ABSTRACT
Childhood primary angiitis of the central nervous system (cPACNS) is a vasculitis of unknown etiology that is confined to the central nervous system (CNS) and can lead to repeated cerebral infarctions if left untreated. Several cases of cPACNS after COVID-19 have been reported. Herein, we present a case of post-vaccination cPACNS. A 9-year-old healthy boy presented with persistent headache and fever after receiving the second COVID-19 vaccine (BNT162b2/Pfizer-BioNtech) dose. Brain magnetic resonance angiography (MRA) performed on the sixth day of symptom onset after vaccination revealed stenosis of the left middle cerebral artery; the patient was referred to our department on the 12th day of symptom onset. Blood tests indicated only minimal evidence of inflammation, whereas cerebrospinal fluid examination indicated pleocytosis. Brain magnetic resonance imaging (MRI) revealed vascular wall thickening and contrast enhancement of the artery with worsened stenosis. We diagnosed the patient as having cPACNS and treated him with three courses of methylprednisolone pulse therapy. The headaches and fever disappeared with improvement of vascular stenosis. The patient has been in remission for more than 1 year since cPACNS onset. This is the first report of a case of cPACNS after mRNA vaccination for COVID-19. Most previous cases of COVID-19-associated cPACNS presented with ischemic stroke. However, the present case could be treated for vasculitis prior to stroke and thus had a favorable prognosis. The mRNA vaccine for COVID-19 differs from other existing vaccines, and further accumulation of data of cases is required to determine adverse CNS reactions.
KEYWORDS: Childhood primary angiitis of the central nervous system, COVID-19 vaccine, mRNA vaccine, vasculitis, methylprednisolone pulse therapy
Introduction
Childhood primary angiitis of the central nervous system (cPACNS) is a rare condition that can cause neurological sequelae or even death. Diagnosis and treatment of cPACNS is challenging because of its variable presentation, limited awareness, and the absence of widely accepted diagnostic and therapeutic standards.1
cPACNS can develop into pediatric acute ischemic stroke (AIS). The estimated incidence rate of pediatric AIS varied from 0.9 to 7.9 per 100,000 person-years until October, 2021.2 However, no data on the incidence rates on cPACNS are available. AIS has emerged as a complication of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative pathogen of coronavirus disease 2019 (COVID-19).3 Although the pathogenesis of AIS associated with COVID-19 remains uncertain, its unique presentation, including involvement of otherwise uncommonly affected vessels, has been described.3 Moreover, several cases of cPACNS after COVID-19 have been reported, and advances in imaging technology have made it possible to recognize vascular wall lesions. However, there have been no previous reports of post-vaccine cPACNS. Here, we report a case of cPACNS that presented the day after the second vaccination with a favorable prognosis. There is no mention of any specific vaccine (in this case, BNT162b2/Pfizer-BioNtech).
