ABSTRACT
A growing number of studies have suggested that traditional Chinese medicine (TCM) plays an essential role in the development and occurrence of liver cancer. However, the function of Ruangan Lidan decoction (RLD) in liver cancer are not yet adequately identified and manifested, which attracted our attention. The key genes related to liver cancer and RLD and the upstream miRNAs of PDK4 were obtained based on bioinformatics analysis, followed by verification of the targeting relationship between miR-9-5p and PDK4. Next, Huh7 cells were treated with RLD to detect cell proliferation, colony formation, migration, invasion, and apoptosis by multiple assays with gain- and loss-of-function experiments. Moreover, subcutaneous transplanted tumor model and lung metastasis model of liver cancer in nude mice were established to further verify the functional role of RLD in liver cancer growth and metastasis via miR-9-5p/PDK4 axis. Bioinformatics analysis found that PDK4 and miR-9-5p were related to liver cancer, and PDK4 may be a downstream regulator of RLD. miR-9-5p could target and inhibit PDK4. In vitro cell experiments demonstrated that RLD suppressed liver cancer cell proliferation, invasion and migration, and promoted apoptosis by inhibiting miR-9-5p expression and promoting PDK4 expression. In vivo animal experiments further confirmed that RLD inhibited liver cancer growth and metastasis via upregulation of miR-9-5p-dependent PDK4. RLD downregulated miR-9-5p and upregulated PDK4 to inhibit the proliferation, migration, invasion, and induce apoptosis, thereby suppressing the growth and metastasis of liver cancer, highlighting a potential novel target for treatment of liver cancer.
KEYWORDS: Liver cancer, Ruangan Lidan decoction, miR-9-5p, PDK4, tumor growth, tumor metastasis
Introduction
Liver cancer ranks as the most frequent cause of cancer deaths globally, and a high incidence of liver diseases can be detected in developing counties.1 There are multiple risk factors for liver cancer, including smoking, obesity, hepatitis B/C virus, fatty liver disease, diabetes, alcohol-related cirrhosis, and diverse dietary exposures.2 Despite the availability of new treatment modalities for patients with liver cancer, the clinical treatment effect is not satisfactory with high recurrence.3,4 Moreover, the widespread metastasis also leads to the poor prognosis of patients with liver cancer.5 Therefore, identifying novel therapeutic strategies for controlling metastasis in liver cancer would be a promising way to improve the prognosis for those patients.
Traditional Chinese medicine (TCM) has been widely used to treat multiple diseases for centuries, and its anti-tumor roles in liver cancer have also been confirmed.6 Ruangan Lidan decoction (RLD), a kind of TCM, comprises Chaihu, Scutellaria baicalensis, Corydalis, turmeric, hypericum japonicum thunb, Sedum sarmentosum, Herba Artemisiae capillaris, Prunella vulgaris, raw oyster, red ginseng, Pinellia ternata, and licorice. These components are verified to exert inhibitory effects on the development of liver cancer. For example, relevant medical research and studies have demonstrated that Xiaochaihu decoction suppresses the progression of liver cancer, acting as a promising drug or complementary drug for liver cancer treatment.7,8 Recent evidence suggests that Scutellaria baicalensis has been widely applied to treat liver disease and as an adjuvant cancer treatment.9 Another study has also explained that polysaccharide extracted from Sedum sarmentosum Bunge serves as a potential natural anti-tumor agent in liver cancer.10 However, the mechanisms of RLD in liver cancer require further investigation. It has been proven in clinical trials that the Ruangan Lidan decoction (RLD) has an inhibitory effect on the occurrence and development of liver cancer. Studies have shown that postoperative patients who take RLD can help prevent the recurrence and metastasis of liver cancer and extend the disease-free survival period.11 Furthermore, RLD can also improve the Karnofsky Performance Status (KPS) of patients with liver cancer undergoing intervention combined with three-dimensional conformal radiotherapy, improve their quality of life, physical function, and psychological state, and reduce their clinical adverse reactions.12
Moreover, based on bioinformatics analysis, pyruvate dehydrogenase kinase 4 (PDK4) was predicted as the downstream regulator of RLD. PDK4 is a mitochondrial protein that regulates the pyruvate dehydrogenase complex by inactivating pyruvate dehydrogenase (PDH) via phosphorylation.13 PDK4 could maintain normal blood glucose levels by coordinating glucose use and fat consumption, which is necessary for metabolic reprogramming.14 The potential tumor suppressor characteristics of PDK4 in liver cancer have also been documented.15 In addition, a previous study has revealed that PDK4 can be targeted by miR-9-5p.16 Intriguingly, the present study also demonstrated that miR-9-5p was a regulator of PDK4. Evidence demonstrates that miRNAs are dysregulated in the formation and progression of liver cancer by mediating cellular processes, including proliferation, apoptosis, and cell-cycle.17 Previous studies have shown that PDK4 is downregulated in the tissue of HCC liver cancer patients, and those with low PDK4 expression have a lower survival rate and a higher recurrence rate.18 In vitro experiments have shown that knocking out PDK4 in HCC liver cancer cells promotes tumor cell proliferation and migration. Another study found that PDK4 expression is associated with the survival and liver function improvement of colon cancer liver metastasis resection patients, and its downregulation predicts poor prognosis.19 In addition, there is also evidence that inhibiting PDK4 expression in HCC liver cancer cells increases the expression of cell cycle protein E1, cell cycle protein A2, and E2F1 protein-mediated cell cycle regulation, thereby promoting tumor cell proliferation.13
A variety of existing studies have confirmed the correlation between miR-9-5p and hepatocellular carcinoma cells (HCC), a type of liver cancer.20,21 Herein, in the present study, we aimed to observe the underlying connection among RLD, miR-9-5p, and PDK4 and explore their effects on the biological properties of liver cancer cells.
Materials and methods
Ethics statement
The animal experiment was approved by the Ethics Committee of Liuzhou Traditional Chinese Medical Hospital. Great efforts were made to minimize the number of animals used in the experiments and their discomfort.
Bioinformatics analysis
The BATMAN-TCM database predicted the RLD-related targets and liver cancer-related genes were collected through the GeneCards and CTD databases with “liver cancer” as the keywords and CTD: Score ≥ 100 and GeneCards: Score ≥ 3.5 as criteria. Finally, the disease and active component targets were introduced into jvenn to generate a Venn diagram with 1 common target screened out.
The liver cancer-related human microarray data GSE74656 was obtained through the Gene Expression Omnibus database containing 5 normal and 10 liver cancer samples. Differential expression analysis of genes in samples was performed using the R language “limma” package. In addition, the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of the target proteins was performed using the Sangerbox database.
The upstream regulatory miRNA of PDK4 was retrieved from the TargetScanHuman 7.1, DIANA TOOLS, and mirDIP databases.
Preparation of traditional Chinese medicine
RLD was composed of 12 g Chaihu (Bupleuri Radix), 12 g Scutellaria baicalensis, 10 g Corydalis, 10 g turmeric, 30 g hypericum japonicum thunb, 30 g Sedum sarmentosum, 30 g Herba Artemisiae capillaris, 15 g Prunella vulgaris, 30 g raw oyster, 10 g red ginseng, 9 g Pinellia ternata, and 6 g licorice. RLD was added with 400 mL water and decocted twice. The supernatant was combined and concentrated into an extract for use.
The obtained extract was filtered with gauze, moved into a 50 mL centrifuge tube, and centrifuged at 5,000 rpm for 10 min in a high-speed centrifuge. The supernatant was transferred into a new centrifuge tube at −80°C overnight. The next day, the liquid was removed and put into a lyophilizer. The obtained powder was weighed and used for cell experiments.
