Abstract
Although it was acknowledged that patients with obsessive‐compulsive disorder (OCD) would exhibit cognitive inflexibility, the underlying neural mechanism has not been fully clarified. Therefore, this study aimed to investigate the neural substrates involved in cognitive inflexibility among individuals with OCD. A total of 42 patients with OCD and 48 healthy controls (HCs) completed clinical assessment and functional magnetic resonance imaging (fMRI) data collection during cued task switching. Behavioral performances and fMRI activation were compared between the OCD group and the HC group. Psychophysiological interactions (PPIs) analyses were applied to explore functional connectivity related to task switching. Pearson correlation was used to investigate the relationships among behavioral performance, fMRI activity, and obsessive‐compulsive symptoms in OCD. The OCD group had a greater switch cost than HCs (χ2 = 5.89, p < .05). A significant difference in reaction time was found during switch (χ2 = 17.72, p < .001) and repeat (χ2 = 16.60, p = .018) between the two groups, while there was no significant difference in group accuracy. Comparison of group differences showed that the OCD group had increased activation in the right superior parietal cortex (rSPL) during task switching, and exhibited increased connectivity of frontoparietal network/default mode network (FPN–DMN; i.e., middle frontal gyrus [MFG]/inferior parietal cortex‐precuneus, MFG‐middle/posterior cingulate gyrus) and within the FPN (inferior parietal cortex‐postcentral gyrus). In the OCD group, the compulsion score was positively correlated with accuracy during switch (r = .405, p = .008, FDRq <.05), and negatively correlated with activation of rSPL (r = −.328, p = .034, FDRq >.05). Patients with OCD had impaired cognitive flexibility and cautious response strategy. The neural mechanism of cognitive inflexibility in OCD may involve increased activation in the rSPL, as well as hyperconnectivity within the FPN and between the FPN and DMN.
Keywords: cognitive flexibility, OCD, psychophysiological interaction, task switching
In this study, we adopt the simple task‐switching paradigm by creating switch and repeat blocks to analyze whole‐brain activation and perform gPPI analysis, to further understand the neural mechanisms of impaired cognitive flexibility in patients with obsessive‐compulsive disorder (OCD). This study found higher switch costs and slower response time in patients with OCD, suggesting that their cognitive inflexibility and strategy of pursuing accuracy at the expense of reaction time. The compensatory activity within the frontoparietal network (FPN) and alterations of interaction between FPN and default mode network may underlie the neural mechanism of difficulties in task switching.

1. INTRODUCTION
It was hypothesized that cognitive flexibility is a relevant psychological mechanism underlying the pathology of obsessive‐compulsive disorder (OCD) (Chamberlain et al., 2007; Robbins et al., 2019). Extensive evidence has revealed that patients with OCD would exhibit impaired cognitive flexibility (Gruner & Pittenger, 2017; Gu et al., 2008; Robbins et al., 2019), which is closely related to persistent intrusive thoughts and rigid compulsive behavior (Robbins et al., 2019). However, the neural mechanism of impaired cognitive flexibility in OCD has not been fully explained. Therefore, it is necessary to clarify the neural mechanism of impaired cognitive flexibility in OCD to provide empirical evidence for understanding the pathogenesis of OCD.
Cognitive flexibility refers to the ability to appropriately and efficiently adjust one's behavior in response to a changing environment, and it is commonly measured by reversal learning, set‐shifting, and cued task‐switching paradigms (Dajani & Uddin, 2015). Cued task‐switching paradigm is usually used in task‐state functional magnetic resonance imaging (fMRI) (Brass et al., 2005; Braver et al., 2003; Gaillard et al., 2021; Sonuga‐Barke & Castellanos, 2007). This paradigm uses explicit cues to guide behaviors (stimulus–response, S‐R), which evaluates individuals' ability to change strategy based on an explicit instruction or cue (Monsell, 2003). Neuroimaging studies identified that task‐switching processes were related to the frontoparietal network (FPN; Brass et al., 2005; Crone et al., 2006; Gruner & Pittenger, 2017) including lateral prefrontal cortex, superior parietal cortex (SPL), inferior parietal cortex (IPL). The inferior frontal gyrus (IFG) plays a crucial role in the activation of task representations, which enables individuals to adjust their behavior in advance to suit a new task environment (Brass et al., 2005). The parietal cortex is related to the response speed and switch costs in behavior performance (Crone et al., 2006). Research also found that the medial prefrontal cortex is essential in task‐switching and the reconfiguration of task sets (Crone et al., 2006; Dajani & Uddin, 2015; Jamadar et al., 2010; Monsell, 2003).
Previous studies on cognitive flexibility using cued task‐switching paradigm showed inconsistent results. Moritz first found no significant difference between patients with OCD and healthy controls (HCs; Moritz et al., 2004). Several studies found that patients with OCD exhibited higher error rates in switch trials, suggesting that patients with OCD had difficulties in task switching (Gu et al., 2008; Stern et al., 2017). However, another study found that the task performance of the OCD group was more accurate but slower than HCs, with greater symptom severity associated with improved accuracy (Remijnse et al., 2013), indicating the preference for accuracy over speed. The inconsistent results may be attributed to differences in experimental design, sample size, or other confounders (gender, medication status, etc.). Previous neuroimaging studies explored potential mechanisms of impaired cognitive flexibility in patients with OCD through cued task‐switching paradigm. Gu et al.'s (2008) research suggested that the imbalance in brain activation between dorsal (dorsolateral prefrontal cortex, anterior cingulate cortex, and caudate) and ventral (ventromedial prefrontal cortex and right orbitofrontal cortex) frontal‐striatal circuits might be associated with impaired task‐switching ability in patients with OCD. Remijnse et al. (2013) also reported increased activation in the putamen, anterior cingulate and insula during event‐related task switching in patients with OCD. A recent study using intrinsic functional connectivity analysis found that patients with OCD exhibited connectivity abnormalities between task‐positive network and default mode network (DMN) when performing a novel attention‐switching task (Stern et al., 2017). However, most studies using event‐related tasks seldom elucidate the mechanism of cognitive inflexibility in OCD from the perspective of functional connectivity. In this case, the block‐designed task can better detect changes in brain activity, especially in simple tasks (Liu, 2012; Shan et al., 2014). The PPI analyses, which explore the relationships between brain regions under different experimental conditions, are more sensitive to block designs (Cisler et al., 2014). There is currently a limited number of block‐designed fMRI and PPI analyses in exploring the neural mechanism of cognitive flexibility in OCD. Additionally, previous studies suggested that gender (Gaillard et al., 2021; Nooyens et al., 2022) and medication status (Nakao et al., 2014; Tükel et al., 2012) may also affect cognitive flexibility, yet few studies have explored the effects of these factors.