Patient presentation
A previously healthy 9-year-old boy developed a left-sided headache and fever 1 day after receiving the second dose of mRNA BNT162b2 COVID-19 vaccine (Pfizer-BioNtech) and 21 days after the first dose. Six days after the onset of fever, brain MRI findings revealed stenosis at the beginning of the left middle cerebral artery. On admission (day 12 after symptom onset), the patient was alert, but he was sometimes irritable due to headaches. His temperature was 38.8°C, pulse rate was 134/min, and respiratory rate was 24/min. Percutaneous oxygen saturation and blood pressure were normal. On examination, meningeal irritations were noted, deep tendon reflexes were normal, and there were no pathological reflexes or symptoms of extremity paralysis. Laboratory findings showed no abnormalities in the blood examination other than an increased erythrocyte sedimentation rate (ESR). Serum cytokine analysis indicated minimal evidence of inflammation: interleukin (IL)-6, 10.9 pg/mL (normal range, <9.5 pg/mL); IL-10, 4.2 pg/mL (<6.8); and interferon (IFN)-γ, <7.1 pg/mL (<21.1). Cerebrospinal fluid (CSF) examination revealed an elevated initial pressure of 35 cmH2O, mononuclear cell count of 212/μL (96.2%), and increased cytokine levels: IL-6, 1684.2 pg/mL (<6.2); IL-10, 42.0 pg/mL (<2.8); and IFN-γ, 233.4 pg/mL (<7.1). His immunoglobulin G index was normal. Polymerase chain reaction (PCR) results for herpes simplex virus (HSV) DNA, varicella zoster virus (VZV) DNA, and oligoclonal bands were all negative. The PCR test result for SARS-CoV-2 in nasopharyngeal swab and CSF was also negative. Electroencephalography results were normal during both the waking and sleep stages. Brain MRI findings (Figure 1) revealed hyperintense areas in the cortex of left cerebral hemispheres on diffusion-weighted images and fluid-attenuated inversion recovery. MRA further showed stenosis in the left internal carotid and left middle cerebral arteries, and peripheral delineation was slightly poor on the affected side. Contrast-enhanced imaging revealed thickening and concentric enhancement of vessel walls. Comparison with images obtained before admission revealed that the disease had progressed. Various tests were negative for secondary central nervous system (CNS) vasculitis, and we confirmed that the patient developed cPACNS after COVID-19 vaccination. Three courses of methylprednisolone pulse therapy and anticoagulation therapies were administered. The patient’s headache and fever improved after the second course, and we switched to oral prednisolone (1.0 mg/kg/day) after the third course. Since then, the patient has been maintained low-dose oral prednisolone (0.1 mg/kg/day) and has been in remission for 1 year since the onset of cPACNS.
Figure 1.
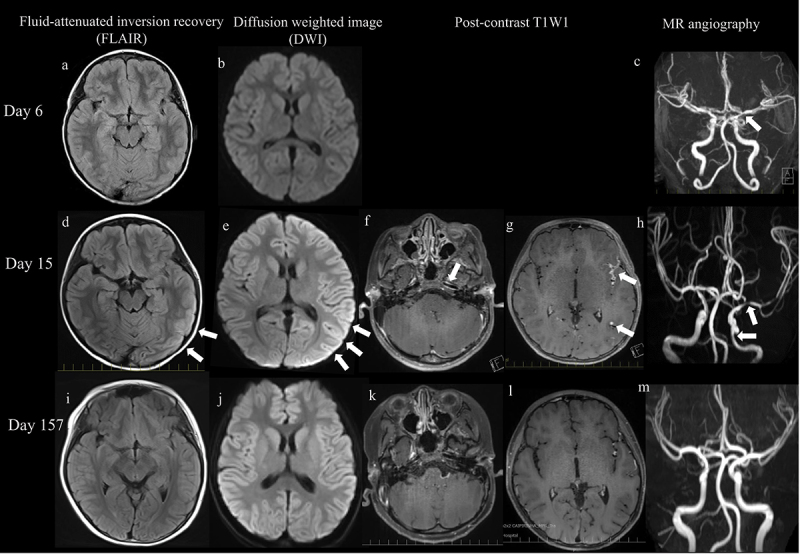
Imaging findings. (a – c): on the 6th day after symptom onset, MRA of the head revealed narrowing of the left middle cerebral artery (c, arrow). Diffusion-weighted images (DWI) and fluid-attenuated inversion recovery (FLAIR) show no lesions. (d,e): on the 15th day after symptom onset, DWI and FLAIR show cortical high-intensity areas in the left cerebral hemisphere (arrow). (f,g): contrast-enhanced effects can be observed in the vessel walls of the left middle cerebral arteries, some of which are nodule-like or concentric (arrow). (h): MRA shows stenosis in the left internal carotid artery and left middle cerebral artery (arrow), and peripheral delineation is slightly poor on the affected side. (i – m): on the 157th day, after symptom onset abnormal findings are noted to have improved.