Clinically, a dose of RLD was decocted in 300 mL water, taken in the morning and evening, respectively, for 1 month. According to the formula of dose conversion between humans and animals, 204 g/60 kg × 60 kg × 0.0026/20 g (human weighing 60 kg and nude mouse weighing 20 g), the clinical equivalent daily gavage dose was 26.52 g/kg, which was set as in the middle dose group of nude mice. At the same time, 0.5 times and 2 times the clinical equivalent dose were set for the low and high dose groups, respectively. With a daily dose of 0.2 mL, RLD extract was prepared in low, medium, and high concentrations of 1.33 g/mL, 2.65 g/mL, and 5.30 g/mL. In order to obtain better experimental results, a high dose of 5.30 g/mL was selected for intragastriction when conducting animal experiments in vivo.
Cell culture
Liver cancer cell lines Hep3B (CM-H169, Shanghai Gaining Biological, Shanghai, China), Huh7 (GDC0134, China Center for Type Culture Collection, Wuhan, China), and normal hepatocytes THLE-2 (CRL-2706, ATCC, USA) were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (11875101, Thermo Fisher Scientific, Rockford, IL, USA) supplemented with 10% fetal bovine serum (FBS; 16140063, Thermo Fisher Scientific), 100 U/mL penicillin, and 100 μg/mL streptomycin, which was placed in an incubator with 5% CO2 at 37°C.
Cell transduction
The core plasmid with (PLKO.1) and auxiliary plasmid (psPAX2, pMD2.G) was inserted into the target gene sequence to construct the lentivirus packaging vector. The lentiviruses were provided by Shanghai Sangon Biotechnology Co. Ltd. (Shanghai, China). And the primer sequence and plasmid construction were also completed by Shanghai Sangon Biotechnology. The lentivirus packaging vector and target vector were transduced into 293T cells (SCSP-502, Chinese Academy of Sciences, Beijing, China) using the Lipofectamine 2000 (11668030, Thermo Fisher Scientific). After incubation for 48 h, the supernatant was collected. The lentivirus in the supernatant after centrifugation was filtered. Cells were transduced with sh-PDK4, miR-9-5p mimic, miR-9-5p inhibitor, and their corresponding negative control (NC) plasmids. The medium was replaced at 8 h after transduction, and the transfection efficiency was observed under a fluorescence microscope at 96 h. Silencing sequences are shown in Table S1.
Logarithmic growth phase Huh7 cells (4 × 10 5 cells/well) were seeded in a 6-well plate. When the cells reached 70–80% confluency, plasmids overexpressing oe-NC or oe-pdk4 (purchased from Gene Pharma, Shanghai, China) were transfected into the cells following the instructions of Lipofectamine 2000 reagent (11668030, Thermo Fisher Scientific, https://www.thermofisher.cn/). The cells were then cultured in a 37°C, 5% CO2 incubator. After 48 hours, the culture medium containing the transfection reagent was discarded. Then, the cells were cultured in a fresh medium for 24–48 hours for subsequent experiments.
RNA extraction and quantification
Total RNA was isolated from Huh7 cells utilizing TRIzol reagent (10296010, Thermo Fisher Scientific). Next, the RNA was quantified by Nanodrop (Nanodrop 3300, Thermo Fisher Scientific). Finally, the miRNA was extracted according to the instructions of the MiPure Cell/Tissue miRNA Kit kit (RC201, Vazyme Nanjing, China). First, the TaqMan reverse transcription reagent (N8080234, Thermo Fisher Scientific) and miRCURY®LNA®RT (Exiqon) were used to reverse mRNA transcription and miRNA. Next, the mRNA and miRNA were quantified using PowerUp SYBR Green premix kit (A25741, Thermo Fisher Scientific) and miRCURY LNA miRNA PCR Starter Kit (339320, Qiagen company, Hilden, Germany). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was an internal reference for mRNA and U6 for miRNA. Primer sequences are shown in Table S2.
Western blot
HCC cells were lysed in cell lysis buffer (C0481, Sigma, Burlington, MA, USA) at 4°C for 30 minutes. Then the cell lysates were collected into a 1.5 mL Eppendorf tube and centrifuged at 12,000×g at 15°C for 4 minutes to collect the supernatant. Protein concentration was determined using the BCA protein concentration determination kit (Beyotime, Shanghai, China). Loading buffer was added to the supernatant and boiled for 5 minutes. Then 20 mg of protein sample was added to a 10% sodium dodecyl sulfate-polyacrylamide gel for electrophoresis, followed by transfer of the gel onto a membrane. The membrane was blocked with 5% skimmed milk powder at room temperature for 1 hour and incubated with primary antibodies against PDK4 (ab134175, 1:10000) and β-actin (ab179467, 1:10000) at 4°C overnight. After washing the membrane three times with TBST, it was incubated with secondary antibodies (goat anti-mouse) (Beijing Chuanggen Biotech Co., Ltd.) at room temperature for 1 hour and then exposed using an enhanced chemiluminescence reagent (Bio-Rad, Shanghai, China). β-actin was used as the internal control, and the gray values of each band were analyzed using Image J software.
Enzyme-linked immunosorbent assay (ELISA)
The ELISA kits were used to detect concentrations of Caspase3 (ab285337, 0.313 ng/mL-20 ng/mL, Abcam, Cambridge, UK), Caspase9 (ab119508, 1.6 ng/mL-100 ng/mL, Abcam), Bcl-2 (ab119506, 0.5 ng/mL-32 ng/mL, Abcam), Bax (ab199080, 12.5 pg/mL-800 pg/mL, Abcam), and PDK4 (EH3544, 1.563 ng/mL-100 ng/mL, Wuhan Feen Biotechnology Co., Ltd., Wuhan, China) after determination of optimal dilution, cells, and tumor tissues were lysed to collect supernatant for ELISA.
Cell counting kit-8 (CCK8) assay
Liver cancer cells were seeded in a 96-well plate (5 × 103 cells/well). After adherence, cells were treated with different concentrations of RLD (40, 20, 10, 5, 2.5, 1, 0.5 mg/mL) for 24 h. Subsequently, cells were incubated with 10 μL CCK8 solution (C0037, Beyotime Biotechnology Co., Shanghai, China) for 24 h. The absorbance value was measured at 450 nm using a microplate reader (5200110, Thermo Fisher Scientific), followed by the calculation of IC50.
Colony formation assay
Cells were seeded in the 6-well plates (400 cells/well). After adherence, cells were treated with different concentrations of RLD (2,4,8 mg/mL) for 14 days. After fixing with 4% paraformaldehyde (P0099, Beyotime), cells were stained with 1% crystal violet (C0121, Beyotime). The stained colonies were photographed under the microscope and the total number of colonies (>50 cells/colonies) was calculated.
Scratch test
Cells were seeded in the 12-well plates. Upon cell, the density reached 80–90%. Next, the scratch test was conducted. Next, a sterile 10 μL pipette tip was used to slowly scratch the plate vertically, at least 3 times per well, followed by rinsing with PBS 1–2 times to remove the floated cells. Next, cells were incubated in the serum-free Minimum Essential Medium (MEM) containing different concentrations of RLD (0.25, 0.5, 1 mg/mL). After 24 h, the cells were observed and photographed, and the proportion of the scratch healing area was calculated to determine the migration ability of the cells.