In summary, this study intended to examine the potential alteration of brain activation and functional connectivity using the block‐designed cued task‐switching paradigm to further understand the neural mechanisms of cognitive flexibility in patients with OCD. The role of gender and medication status were also explored as potential moderators in this study. This study will provide empirical evidence to provide further insights into the pathogenesis of OCD from a cognitive flexibility perspective.
2. MATERIALS AND METHODS
2.1. Participants
Forty‐two patients diagnosed with OCD were recruited from outpatient clinics affiliated with the Second Xiangya Hospital of Central South University in Changsha, Hunan, China. All patients met the DSM‐V criteria for OCD and were diagnosed by two experienced psychiatrists using the Structured Clinical Interview for DSM‐IV. The inclusion criteria were: (1) meeting the OCD diagnostic criteria in DSM‐V (2) ≥16 years old; (3) ≥9 years of education; (4) no history or current diagnosis of any other psychiatric disorders such as schizophrenia, substance dependence or abuse, post‐traumatic stress disorder; and (5) right‐handed. In the current study, 22 patients were drug naïve and 3 were unmedicated for at least 12 weeks. Seventeen patients were taking medication at a stable dosage: 14 were on selective serotonin reuptake inhibitor (SSRI), one was on selective serotonin and norepinephrine reuptake inhibitor (SSNRI), one was taking both SSRI and SSNRI, and one was on SSRI along with atypical antipsychotics. Forty‐eight HCs were also recruited from the community and matched for age, gender, and years of education. The inclusion criteria were: (1) ≥16 years old; (2) ≥9 years of education; (3) no history or current diagnosis of any psychiatric disorders; and (4) right‐handed. The exclusion criteria for all participants were: (1) a history of major medical or neurological problems (e.g., hypothyroidism, seizure disorder, or brain injury); (2) MRI contraindication; (3) being pregnant, lactating, or preparing for pregnancy; and (4) inability to cooperate with the MRI procedure. No participant was excluded due to excessive head motion in any direction more than 2.0 or/and 2.0 mm.
The Center for Epidemiologic Studies Depression Scale (CES‐D) assessed the depression level (Park & Yu, 2021), and The State Anxiety Inventory (SAI) assessed the anxiety level of all participants (Knowles & Olatunji, 2020; Spielberger et al., 1970). The Yale–Brown Obsessive‐Compulsive Scale (Y‐BOCS) assessed the severity of obsessive‐compulsive symptoms of patients with OCD (Goodman et al., 1989). All participants were required to undergo MRI scans while performing the task‐switching paradigm. This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. All participants learned about the study procedure and provided informed consent.
2.2. Task paradigm
The cued task‐switching paradigm would evaluate participants' cognitive flexibility (Figure 1; Monsell, 2003), and this paradigm contains cues of shape (circle and square) and color (red and blue). The stimulus included “red square,” “blue square,” “red circle,” and “blue circle.” Participants were required to identify shapes or colors. During each trial, participants had to make a response according to the cue (shape or color) and press the left and right buttons, respectively. There were two types of blocks: repeat block and switch block. During repeat (shape/color) blocks, participants were only asked to react to one cue. During switch blocks, participants were required to switch between the two different cues and respond accordingly.
FIGURE 1.

Cued task switching paradigm. Shape block and color block belong to task‐repeat, switch block belongs to task‐switch.
The whole experiment consists of three runs. The first run contains two shape blocks and two color blocks, presented in a pseudo‐random order. The last two runs started with either a shape or color block, followed by two switch blocks. There was a 10‐s fixation before the start of each block. Each block consists of 16 trials, and each trial lasts for 3000 ms. At the beginning of each trial, a fixation was presented for 200 ms, followed by a word cue (“shape” or “color”) for 150 ms, and then a target stimulus appeared for 2650 ms. Prior to the MRI scanning, each participant learned and practiced stimulus–response mapping until they were familiar with the rules. The entire task lasted 11.6 min.
2.3. MRI data acquisition
The neuroimaging acquisition was performed on a Siemens Skyra 3‐T magnetic resonance scanner at the Second Xiangya Hospital of Central South University. High‐resolution brain structural images were obtained by using a T1‐weighted sagittal magnetization‐prepared, rapid acquisition gradient‐echo sequence. The sequence parameters were as follows: repetition time (TR) = 1900 ms, echo time (TE) = 2.01 ms, flip angle (FA) = 9°, field of view (FOV) = 256 mm × 256 mm, slice thickness = 1.0 mm, matrix = 320 × 320, voxel size = 1.0 × 1.0 × 1.0 mm3 and 176 slices covering the whole brain. Three runs of functional data were acquired during the cued task‐switching paradigm using a T2*‐weighted EPI pulse sequence (TR = 2000 ms, TE = 30 ms, field of view = 256 mm × 256 mm, 32 slices with no gaps, voxel size = 1.0 × 1.0 × 1.0 mm3, slice thickness = 4.0 mm, 3 min 52 s per run) with 116 frames per run.
