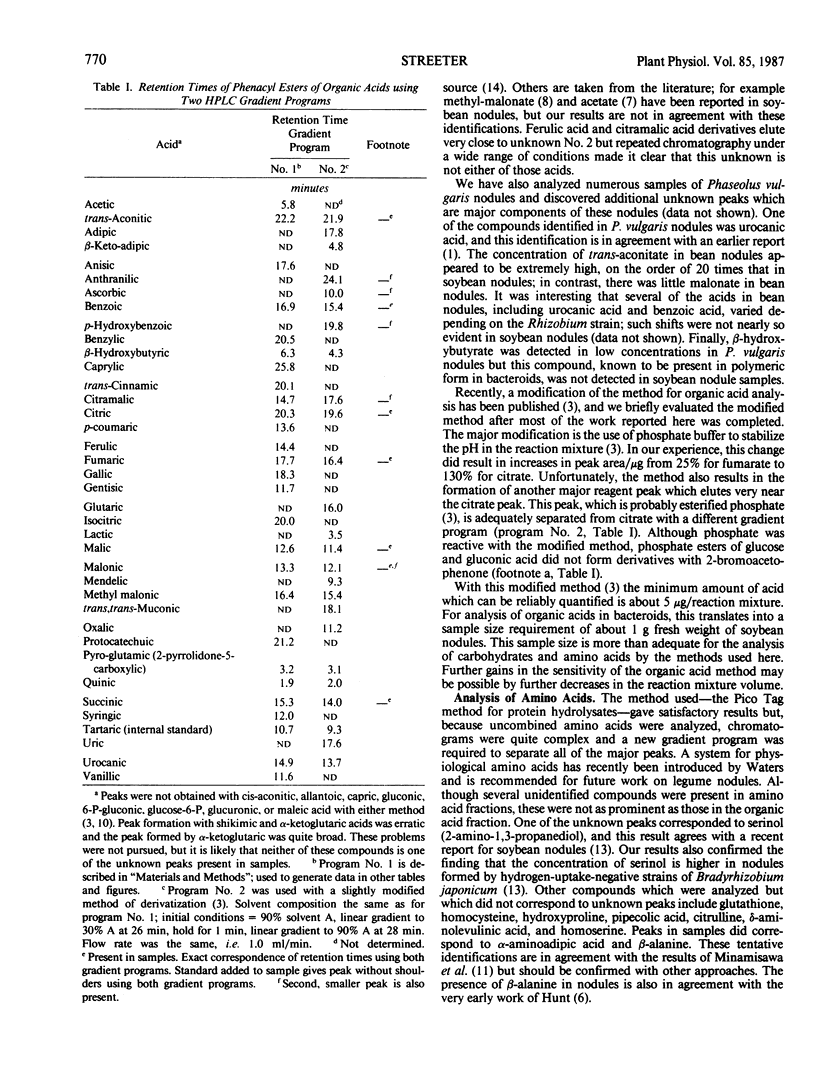
Abstract
Metabolites in Bradyrhizobium japonicum bacteroids and in Glycine max (L.) Merr. cytosol from root nodules were analyzed using an isolation technique which makes it possible to estimate and correct for changes in concentration which may occur during bacteroid isolation. Bacteroid and cytosol extracts were fractionated on ion-exchange columns and were analyzed for carbohydrate composition using gas-liquid chromatography and for organic acid and amino acid composition using high performance liquid chromatography. Analysis of organic acids in plant tissues as the phenacyl derivatives is reported for the first time and this approach revealed the presence of several unknown organic acids in nodules. The time required for separation of bacteroids and cytosol was varied, and significant change in concentration of individual compounds during the separation of the two fractions was estimated by calculating the regression of concentration on time. When a statistically significant slope was found, the true concentration was estimated by extrapolating the regression line to time zero. Of 78 concentration estimates made, there was a statistically significant (5% level) change in concentration during sample preparation for only five metabolites: glucose, sucrose, and succinate in the cytosol and d-pinitol and serine in bacteroids. On a mass basis, the major compounds in bacteroids were (descending order of concentration): myo-inositol, d-chiro-inositol, α,α-trehalose, sucrose, aspartate, glutamate, d-pinitol, arginine, malonate, and glucose. On a proportional basis (concentration in bacteroid as percent of concentration in bacteroid + cytosol fractions), the major compounds were: α-aminoadipate (94), trehalose (66), lysine (58), and arginine (46). The results indicate that metabolite concentrations in bacteroids can be reliably determined.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Davis L. C., Nordin P. Sugar and organic Acid constituents in white clover. Plant Physiol. 1983 Aug;72(4):1051–1055. doi: 10.1104/pp.72.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grushka E., Durst H. D., Kikta E. J., Jr Liquid chromatographic separation and detection of nanogram quantities of biologically important dicarboxylic acids. J Chromatogr. 1975 Oct 29;112:673–679. doi: 10.1016/s0021-9673(00)99996-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mentasti E., Gennaro M. C., Sarzanini C., Baiocchi C., Savigliano M. Derivatization, identification and separation of carboxylic acids in wines and beverages by high-performance liquid chromatography. J Chromatogr. 1985 Mar 29;322(1):177–189. doi: 10.1016/s0021-9673(01)97670-8. [DOI] [PubMed] [Google Scholar]
- Redgwell R. J. Fractionation of plant extracts using ion-exchange Sephadex. Anal Biochem. 1980 Sep 1;107(1):44–50. doi: 10.1016/0003-2697(80)90489-3. [DOI] [PubMed] [Google Scholar]
- Salminen S. O., Streeter J. G. Involvement of glutamate in the respiratory metabolism of Bradyrhizobium japonicum bacteroids. J Bacteriol. 1987 Feb;169(2):495–499. doi: 10.1128/jb.169.2.495-499.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Accumulation of alpha,alpha-trehalose by Rhizobium bacteria and bacteroids. J Bacteriol. 1985 Oct;164(1):78–84. doi: 10.1128/jb.164.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Asparaginase and asparagine transaminase in soybean leaves and root nodules. Plant Physiol. 1977 Aug;60(2):235–239. doi: 10.1104/pp.60.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. A micromethod for the purification and quantification of organic acids of the tricarboxylic acid cycle in plant tissues. Anal Biochem. 1979 May;95(1):311–315. doi: 10.1016/0003-2697(79)90221-5. [DOI] [PubMed] [Google Scholar]
- Stumpf D. K., Burris R. H. Organic Acid contents of soybean: age and source of nitrogen. Plant Physiol. 1981 Nov;68(5):989–991. doi: 10.1104/pp.68.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T. C., Faris M. A., Macdowall F. D. Pathways of Nitrogen Metabolism in Nodules of Alfalfa (Medicago sativa L.). Plant Physiol. 1986 Apr;80(4):1002–1005. doi: 10.1104/pp.80.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]