Transwell assay
Transwell invasion assay was performed using the Transwell chamber (8 μm, 3422, Corning Glass Works, Corning, NY, USA). Matrigel matrix gel (1: 5) diluted with the precooled serum-free medium was added vertically to the upper chamber of the Transwell chamber, which was placed in a box at 37°C for 30 min. The chamber could be used until the matrix gel was solidified. The solidified upper chamber was added with 100 μL cell suspension (1 × 105 cells/chamber), and the lower chamber was added with 600 μL MEM containing 20% FBS. The cells not passing through the membrane in the upper chamber were gently wiped with a cotton swab after 24 h. The migrated cells in the lower chamber were fixed with ethanol for 20 min at room temperature and stained with crystal violet for 20 min. Cells were observed and photographed using a microscope, and five fields were randomly counted to calculate the average number of cells for each section.
Flow cytometry for apoptosis
Huh7 cells in the logarithmic growth phase were seeded in the 6-well plates (3 × 105 cells/well). Upon cell, the density reached 70–80%. Cells were treated with different concentrations of RLD (2,4,8 mg/mL). Cell apoptosis detection was performed according to the instructions of Annexin V- fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (C1062S, Beyotime) utilizing a flow cytometer (BD FACSAris III, NJ, USA). The lower right and upper right quadrants indicate apoptotic cells.
Flow cytometry for cell cycle
Huh7 cells in the logarithmic growth phase were seeded in the 6-well plates (3 × 105 cells/well). Upon cell, the density reached 70–80%. Cells were treated with different concentrations of RLD (2,4,8 mg/mL) for 24 h. First, cells were harvested, centrifuged at 1000 rpm for 3–5 min, resuspended with 1 mL precooled PBS in an ice bath, and transferred to 1.5 mL EP tubes. After further centrifugation, cells were precipitated to extract the supernatant. Next, cells were added with 1 mL precooled 70% ethanol in an ice bath, gently triturated, mixed, and fixed at 4°C for 0.5–2 h. Next, cells were centrifuged at 3000 rpm for 3–5 min, precipitated, stained with the 0.5 mL prepared PI staining solution (C1052, Beyotime), resuspended, and incubated at 37°C, avoiding light exposure for 30 min. The cell cycle was detected by a flow cytometer (BD FACSAris III).
Measurement of reactive oxygen species (ROS)
Cells were seeded in the 6-well plates. Upon cell, the density reached 70–80%. Cells were treated with different concentrations of RLD (2,4,8 mg/mL) for 24 h. After that, cells were incubated with 1 mL DCFH-DA (10 mm/L) (S0033S, Beyotime) at 37°C for 30 min and washed with serum-free medium three times. ROS was detected using flow cytometry at an excitation wavelength of 488 nm and emission wavelength of 525 nm.
Measurement of mitochondrial membrane potential
Upon cell density reaching 70–80%, cells in the 6-well plates were treated with different concentrations of RLD (2,4,8 mg/mL) for 24 h. After that, cells were collected in EP tubes and resuspended with 0.5 mL cell culture medium. The JC-1 staining solution was prepared according to the instructions of the mitochondrial membrane potential detection kit (JC-1) (C2006, Beyotime). First, each tube was added with 0.5 mL JC-1 staining working solution, mixed, and incubated for 20 min. Next, cells were centrifuged at 600 g at 4°C for 3–4 min and precipitated, and the cell supernatant was discarded. Next, the cells were resuspended with 1 mL JC-1 staining buffer, centrifuged at 600 g at 4°C for 3–4 min again, and precipitated. After removing the cell supernatant, cells were resuspended with the appropriate JC-1 staining buffer. Mitochondrial membrane potential was detected using flow cytometry at an excitation wavelength of 488 nm and emission wavelength of 525 nm.
Mitochondrial respiratory function of cells
Cells were seeded in a 24-well plate (Seahorse Bioscience) (5 × 104 cells/well). Cells were carefully washed with PBS, replaced with XF basal medium pH7.4 (Seahorse Bioscience), and incubated for 1 h at 37°C. After baseline measurement, RLD (2,4,8 mg/mL) was successively added to each well for measurement of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) using an Extracellular Flux analyzer (Xfe24, Seahorse Bioscience, Beijing, China).
Dual-luciferase reporter gene assay
The PDK4 3’-untranslated region (3’−UTR) fragment containing the miR-9-5p binding site as a PmirGLO-PDK4-wild type (WT) and PmirGLO-PDK4-mutant (MUT) was inserted into the PmirGLO vector. Then, the miR-9-5p mimic and the NC plasmids were co-transduced into 293T cells (SCSP-502, CAS), respectively. Cells were lysed after 24 h and centrifuged at 12,000 rpm for 1 min. Subsequently, luciferase activity was measured using a luciferase system (Dual-Luciferase® Reporter Assay System, E1910, Promega, Madison, WI, USA). The ratio of firefly luciferase activity to renilla luciferase activity was considered the luciferase activity, with renilla luciferase activity as the internal reference.
In vivo animal experiments
Thirty-two male BALB/c nude mice (aged 4–5 weeks, Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) were housed in a specific pathogen-free (SPF) animal laboratory individually at 22–25°C with humidity of 60–65% with a 12-h light/dark cycle. The mice were given ad libitum access to food and water. The experiment was conducted after acclimatization for one week.
The Huh7 cells were transduced with sh-NC + DMSO, sh-NC + RLD (5.30 g/mL), sh-PDK4 + DMSO, or sh-PDK4 + RLD (5.30 g/mL).
Subcutaneous transplanted tumor model in nude mice: Huh7 cell suspension (1 × 107 cells/mL) and 50 μL Matrigel solution were injected into the left upper back of nude mice (n = 8). After inoculation, tumor growth was observed and recorded, tumor size was measured with a vernier caliper once every 5 days, and tumor volume was recorded and calculated with tumor volume = (a × b2/2 (a = length, b = width). In the RLD efficacy experiment, when the tumor volume reached 50 mm3, nude mice were subjected to a gavage of 0.2 mL RLD at 5.3 g/mL/day. After administration for 25 days, nude mice were euthanized. Then, the tumors were obtained and weighed.
Lung metastasis model in nude mice: The lung metastasis model of liver cancer was constructed by injecting cells into nude mice via the tail vein. Nude mice were subjected to gavage of 0.2 mL RLD at 5.3 g/mL/day for 60 days. Next, nude mice were euthanized, followed by the detection of lung metastasis using hematoxylin & eosin (H&E) staining.
H&E staining
Tumor tissues were dehydrated, fixed with xylene, embedded with paraffin, and sliced into sections. The sections were washed in xylene I, xylene II, absolute ethanol I, absolute ethanol II, and alcohol of different concentrations. First, the sections were stained with hematoxylin solution for 5 min and washed with tap water for back to blue. After adding 1% hydrochloride alcohol to differentiate for 2–5 s, the sections were washed with running water, stained with eosin solution for 1 min, and washed with running water again. Next, the sections were placed in absolute ethanol and xylene for 5 min, dried in the air, and photographed under a microscope.
Immunohistochemical staining
The paraffin-embedded sections of tumor tissues from nude mice were dewaxed to water and dehydrated, followed by antigen retrieval at 121°C for 5 min. After incubation with 3% H2O2 to inactivate the endogenous peroxidase, the sections were blocked with 10% normal goat serum and probed with PDK4 (rabbit polyclonal antibody, A13337, 1: 50, Abclonal, Wuhan, China) and Ki67 (rabbit monoclonal antibody, A2094, 1: 50, Abclonal) at 4°C overnight. After washing with PBS three times, the sections were re-probed with secondary antibodies goat anti-rabbit IgG (A0208, Beyotime) for 1 h. Finally, the sections were developed by DAB for 5–10 min and counterstained with hematoxylin for 2 min, followed by sealing and observation under the microscope.