2.4. Behavioral performances analyses
Two sample t tests and Chi‐square tests were used to compare the demographic and clinical characteristics of the OCD and HC groups. Normal distributions of reaction time (RT) and accuracy (ACC) were tested by the Shapiro–Wilk test (Zhou & Shao, 2014). The RT and ACC were non‐normally distributed in this study (ps <.05), then the median tests were performed to compare the group differences. All participants were divided into two groups based on whether they were above the median. The Chi‐square test was used to compare the difference in behavioral performance. The switch cost was calculated by RT under switch conditions minus RT under repeat conditions and was compared by median test. The differences in behavioral performances were also compared across gender and medication status (see Supporting Information, S1).
2.5. Functional imaging data analysis
The DPARSF 5.2 was used to preprocess the imaging data (Yan et al., 2016), including the process of motion correction, normalization, and smoothness. Spatial normalization into standard MNI space was performed using a T1 image of each subject. The images were spatially smoothed using an 8 mm FWHM Gaussian kernel. First‐level general linear models were constructed for each participant using SPM12 (https://www.fil.ion.ucl.ac.uk/spm). Three event types (repeat, switch, and fixation) were defined at the first level of analysis. First, to determine the task‐switching‐related brain activation, we performed a one‐sample t test on switch minus repeat (switch‐repeat) contrast images of individuals in the OCD and HC groups. Second‐level analysis used two‐sample t tests, the differences between the two groups after controlling for age, gender, education, depression, and anxiety levels. The results were reported at an initial p value <.001, corrected for family‐wise error at the cluster level (p < .001, FWEp <.05). Group difference analysis was restricted to the regions of interest (ROIs) of bilateral IFG, middle frontal gyrus (MFG), SPL, and IPL (p < .05, voxel‐level FWEp <.05, small volume corrected) according to previous research (Gruner & Pittenger, 2017) and our findings of task‐switching‐related brain activation. All masks were created in the anatomical automatic labeling (AAL) atlas (Tzourio‐Mazoyer et al., 2002). Here, we created the IFG mask by combining the inferior frontal gyrus, triangle part, inferior frontal gyrus, and opercular part from the AAL atlas (Tzourio‐Mazoyer et al., 2002). All anatomically defined masks were created using the WFU_pickatlas (Tzourio‐Mazoyer et al., 2002). The generalized form of task‐dependent psychophysiological interactions analysis (gPPI; www.nitrc.org/progjects/gppi) was applied to examine task‐modulated connectivity through fMRI data (Akkermans et al., 2018; McLaren et al., 2012). We used the group‐constrained subject‐specific approach (Julian et al., 2012) to define seed ROIs based on subject‐specific functional activation that was restricted to the task‐switching‐related brain mask (left IFG, MFG, SPL, and IPL; Kuhnke et al., 2021; Nieto‐castañón & Fedorenko, 2012). To determine the ROIs of each participant in the gPPI analyses, we used task‐switching‐related brain activation maps of each group and found the individualized peak point within each brain region (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). Based on the individualized peak point of the main effect of the task (switch‐repeat), several spherical ROIs were created with a diameter of 6 mm. Then the PPI signal of each brain area from these spherical seeds was constructed. Two sample t test was used to compare the PPI signals between the two groups after controlling age, gender, education, C‐ESD, and SAI scores (p < .001, cluster‐level FWEp <.05). The differences in signals of activation and PPI were also compared across gender and medication status (see Supporting Information, S1).
2.6. Correlation analyses
Correlation analyses were performed between the severity of obsessive‐compulsive symptoms and behavioral performance/altered brain activation/PPI results in the OCD group. The results were reported after false discovery rate (FDR) was corrected for multiple comparisons. The medication status and gender were tested as moderators in these relationships (see Supporting Information, S1).
3. RESULTS
3.1. Demographic and clinical data
As shown in Table 1, there were no significant differences in age, gender and education between the OCD and HC groups. Patients with OCD scored higher in CES‐D and SAI than HCs.
TABLE 1.
Differences of demographic and clinical variables between OCD and HC groups.
| OCD | HC | (χ2/t) | p | |
|---|---|---|---|---|
| Age (years) | 21.86 ± 4.85 | 20.65 ± 2.10 | 1.49 | .142 |
| Gender (male/female) | 25/17 | 21/27 | 2.23 | .135 |
| Education (years) | 13.62 ± 2.09 | 14.31 ± 1.93 | −1.63 | .104 |
| CES‐D | 45.17 ± 11.49 | 34.75 ± 8.57 | 4.91 | <.001 |
| SAI | 46.00 ± 8.78 | 34.13 ± 7.76 | 6.81 | <.001 |
| Y‐BOCS | 18.87 ± 5.90 | ‐ | ‐ | ‐ |
| Obsession | 9.34 ± 3.34 | ‐ | ‐ | ‐ |
| Compulsion | 9.46 ± 4.08 | ‐ | ‐ | ‐ |
Abbreviations: CES‐D, the score of the Center for Epidemiologic Studies Depression Scale; HC, healthy control; OCD, obsessive‐compulsive disorder; SAI, the score of the state anxiety scale; Y‐BOCS, Yale–Brown Obsessive‐Compulsive Scale.
3.2. Behavioral performance
The switch cost of the OCD group was significantly higher than the HC group (see Table 2). As shown in Table 2, there was no significant difference in accuracy during switch and repeat conditions between the OCD and HC groups. Patients with OCD exhibited longer RT during switch and repeat conditions. There was no significant effect of medication status and gender on behavioral performances (see Supporting Information, S1).
TABLE 2.
Comparison of behavioral performance (median) between OCD and HC groups.
| OCD (n = 42) | HC (n = 48) | χ2 | ||
|---|---|---|---|---|
| Switch cost (RTswitch‐RTrepeat) | 167.45 | 146.27 | 5.89* | |
| RT | Switch | 917.70 | 870.80 | 17.72** |
| Repeat | 765.86 | 708.70 | 16.10** | |
| ACC | Switch | 0.94 | 0.94 | 0.04 |
| Repeat | 0.96 | 0.94 | 1.32 | |
Note: The median of response time and accuracy were compared between two groups because of the non‐normally distribution.
