Abstract
Objective:
Osteoarthritis (OA) is a leading cause of chronic pain, yet OA pain management remains poor. Age is the strongest predictor of OA development, and mechanisms driving OA pain are unclear. The goal of this study was to characterize age-associated changes in knee osteoarthritis, pain-related behaviors, and dorsal root ganglia (DRG) molecular phenotypes in mice of both sexes.
Methods:
Male or female C57BL/6 mice 6- or 20-months old were evaluated for histopathologic knee OA, pain-related behaviors, and L3-L5 DRG immune characterization via flow cytometry. DRG gene expression in aged mice and humans was also examined.
Results:
Twenty-month old male mice had worse cartilage degeneration than 6-month old mice. Older female knees showed increased cartilage degeneration, but to a lesser degree than males. Older mice of both sexes had worse mechanical allodynia, knee hyperalgesia, and grip strength compared to younger mice. For both sexes, DRGs from older mice showed decreased CD45+ cells, and a significant increase in F4/80+ macrophages and CD11c+ dendritic cells. Older male DRGs showed increased expression of Ccl2 and Ccl5 and older female DRGs showed increased Cxcr4 and Ccl3 compared to 6-month DRGs, among other differentially expresssed genes. Human DRG analysis from six individuals >80 years old revealed elevated CCL2 in males compared to females, whereas CCL3 was higher in female DRGs.
Conclusions:
Here we show that aging in male and female mice is accompanied by mild knee OA, mechanical sensitization, and changes to immune cell populations in the DRG, suggesting novel avenues for development of OA therapies.
Introduction
Risk factors for osteoarthritis (OA), the most prevalent joint disease, include age, female sex, obesity, and joint trauma (1). Age is one of the strongest predictors of OA development (1, 2), and after the age of 50, radiographic joint changes and joint pain become more common (3). It has been well studied that aging is associated with cartilage changes that contribute to the development of OA, including, but not limited to reduced cartilage thickness, increased proteolytic activity, and cellular senescence with abnormal secretory profiles (4). However, less is known about the mechanisms that promote chronic OA pain and how the aging process is involved. Chronic pain is the main reason OA patients seek medical care, yet therapies to address OA pain are inadequate. Existing drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs) often come with serious side effects when used chronically, especially in an older population (5). In addition, studies have found weak correlations between radiographic knee OA and pain severity, but imaging of soft tissues showed that synovitis and bone marrow lesions correlated with pain (6). Therefore, there is a need for a deeper understanding of mechanisms that drive joint pain associated with aging to identify new analgesic targets.
Both peripheral (7, 8) and central (9) sensitization contribute to chronic pain development. The cell bodies of specialized pain-sensing neurons called nociceptors reside in the dorsal root ganglia (DRG). Nociceptive neurons extend an axon to the periphery, e.g., the joint, and another to the dorsal horn of the spinal cord. From the DRG, the pain signal is transmitted to the dorsal horn, where the first synapse occurs, then second-order neurons project to supraspinal regions, and the signal is relayed to higher regions of the brain, where pain perception occurs (reviewed in (8) ). Peripheral sensitization is influenced by neuroinflammation in the DRG (10, 11), and while an important role for immune cells in modifying pain sensitization has been suggested, very little is known in the context of age-associated OA. Older mice have been reported to have decreased locomotor activity, motor function, acoustic startle response, social behavior, and depression-related behavior (12). In the monoiodoacetate (MIA) model of OA, differences in the development of referred mechanical allodynia and microglial responses in the spinal cord have been reported in young vs. old mice, suggesting potentially different sensitization mechanisms with age (13).
Most experimental animal studies of OA use surgical or chemical methods to cause OA in young animals (reviewed in (14)). These models offer many benefits, including controlling variability and low cost, but they may not precisely represent the pathological mechanisms involved in OA associated with aging. Therefore, studying naturally occurring OA in aged animals may uncover different mechanisms contributing to progression of OA joint damage and associated pain. The goal of this study was to examine aging-associated characteristics of 6-month and 20-month old male and female C57BL/6 mice. These ages were chosen because a 6-month old mouse roughly translates to a mature adult human (approximately 30-40 years old), while a 20-month old mouse correlates with an elderly human (approximately 60-70 years old) (15). To our knowledge, this is the first study to simultaneously measure sex-specific features of aging that include knee joint degeneration, pain-related behaviors, and immune and molecular phenotypes of knee-innervating DRGs.
Methods
Animals
Wildtype (WT) C57BL/6J male mice were bred in house and aged to either 6 months (n=10) or 20 months old (n=12). WT C57BL/6J male mice aged 10 weeks (n=10) were purchased from Jackson Laboratory. WT C57BL/6J female mice aged 10 weeks (n=10), 6 months (n=6) or 20 months (n=6) were purchased from Jackson Laboratory. In each age group, each mouse was subjected to behavioral testing, joint histology, and DRG and peripheral blood flow cytometry with the exception of 10-week old animals that were only evaluated for behavioral testing. The DRGs used for immune cell characterization via flow cytometric analysis were the same mice that were evaluated for behavioral testing and for joint histology (Suppl. Fig. 1). Animals housed at Rush had unrestricted access to food and water and were kept on a 12-hour light cycle. Purchased animals from Jackson Labs were acclimated for 4 weeks upon arrival before beginning behavioral testing.
For DRG gene expression analyses, 6-, 12-, 18- and 24-month-old male and female C57BL/6N mice were obtained from the National Institute on Aging Aged Rodent Colony and housed at Oklahoma Medical Research Foundation for a minimum of one week with unrestricted access to food and water prior to euthanasia and tissue collection. All animal experiments were approved by the respective Institutional Animal Care and Use Committees.
Behavioral studies
Independent sets of mice were evaluated at 10 weeks (n=10 for both males and females), 6 months (n=10 males, n=6 females), or 20 months (n=12 males, n=6 females) for the following behaviors: mechanical allodynia, knee hyperalgesia, and grip strength (Suppl. Fig. 1). Mechanical allodynia in the hind paw was measured by von Frey testing. Knee hyperalgesia was measured by pressure application measurement (PAM) testing, and grip strength testing was done on a square grid device using a grip force meter and normalized to mouse body weight. Only one behavioral test was performed per day, and animals were allowed to rest at least one day between different tests. Blinding was not feasible as the mice were tested as they came of age. Please refer to supplemental methods for more detail.
Histology
Following behavioral testing, 6-month (n=10) and 20-month old (n=12) male and 6-month (n=6) and 20-month (n=6) female mice were sacrificed to collect right-side knees for histologic analysis, and peripheral blood and DRGs were collected for flow cytometry as described below. The knees were fixed in 10% natural buffered formalin, decalcified in EDTA for 3 weeks, and embedded in paraffin. Sections from the center of the joint were stained with 0.1% Safranin O/Fast Green for the evaluation of cartilage damage based on Osteoarthritis Research Society International recommendations. We also measured chondrophyte/osteophyte width via an ocular micrometer if present. Synovial pathology was also evaluated. Please see supplemental methods for more details.