Statistical analysis
Data were analyzed using the SPSS 21.0 software (IBM, Armonk, NY, USA). All quantitative data are presented as mean ± standard deviation. The data between the two groups were analyzed by unpaired t-test, and the comparison of data among multiple groups was tested by one-way analysis of variance (ANOVA). In addition, two-factor ANOVA was used to compare data at different time points, and repeated measures ANOVA was used to compare tumor data at different time points. p < .05 was considered statistically significant.
Results
RLD inhibits liver cancer cell proliferation, invasion, and migration and promotes apoptosis
At first, the effects of RLD on the biological characteristics of liver cancer cells were explored. Human liver cancer cells Huh7 and Hep3B, as well as human non-small cell lung cancer cells A549 and human prostate cancer cells DU-145, were treated with different concentrations of RLD. CCK8, colony formation assay, scratch test, and Transwell assay exhibited that RLD treatment could inhibit cancer cell proliferation, colony formation, migration, and invasion in a concentration-dependent manner (Figure 1a-d, Fig. S1A-D, Fig. S2A).
Figure 1.

Effects of different concentrations of RLD on mitochondrial membrane potential, proliferation, migration, invasion, and apoptosis of Huh7 cells. Huh7 cells were treated with RLD of different concentrations. a, Huh7 cell proliferation detected by CCK8 assay. b, Huh7 cell colony formation detected by colony formation assay. c, Huh7 cell migration detected by scratch test. d, Huh7 cell invasion detected by Transwell assay. e, Huh7 cell apoptosis detected by flow cytometry. f, Huh7 cell cycle detected by flow cytometry. g, levels of apoptosis-related factors in Huh7 cells measured by ELISA. h, OCR level in Huh7 cells measured by Seahorse analyzer. i, ECAR level in Huh7 cells measured by Seahorse analyzer. j, ROS level in Huh7 cells measured by flow cytometry. k, mitochondrial membrane potential in Huh7 cells detected by flow cytometry. *p < .05, **p < .001. All cell experiments were performed in triplicate.
Moreover, flow cytometry exhibited that RLD treatment promoted apoptosis of Huh7 and Hep3B cells, increased the proportion of cells at the G0/G1 phase, and reduced the proportion of cells at the S phase; moreover, apoptosis was enhanced, and the proportion of cells at G0/G1 phase was elevated gradually with the increase of RLD concentration (Figure 1e–f, Fig. S1E-F, Fig. S2A). ELISA showed that RLD treatment decreased Bcl-2 levels and increased Caspase 3, Caspase 9, and Bax levels in Huh7 and Hep3B cells (Figure 1g, Fig. S1G).
In addition, it was also found that OCR level was elevated, ROS and ECAR levels and mitochondrial membrane potential were decreased in Huh7 and Hep3B cells treated with RLD, and with the increase of RLD concentration, the changes mentioned above were more obvious (Figure 1h–k, Fig. S1H-K, Fig. S3A).
These findings indicated that RLD treatment could reduce the ROS level and decrease the mitochondrial membrane potential, thus inhibiting the malignant features of liver cancer cells. Furthermore, as the concentration of RLD treatment increased, the effect was better, so the concentration of 8 mg/mL was selected in the subsequent experiments.
RLD upregulates PDK4 expression to suppress liver cancer cell malignant behaviors
Through differential analysis of liver cancer-related microarray dataset GSE74656, 1080 differentially expressed genes (DEGs) (651 upregulated DEGs and 429 downregulated DEGs), and the top 50 DEGs were selected to draw heatmap (Fig. S4A-B). Then, 404 and 5284 liver cancer targets were predicted by CTD and GeneCards databases, respectively, and the obtained targets and the DEGs in GSE74656 were intersected to obtain 88 differential genes (Fig. S4C). Finally, STRING was utilized to construct a PPI network of intersected genes, which displayed that PDK4 was at the center and interacted with IRS1, ACADL, EHHADH, and ACADM (Figure 2a).
Figure 2.

Effect of RLD-mediating PDK4 expression on the biological properties of liver cancer cells. a, PPI network of intersected genes constructed by STRING. b, KEGG pathway enrichment analysis of differential genes. c, box plot of PDK4 expression in liver cancer and normal tissues in microarray data GSE74656 (the left box represents the expression in normal tissues, and the right box represents the expression in liver cancer tissues). d, Venn diagram of intersection between active ingredients and disease-related genes and Upset diagram of active ingredients of RLD. Huh7 cells were treated with RLD (8 mg/mL) and transduced with sh-PDK4. e, Huh7 cell proliferation detected by CCK8 assay. f, Huh7 cell colony formation detected by colony formation assay. g, Huh7 cell migration detected by scratch test. h, Huh7 cell invasion detected by Transwell assay. i, Huh7 cell apoptosis detected by flow cytometry (the lower right and upper right quadrants represent apoptotic cells). j, Huh7 cell cycle detected by flow cytometry. k, levels of apoptosis-related factors in Huh7 cells measured by ELISA. l, OCR level in Huh7 cells measured by Seahorse analyzer. M, ECAR level in Huh7 cells measured by Seahorse analyzer. n, ROS level in Huh7 cells measured by flow cytometry. o, mitochondrial membrane potential in Huh7 cells detected by flow cytometry. *p < .05 indicates comparison with Huh7 cells treated with sh-NC + DMSO; #p < .05 vs. Huh7 cells treated with sh-NC + RLD. All cell experiments were performed in triplicate.
Figure 2.

(Continued).
To further explore its molecular function, KEGG enrichment analysis of 88 differential genes was performed, which presented that the candidate genes were mainly enriched in entries of the Cell Cycle, p53 signaling pathway, AMPK signaling pathway, Cellular senescence, Transcriptional misregulation in cancer, and Apoptosis (Figure 2b). Moreover, through differential analysis of GSE74656, poorly expressed PDK4 was found (Figure 2c). Additionally, the BATMAN-TCM database was used to screen 12 active ingredient targets of TCM in RLD. 1 target PDK4 was obtained after intersecting with 88 differential genes (Figure 2d).
Next, RT-qPCR confirmed the reduced PDK4 expression in Huh7 and Hep3B cells, and it was significantly reduced in Huh7 cells (Fig. S4D). Thus, Huh7 cells were selected for the subsequent experiments. RT-qPCR also displayed that PDK4 expression was elevated in Huh7 cells treated with RLD (Fig. S4E). In addition, Western blot results also showed that RLD treatment significantly increased the protein expression of PDK4 compared to the DMSO group.
Next, we further investigated whether RLD regulates the biological functions of liver cancer by regulating PDK4 expression. We transfected sh-PDK4 and oe-PDK4 into Huh7 cells and treated them with RLD (8 mg/mL). RT-qPCR and Western blot results showed that PDK4 expression decreased after sh-PDK4 transfection and increased after oe-PDK4 transfection, indicating successful transfection (Fig. S4F). CCK8 assay results showed that compared with the sh-NC+DMSO group, the sh-NC+RLD group inhibited Huh7 cell proliferation, while the sh-PDK4+DMSO group promoted Huh7 cell proliferation. Compared with the sh-NC+RLD group, the sh-PDK4+RLD group significantly promoted Huh7 cell proliferation. Compared with the OE-NC+DMSO group, the oe-PDK4+DMSO group and OE-NC+RDL group inhibited Huh7 cell proliferation, while the oe-PDK4+RDL group further significantly inhibited Huh7 cell proliferation. Colony formation assay results showed that compared with the sh-NC+DMSO group, the sh-NC+RLD group inhibited Huh7 colony formation, while the sh-PDK4+DMSO group promoted Huh7 colony formation. Compared with the sh-NC+RLD group, the sh-PDK4+RLD group significantly promoted Huh7 colony formation (Figure 2e–f, Fig. S2B(a)). Cell scratch and Transwell assay results showed that compared with the sh-NC+DMSO group, the sh-NC+RLD group inhibited Huh7 cell migration and invasion, while the sh-PDK4+DMSO group promoted Huh7 cell migration and invasion. Compared with the sh-NC+RLD group, the sh-PDK4+RLD group promoted Huh7 cell migration and invasion (Figure 2g–h, Fig. S2B(b-c)).