Abbreviations: ACC, accuracy; HC, healthy control; OCD, obsessive‐compulsive disorder; RT, response time.
Significance level of .05.
Significance level of .01.
3.3. Brain activation
The HC group exhibited increased BOLD signals in the left FPN (Figure 2a), including the left supplementary motor area (SMA), IFG, MFG, mSFG, IPL, SPL, and precentral gyrus, and exhibited decreased BOLD signals in the DMN (bilateral ventromedial prefrontal cortex, right superior occipital gyrus, right middle temporal gyrus, right medial orbital frontal cortex, left medial anterior cingulate cortex and left parahippocampal gyrus), and right caudate in contrast of switch‐repeat (Figure 2b). In addition to the left FPN, patients with OCD also exhibited increased activation in the right FPN and bilateral insula in contrast to switch‐repeat, while no brain region exhibited decreased activation in contrast to switch‐repeat.(as shown in Figure 2c, Table 3). Group difference analysis restricted to the priori ROIs showed increased activation in right SPL in contrast to switch‐repeat in patients with OCD (voxel‐level FWEp <.05, small volume corrected, Figure 2d). No significant group difference was found in other ROIs, and there were no significant effects of medication status and gender on brain activation (see Supporting Information, S1).
FIGURE 2.

Activations in contrast to switch‐repeat in the healthy controls and in the patients with OCD. The activation is mapped on the MNI template. Color bars represent the T value. (a), activations of switch > repeat in healthy controls; (b) activations of switch < repeat in healthy controls; (c) activations of switch > repeat in patients with OCD; and (d) group difference of switch > repeat restricting to the priori ROIs. OCD, obsessive‐compulsive disorder; ROI, region of interest.
TABLE 3.
Main effect (switch vs. repeat) brain regions in OCD and healthy control (HC) groups.
| L/R | BA | Peak coordinates | T/F | Cluster size (voxel) | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| OCD patients (switch > repeat) | |||||||
| Supplementary motor area | R | 32/6 | 9 | 21 | 45 | 4.30 | 123 |
| Superior frontal gyrus, medial | R | 0 | 21 | 42 | 4.26 | ||
| Inferior parietal cortex, extending to superior parietal cortex | L | 40 | −21 | −69 | 57 | 5.68 | 832 |
| R | 40 | 39 | −45 | 33 | 4.12 | 224 | |
| Inferior frontal gyrus | L | 48 | −48 | 21 | 21 | 5.63 | 748 |
| Insula | L | 47 | −33 | 21 | −6 | 4.33 | 96 |
| R | 33 | 21 | −3 | 4.73 | 106 | ||
| Middle frontal gyrus | R | 45/48 | 45 | 36 | 27 | 5.08 | 363 |
| HCs (switch > repeat) | |||||||
| Supplementary motor area | L | 6/32 | −6 | 18 | 45 | 5.03 | 518 |
| Inferior frontal gyrus | 6/44/48 | −45 | 6 | 18 | 4.69 | ||
| Middle frontal gyrus, extending to superior frontal gyrus, medial | 6/8 | −24 | 8 | 58 | 4.49 | ||
| Precentral gyrus | 6/44 | −49 | 3 | 42 | 4.21 | ||
| Inferior parietal cortex, extending to superior parietal cortex | L | 7/40 | −51 | −42 | 45 | 4.89 | 409 |
| HCs (switch < repeat) | |||||||
| Superior frontal gyrus, medial | L | 10 | −6 | 48 | 42 | 4.85 | 473 |
| R | 0 | 54 | 36 | 5.70 | |||
| Superior occipital gyrus | R | 18 | 21 | −93 | 21 | 5.21 | 167 |
| Middle temporal gyrus | R | 37 | 42 | −60 | 3 | 4.60 | 151 |
| Rolandic operculum | L | 48 | −45 | −12 | 15 | 4.91 | 128 |
| Orbital frontal cortex | R | 10 | 3 | 45 | −8 | 6.11 | 888 |
| L | 0 | 41 | −13 | 5.14 | |||
| Caudate | R | 25 | 9 | 18 | 3 | 5.49 | |
| Anterior cingulate cortex | L | 11 | −3 | 33 | −6 | 5.18 | |
| Middle temporal gyrus, extending to Parahippocampal gyrus | L | 20 | 54 | −9 | −24 | 4.76 | 784 |
| OCD > HC (switch > repeat, ROI analysis) | |||||||
| Superior parietal cortex | R | 7 | 24 | −69 | 48 | 3.81 | 7 |
Abbreviations: BA, Brodmann's area; L, left; OCD, obsessive‐compulsive disorder; R, right; ROI, region of interest.
3.4. gPPI analysis
Patients with OCD exhibited increased connectivity between the FPN and DMN (left MFG‐bilateral posterior/middle cingulate gyrus, left MFG‐left middle occipital cortex, left MFG‐right precuneus) and within FPN (left IPL‐left precuneus) in contrast of switch‐repeat than HCs (see Figure 3, Table 4).
FIGURE 3.

Increased PPI results in OCD. Color bars represent the T value. IPL, inferior parietal cortex; MFG, middle frontal gyrus; OCD, obsessive‐compulsive disorder; PPI, psychophysiological interaction.
TABLE 4.
gPPI differences between OCD and HC groups (OCD > HC).
| ROIs | L/R | BA | Peak coordinates | T | Cluster size | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| left MFG | Middle occipital cortex | L | 18/19 | −24 | −72 | 15 | 4.24 | 113 |
|
Middle cingulate gyrus ext. posterior cingulate gyrus |
L/R | 23/31 | 6 | −33 | 18 | 5.11 | 451 | |
| Precuneus | R | 23/26 | 15 | −66 | 42 | 4.10 | 154 | |
| left IPL | Postcentral gyrus | L | 43 | −42 | −21 | 24 | 4.72 | 90 |
| Middle cingulate gyrus ext. posterior cingulate gyrus | L | 3/40 | −9 | −21 | 48 | 4.21 | 139 | |
| Precuneus | L | 7 | 0 | −60 | 51 | 4.26 | 95 | |
Abbreviations: BA, Brodmann's area; HC, healthy control; IFG, inferior frontal gyrus; IPL, inferior parietal cortex; L, left; MFG, middle frontal gyrus; OCD, obsessive‐compulsive disorder; R, right; ROI, region of interest.