Mouse DRG qPCR analysis
Immediately following euthanasia, L3-L5 DRGs were harvested from the left and right side, immediately placed in TRIzol® Reagent (Ambion), and stored at −80°C until homogenization. Samples were mechanically homogenized in TRIzol, and RNA was isolated using RNeasy mini kit (Qiagen) following manufacturer instructions. RNA (224 ng) was reverse transcribed to cDNA, pre-amplified, and loaded at 1:20 dilution into a custom Fluidigm DELTAgene Assay 96.96 Dynamic Array IFC for automated PCR reactions run on BioMark HD (Fluidigm), with 88 genes assessed per sample. Standard QC steps were applied using melting curve analysis, reference gene normalization, and sample amplification. For male mice, 31 samples passed QC evaluation with n=8 for 6-month, n=8 for 12-month, n=7 for 18-month, and n=8 for 24-month old mice. For female mice, 30 samples passed QC evaluation with n=6 for 6-month and n=8 for 12-, 18-, and 24-month old mice.
Flow cytometry
To yield at least 1 million cells for flow cytometry, 6 DRGs were needed. For males, DRGs from 2 mice were pooled such that L3-L5 DRGs of either the right or left side from two mice were collected (3 DRGs per mouse = total 6 DRGs), i.e., when the mouse number n=10, flow cytometry sample number n=5. Lumbar levels 3-5 were chosen based on previous reports showing knee and hindpaw innervation via retrograde labeling (16). Trends were similar on right and left sides, thus, flow cytometry plots show right side only data for males. Based on this finding, for females, we pooled right and left side L3-L5 DRGs together for one mouse per sample (n=6). After dissection of the L3-L5 knee-innervating DRGs, tissue was digested with collagenase type IV (1.6mg/mL) and DNase I (200ug/mL) for 1 hour shaking at 37°C. Following digestion, cells were counted, and 1 million cells were stained with an immune cell panel of anti-mouse antibodies (see Supplemental methods). Sample data were acquired through an LSR Fortessa flow cytometer. Flow cytometry analysis was completed with FlowJo software (version 10). A representative gating strategy is shown in Suppl. Figs. 2,3 (DRG) and Suppl. Fig. 4 (Peripheral blood).
Human DRG in situ hybridization
Human DRGs came from participants in the Religious Orders Study (ROS) or Rush Memory and Aging Project (MAP) (17). Both studies were approved by an Institutional Review Board of Rush University Medical Center. All participants signed an informed consent, Anatomic Gift Act, and a repository consent to allow their resources to be shared. DRGs were removed postmortem and flash frozen as part of the spinal cord removal. Clinical characteristics of the human DRGs can be found in Suppl. Table 1.
The human DRGs, collected from upper lumbar level spine, were embedded in optimal cutting temperature (OCT) compound and cryo-sectioned (20 μm). RNAscope Multiplex Fluorescent V2 Assay was performed according to Advanced Cell Diagnostics (ACD) Bio instructions with minor modifications, see supplemental methods. Targets included human CCL2 (#423811) and CCL3 (#455331). Quantification was performed using Radial Symmetry-fluorescent in-situ hydrization (RS-FISH) spot detection software (18). Analysis was performed in a blinded manner to count the positive signal (number of dots per area) in five different areas per section at 60x magnification.
Statistical analysis
Sample sizes were chosen based on published data (19) – we calculated that to achieve 80% power with α = 0.05, n > 5 mice would be needed to detect changes in cartilage pathology in male C57BL/6 mice from age 6 months to age 20 months. Tests for normal distribution were performed and non-parametric testing was performed if data did not pass the test. For behavioral tests, ordinary one-way ANOVA was used for comparison of multiple groups with Tukey post test. For mechanical allodynia, since the von Frey fibers are on a log scale, the data was first log-transformed. For histology and flow cytometry, statistical analysis was done by Mann-Whitney test. qPCR data were assessed by Kruskal-Wallis test and Mann-Whitney U-test. RNAscope data were compared by unpaired, two-tailed t-test. Mean +/− standard error of the mean (SEM) is shown in all graphs and p values are stated on each graph. P values were considered significant if less than 0.05. Statistical calculations were performed using GraphPad Prism 9.
Results
Knee osteoarthritis in aged mice
We first aimed to assess whether natural aging was associated with histopathologic development of OA. Knees from male and female mice showed no cartilage degeneration in either compartment at the age of 6 months. By 20 months, male mice had significant cartilage damage, both in the medial and lateral compartments (Fig.1A–C), consistent with previous literature (19). Female mice developed cartilage degeneration in the medial and lateral compartments by 20 months, but to a much lesser degree than male mice (Fig. 1H–J). In addition, the knees of 20-month old male mice showed small osteophytes (less than 100 μm in width), while no osteophytes were present in 6-month old male mice (Fig. 1D). Knees from female mice showed small chondrophytes/osteophytes at both 6 and 20 months (less than 50 μm in width) (Fig. 1K). Representative Safranin-O images per group (i.e., 6-month, 20-month, male or female knees) are shown in Suppl. Fig. 5. To show the wide range of cartilage degeneration seen in male mice, we show images from 20-month old male mice that have low-to-high degeneration scores on either the medial or lateral sides in Suppl. Fig. 6A. Representative images of chondrocytes and osteophytes are shown in Suppl. Fig. 6B–D.
Fig. 1.