Discussion
This is the first report of a case with cPACNS after mRNA vaccination for COVID-19. The criteria for cPACNS diagnosis are as follows: 1) neurological and psychiatric symptoms that cannot be explained by other diseases; 2) angiographic or pathological findings of vasculitis in the CNS; 3) systemic vasculitis and similar diseases that cannot be explained by other pathologies.4 Although a brain biopsy was not performed in this case, there was minimal evidence of systemic inflammation, and contrast-enhancing cerebral vessel walls of brain MRI revealed CSF pleocytosis and elevated cytokine levels, indicative of inflammation in the CNS.
There are currently no evidence-based guidelines for the treatment of cPACNS; however, based on previous reports, combined therapies with immunosuppressive and anticoagulant agents have been used. The initial therapy comprises corticosteroids, followed by cyclophosphamide, depending on the course of therapy; intravenous immunoglobulin has also been reported to be effective.5 In the present case, methylprednisolone pulse therapy was initiated in the early phase because of progressive vascular stenosis on brain imaging, and the patient responded well to treatment.
There have been no reports of cPACNS due to COVID-19 vaccine; however, there have been eight reported cases of pediatric CNS vasculitis after COVID-19, including four male and four female patients aged between 1 and 16 years (Table 1).6–13 CNS vasculitis after COVID-19 occurred from 8 days to 2 months after SARS-CoV-2 infection. Evidence of either positive CSF PCR or antibody for SARS-CoV-2 was seen in two of these patients (patient 4 and 2, respectively).8,10 Separately, in four cases (patient 3, 6–8),9,12,13 systemic inflammation, inconsistent with multisystem inflammatory syndrome in children, was suspected as a coexisting or underlying condition, and the remaining patients were considered vasculitis confined to the cerebral vessels. Furthermore, focal stenosis of the cerebral artery was confirmed using MRI in six patients, and vasculitis was confirmed in five patients based on vessel wall thickening and/or enhancement in vessel wall imaging analysis (patient 3, 5, and 6)9,11,12 or histological methods (patient 2 and 8).8,13 Unlike in the present case, CSF pleocytosis has not been described, except for one case with comorbidity of bacterial meningitis. The prognosis is variable – complete recovery, neurological sequelae, or death – despite the induction of immunomodulatory therapy.
Table 1.
Cases of cerebral vasculitis after SARS-CoV-2 infection in patients under the age of 18 years.
| Patient | Age at onset | Sex | Period from infection to vasculitis | Symptoms associated with vasculitis | SARS-CoV-2 |
Laboratory data | CSF examination | Imaging or histological findings suggesting cerebral vasculitis | Comorbidities or complications | Treatment | Prognosis | Reports [ref.] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | PCR | Antibody | |||||||||||
| 1 | 1 | Female | 2 months | Irritability, unable to walk, left hemiparesis | negative | positive (serum) | normal | n.d. | Brain MRI: subacute infarct in the right MCA territory; severe focal stenosis in the M1 segment of the right MCA. | None | mPSL, Nimodipine, ASA | Improved | Avital D.7 |
| 2 | 8 | Female | 2 weeks | Left hemiparesis | positive (NP), negative (brain) | positive (serum, CSF) | CRP 0.8 mg/dL, ESR 11 mm/h | Cells 3/µL, protein 21 mg/dL, glucose 72 mg/dL; negative PCR panel including HSV and VZV | Brain MRI: a right frontal lobe enhancing lesion with vasogenic edema, histology: vasculitis (infarct-like necrosis with perivascular lymphohistiocytic inflammatory infiltrates) | Secondary macrophage activation syndrome | mPSL, IVIG, plasmapheresis, CYC, RTX | Deceased | Poisson KE.8 |