3.5. Correlation analysis between behavioral performance and brain activity
Within the OCD group, the severity of compulsive was positively correlated with accuracy during switch condition (r = .405, p = .008, FDRq <.05, see Figure 4). The correlation between the Y‐BOCS total score and accuracy during switch condition showed a trend toward significance (p = .057, r = .296). No other association between ACC/RT and obsessive‐compulsive symptoms was found. The BOLD signals of the right SPL, which exhibited differences between the two groups, showed a negative correlation with the severity of compulsion (r = −.328, p = .034, FDRq >.05). No significant correlation was found between obsessive‐compulsive symptoms and PPI results. The moderating effects of medication status and gender were not significant in these relationships.
FIGURE 4.
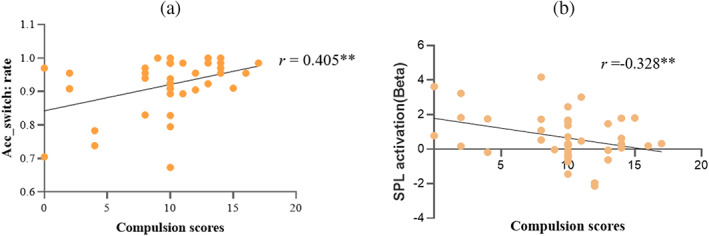
Scatter plot by obsessive‐compulsive symptoms, behavioral performance, and fMRI activity in OCD group (a) correlation between accuracy during switch and compulsion; (b) correlation between altered superior parietal cortex (SPL) and compulsion; r, correlation coefficient between compulsion scores and accuracy rate of switch block; Acc_switch, the accuracy during task‐switch; fMRI, functional magnetic resonance imaging; OCD, obsessive‐compulsive disorder. **Significance level of .01.
4. DISCUSSION
This study recruited a relatively large sample to explore the neurobiology underlying cognitive inflexibility in OCD through a block‐designed cued task‐switching paradigm. The behavioral performance showed that patients with OCD had higher switch costs and longer reaction times. Neuroimaging results found that patients with OCD had increased SPL activation, increased PPIs of FPN–DMN, and within FPN during task‐switching compared with HCs.
4.1. Longer reaction time in patients with OCD
Analyses of behavioral performance showed impaired cognitive flexibility and a tendency toward high accuracy in patients with OCD. First, patients with OCD had greater switch costs than HCs, which suggested an inflexible pattern of response strategies and difficulty in task switching in OCD. The difficulty in task switching may be related to the dysfunction of transient control and inhibition (Gruner & Pittenger, 2017; Klawohn et al., 2016; Remijnse et al., 2013). Previous studies also found that patients with OCD showed less adaptation to different contexts than HCs (Klawohn et al., 2016; Riesel et al., 2019). In addition to the higher switch cost, patients with OCD also had longer average reaction times, which may reflect their cautiousness and error‐avoidant response style (Klawohn et al., 2016; Riesel et al., 2019). Patients with OCD was also found to exhibit slower response than controls on a variety of tasks. For example, Remijnse et al. (2013) found a prolonged RT in OCD during task switching, suggesting that patients with OCD applied a strategic tradeoff between speed and accuracy. Consistent with previous research, this study revealed that the speed‐accuracy tradeoff strategy in patients with OCD comes at the expense of reaction time. Correlational analysis showed that the accuracy of task‐switch increased with the severity of compulsion, further supporting that the pursuit of accuracy was closely related to the severity of symptoms.
4.2. Compensatory mechanism of FPN
Neuroimaging results suggested that patients with OCD had more activated brain regions than HCs, according to the switch‐repeat contrast images. The HCs showed increased BOLD signals in left FPN, including SMA, IPL, and IFG. In addition to the left FPN, patients with OCD also exhibited increased activation in the right FPN, especially in the SPL. It was reported that the SPL was involved in the task reconfiguration and transient control process (Braver et al., 2003; Gruner & Pittenger, 2017), and the signal of SPL had a negative correlation with the severity of compulsion in this study. This suggests that the increased activation in the SPL may be a compensatory mechanism. However, such abnormal activation related to task switching in OCD in this study was inconsistent with the study of Gu et al., which may be caused by different task designs (event vs. block) and the sample composition (sample size and comorbidity). Results of gPPI analysis further verified the enhanced functional connectivity of the inferior parietal cortex‐postcentral gyrus during switch condition, indicating the synchronicity of brain activity within FPN was enhanced in patients with OCD. The enhanced connectivity within FPN in patients with OCD was also observed in De Vries's research on working memory tasks, which indicated that compensatory frontoparietal brain activity in patients with OCD maintains task performance under low task loads, but not enough to maintain performance under high task loads (de Vries et al., 2014). Patients with OCD also showed increased activity in the bilateral insula, which is consistent with previous research (Remijnse et al., 2013). It was found that hyperactive insula is associated with more perception of error‐related signals (Tomiyama et al., 2022), which provided evidence for avoiding mistakes in OCD. Therefore, patients with OCD would adopt a cautious response strategy at the expense of reaction time. Taken together, patients with OCD have compensatory activity within FPN, improving accuracy at the expense of reaction time.