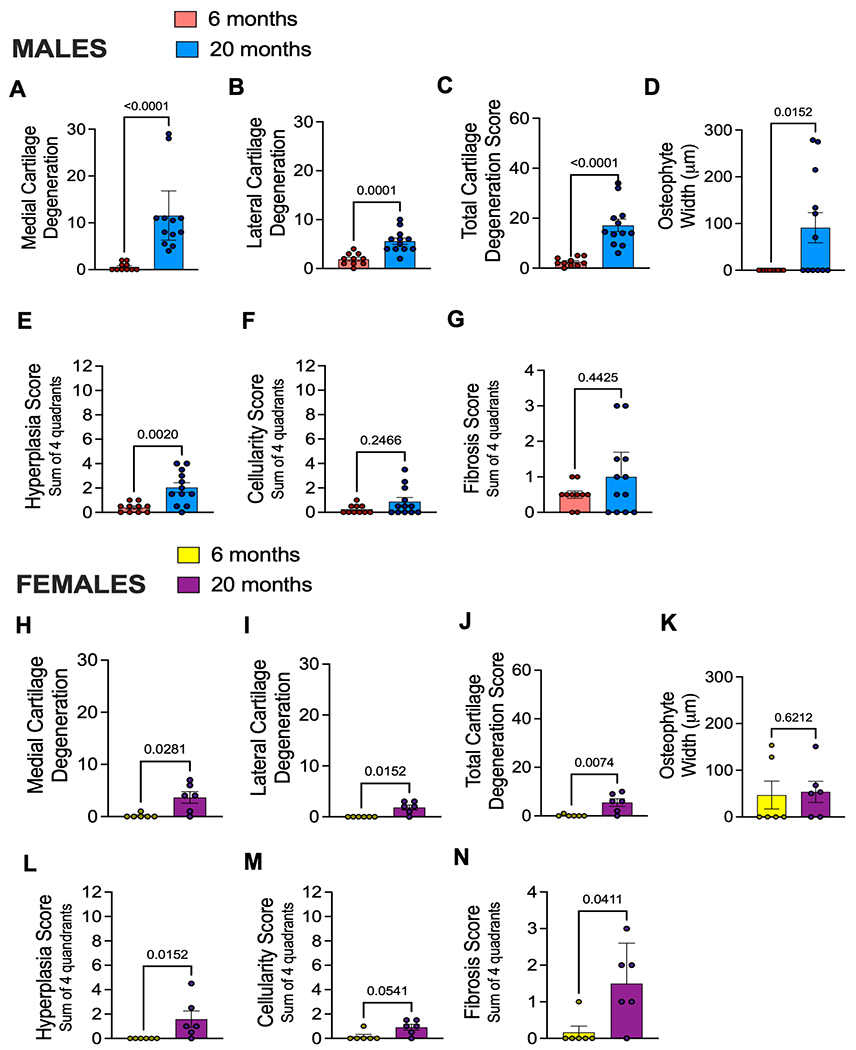
Aging is associated with mild knee osteoarthritis in mice. (A) Medial cartilage degeneration scores for male mice right knees aged 6 months (n=10; Mean ± SEM = 0.600 ± 0.267) or 20 months (n=12; Mean ± SEM = 11.5 ± 2.39) old as determined by OARSI scoring methods; (B) lateral cartilage degeneration for males aged 6 months (n=10; Mean ± SEM = 1.9 ± 0.3786) or 20 months (n=12; Mean ± SEM = 5.58 ± 0.656) ; (C) Total cartilage degeneration score for males aged 6 months (n=10; Mean ± SEM = 2.5 ± 0.543 or 20 months (n=12; Mean ± SEM = 17.13 ± 2.49) plotted as sum of medial and lateral compartments; (D) Osteophyte width for males plotted in micron size found in either medial or lateral compartments of the knee; (E) Synovitis hyperplasia score for males showing an average of two blinded scores (maximum score is 12); (F) Synovitis cellularity score for males showing an average of two blinded scores (maximum score is 12); (G) Synovitis fibrosis score for males showing an average of two blinded scores (maximum score is 4); (H – N) Same as in (A – G) but shown for female mice aged 6 months (n=6; Medial Mean ± SEM = 0.1667 ± 0.1667; Lateral Mean ± SEM = 0.0 ± 0.0; Total Mean ± SEM = 0.1667 ± 0.1667) or 20 months old (n=6; Medial Mean ± SEM = 3.667 ± 1.116; Lateral Mean ± SEM = 1.884 ± 0.4773; Total Mean ± SEM = 5.50 ± 1.586). Statistical analysis by Mann-Whitney test. Significant if p < 0.05. Error bars show Mean ± SEM.
No synovial pathology was detected in either male or female mice at the age of 6 months. However, mice of both sexes showed mild synovial pathology at 20 months (Fig. 1E–G & 1L–N). Both male and female mice showed significant hyperplasia in the lining layer of synovium compared to younger mice (Fig. 1E+L). No significant changes were detected in cell density of the subintimal layer of male and female mice, yet a trend of increased cellularity was detected in old female mice (Fig. 1M). In addition, older female mice showed fibrotic subintimal synovium compared to younger counterparts (Fig. 1N). Synovial pathologies in each anatomical location (e.g., medial tibial, medial femoral, etc.) are shown in Suppl. Fig. 7. Overall, these results suggest mild joint degeneration occurs naturally with aging in both sexes.
Behavior changes in mice with aging-associated osteoarthritis
We evaluated behaviors indicative of mechanical sensitization in 10-week, 6-month, or 20-month old mice. Mechanical allodynia of the hind paw progressively developed with age in both sexes (shown in Fig. 2A+D). Assessment of knee hyperalgesia revealed no difference in knee withdrawal threshold between 10-week old and 6-month old mice, but knee hyperalgesia developed by 20 months of age compared to 6-month old mice, again in both sexes (shown in Fig. 2B+E). These results suggest aging and naturally occurring knee joint damage are accompanied by increased mechanical sensitization in the hind limb. Finally, we evaluated grip strength, which is a measure of deep tissue hyperalgesia and indicative of muscle weakness, neuromuscular dysfunction and/or painful movements (20). There was a progressive decline in grip strength with age in both sexes, similar to the trend seen in the hind paw mechanical allodynia assay (shown in Fig. 2C+F). Grip strength data were normalized to mouse bodyweights, which are shown in Suppl. Fig. 8.
Fig. 2.
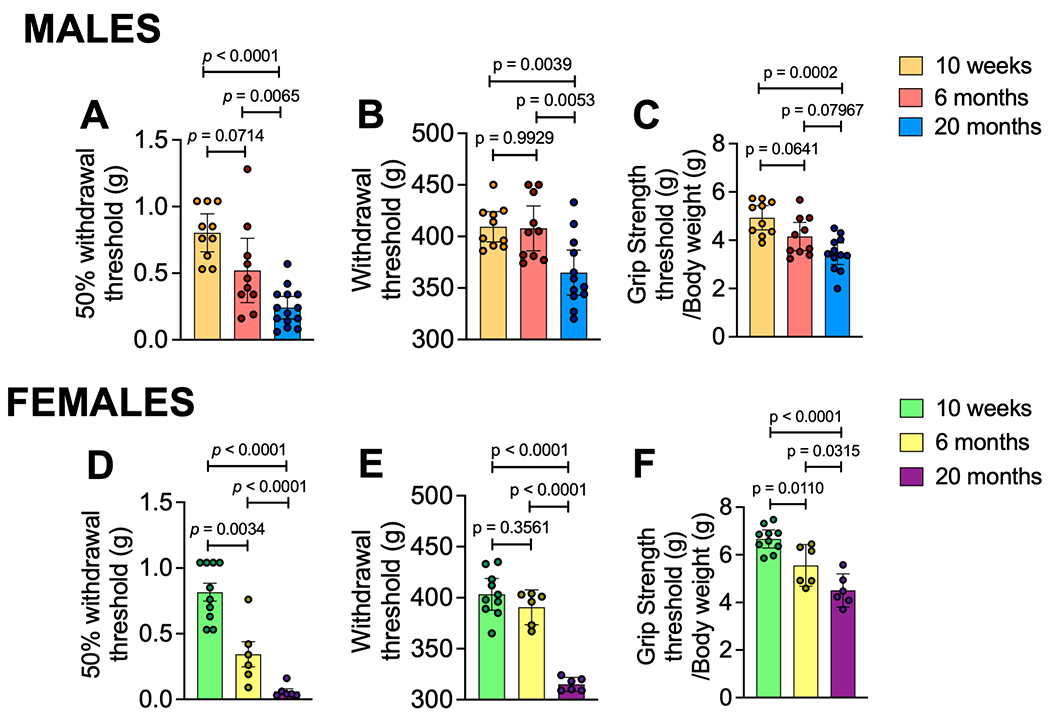
Aging is associated with development of pain-related behaviors. (A) Mechanical allodynia by von Frey fiber testing; (B) Knee hyperalgesia by pressure application measurement testing; (C) Grip strength by square grid grip testing of all four paws observed in 10-week (n=10), 6-month (n=10), or 20-month (n=12) old male mice. (D - F) Same as in (A - C) but for 10-week (n=10), 6-month (n=6), or 20-month (n=6) old females. Statistical analysis by Ordinary One-Way ANOVA test with Tukey post test. Mechanical allodynia values were first log transformed before running statistical analysis as the von Frey fibers are on a log scale. Significant if p < 0.05. Error bars show mean±SEM.