| 3 | 8 | Female | 3 weeks | Right hemiplegia, language impairment | negative | positive | CRP 2.7 mg/mL, IL-6 3.2 pg/mL | n.d. | Brain MRI: proximal left M1 occlusion; thick concentric mural enhancement of left ICA | Iron deficiency anemia (blood transfusion) | mPSL, ASA | Walk with support | Appavu B.9 |
| 4 | 12 | Male | n.d. | Generalized seizures, right hemiparesis, and dysarthria | positive (NP, CSF) | n.d. | CRP 0.3 mg/dL, ESR 45 mm/h | No white blood cells, protein 21 mg/dL, glucose 62 mg/dL; negative PCR for HSV and VZV | Brain MRI: acute infarction, along with focal irregular narrowing and banding of the proximal M1 segment of the left MCA. | None | Diazepam | Right hemiparesis | Mirzaee SMM.10 |
| 5 | 13 | Female | 2 months | Persistent headache, speech difficulty, right hemiparesis | positive (NP) | positive (serum) | normal | normal; negative PCR for multiple viruses including HSV and VZV | Brain MRI: acute-subacute infarcts in the left MCA vascular territory; focal moderate stenosis within the M1 segment of the left MCA; wall thickening and marked, concentric enhancement | None | n.d. | Improved | Gulko E.11 |
| 6 | 16 | Male | 8 days (17 days) | Fever, neck stiffness, stupor (right hemiplegia, aphasia) | positive (NP), negative (CSF) | n.d. | CRP 25 mg/dL, IL-6 15.6 pg/mL | Cells 1566/µL (76% neutrophils), protein 500 mg/dL, glucose 27 mg/dL | Brain MRI: vessel wall enhancement and stenosis of the left ICA, MCA, and anterior cerebral artery; arterial ischemic stroke in the left MCA territory and left ophthalmic vein thrombosis. | Bacterial meningitis, Lemierre syndrome | antibiotics, CS, TCZ, ASA | Deceased | de Marcellus C.12 |
| 7 | 16 | Male | 30 days | Lethargy, decreased responsiveness, right hemiparesis, global aphasia | positive (NP, infection), negative (NP, vasculitis) | n.d. | CRP 13.6 mg/mL, ESR 59 mm/h, positive lupus anticoagulant | n.d. | Brain MRI: complete left MCA territory infarction, irregularity of left M1, and occlusion of left MCA bifurcation; no mural enhancement | Multiple organ system dysfunctions | Heparin, Enoxaparin | Dysarthria, aphasia, right hemiparesis | Appavu B.9 |
| 8 | 16 | Male | n.d. | Cardiopulmonary arrest | positive (NP) | n.d. | n.d. | n.d. | Histology: vasculitis in medium-sized vessels | Idiopathic cardiomegaly, thrombotic microangiopathy in the lung | n.p. | Deceased | Daisley Jr H.13 |
| Present case | 9 | Male | no infection | Left-sided headache, irritability, fever | negative (NP) | n.p. | CRP 0.06 mg/dL, ESR 52 mm/h | Cells 212/µL, protein 69 mg/dL, glucose 38 mg/dL; negative PCR for SARS-CoV-2, HSV, and VZV | Brain MRI: cortical high-intensity areas in the left cerebral hemispheres; stenosis in the left ICA and MCA; vessel wall thickening and enhancement | None | IVMP, PSL, ASA | No neurological sequelae | |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; NP, nasopharyngeal; CSF, cerebrospinal fluid; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; n.d., not described; n.p., not performed; CS, corticosteroid; TCZ, tocilizumab; ASA, acetylsalicylic acid; CYC, cyclophosphamide; RTX, rituximab; IVIG, intravenous immunoglobulin; IFNX, infliximab; HQ, hydroxychloroquine; IVMP, intravenous methylprednisolone; mPSL: methylprednisolone; PSL, prednisolone; MCA, middle cerebral artery; ICA, internal caroid artery; HSV, herpes simplex virus; VZV, varicella zoster virus; PCR, polymerase chain reaction; IL, interleukin.