4.3. Decreased connectivity of FPN–DMN
Our results indicated that HCs exhibited decreased activation of the regions in the DMN in contrast to switch‐repeat, while patients with OCD did not show decreased activation in any brain region. It could be explained that FPN was found to be a task‐positive network where activity increases with increased cognitive demands during task performance (Nikolaidis et al., 2022; Uddin et al., 2009). A previous study also stated that DMN shows anticorrelation with FPN and is associated with improved task performance (DeSerisy et al., 2021; Kelly et al., 2008; Uddin et al., 2009; Wen et al., 2013). In addition, according to Default Mode Interference Hypothesis (Sonuga‐Barke & Castellanos, 2007; Soursa et al., 2013), the FPN should adequately deactivate the DMN, which was also observed in the HC group during the switch task in our study. In terms of patients with OCD, FPN showed reduced deactivation of the DMN, which is also consistent with previous research. Therefore, it can be speculated that patients with OCD are unable to completely disengage from mentally generated thoughts or scenarios when performing cognitive or daily tasks (Stern et al., 2012). The gPPI analysis further suggested the anticorrelation between FPN and DMN was reduced in patients with OCD. Consistently, a previous meta‐analysis also identified alterations in the relationship between FPN and DMN in patients with OCD (Gürsel et al., 2018; Pittenger, 2017). Stern et al. (2011, 2017) found that FPN was positively correlated with DMN regions, suggesting that changing the interactions between FPN and DMN may contribute to the OCD phenotype. Fan et al. (2017) also suggested that patients with OCD had difficulties in switching task‐positive and task‐negative networks. Therefore, besides the above resting‐state fMRI studies, which found the alteration of interactions between FPN and DMN in OCD, our study provided further evidence for the hyperconnectivity between FPN and DMN in OCD from the perspective of task‐state fMRI.
4.4. Effect of medication status and gender
The moderating effects of medication status and gender were not significant in the correlational analyses. Meanwhile, there was no significant group difference in behavioral performance and fMRI results between medicated and unmedicated individuals with OCD, and across gender. Therefore, results regarding the effect of medication status and gender were excluded.
In summary, this study found higher switch costs and slower response time in patients with OCD, suggesting that their cognitive inflexibility and strategy of pursuing accuracy at the expense of reaction time. The compensatory activity within FPN and alterations of interaction between FPN and DMN may underlie the neural mechanism of difficulties in task switching. In addition, gender and medication status did not show a significant moderating effect.
5. CONCLUSION
This study used block designed cued task‐switching paradigm to explore cognitive flexibility and its neural substrate in patients with OCD. This study found that patients with OCD tend to have difficulties in task switching and cautious response strategy. The compensatory activity within FPN and alteration of the interaction of FPN–DMN may underly the neural mechanism of impaired cognitive flexibility.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
DATA S1. Supporting Information.
ACKNOWLEDGMENTS
The authors are grateful to all the subjects for their participation. This work was supported by the National Natural Science Foundation of China (grant numbers 31871112 and 82171532), the Hunan Provincial Innovation Foundation for Postgraduate (grant number, CX20220314), and the Fundamental Research Funds for the Central Universities of Central South University (grant number 2022ZZTS0229).
Liu, Q. , Gao, F. , Wang, X. , Xia, J. , Yuan, G. , Zheng, S. , Zhong, M. , & Zhu, X. (2023). Cognitive inflexibility is linked to abnormal frontoparietal‐related activation and connectivity in obsessive‐compulsive disorder. Human Brain Mapping, 44(16), 5460–5470. 10.1002/hbm.26457
Contributor Information
Mingtian Zhong, Email: ztomorrow@126.com.
Xiongzhao Zhu, Email: xiongzhaozhu@csu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Akkermans, S. E. A. , Luijten, M. , van Rooij, D. , Franken, I. H. A. , & Buitelaar, J. K. (2018). Putamen functional connectivity during inhibitory control in smokers and non‐smokers. Addiction Biology, 23(1), 359–368. 10.1111/adb.12482 [DOI] [PubMed] [Google Scholar]
- Brass, M. , Derrfuss, J. , Forstmann, B. , & von Cramon, D. Y. (2005). The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Sciences, 9(7), 312–314. 10.1016/j.tics.2005.05.013 [DOI] [PubMed] [Google Scholar]
- Braver, T. S. , Reynolds, J. R. , & Donaldson, D. I. (2003). Neural mechanisms of transient and sustained cognitive control during task switching. Neuron, 39, 713–726. [DOI] [PubMed] [Google Scholar]
- Chamberlain, S. R. , Fineberg, N. A. , Menzies, L. A. , Blackwell, A. D. , Bullmore, E. T. , Robbins, T. W. , & Sahakian, B. J. (2007). Impaired cognitive flexibility and motor inhibition in unaffected first‐degree relatives of patients with obsessive‐compulsive disorder. American Journal of Psychiatry, 164(2), 335–338. 10.1176/ajp.2007.164.2.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler, J. M. , Bush, K. , & Steele, J. S. (2014). A comparison of statistical methods for detecting context‐modulated functional connectivity in fMRI. NeuroImage, 84, 1042–1052. 10.1016/j.neuroimage.2013.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone, E. A. , Wendelken, C. , Donohue, S. E. , & Bunge, S. A. (2006). Neural evidence for dissociable components of task‐switching. Cerebral Cortex, 16(4), 475–486. 10.1093/cercor/bhi127 [DOI] [PubMed] [Google Scholar]