Gene expression analysis of aged mice L3-L5 DRGs
To assess DRG immune changes in aged mice on a molecular level, RNA was extracted from DRGs collected from mice of different ages (6, 12, 18, and 24 months). There were 88 genes assessed and results are shown in Suppl. Table 2 (males) and Suppl. Table 3 (females). In male mice, there was significantly increased mRNA expression of Ccl5, Tacr1, and Ccl2 and significantly decreased Gss gene expression at 24 months compared to 6 months (Suppl. Table 2). In females, Cxcr4, Hgf, Tacr1, Il1rn, Runx3, Scn9a, Ccl3, Kit, Mrgprd, Cx3cr1, Ptk2b, Trpv1, Ntrk1, Trpa1, Tnf, Gclc, Gsr, Npy, and Scn10a were significantly increased at 24 months compared to 6 months, while Gstsa4, Prkcq, and Gstm1 gene expression were decreased (Suppl. Table 3). These data suggest a role for chemokine signaling and immune cell communication within the DRG in aging-associated spontaneous OA.
DRG immune cell characterization of aged mice
To investigate the presence of immune cells in the DRG in more detail, flow cytometry of DRGs was performed. Changes in a number of cell types were seen in 20-month old mice compared to younger (6 month) mice in both males and females. In particular, 20-month old mice had a decreased total relative frequency of CD45+ cells (significant in males shown in Fig. 3A and trending in females shown in Fig. 4A). In contrast, a significant increase in F4/80+ macrophages in both males and females (Fig. 3B and Fig. 4B) and a significant increase in CD11c+ dendritic cells in both males and females (Figs. 3C and 4C) was observed in 20-month old mice compared to younger (6 month) mice. For both sexes, there were no significant differences in relative frequencies of other cell types, including CD3+ T cells (Figs. 3D and 4D), CD11b+ myeloid cells (Figs. 3E and 4E), Ly6Chi monocytes (Figs. 3F and 4F), and Ly6G+ granulocytes (Figs. 3G and 4G). In females, there was no difference in proportion of Ly6C- cells between DRGs from 20-month old mice compared to 6-month old mice (Fig. 4H), but this population significantly increased in DRGs from older males (Fig. 3H). Additionally, there was no significant difference in the frequency of CCR2+ cells between younger and older male DRGs (Fig. 3I); however, there was a significant decrease in the relative frequency of CCR2+ cells in DRGs from 20-month old female mice compared to 6-month old female mice (Fig. 4I).
Fig. 3.
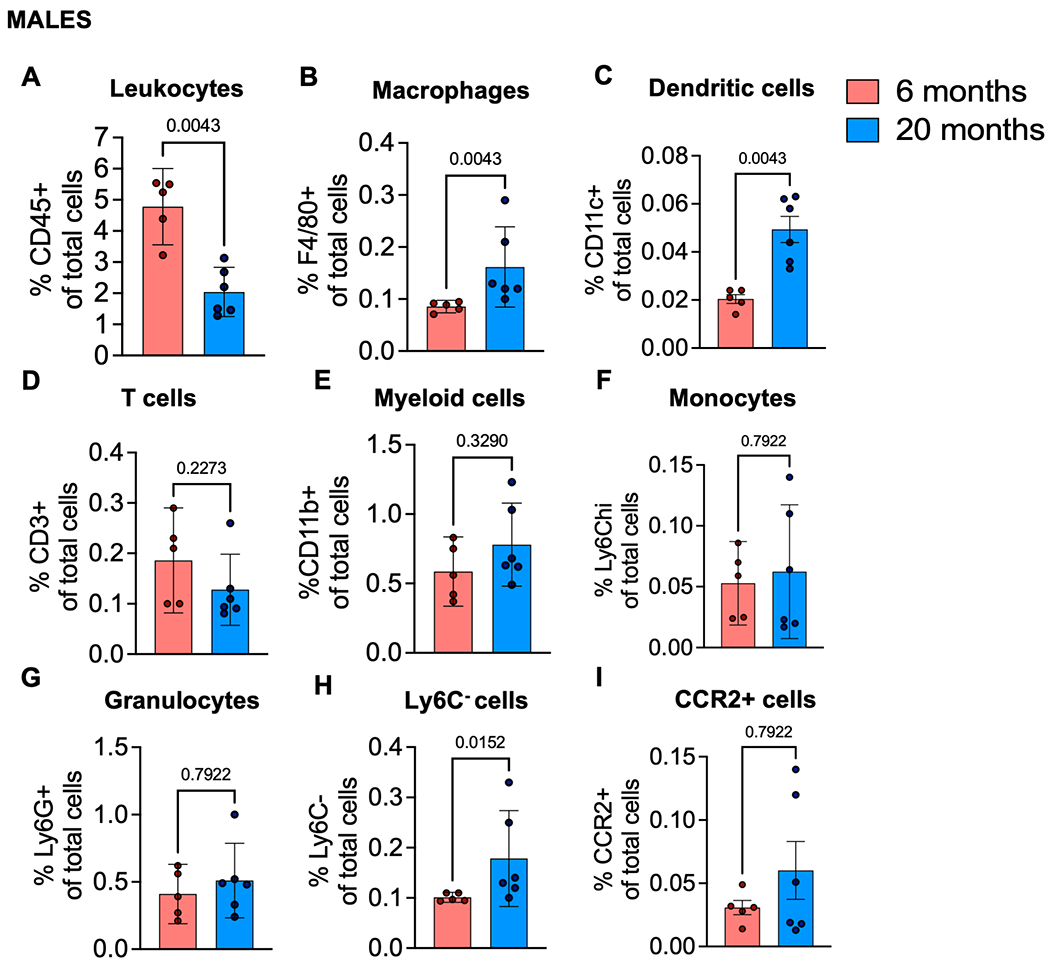
Immune cell populations in dorsal root ganglia of aged male mice. (A) Frequency of CD45+ leukocytes; (B) F4/80+ macrophages; (C) CD11c+ Dendritic cells; (D) CD3+ T cells; (E) CD11b+ myeloid cells; (F) Ly6Chi monocytes; (G) Ly6G+ granulocytes; (H) Ly6C-antigen presenting precursor cells; (I) CCR2+ cells in 6-month (n=5), or 20-month (n=6) old male mice. Flow cytometry gating strategy in Suppl. Fig. 2. Statistical analysis by Mann-Whitney test. Significant if p < 0.05. Error bars show mean±SEM.
Fig. 4.