In the present case, marked CNS inflammation characterized by elevated IFN-γ was observed. Although CSF cytokine analyses in CNS vasculitis have been reported,4 IFN-γ profiles have not yet been described. Elevated CSF IL-6 level indicates underlying conditions of CNS inflammation, which are typically observed in infectious or noninfectious autoimmune CNS diseases. CSF IFN-γ levels are often elevated in cases of CNS infection, including viral and bacterial invasion. A previous in vivo study demonstrated that SARS-CoV-2 induced co-localization of viral spike protein with angiotensin-converting enzyme 2 (ACE2) in the cerebrovascular wall in human ACE2 transgenic mice.9,14 VZV is known to be detectable in a certain population of patients with unilateral cerebral vasculitis.15 The relationship between VZV reactivation and the trigeminal nerve may likely explain unilateral vasculitis.16 The presence of pathogens in CSF or the latent period of infection provides evidence for both the infectious and autoimmune origin hypotheses for COVID-19-associated cPACNS (Table 1). However, viruses, including VZV and SARS-CoV-2, were not detected in the present case, although it is difficult to completely rule out a potential role of CNS co-infections. At present, there is insufficient evidence to draw any solid conclusions. In addition, the elevation in ESR is questionable, since in PACNS inflammatory markers are commonly normal. In general, ESR is not elevated in patients of cPACNS, but in Patients 2 and 4 in this paper (Table 1), ESR is elevated, although CRP is not increased. It is possible that the ESR is elevated due to a common effect of COVID-19 and vaccines. It is very important to screen for autoimmune and infectious diseases.
Although stroke did not occur at the onset of cPACNS in the present case, other common clinical symptoms were observed. Fever (7%) and headache (28%) are the most frequently reported adverse reactions to COVID-19 vaccine.17 When these symptoms persist after COVID-19 vaccination, a brain MRI scan is recommended to exclude cPACNS. The COVID-19 vaccine is different from existing vaccines in that it uses a modified uridine mRNA. In this system, non-replicating mRNA is taken up by host cells, which subsequently transiently express the encoded SARS-CoV-2 spike protein.18 We speculate that the mRNA vaccine may have caused a response similar to COVID-19-associated vasculitis. It is unclear whether early treatment was successful and no sequelae were observed or whether vasculitis after COVID-19 vaccination was less severe than cPACNS caused after COVID-19. From a neurologic perspective, mRNA vaccines are extremely safe.19 Further case studies are needed to determine whether cPACNS after COVID-19 vaccination has a unique mechanism and a better prognosis.
Acknowledgments
We would like to sincerely thank the patient and his family members for their participation in this study. We also thank Editage for English language editing.
Funding Statement
This work was supported in part by JSPS KAKENHI [Grant Number 20K10937].
Authors contributions
TA analyzed the data and wrote the manuscript. SH analyzed the data. MH and MT designed the study, cared for the patients, analyzed the data, and wrote the manuscript. TM, YK, YA, and TI cared for the patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Consent for publication
Consent from the patient and his parents was obtained for the publication of the patient’s detailed medical histories and examination dates.
References
- 1.Quan AS, Brunner J, Rose B, Smitka M, Hahn G, Pain CE, Häfner R, Speth F, Gerstl L, Hedrich CM.. Diagnosis and treatment of angiography positive medium to large vessel childhood primary angiitis of central nervous system (p-cPACNS): an international survey. Front Pediatr. 2021;9:654537. doi: 10.3389/fped.2021.654537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao L, Lim M, Nguyen D, Bowe S, MacKay MT, Stojanovski B, Moodie M. The incidence of pediatric ischemic stroke: a systematic review and meta-analysis. Int J Stroke. 2023;18(7):765–6. doi: 10.1177/17474930231155336. [DOI] [PubMed] [Google Scholar]