- Dajani, D. R. , & Uddin, L. Q. (2015). Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends in Neuroscience, 38(9), 571–578. 10.5040/9781350152892.ch-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, F. E. , de Wit, S. J. , Cath, D. C. , van der Werf, Y. D. , van der Borden, V. , van Rossum, T. B. , van Balkom, A. J. L. M. , van der Wee, N. J. A. , Veltman, D. J. , & van den Heuvel, O. A. (2014). Compensatory frontoparietal activity during working memory: An endophenotype of obsessive‐compulsive disorder. Biological Psychiatry, 76(11), 878–887. 10.1016/j.biopsych.2013.11.021 [DOI] [PubMed] [Google Scholar]
- DeSerisy, M. , Ramphal, B. , Pagliaccio, D. , Raffanello, E. , Tau, G. , Marsh, R. , Posner, J. , & Margolis, A. E. (2021). Frontoparietal and default mode network connectivity varies with age and intelligence. Developmental Cognitive Neuroscience, 48, 100928. 10.1016/j.dcn.2021.100928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J. , Zhong, M. , Gan, J. , Liu, W. , Niu, C. , Liao, H. , Zhang, H. , Yi, J. , Chan, R. C. K. , Tan, C. , & Zhu, X. (2017). Altered connectivity within and between the default mode, central executive, and salience networks in obsessive‐compulsive disorder. Journal of Affective Disorders, 223, 106–114. 10.1016/j.jad.2017.07.041 [DOI] [PubMed] [Google Scholar]
- Gaillard, A. , Fehring, D. J. , & Rossell, S. L. (2021). A systematic review and meta‐analysis of behavioural sex differences in executive control. European Journal of Neuroscience, 53(2), 519–542. 10.1111/ejn.14946 [DOI] [PubMed] [Google Scholar]
- Goodman, W. K. , Price, L. H. , Rasmussen, S. A. , Mazure, C. , Fleischmann, R. L. , Hill, C. L. , Heninger, G. R. , & Charney, D. S. (1989). The Yale‐Brown obsessive compulsive scale: I. Development, use, and reliability. Archives of General Psychiatry, 46(11), 1006–1011. 10.1001/archpsyc.1989.01810110048007 [DOI] [PubMed] [Google Scholar]
- Gruner, P. , & Pittenger, C. (2017). Cognitive inflexibility in obsessive‐compulsive disorder. Neuroscience, 345, 243–255. 10.1016/j.neuroscience.2016.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, B. M. , Park, J. Y. , Kang, D. H. , Lee, S. J. , Yoo, S. Y. , Jo, H. J. , Choi, C. H. , Lee, J. M. , & Kwon, J. S. (2008). Neural correlates of cognitive inflexibility during task‐switching in obsessive‐compulsive disorder. Brain, 131(1), 155–164. 10.1093/brain/awm277 [DOI] [PubMed] [Google Scholar]
- Gürsel, D. A. , Avram, M. , Sorg, C. , Brandl, F. , & Koch, K. (2018). Frontoparietal areas link impairments of large‐scale intrinsic brain networks with aberrant fronto‐striatal interactions in OCD: A meta‐analysis of resting‐state functional connectivity. Neuroscience and Biobehavioral Reviews, 87, 151–160. 10.1016/j.neubiorev.2018.01.016 [DOI] [PubMed] [Google Scholar]
- Jamadar, S. , Michie, P. , & Karayanidis, F. (2010). Compensatory mechanisms underlie intact task‐switching performance in schizophrenia. Neuropsychologia, 48(5), 1305–1323. 10.1016/j.neuropsychologia.2009.12.034 [DOI] [PubMed] [Google Scholar]
- Julian, J. B. , Fedorenko, E. , Webster, J. , & Kanwisher, N. (2012). An algorithmic method for functionally defining regions of interest in the ventral visual pathway. NeuroImage, 60(4), 2357–2364. 10.1016/j.neuroimage.2012.02.055 [DOI] [PubMed] [Google Scholar]
- Kelly, A. M. C. , Uddin, L. Q. , Biswal, B. B. , Castellanos, F. X. , & Milham, M. P. (2008). Competition between functional brain networks mediates behavioral variability. NeuroImage, 39(1), 527–537. 10.1016/j.neuroimage.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Klawohn, J. , Endrass, T. , Preuss, J. , Riesel, A. , & Kathmann, N. (2016). Modulation of hyperactive error signals in obsessive‐compulsive disorder by dual‐task demands. Journal of Abnormal Psychology, 125(2), 292–298. 10.1037/abn0000134 [DOI] [PubMed] [Google Scholar]
- Knowles, K. A. , & Olatunji, B. O. (2020). Specificity of trait anxiety in anxiety and depression: Meta‐analysis of the State‐Trait Anxiety Inventory. Clinical Psychology Review, 82, 101928. 10.1016/j.cpr.2020.101928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnke, P. , Kiefer, M. , & Hartwigsen, G. (2021). Task‐dependent functional and effective connectivity during conceptual processing. Cerebral Cortex, 31(7), 3475–3493. 10.1093/cercor/bhab026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T. T. (2012). The development of event‐related fMRI designs. NeuroImage, 62(2), 1157–1162. 10.1016/j.neuroimage.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren, D. G. , Ries, M. L. , Xu, G. , & Johnson, S. C. (2012). A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61(4), 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell, S. (2003). Task switching. Trends in Cognitive Sciences, 7(3), 134–140. 10.1016/S1364-6613(03)00028-7 [DOI] [PubMed] [Google Scholar]
- Moritz, S. , Hübner, M. , & Kluwe, R. (2004). Task switching and backward inhibition in obsessive‐compulsive disorder. Journal of Clinical and Experimental Neuropsychology, 26(5), 677–683. 10.1080/13803390409609791 [DOI] [PubMed] [Google Scholar]
- Nakao, T. , Okada, K. , & Kanba, S. (2014). Neurobiological model of obsessive‐compulsive disorder: Evidence from recent neuropsychological and neuroimaging findings. Psychiatry and Clinical Neurosciences, 68(8), 587–605. 10.1111/pcn.12195 [DOI] [PubMed] [Google Scholar]