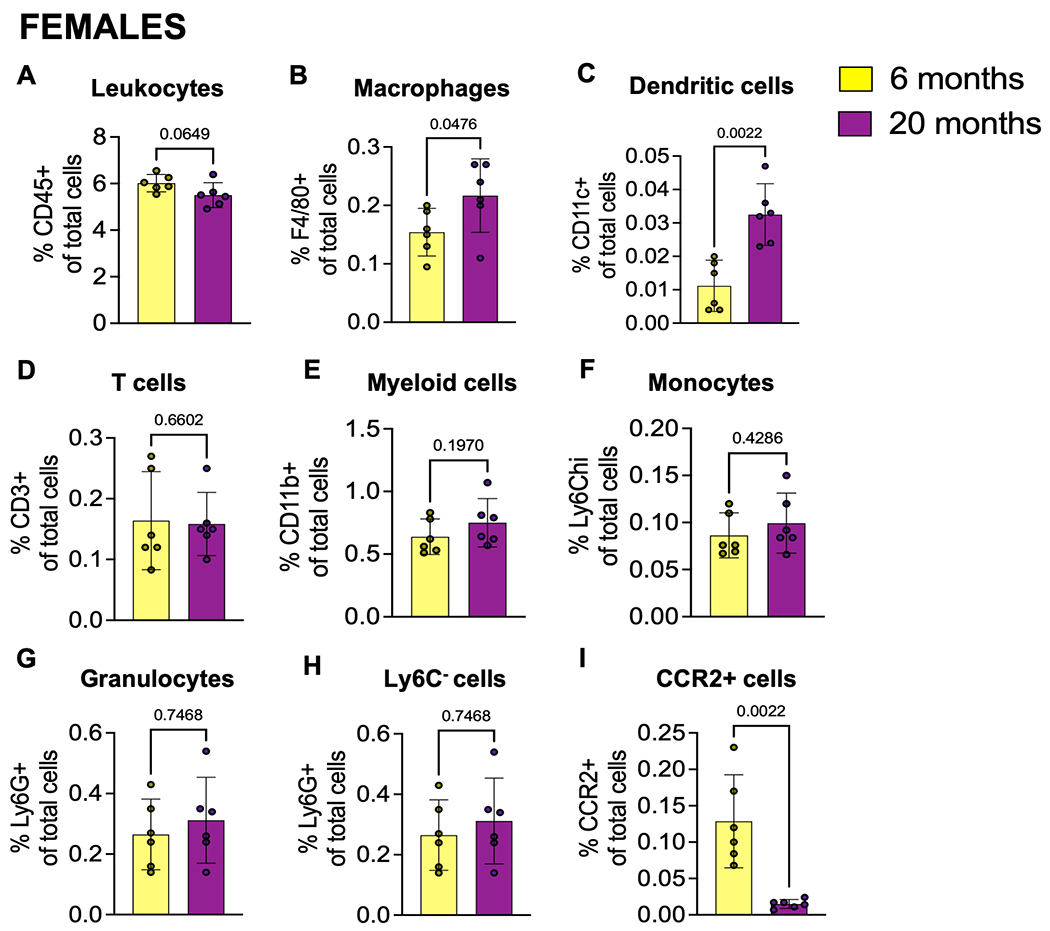
Immune cell populations in dorsal root ganglia of aged female mice. (A) Frequency of CD45+ leukocytes; (B) F4/80+ macrophages; (C) CD11c+ Dendritic cells; (D) CD3+ T cells; (E) CD11b+ myeloid cells; (F) Ly6Chi monocytes; (G) Ly6G+ granulocytes; (H) Ly6C-antigen presenting precursor cells; (I) CCR2+ cells in 6-month (n=6), or 20-month (n=6) old female mice. Flow cytometry gating strategy in Suppl. Fig. 2. Statistical analysis by Mann-Whitney test. Significant if p < 0.05. Error bars show mean±SEM.
We also evaluated macrophage subtypes within the DRG to assess macrophage activation and characterize if they are more pro- or anti-inflammatory. To discriminate between these functional phenotypes, we stained for CD163 and CD206 (also known as mannose-C-type lectin receptor-1 (MRC1)), scavenger receptors associated with an anti-inflammatory phenotype, and CX3CR1 and MHCII, receptors associated with an activated, pro-inflammatory phenotype (21). We found a significant increase in CX3CR1+ macropahges, a trending increase in MHCII+, and CD206+ macrophages, and no change in CD163+ macrophages in male mice at 20 months compared to 6 months (Fig. 5A–D). There were no significant changes in the number of these macrophage subtypes in DRGs from female aged mice (Fig. 5E–H).
Fig. 5.
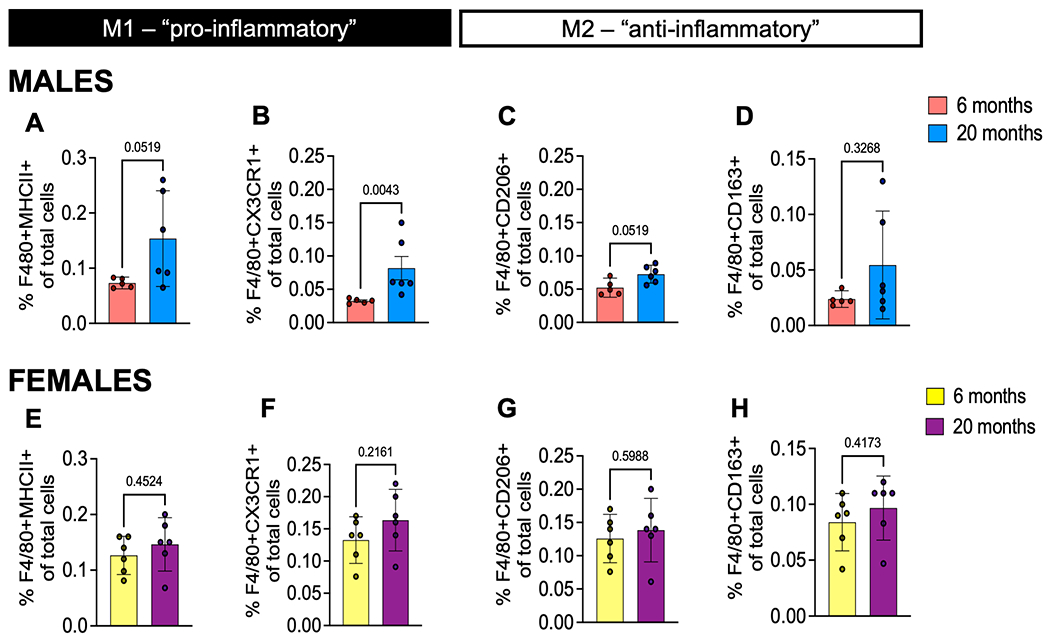
Macrophage phenotypes in male and female aged DRGs. (A) Frequency of F4/80+MHCII+ pro-inflammatory macrophages; (B) F4/80+CX3CR1+ pro-inflammatory macrophages; (C) F4/80+CD206+ anti-inflammatory macrophages; (D) F4/80+CD163+ anti-inflammatory macrophages from 6-month (n=5) or 20-month old (n=6) male mice DRGs. (E - H) Same as in (A - D) but for 6-month or 20-month old female mice (n=6) DRGs. Flow cytometry gating strategy in Suppl. Fig. 3. Statistical analysis by Mann-Whitney test. Significant if p < 0.05. Error bars show mean±SEM.