- 3.Vogrig A, Gigli GL, Bnà C, Morassi M. Stroke in patients with COVID-19: clinical and neuroimaging characteristics. Neurosci Lett. 2021;743:135564. doi: 10.1016/j.neulet.2020.135564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smitka M, Bruck N, Engellandt K, Hahn G, Knoefler R, von der Hagen M. Clinical perspective on primary angiitis of the central nervous system in Childhood (cPACNS). Front Pediatr. 2020;8:281. doi: 10.3389/fped.2020.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishida H, Kumada S, Komori T, Takai K, Mori H, Morino M, Suzuki H, Mashimo H, Inoue K, Arisaka A, et al. IVIG in childhood primary angiitis of the central nervous system: a case report. Brain Dev. 2020;42(9):675–9. doi: 10.1016/j.braindev.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Batu ED, Sener S, Ozen S. COVID-19 associated pediatric vasculitis: a systematic review and detailed analysis of the pathogenesis. Semin Arthritis Rheum. 2022;55:152047. doi: 10.1016/j.semarthrit.2022.152047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avital D, Peretz S, Perlow E, Konen O, Inbar E, Bulkowstein Y, Nahum E, Aharoni S, Vig LC, Nevo Y, et al. Clinical improvement of a toddler with COVID-19 focal cerebral arteriopathy possibly due to intra-arterial nimodipine. J Paediatr Neurol. 2022;40:40–3. doi: 10.1016/j.ejpn.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poisson KE, Zygmunt A, Leino D, Fuller CE, Jones BV, Haslam D, Staat MA, Clay G, Ting TV, Wesselkamper K, et al. Lethal pediatric cerebral vasculitis triggered by severe acute respiratory syndrome coronavirus 2. Pediatr Neurol. 2022;127:1–5. doi: 10.1016/j.pediatrneurol.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appavu B, Deng D, Dowling MM, Garg S, Mangum T, Boerwinkle V, Abruzzo T. Arteritis and large vessel occlusive strokes in children after COVID-19 infection. Pediatrics. 2021;147(3):e2020023440. doi: 10.1542/peds.2020-023440. [DOI] [PubMed] [Google Scholar]
- 10.Mirzaee SMM, Gonçalves FG, Mohammadifard M, Tavakoli SM, Vossough A. Focal cerebral arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297(2):E274–E5. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulko E, Overby P, Ali S, Mehta H, Al-Mufti F, Gomes W. Vessel wall enhancement and focal cerebral arteriopathy in a pediatric patient with acute infarct and COVID-19 infection. AJNR Am J Neuroradiol. 2020;41(12):2348–50. doi: 10.3174/ajnr.A6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Marcellus C, Dupic L, Roux CJ, El Aouane El Ghomari I, Parize P, Luscan R, Moulin F, Kossorotoff M. Case report: cerebrovascular events associated with bacterial and SARS-CoV-2 infections in an adolescent. Front Neurol. 2021;12:606617. doi: 10.3389/fneur.2021.606617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daisley H Jr, Rampersad A, Daisley M, Ramdin A, Acco O, Narinesingh F, Humphrey O. COVID-19: a closer look at the pathology in two autopsied cases. Is the pericyte at the center of the pathological process in COVID-19? Autops Case Rep. 2021;11:e2021262. doi: 10.4322/acr.2021.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Zhou L, Bao L, Liu J, Zhu H, Lv Q, Liu R, Chen W, Tong W, Wei Q, et al. SARS-CoV-2 crosses the blood–brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Target Ther. 2021;6(1):337. doi: 10.1038/s41392-021-00719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearn ND, Mackay MT. Focal cerebral arteriopathy and childhood stroke. Curr Opin Neurol. 2020;33(1):37–46. doi: 10.1097/WCO.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 16.Cornet MC, Grose C, Vexler Z, Wu YW, Fullerton HJ. The role of infection and inflammation in the pathogenesis of pediatric arterial ischemic stroke. Semin Pediatr Neurol. 2022;44:100995. doi: 10.1016/j.spen.2022.100995. [DOI] [PubMed] [Google Scholar]
- 17.Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, Barnett ED, Muñoz FM, Maldonado Y, Pahud BA, Clinical Trial Group, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386(1):35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Grimshaw M, Ceballos-Liceaga SE, Hernández-Vanegas LE, Núñez I, Hernández-Valdivia N, Carrillo-García DA, Michel-Chávez A, Galnares-Olalde JA, Carbajal-Sandoval G, Del Mar Saniger-Alba M, et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: a nationwide descriptive study. Clin Immunol. 2021;229:108786. doi: 10.1016/j.clim.2021.108786. [DOI] [PMC free article] [PubMed] [Google Scholar]