- Nieto‐castañón, A. , & Fedorenko, E. (2012). Subject‐specific functional localizers increase sensitivity and functional resolution of multi‐subject analyses. NeuroImage, 63(3), 1646–1669. 10.1016/j.neuroimage.2012.06.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis, A. , He, X. , Pekar, J. , Rosch, K. , & Mostofsky, S. H. (2022). Frontal corticostriatal functional connectivity reveals task positive and negative network dysregulation in relation to ADHD, sex, and inhibitory control. Developmental Cognitive Neuroscience, 54, 101101. 10.1016/j.dcn.2022.101101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooyens, A. C. J. , Wijnhoven, H. A. H. , Schaap, L. S. , Sialino, L. D. , Kok, A. A. L. , Visser, M. , Verschuren, W. M. M. , Picavet, H. S. J. , & van Oostrom, S. H. (2022). Sex differences in cognitive functioning with aging in the Netherlands. Gerontology, 68(9), 999–1009. 10.1159/000520318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S. H. , & Yu, H. Y. (2021). How useful is the center for epidemiologic studies depression scale in screening for depression in adults? An updated systematic review and meta‐analysis. Psychiatry Research, 302, 114037. 10.1016/j.psychres.2021.114037 [DOI] [PubMed] [Google Scholar]
- Pittenger, C. (2017). Obsessive‐compulsive disorder: Phenomenology, pathophysiology, and treatment. Oxford University Press. 10.1093/med/9780190228163.001.0001 [DOI] [Google Scholar]
- Remijnse, P. L. , van den Heuvel, O. A. , Nielen, M. M. A. , Vriend, C. , Hendriks, G. , Hoogendijk, W. J. G. , Uylings, H. B. M. , & Veltman, D. J. (2013). Cognitive inflexibility in obsessive‐compulsive disorder and major depression is associated with distinct neural correlates. PLoS One, 8(4), e59600. 10.1371/journal.pone.0059600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesel, A. , Kathmann, N. , & Klawohn, J. (2019). Flexibility of error‐monitoring in obsessive‐compulsive disorder under speed and accuracy instructions. Journal of Abnormal Psychology, 128(7), 671–677. 10.1037/abn0000463 [DOI] [PubMed] [Google Scholar]
- Robbins, T. W. , Vaghi, M. M. , & Banca, P. (2019). Obsessive‐compulsive disorder: Puzzles and prospects. Neuron, 102(1), 27–47. 10.1016/j.neuron.2019.01.046 [DOI] [PubMed] [Google Scholar]
- Shan, Z. Y. , Wright, M. J. , Thompson, P. M. , McMahon, K. L. , Blokland, G. G. A. M. , De Zubicaray, G. I. , Martin, N. G. , Vinkhuyzen, A. A. E. , & Reutens, D. C. (2014). Modeling of the hemodynamic responses in block design fMRI studies. Journal of Cerebral Blood Flow and Metabolism, 34(2), 316–324. 10.1038/jcbfm.2013.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga‐Barke, E. J. S. , & Castellanos, F. X. (2007). Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neuroscience and Biobehavioral Reviews, 31(7), 977–986. 10.1016/j.neubiorev.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Soursa, C. , Zhuo, J. , Janowich, J. , Aarabi, B. , Shanmuganathanb, K. , & Gullapalli, R. P. (2013). Default mode network interference in mild traumatic brain injury—A pilot resting state study. Brain Research, 1537, 201–215. 10.1016/j.brainres.2013.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , & Lushene, R. E. (1970). Manual for the state‐trait anxiety inventory .
- Stern, E. R. , Fitzgerald, K. D. , Welsh, R. C. , Abelson, J. L. , & Taylor, S. F. (2012). Resting‐state functional connectivity between fronto‐parietal and default mode networks in obsessive‐compulsive disorder. PLoS One, 7(5), e36356. 10.1371/journal.pone.0036356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, E. R. , Muratore, A. F. , Taylor, S. F. , Abelson, J. L. , Hof, P. R. , & Goodman, W. K. (2017). Switching between internally and externally focused attention in obsessive‐compulsive disorder: Abnormal visual cortex activation and connectivity. Psychiatry Research: Neuroimaging, 265, 87–97. 10.1016/j.pscychresns.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, E. R. , Welsh, R. C. , Fitzgerald, K. D. , Gehring, W. J. , Lister, J. J. , Himle, J. A. , Abelson, J. L. , & Taylor, S. F. (2011). Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive‐compulsive disorder. Biological Psychiatry, 69(6), 583–591. 10.1016/j.biopsych.2010.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama, H. , Murayama, K. , Nemoto, K. , Hasuzawa, S. , Mizobe, T. , Kato, K. , Matsuo, A. , Ohno, A. , Kang, M. , Togao, O. , Hiwatashi, A. , Ishigami, K. , & Nakao, T. (2022). Alterations of default mode and cingulo‐opercular salience network and frontostriatal circuit: A candidate endophenotype of obsessive‐compulsive disorder. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 116, 110516. 10.1016/j.pnpbp.2022.110516 [DOI] [PubMed] [Google Scholar]
- Tükel, R. , Gürvit, H. , Ertekin, B. A. , Oflaz, S. , Ertekin, E. , Baran, B. , Kalem, Ş. A. , Kandemir, P. E. , Özdemiroǧlu, F. A. , & Atalay, F. (2012). Neuropsychological function in obsessive‐compulsive disorder. Comprehensive Psychiatry, 53(2), 167–175. 10.1016/j.comppsych.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , Mazoyer, B. , & Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Uddin, L. Q. , Kelly, A. M. C. , Biswal, B. B. , Castellanos, F. X. , & Milham, M. P. (2009). Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping, 30(2), 625–637. 10.1002/hbm.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, X. , Liu, Y. , Yao, L. , & Ding, M. (2013). Top‐down regulation of default mode activity in spatial visual attention. Journal of Neuroscience, 33(15), 6444–6453. 10.1523/JNEUROSCI.4939-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. G. , Wang, X.‐D. , Zuo, X. N. , & Zang, Y. F. (2016). DPABI: Data Processing & Analysis for (Resting‐State) Brain Imaging. Neuroinformatics, 14(3), 339–351. 10.1007/s12021-016-9299-4 [DOI] [PubMed] [Google Scholar]
- Zhou, M. , & Shao, Y. (2014). A powerful test for multivariate normality. Journal of Applied Statistics, 41(2), 351–363. 10.1080/02664763.2013.839637.A [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DATA S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