Additionally, to assess any systemic immune changes in naturally aged mice, we examined the circulating immune cells in peripheral blood of aged mice. In both males and females there was a significant decrease in total CD45+ leukocytes in 20-month-old blood compared to 6-month-old blood (Suppl. Figs. 9A and 9J). There were no significant changes in myeloid cells, granulocytes, monocytes, Ly6Clo or Ly6C- cells in circulation, for both sexes (Suppl. Figs. 9B–F and 9K–N), except a slight increase in Ly6C- cells circulating in male mice (Suppl. Fig. 9O). There were no changes in T cell populations in females (Suppl. Fig. 9G–I), but significantly decreased frequencies of CD3+, CD4+, and CD8+ T cells in peripheral blood of male mice (Suppl. Fig. 9P–R). This is consistent with reported literature where it has been reported that peripheral blood CD4+ T cells and CD8+ T cells declined in male C57BL/6 mice by the age of 20-26 months (22). A representative gating strategy for peripheral blood flow cytometry depicting how this data was acquired and analyzed is shown in Suppl. Fig. 4.
In situ hybridization of aged human DRGs
To further appreciate the translational relevance of immune-related genes in aged DRGs, we used RNAscope to assess the expression of chemokines CCL2 and CCL3 in human DRGs (n=3/sex). We chose these genes because we observed both genes differentially regulated in the mouse DRG gene expression analyses. We assessed 3 male and 3 female human DRGs with an average age at death of 90.9 years and average BMI of 25 (Suppl. Table 1). There was expression of both CCL2 and CCL3 in male and female human DRGs (Fig. 6 A, B, D, and E). Interestingly, after quantifying this signal in a blinded manner, we observed higher CCL2+ signal in males compared to females (Fig. 6C), whereas there was higher CCL3+ signal in females compared to males (Fig. 6F). Together with the mouse data, this suggests a role for chemokine signaling in aging associated DRG changes and perhaps a sexual dimorphism in how these signals change over time.
Fig. 6.
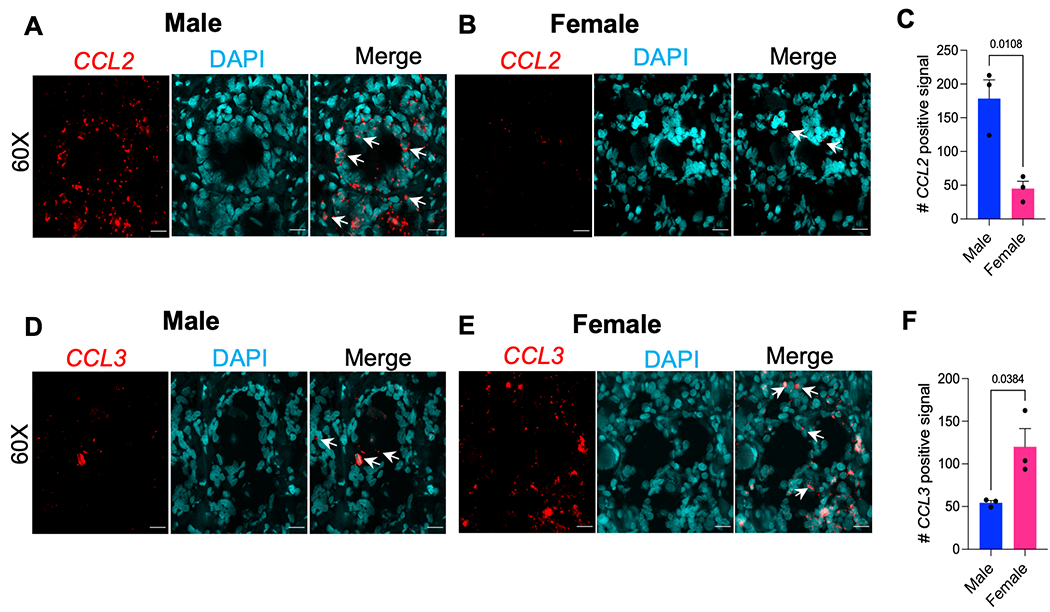
Expression of CCL2 and CCL3 in male and female human dorsal root ganglia. (A-C) RNAscope used to quantify the expression of CCL2 or (D-F) CCL3 in serial sections of human dorsal root ganglia (n=6 donors, three male, three female); Mean±SEM. (A-B, D-E) Representative sections of (A, D) male and (B, E) female human DRG. 60X magnification (scale bar = 25 μm). White arrows indicate examples of positive signal. (C, F) RNAscope signal was quantified using RS-FISH in 5-6 60X images and averaged per donor. Significant if, p < 0.05, by unpaired two-tailed t-test.
Discussion
Aging is a predominant risk factor for nontraumatic OA development (2). To our knowledge, there have been no studies concurrently evaluating pain behavior, joint damage, and immune cell changes in the DRG in naturally aged mice. While results shown here are correlative, they are consistent with degenerative knee changes being associated with age-related pain phenotypes such as mechanical sensitization as well as DRG molecular and immune changes. Major novel findings include increased mechanical sensitization with age in both sexes, worse cartilage degeneration in aged males compared to females, changes in DRG immune cells, such as increased macrophages and dendritic cells in aged DRGs of both sexes, and significantly decreased CCR2+ cells only in older female DRGs. In addition, DRG gene expression changes with age and there is a sexual dimorphism of chemokine expression in both mice and human DRGs.
Here, we saw increased histopathologic signs of OA in aged male and female mice, with worse cartilage degernation in males than in females, yet both sexes had similar levels of synovial pathology. This supports previous reports that have evaluated naturally occurring mouse OA joint degeneration in males (19, 23–27). McNulty et al. reported that older male mice had significantly thinner articular cartilage compared to younger mice (19). In addition, collagen-rich tissues are pre-disposed to common age-related disorders, such as OA, and studies reported age-associated changes in cartilage matrix protein content, such as reduced protein incorporation in all tissues (cartilage, bone, skin), dynamic turnover of matrisome, and reduced collagen synthesis in bone of older mice, compared to younger mice (23, 28). Here, we found mild synovitis and osteophyte presence in both male and female 20-month mice. One previous report showed no synovial hyperplasia and grade 2 and grade 3 osteophytes in 18-month male mice (29). Other studies showed age-related changes in the meniscus (30). Notably, here we found worse OA in male knee joints compared to female, which is opposite of the human OA trend, where females experience increased incidence and severity of OA (2).
Results reported here are consistent with previous studies showing that aging is correlated with a decline in spontaneous locomotion in mice (12) (31). Tran et al. observed a decline in spontaneous locomotion in 22-month compared to 3-month old mice for both sexes (31). They also found a greater age-related decline in males than in females with respect to climbing and rearing. Shoji and colleagues reported age-related behavioral changes in a wide range of behaviors from young adulthood to middle age in male mice. They found decreased locomotor activity and increased anxiety-like behavior from young adult to middle aged mice (12). Here, we did not evaluate spontaneous locomotion or anxiety, but we found that mechanical allodynia, knee hyperalgesia, and grip strength worsen with age in both males and females. This result is consistent with a previous report in a partial menisectomy model of OA, where young female mice developed pain-like behavior at the same time as young male mice despite reduced chondropathy in females compared to males (32). Peripheral neuropathy may also partially explain increased mechanical sensitivity with age, however, previous studies have reported that the decline in epidermal innervation occurs in C57BL/6 mice older (>22 months of age) (33, 34) than the ones chosen for this study.