Flow cytometry presented that RLD treatment promoted apoptosis of Huh7 cells, increased the proportion of cells at the G0/G1 phase, and reduced the proportion of cells at the S phase, while downregulation of PDK4 exerted the opposite effects, and these effects were further reinforced by RLD treatment and PDK4 knockdown (Figure 2i–j, Fig. S2B). In addition, ELISA showed that RLD treatment decreased Bcl-2 levels and increased levels of Caspase 3, Caspase 9, and Bax in Huh7 cells, while depleted PDK4 elevated Bcl-2 levels and reduced levels of Caspase 3, Caspase 9, and Bax; relative to sh-NC + RLD treatment, cells treated with both RLD and PDK4 knockdown showed elevated Bcl-2 level and reduced levels of Caspase 3, Caspase 9, and Bax (Figure 2k).
It was also found that OCR level was elevated, ROS and ECAR levels and mitochondrial membrane potential were decreased in Huh7 cells treated with RLD, while these trends were reversed by PDK4 knockdown; relative to sh-NC + RLD treatment, sh-PDK4 + RLD treatment led to reduced OCR level, and increased ROS and ECAR levels and mitochondrial membrane potential (Figure 2l–o, Fig. S3B).
The obtained data suggested that silencing of PDK4 in liver cancer cells could reverse the inhibitory effects of RLD treatment on cell proliferation, migration, and invasion.
miR-9-5p promotes liver cancer cell proliferation, invasion, and migration and inhibits apoptosis by targeting PDK4
The upstream regulatory miRNAs of PDK4 were retrieved from the TargetScanHuman 7.1, DIANA TOOLS, and mirDIP databases, and 32, 17, and 18 miRNAs were obtained, respectively. After the intersection of the miRNAs from three databases using the jvenn website, miR-9-5p was obtained (Figure 3a). The binding sites between miR-9-5p and PDK4 were identified and confirmed by TargetScanHuman 7.1 (Figure 3b).
Figure 3.
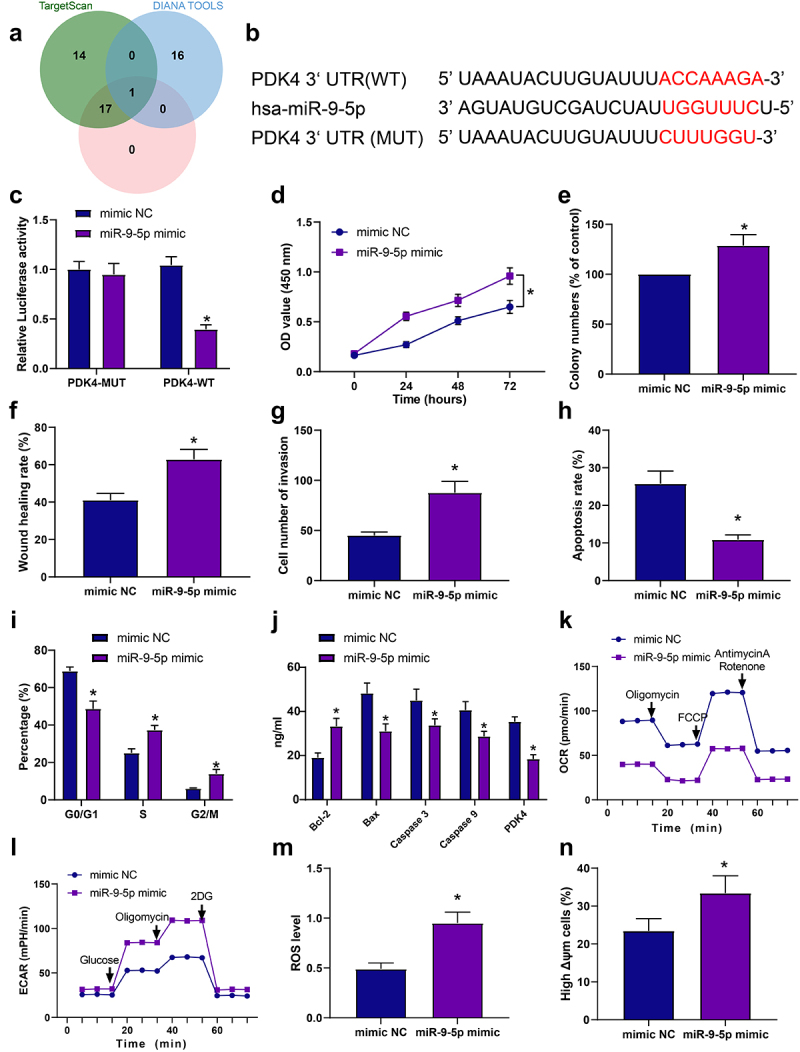
miR-9-5p promotes malignant features of liver cancer cells by targeting PDK4. a, Venn diagram of the upstream miRnas of PDK4 predicted in the three databases. b, the targeting relationship between miR-9-5p and PDK4 verified by TargetScanHuman 7.1. c, the effect of miR-9-5p on luciferase activity of PDK4 promoter detected by dual-luciferase reporter gene assay. Huh7 cells were transduced with miR-9-5p mimic. d, Huh7 cell proliferation detected by CCK8 assay. e, Huh7 cell colony formation detected by colony formation assay. f, Huh7 cell migration detected by scratch test. g, Huh7 cell invasion detected by Transwell assay. H, Huh7 cell apoptosis detected by flow cytometry (the lower right and upper right quadrants represent apoptotic cells). i, Huh7 cell cycle detected by flow cytometry. j, levels of apoptosis-related factors in Huh7 cells measured by ELISA. k, OCR level in Huh7 cells measured by Seahorse analyzer. l, ECAR level in Huh7 cells measured by Seahorse analyzer. m, ROS level in Huh7 cells measured by flow cytometry. n, mitochondrial membrane potential in Huh7 cells detected by flow cytometry. *p < .05 vs. Huh7 cells transduced with mimic NC. All cell experiments were performed in triplicate.
Dual-luciferase reporter gene assay was employed to verify further the targeting relationship between miR-9-5p and PDK4, which showed that luciferase activity of PDK4-WT was inhibited by miR-9-5p mimic. However, no change was found in the luciferase activity of PDK4-MUT (Figure 3c). Next, Huh7 cells were transduced with miR-9-5p mimic or miR-9-5p inhibitor, and RT-qPCR exhibited that miR-9-5p expression was elevated in Huh7 cells transduced with miR-9-5p mimic. At the same time, it was decreased in Huh7 cells transduced with miR-9-5p inhibitor, indicating successful cell transduction (Fig. S5A-B).
