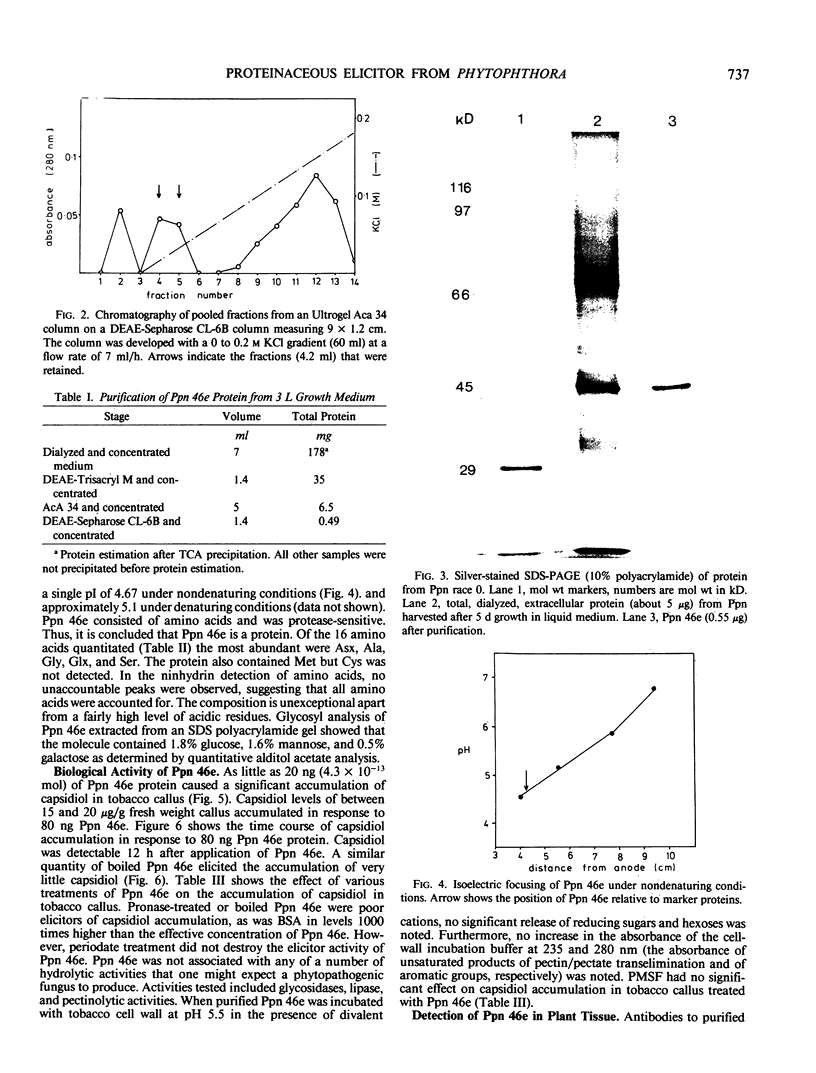
The most abundant extracellular protein produced by Phytophthora parasitica var nicotianae at early stages of rapid growth in culture has a molecular weight of 46 kilodaltons and has been designated Ppn 46e. Culture conditions for the production of this protein have been optimized and the protein has been purified by gel filtration and ion-exchange chromatography. Ppn 46e is a soluble, acidic protein (pI 4.67). The amino acids Asx (aspartic acid or asparagine), alanine, glycine, Glx (glutamic acid or glutamine), and serine are the most abundant at 13.4%, 12.3%, 12.1%, 9.3%, and 9.3% of the residues, respectively. The purified protein is, by weight, 1.8% glucose, 1.6% mannose, and 0.5% galactose. A bioassay for Ppn 46e based on tobacco callus has been developed. In this assay as little as 20 nanograms (4.3 × 10−13 mole) Ppn 46e causes the accumulation of the sesquiterpenoid phytoalexin, capsidiol, as estimated by gas chromatography. Levels of capsidiol of 25 micrograms per gram fresh weight were elicited by 80 nanograms Ppn 46e per callus piece. Pretreatment of the protein with either pronase or by boiling resulted in a loss of elicitor activity. Periodate treatment, which inactivates glucan elicitors, did not affect the ability of Ppn 46e to cause capsidiol accumulation. Monospecific antibodies to Ppn 46e were raised in mice. Western blotting experiments employing these antibodies showed that Ppn 46e was present in infected tobacco plants. Dot blotting experiments revealed the presence of the Ppn 46e epitope(s) in Phytophthora megasperma, P. cactorum, P. cinnamomi, and P. infestans but not in Fusarium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Finelli F., Berger N., Albersheim P. Host-Pathogen Interactions: IX. Quantitative Assays of Elicitor Activity and Characterization of the Elicitor Present in the Extracellular Medium of Cultures of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):751–759. doi: 10.1104/pp.57.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of Casbene Synthetase Activity in Castor Bean : THE ROLE OF PECTIC FRAGMENTS OF THE PLANT CELL WALL IN ELICITATION BY A FUNGAL ENDOPOLYGALACTURONASE. Plant Physiol. 1982 May;69(5):1181–1188. doi: 10.1104/pp.69.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. R., Lyon G. D., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XXV. Endopolygalacturonic Acid Lyase from Erwinia carotovora Elicits Phytoalexin Accumulation by Releasing Plant Cell Wall Fragments. Plant Physiol. 