There are many types of immune cells involved in neuroimmune crosstalk and function (11), including dendritic cells, macrophages, and T cells. Studies have indicated that macrophages skew toward an inflammatory phenotype as tissues age, which may be a natural part of aging (35–37). OA has a prolonged inflammatory phase and lack of repair and remodeling, which may contribute to the development of OA pain (38). Mediators secreted from macrophages and other immune cells, such as resolvins, cytokines, and chemokines, contribute to the resolution of inflammation (39). Moreover, our study is in line with previous findings in other models demonstrating an important role for DRG macrophages in mediating osteoarthritis pain (40). Raoof and colleagues showed M1-like macrophages accumulate in the DRG, depletion of macrophages intrathecally resolved OA pain in male and female mice, and altering macrophage signaling to an M2-like phenotype using IL-4/IL-10 fusion protein attenuated OA pain in the MIA model (40). Interestingly, blockade of CCL3 has resulted in diminished pain in a model of neuropathic pain (41), and both CCL2 and CCL3 have been shown to regulate pelvic pain (42). In other animal models of OA, we and others have found a major role for CCL2/CCR2 signaling in mediating OA pain (43–46), an important chemokine receptor for monocyte/macrophage trafficking and signaling.
Interestingly, sexual dimorphism in the age-related decline of several mouse behaviors has been reported (31), and there are known transcriptional and translational sex differences in mouse sensory neurons (47, 48). Here we studied both sexes and found a greater increase in DRG macrophages in aged males than females and this coincided with a significant decrease in CCR2+ cells in aged female DRGs. Furthermore, we saw significant increases in MHCII+, CX3CR1+, and CD206+ macrophages in male DRGs, while females had no changes in these populations, suggesting a more activated macrophage phenotype in males than females. In contrast, T cells are thought to play a more important role in female pain development (49). There have been studies of lymphoid compartments in aged mice (22), and here we show results consistent with the literature of decreased peripheral blood CD4+ T cells only in older males (22). Additionally, we found increased gene expression of CCL2 and CCL3 in male and female human DRGs, respectively, which supports previous reports showing significant sex differences in genes related to immune pathways in human DRGs (50).
While this study of aged mouse knee OA, pain-like behaviors, and DRG immune phenotyping elucidates the complex neuro-immune interactions involved in aging-associated OA pain, it comes with some limitations. We were limited in the number of markers we could evaluate via flow cytometry. We sought to understand these data with other data sets, such as gene expression data of mice DRGs, which showed increased immune cell recruitment chemokines, e.g., Ccl2 and Ccl3. Furthermore, we used human DRGs to validate these mouse gene expression analyses providing clinical relevance of these findings. Finally, when studying aging there are always confounding variables that may alter interpretation of findings, such as aging-related obesity and other disorders, e.g., cataracts, dermatitis, etc., however mice studied here did not have any outward signs of disease. Nonetheless, our study should be considered an observation of features in the knee joint and DRG that accompany aging.
In summary, this study demonstrates that in mice, aging is accompanied with mild knee osteoarthritis, increased pain-related behaviors, and distinct DRG immune phenotypes, with important differences between sexes. Findings we report here may lead to development of better targeted therapies and analgesic options for OA pain, especially for the aging population. Future research will aim to better define how neuroimmune communication contributes to OA pain in both mice and humans.
Supplementary Material
Acknowledgements
We would like to thank the Rush University Flow Cytometry Core and the OMRF Arthritis and Clinical Immunology Cores. We would like to thank Nirupa Jayaraj for technical assistance. We thank the study participants and staff of the Rush Alzheimer’s Disease Center funded through NIH grants P30AG10161, R01AG17917, R01AG24490. ROSMAP resources can be requested at https://www.radc.rush.edu.
Role of Funding Source
This work was supported by National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Grants. T32AR073157 and F32AR081129 (TG), R01AR077019 (REM), R01AR064251, R01AR060364, P30AR079206 (AMM), R01AR075737 and I01BX004912 from the VA (CRS). Rheumatology Research Foundation Innovative Research Award (AMM and MJW). R01AG049058 from the NIH National Institute on Aging and I01BX004666 and I01BX004882 from the Department of Veterans Affairs (TMG).
Footnotes
Conflict of Interest
The following authors declare no conflicts of interest: TG, AMO, SI, MJW, JL, EBP, CRS, and TMG. AMM received consulting fees from Asahi Kasei Pharma Corporation, Eli Lilly, Pfizer, and Collegium Pharma. REM serves as an Associate Editor of Arthritis & Rheumatology.