To further explore the molecular mechanism of miR-9-5p and PDK4 in the development of liver cancer, RT-qPCR was adopted to assess miR-9-5p expression in human normal liver cell lines THLE-2 and liver cancer cell lines (Huh7, Hep3B), which presented that miR-9-5p expression was elevated in liver cancer cell lines. It was higher in Huh7 cells (Fig. S5C). After treatment with RLD, miR-9-5p expression was reduced in Huh7 cells (Fig. S5D).
Moreover, upregulation of miR-9-5p induced Huh7 cell proliferation, colony formation, migration, and invasion (Figure 3d–g, Fig. S2C). Flow cytometry and ELISA revealed that restored miR-9-5p inhibited apoptosis, reduced the proportion of cells at G0/G1 phase and levels of Caspase 3, Caspase 9, and Bax, and increased the proportion of cells at S phase and Bcl-2 level (Figure 3h–j, Fig. S2C). Furthermore, overexpression of miR-9-5p elevated ROS and ECAR levels and mitochondrial membrane potential but diminished the level of OCR in Huh7 cells (Figure 3k–n, Fig. S3C).
These findings elucidated that miR-9-5p suppressed PDK4 expression to induce liver cancer cell proliferation, invasion, and migration and repress apoptosis of Huh7 cells.
RLD inhibits miR-9-5p and promotes PDK4 to suppress liver cancer cell proliferation, migration, and invasion and induce apoptosis
To further investigate the effect of RLD-regulating miR-9-5p/PDK4 axis on the biological functions of liver cancer cells, Huh7 cells were treated with RLD and transduced with miR-9-5p mimic. RT-qPCR exhibited decreased miR-9-5p expression and elevated PDK4 expression in Huh7 cells after RLD treatment, while these trends were reversed by miR-9-5p overexpression alone or combined with RLD treatment (Figure 4a). Functional assays displayed that RLD treatment could inhibit Huh7 cell proliferation, colony formation, migration, and invasion, while upregulation of miR-9-5p alone or combined with RLD treatment led to opposite findings (Figure 4b–e).
Figure 4.

RLD suppresses liver cancer cells’ malignant features by regulating miR-9-5p-mediated PDK4. Huh7 cells were treated with RLD and transduced with miR-9-5p mimic. a, expression of miR-9-5p and PDK4 in Huh7 cells determined by RT-qPCR. b, Huh7 cell proliferation detected by CCK8 assay. C, Huh7 cell colony formation detected by colony formation assay. d, Huh7 cell migration detected by scratch test. e, Huh7 cell invasion detected by Transwell assay. f, Huh7 cell apoptosis detected by flow cytometry (the lower right and upper right quadrants represent apoptotic cells). g, Huh7 cell cycle detected by flow cytometry. h, levels of apoptosis-related factors in Huh7 cells measured by ELISA. I, OCR level in Huh7 cells measured by Seahorse analyzer. j, ECAR level in Huh7 cells measured by Seahorse analyzer. k, ROS level in Huh7 cells measured by flow cytometry. l, mitochondrial membrane potential in Huh7 cells detected by flow cytometry. *p < .05 vs. Huh7 cells treated with mimic NC + DMSO. #p < .05 vs. Huh7 cells treated with mimic NC + RLD. All cell experiments were performed in triplicate.
Flow cytometry presented that RLD treatment promoted apoptosis of Huh7 cells, increased the proportion of cells at the G0/G1 phase, and reduced the proportion of cells at the S phase, while these effects were counteracted by restored miR-9-5p alone or combined with RLD treatment (Figure 4f–g, Fig. S2D). In addition, ELISA showed that RLD treatment decreased Bcl-2 level and increased Caspase 3, Caspase 9, Bax, and PDK4 in Huh7 cells, while opposing trends were witnessed upon overexpression of miR-9-5p alone or combined with RLD treatment (Figure 4h).
It was also found that elevated OCR levels and reduced ROS and ECAR levels and mitochondrial membrane potential in Huh7 cells treated with RLD, while these effects were abolished by upregulated miR-9-5p alone or combined with RLD treatment (Figure 4i–l, Fig. S3D).
Thus, we suggested that RLD suppressed liver cancer cell proliferation, migration, and invasion and induced apoptosis by downregulating miR-9-5p and upregulating PDK4.
RLD represses liver cancer growth and metastasis by upregulating miR-9-5p-dependent PDK4
To further verify the effects of RLD on liver cancer in nude mice, we constructed a subcutaneous tumor model. The results found that RLD treatment reduced tumor volume and weight, while silencing of PDK4 increased tumor volume and weight, and both RLD treatment and PDK4 knockdown strengthened these trends (Figure 5a–b). Furthermore, RT-qPCR exhibited that RLD treatment decreased miR-9-5p expression and elevated PDK4 expression in tumor tissues, while PDK4 expression was reduced and miR-9-5p expression showed no difference in tumor tissues of nude mice injected with sh-PDK4 + DMSO or sh-PDK4 + RLD (Figure 5c). ELISA and immunohistochemical staining unfolded that RLD treatment decreased levels Bcl-2 and Ki67 and increased levels of Caspase 3, Caspase 9, Bax, and PDK4 in tumor tissues of nude mice while silencing of PDK4 alone or combined with RLD treatment resulted in contrary findings (Figure 5d–f). Furthermore, H&E staining demonstrated that relative to sh-NC + DMSO treatment, sh-NC + RLD treatment reduced lung tumor metastasis, while sh-PDK4 + DMSO treatment increased lung tumor metastasis; in comparison to sh-NC + RLD treatment, sh-PDK4 + RLD treatment induced increased lung tumor metastasis in nude mice (Figure 5g).
Figure 5.

RLD blocks liver cancer growth and metastasis by regulating miR-9-5p-mediated PDK4. Nude mice were injected with Huh7 cells transduced with sh-NC + DMSO, sh-NC + RLD, sh-PDK4 + DMSO, or sh-PDK4 + RLD . a, tumor volume of nude mice. b, tumor weight of nude mice. c, expression of miR-9-5p and PDK4 in tumor tissues of nude mice determined by RT-qPCR. d, levels of apoptosis-related factors in tumor tissues of nude mice measured by ELISA. e-f, expression of Ki67 and PDK4 in tumor tissues of nude mice determined by immunohistochemical staining (scale bar: 50 μm). g, lung tumor metastasis in nude mice detected by H&E staining (scale bar: 50 μm). *p < .05 vs. nude mice injected with Huh7 cells transduced with sh-NC + DMSO. #p < .05 vs. nude mice injected with Huh7 cells transduced with sh-NC + RLD. n = 8.
It can be concluded that RLD inhibited liver cancer growth and metastasis via the miR-9-5p/PDK4 axis.
Discussion
Metastasis is the main cause of cancer-related death, and metastatic liver disease accounts for 95% of all hepatic cancers, showing the features of difficulty in treatment.22 TCM has been reported to be used for the clinical treatment of liver cancer.3 The present study focused on one TCM, RLD, in liver cancer. Based on observations and evaluations made during the study, it was suggested that RLD inhibited malignant features in vitro and growth and metastasis of liver cancer in vivo by downregulating miR-9-5p and upregulating PDK4.
The current study initially proved that RLD could suppress malignant phenotypes of liver cancer cells by elevating Caspase 3, Caspase 9, and Bax levels and reducing the Bcl-2 level. It is known that Bax, Caspase 3, and Caspase 9 are pro-apoptotic factors, and Bcl-2 acts as an anti-apoptotic factor in liver cancer cells.23,24 Many studies have suggested that TCM acts as a popular anti-tumor therapy for liver cancer by inducing tumor cell apoptosis.8,25 A variety of existing studies have confirmed that components of RLD play vital roles in cellular biological processes in liver cancer. For instance, Prunella vulgaris and Red Ginseng suppress cell invasion and migration in liver cancer cells.26,27 In addition, Scutellaria baicalensis Georgi and Licorice have been verified to repress the progression of liver cancer by enhancing apoptosis and cell cycle arrest.28,29 However, the effects of RLD on the cell functions of liver cancer remain to be elucidated.