1984 Jan;74(1):52–60. doi: 10.1104/pp.74.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster H., Rasched I. Purification and Characterization of Extracellular Pectinesterases from Phytophthora infestans. Plant Physiol. 1985 Jan;77(1):109–112. doi: 10.1104/pp.77.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. G., Bonhoff A., Grisebach H. Quantitative Localization of the Phytoalexin Glyceollin I in Relation to Fungal Hyphae in Soybean Roots Infected with Phytophthora megasperma f. sp. glycinea. Plant Physiol. 1985 Mar;77(3):591–601. doi: 10.1104/pp.77.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L., Wheeler S., Loomis W. F. Antigenic determinants shared by lysosomal proteins of Dictyostelium discoideum. Characterization using monoclonal antibodies and isolation of mutations affecting the determinant. J Biol Chem. 1984 Aug 25;259(16):10633–10640. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lever M. A new reaction for colorimetric determination of carbohydrates. Anal Biochem. 1972 May;47(1):273–279. doi: 10.1016/0003-2697(72)90301-6. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Peled N., Krenz M. C. A new assay of microbial lipases with emulsified trioleoyl glycerol. Anal Biochem. 1981 Apr;112(2):219–222. doi: 10.1016/0003-2697(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Ried J. L., Collmer A. Activity stain for rapid characterization of pectic enzymes in isoelectric focusing and sodium dodecyl sulfate-polyacrylamide gels. Appl Environ Microbiol. 1985 Sep;50(3):615–622. doi: 10.1128/aem.50.3.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. K., McNeil M., Albersheim P. The primary structures of one elicitor-active and seven elicitor-inactive hexa(beta-D-glucopyranosyl)-D-glucitols isolated from the mycelial walls of Phytophthora megasperma f. sp. glycinea. J Biol Chem. 1984 Sep 25;259(18):11321–11336. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sreekrishna K., Jones C. E., Guetzow K. A., Prasad M. R., Joshi V. C. A modified method for amino acid analysis with ninhydrin of Coomassie-stained proteins from polyacrylamide gels. Anal Biochem. 1980 Mar 15;103(1):55–57. doi: 10.1016/0003-2697(80)90235-3. [DOI] [PubMed] [Google Scholar]
- Stekoll M., West C. A. Purification and Properties of an Elicitor of Castor Bean Phytoalexin from Culture Filtrates of the Fungus Rhizopus stolonifer. Plant Physiol. 1978 Jan;61(1):38–45. doi: 10.1104/pp.61.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]