Moreover, the obtained data in this study confirmed that RLD could elevate PDK4 expression to restrain the progression of liver cancer, as manifested by increased OCR and decreased ECAR ROS levels and mitochondrial membrane potential in liver cancer cells. Cancer cell metabolism is a biology field that provides a novel option for cancer treatments. ECAR and OCR are the biomarkers to assess the glycolysis level in human cancer.30 Mitochondria are the major contributors to the secretion of ROS, which is implicated in human cancers.31 The prohibitory roles of PDK4 in glucose oxidation associated with mitochondrial dysfunction have been proved.32 A prior study has proved that PDK4 within the mitochondria is related to tumor proliferation due to its effects on glucose metabolic pathways.11 Numerous studies have also indicated that PDK4 is poorly expressed in HCC cells, and upregulated PDK4 is capable of suppressing the progression of HCC,12,13 which is consistent with our findings.
Furthermore, a recent study has suggested that miR-9-5p directly targets PDK4 and suppresses its expression, which induces malignant phenotype, mitochondrial activity, and energy metabolism. In addition, it represses apoptosis in HCC cells, thereby promoting the development of HCC,14 which aligns with our results. Accumulating evidence has revealed that miRNAs are implicated in the biological processes of liver cancer cells by targeting gene expression.16,33 Multiple studies have elucidated that miR-9-5p is highly expressed in HCC cells, which stimulates malignant phenotypes and curbs apoptosis of HCC cells,17,34,35 suggesting that downregulation of miR-9-5p could inhibit the progression of liver cancer. Both in vitro and in vivo experiments in the present study have validated that RLD inhibited miR-9-5p and promoted PDK4 to suppress malignant features and induce apoptosis, leading to the inhibition of liver cancer growth and metastasis.
Conclusion
In conclusion, the findings in this study have demonstrated that RLD curbs the malignant features of liver cancer cells via upregulation of miR-9-5p-dependent PDK4 (Figure 6). It is hoped that studying the RLD-mediating miR-9-5p/PDK4 axis mechanism will stimulate interest in prospective studies. These will lead to the discovery novel and effective therapeutic targets for liver cancer treatment. The present study has contributed to this process by uncovering the role of RLD as a potential therapeutic target.
Figure 6.

Molecular mechanism of RLD regulating miR-9-5p/PDK4 axis to inhibit the growth and metastasis of liver cancer.
Supplementary Material
Biographies
Tao Si, Doctor, Chief Physician, Research Direction: Integrated Traditional Chinese and Western Medicine Treatment for Malignant Tumors.
Liyin Huang, Master student, Resident Physician, Research on the Prevention and Treatment of Tumor Diseases.
Ting Liang, Master, Resident Physician, Research Direction: Integrated Traditional Chinese and Western Medicine Treatment for Malignant Tumors.
Ping Huang, Doctoral student, Attending Physician, Research Direction: Radiotherapy for Malignant Tumors.
Hongyu Zhang, Master of Medicine, Associate Chief Technician, Research Direction: Clinical Laboratory Technology and Diagnosis. He has published 3 SCI papers and participated in 2 National Natural Science Foundation of China.
Mingmin Zhang, Master, Resident Physician, Research Direction: Integrated Traditional Chinese and Western Medicine Treatment for Malignant Tumors.
Xiaoling Zhou, Doctor, Chief Physician, Research Direction: Integrated Traditional Chinese and Western Medicine Treatment for Digestive System Diseases.
Funding Statement
This study was supported by National Natural Science Foundation of China (No. 82060856) and Natural Science Foundation of Guangxi Zhuang Autonomous Region (No. 2020JJA140194).
Abbreviations
- CCK8
Cell counting kit-8
- ELISA
Enzyme-linked immunosorbent assay
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HCC
hepatocellular carcinoma cells
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MEM
Minimum Essential Medium
- NC
negative control
- PDH
pyruvate dehydrogenase
- PDK4
pyruvate dehydrogenase kinase 4
- PI
propidium iodide
- RLD
Ruangan Lidan decoction
- TCM
Traditional Chinese medicine
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting this study’s findings are available on request from the corresponding author.
Credit author statement
Tao Si, Liyin Huang, and Ting Liang wrote the paper and conceived and designed the experiments; Ping Huang and Hongyu Zhang analyzed the data; Mingmin Zhang and Xiaoling Zhou collected and provided the sample for this study. All authors have read and approved the final submitted manuscript.
Consent for publication
Consent for publication was obtained from the participants.
Ethics statement
The animal experiment was also approved by the Ethics Committee of Liuzhou Traditional Chinese Medical Hospital. Great efforts were made to minimize the number of animals used in the experiments and their discomfort.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384047.2023.2246198
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–14. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188314. doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao X, Hao J, Chen S. Network pharmacology-based strategy for predicting therapy targets of Traditional Chinese medicine Xihuang Pill on liver cancer. Evid Based Complement Alternat Med. 2020;2020:6076572. doi: 10.1155/2020/6076572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q, Chen Z, Cao S, Guo B, Chen Y, Feng Z, Wang J, Guo G, Chen X, Huang X. Role of CircRNAs_100395 in proliferation and metastases of liver cancer. Med Sci Monit. 2019;25:6181–6192. doi: 10.12659/MSM.915963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang XY, Huang ZL, Huang J, Xu B, Huang XY, Xu YH, Zhou J, Tang ZY. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39(1):20. doi: 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao X, Wang Y, Li Y, Wang Y, Yan M, Sun H, Chen S, Pan X. Huganpian, a traditional Chinese medicine, inhibits liver cancer growth in vitro and in vivo by inducing autophagy and cell cycle arrest. Biomed Pharmacother. 2019;120:109469. doi: 10.1016/j.biopha.2019.109469. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Xie M, Gan Y. Effect of Xiaochaihu decoction and different herbal formulation of component on inhibiting H22 liver cancer in mice and enhancing immune function. Zhongguo Zhong Yao Za Zhi. 2008;33(9):1039–1044. [PubMed] [Google Scholar]
- 8.Dai M, Yang YW, Guo WH, Wang FL, Xiao GM, Li YM, Yang HZ. Addition and subtraction theory of TCM using Xiao-Chaihu-decoction and naturopathy in predicting survival outcomes of primary liver cancer patients: A Prospective cohort study. Evid Based Complement Alternat Med. 2016;2016:4723530. doi: 10.1155/2016/4723530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei T, Yan M, Huang Y, Wei Y, Martin C, Zhao Q. Specific flavonoids and their biosynthetic pathway in Scutellaria baicalensis. Front Plant Sci. 2022;13:866282. doi: 10.3389/fpls.2022.866282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Bi C, Chen Q, Xu H, Shi H, Li X. Structure elucidation of arabinogalactoglucan isolated from Sedum sarmentosum Bunge and its inhibition on hepatocellular carcinoma cells in vitro. Int J Biol Macromol. 2021;180:152–160. doi: 10.1016/j.ijbiomac.2021.03.051. [DOI] [PubMed] [Google Scholar]
- 11.Si TNX, Yang J, Feng XB. Effect of Ruangan Lidan decoction on disease-free survival of postoperative patients with hepatocellular carcinoma. J Changchun Univ Tradit Chinese Med. 2015;31(1):145–148. [Google Scholar]
- 12.Ning X, Si T, Feng X. Effect of Ruangan Lidan decoction on the quality of life in patients with hepatocellular carcinoma received three-dimensional conformal radiotherapy in joint. Hebei J Tradit Chin Med. 2014;36(7):976–978. [Google Scholar]
- 13.Zhang X, Wu J, Choiniere J, Yang Z, Huang Y, Bennett J, Wang L. Arsenic silences hepatic PDK4 expression through activation of histone H3K9 methylatransferase G9a. Toxicol Appl Pharmacol. 2016;304:42–47. doi: 10.1016/j.taap.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Tran M, Wang L, Shin DJ, Wu J. PDK4-deficiency reprograms intrahepatic glucose and lipid metabolism to facilitate liver regeneration in mice. Hepatol Commun. 2020;4(4):504–517. doi: 10.1002/hep4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choiniere J, Wu J, Wang L. Pyruvate dehydrogenase kinase 4 deficiency results in expedited cellular proliferation through E2F1-mediated increase of cyclins. Mol Pharmacol. 2017;91(3):189–196. doi: 10.1124/mol.116.106757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Si T, Ning X, Zhao H, Zhang M, Huang P, Hu Z, Yang L, Lin L. microRNA-9-5p regulates the mitochondrial function of hepatocellular carcinoma cells through suppressing PDK4. Cancer Gene Ther. 2021;28(6):706–718. doi: 10.1038/s41417-020-00253-w. [DOI] [PubMed] [Google Scholar]
- 17.Linck-Paulus L, Hellerbrand C, Bosserhoff AK, Dietrich P. Dissimilar appearances are deceptive–common microRNAs and therapeutic strategies in liver cancer and melanoma. Cells. 2020;9(1):114. doi: 10.3390/cells9010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Wang S, Ruan H, Li B, Cheng Z, He J, Zuo Q, Yu C, Wang H, Lv Y, et al. Downregulation of PDK4 increases lipogenesis and associates with poor prognosis in hepatocellular carcinoma. J Cancer. 2019;10(4):918–926. doi: 10.7150/jca.27226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strowitzki MJ, Radhakrishnan P, Pavicevic S, Scheer J, Kimmer G, Ritter AS, Tuffs C, Volz C, Vondran F, Harnoss JM, et al. High hepatic expression of PDK4 improves survival upon multimodal treatment of colorectal liver metastases. Br J Cancer. 2019;120:675–688. doi: 10.1038/s41416-019-0406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Chen JY, Zhong Y, Xie L, Li JS. lncRNA MEG3 inhibits the growth of hepatocellular carcinoma cells by sponging miR-9-5p to upregulate SOX11. Braz J Med Biol Res. 2019;52(10):e8631. doi: 10.1590/1414-431X20198631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong X, Wang F, Xue Y, Lin Z, Song W, Yang N, Li Q. MicroRNA‑9‑5p downregulates Klf4 and influences the progression of hepatocellular carcinoma via the AKT signaling pathway. Int J Mol Med. 2019;43(3):1417–1429. doi: 10.3892/ijmm.2019.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma C, Zhang Q, Greten TF. Mdscs in liver cancer: A critical tumor-promoting player and a potential therapeutic target. Cell Immunol. 2021;361:104295. doi: 10.1016/j.cellimm.2021.104295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Liu Z, Chen S, Li H, Dong L, Fu X. A new discovery: Total Bupleurum saponin extracts can inhibit the proliferation and induce apoptosis of colon cancer cells by regulating the PI3K/Akt/mTOR pathway. J Ethnopharmacol. 2022;283:114742. doi: 10.1016/j.jep.2021.114742. [DOI] [PubMed] [Google Scholar]
- 24.Mitupatum T, Aree K, Kittisenachai S, Roytrakul S, Puthong S, Kangsadalampai S, Rojpibulstit P. mRNA expression of Bax, Bcl-2, p53, Cathepsin B, Caspase-3 and Caspase-9 in the HepG2 cell line Following Induction by a novel monoclonal Ab Hep88 mAb: Cross-talk for paraptosis and apoptosis. Asian Pac J Cancer Prev. 2016;17(2):703–712. doi: 10.7314/apjcp.2016.17.2.703. [DOI] [PubMed] [Google Scholar]
- 25.Li T, Zhang X, Xu S, Cheng H, Zhang H. Effectiveness and safety of adjunctive traditional Chinese medicine therapy for primary liver cancer patients: A protocol for systematic review and meta analysis. Med. 2020;99(31):e21281. doi: 10.1097/MD.0000000000021281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Huang CY, Tsai CY, Lu SY, Chiu CC, Fang K. The aqueous extract of Prunella vulgaris suppresses cell invasion and migration in human liver cancer cells by attenuating matrix metalloproteinases. Am J Chin Med. 2012;40(3):643–656. doi: 10.1142/S0192415X12500486. [DOI] [PubMed] [Google Scholar]
- 27.Kwon HJ, Lee S, Lee HH, Cho H, Jung J. Korean red ginseng enhances Immunotherapeutic effects of NK cells via eosinophils in metastatic liver cancer model. Nutrients. 2021;14(1). doi: 10.3390/nu14010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Q, Zhang W, Wu Z, Tian X, Xiang J, Li L, Li Z, Peng X, Wei S, Ma X, et al. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol Res. 2021;165:105444. doi: 10.1016/j.phrs.2021.105444. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Wahab AA, Effat H, Mahrous EA, Ali MA, Al-Shafie TA. A licorice roots extract Induces apoptosis and cell cycle arrest and Improves metabolism via regulating MiRNAs in liver cancer cells. Nutr Cancer. 2021;73(6):1047–1058. doi: 10.1080/01635581.2020.1783329. [DOI] [PubMed] [Google Scholar]
- 30.Sun Z, Tan Z, Peng C, Yi W. HK2 is associated with the Warburg effect and proliferation in liver cancer: Targets for effective therapy with glycyrrhizin. Mol Med Rep. 2021;23(5). doi: 10.3892/mmr.2021.11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang CW, Lo JF, Wang XW. Roles of mitochondria in liver cancer stem cells. Differentiation. 2019;107:35–41. doi: 10.1016/j.diff.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SJ, Jeong JY, Oh CJ, Park S, Kim JY, Kim HJ, Doo Kim N, Choi YK, Do JY, Go Y, et al. Pyruvate dehydrogenase kinase 4 promotes vascular calcification via SMAD1/5/8 Phosphorylation. Sci Rep. 2015;5:16577. doi: 10.1038/srep16577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghafouri-Fard S, Honarmand Tamizkar K, Hussen BM, Taheri M. MicroRNA signature in liver cancer. Pathol Res Pract. 2021;219:153369. doi: 10.1016/j.prp.2021.153369. [DOI] [PubMed] [Google Scholar]
- 34.Shu Z, Gao F, Xia Q, Zhang M. MiR-9-5p promotes cell proliferation and migration of hepatocellular carcinoma by targeting CPEB3. Biomark Med. 2021;15(2):97–108. doi: 10.2217/bmm-2020-0322. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Cui M, Cheng D, Qu F, Yu J, Wei Y, Cheng L, Wu X, Liu X. miR-9-5p facilitates hepatocellular carcinoma cell proliferation, migration and invasion by targeting ESR1. Mol Cell Biochem. 2021;476(2):575–583. doi: 10.1007/s11010-020-03927-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are available on request from the corresponding author.