References
- 1.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30(2):184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. 2013;25(1):108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51(2):241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–89. [DOI] [PubMed] [Google Scholar]
- 6.Neogi T. Structural correlates of pain in osteoarthritis. Clin Exp Rheumatol. 2017;35 Suppl 107(5):75–8. [PubMed] [Google Scholar]
- 7.Vincent TL. Peripheral pain mechanisms in osteoarthritis. Pain. 2020;161 Suppl 1:S138–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malfait AM, Miller RE, Miller RJ. Basic Mechanisms of Pain in Osteoarthritis: Experimental Observations and New Perspectives. Rheum Dis Clin North Am. 2021;47(2):165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clauw DJ, Hassett AL. The role of centralised pain in osteoarthritis. Clin Exp Rheumatol. 2017;35 Suppl 107(5):79–84. [PubMed] [Google Scholar]
- 10.Geraghty T, Winter DR, Miller RJ, Miller RE, Malfait AM. Neuroimmune interactions and osteoarthritis pain: focus on macrophages. Pain Rep. 2021;6(1):e892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hore Z, Denk F. Neuroimmune interactions in chronic pain - An interdisciplinary perspective. Brain Behav Immun. 2019;79:56–62. [DOI] [PubMed] [Google Scholar]
- 12.Shoji H, Takao K, Hattori S, Miyakawa T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain. 2016;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogbonna AC, Clark AK, Malcangio M. Development of monosodium acetate-induced osteoarthritis and inflammatory pain in ageing mice. Age (Dordr). 2015;37(3):9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alves-Simoes M. Rodent models of knee osteoarthritis for pain research. Osteoarthritis Cartilage. 2022;30(6):802–14. [DOI] [PubMed] [Google Scholar]
- 15.Fox James G. SWB, Davidson Muriel T., Newcomer Christian E., Quimby Fred W., Smith Abigail L.. The Mouse in Biomedical Research. The Mouse in Biomedical Research. 2nd ed: Elsevier, Inc.; 2007. p. 637–68. [Google Scholar]
- 16.Miller RE, Kim YS, Tran PB, Ishihara S, Dong X, Miller RJ, et al. Visualization of Peripheral Neuron Sensitization in a Surgical Mouse Model of Osteoarthritis by In Vivo Calcium Imaging. Arthritis Rheumatol. 2018;70(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahry E, Breimann L, Zouinkhi M, Epstein L, Kolyvanov K, Mamrak N, et al. RS-FISH: precise, interactive, fast, and scalable FISH spot detection. Nat Methods. 2022;19(12):1563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNulty MA, Loeser RF, Davey C, Callahan MF, Ferguson CM, Carlson CS. Histopathology of naturally occurring and surgically induced osteoarthritis in mice. Osteoarthritis Cartilage. 2012;20(8):949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montilla-Garcia A, Tejada MA, Perazzoli G, Entrena JM, Portillo-Salido E, Fernandez-Segura E, et al. Grip strength in mice with joint inflammation: A rheumatology function test sensitive to pain and analgesia. Neuropharmacology. 2017;125:231–42. [DOI] [PubMed] [Google Scholar]
- 21.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Flurkey K, Harrison DE. A reduced peripheral blood CD4(+) lymphocyte proportion is a consistent ageing phenotype. Mech Ageing Dev. 2002;123(2-3):145–53. [DOI] [PubMed] [Google Scholar]
- 23.Ariosa-Morejon Y, Santos A, Fischer R, Davis S, Charles P, Thakker R, et al. Age-dependent changes in protein incorporation into collagen-rich tissues of mice by in vivo pulsed SILAC labelling. Elife. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeser RF, Olex AL, McNulty MA, Carlson CS, Callahan MF, Ferguson CM, et al. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis Rheum. 2012;64(3):705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scharstuhl A, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Loss of transforming growth factor counteraction on interleukin 1 mediated effects in cartilage of old mice. Ann Rheum Dis. 2002;61(12):1095–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mistry D, Oue Y, Chambers MG, Kayser MV, Mason RM. Chondrocyte death during murine osteoarthritis. Osteoarthritis Cartilage. 2004;12(2):131–41. [DOI] [PubMed] [Google Scholar]
- 27.van der Kraan PM, Stoop R, Meijers TH, Poole AR, van den Berg WB. Expression of type X collagen in young and old C57Bl/6 and Balb/c mice. Relation with articular cartilage degeneration. Osteoarthritis Cartilage. 2001;9(2):92–100. [DOI] [PubMed] [Google Scholar]
- 28.Kobak KA, Batushansky A, Borowik AK, Lopes EPB, Peelor Iii FF, Donovan EL, et al. An In Vivo Stable Isotope Labeling Method to Investigate Individual Matrix Protein Synthesis, Ribosomal Biogenesis, and Cellular Proliferation in Murine Articular Cartilage. Function (Oxf). 2022;3(2):zqac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong AR, Carlson CS, Rendahl AK, Loeser RF. Optimization of histologic grading schemes in spontaneous and surgically-induced murine models of osteoarthritis. Osteoarthritis Cartilage. 2021;29(4):536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwok J, Onuma H, Olmer M, Lotz MK, Grogan SP, D’Lima DD. Histopathological analyses of murine menisci: implications for joint aging and osteoarthritis. Osteoarthritis Cartilage. 2016;24(4):709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran T, Mach J, Gemikonakli G, Wu H, Allore H, Howlett SE, et al. Male-Female Differences In The Effects Of Age On Performance Measures Recorded For 23 Hours In Mice. J Gerontol A Biol Sci Med Sci. 2021. [DOI] [PubMed] [Google Scholar]
- 32.von Loga IS, Batchelor V, Driscoll C, Burleigh A, Chia SL, Stott B, et al. Does Pain at an Earlier Stage of Chondropathy Protect Female Mice Against Structural Progression After Surgically Induced Osteoarthritis? Arthritis Rheumatol. 2020;72(12):2083–93. [DOI] [PubMed] [Google Scholar]
- 33.Gavini CK, Elshareif N, Aubert G, Germanwala AV, Calcutt NA, Mansuy-Aubert V. LXR agonist improves peripheral neuropathy and modifies PNS immune cells in aged mice. J Neuroinflammation. 2022;19(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaliappan S, Simone DA, Banik RK. Nonlinear Inverted-U Shaped Relationship Between Aging and Epidermal Innervation in the Rat Plantar Hind Paw: A Laser Scanning Confocal Microscopy Study. J Pain. 2018;19(9):1015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oishi Y, Manabe I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech Dis. 2016;2:16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stahl EC, Haschak MJ, Popovic B, Brown BN. Macrophages in the Aging Liver and Age-Related Liver Disease. Front Immunol. 2018;9:2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deczkowska A, Matcovitch-Natan O, Tsitsou-Kampeli A, Ben-Hamo S, Dvir-Szternfeld R, Spinrad A, et al. Mef2C restrains microglial inflammatory response and is lost in brain ageing in an IFN-I-dependent manner. Nat Commun. 2017;8(1):717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20(5):565–72. [DOI] [PubMed] [Google Scholar]
- 39.Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, et al. Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain. 2014;15(5):496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raoof R, Martin Gil C, Lafeber F, de Visser H, Prado J, Versteeg S, et al. Dorsal Root Ganglia Macrophages Maintain Osteoarthritis Pain. J Neurosci. 2021;41(39):8249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pawlik K, Ciechanowska A, Ciapala K, Rojewska E, Makuch W, Mika J. Blockade of CC Chemokine Receptor Type 3 Diminishes Pain and Enhances Opioid Analgesic Potency in a Model of Neuropathic Pain. Front Immunol. 2021;12:781310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quick ML, Mukherjee S, Rudick CN, Done JD, Schaeffer AJ, Thumbikat P. CCL2 and CCL3 are essential mediators of pelvic pain in experimental autoimmune prostatitis. Am J Physiol Regul Integr Comp Physiol. 2012;303(6):R580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishihara S, Obeidat AM, Wokosin DL, Ren D, Miller RJ, Malfait AM, et al. The role of intra-articular neuronal CCR2 receptors in knee joint pain associated with experimental osteoarthritis in mice. Arthritis Res Ther. 2021;23(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miotla Zarebska J, Chanalaris A, Driscoll C, Burleigh A, Miller RE, Malfait AM, et al. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthritis Cartilage. 2017;25(3):406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longobardi L, Temple JD, Tagliafierro L, Willcockson H, Esposito A, D’Onofrio N, et al. Role of the C-C chemokine receptor-2 in a murine model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2017;25(6):914–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A. 2012;109(50):20602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tavares-Ferreira D, Ray PR, Sankaranarayanan I, Mejia GL, Wangzhou A, Shiers S, et al. Sex Differences in Nociceptor Translatomes Contribute to Divergent Prostaglandin Signaling in Male and Female Mice. Biol Psychiatry. 2022;91(1):129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mecklenburg J, Zou Y, Wangzhou A, Garcia D, Lai Z, Tumanov AV, et al. Transcriptomic sex differences in sensory neuronal populations of mice. Sci Rep. 2020;10(1):15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mapplebeck JCS, Beggs S, Salter MW. Sex differences in pain: a tale of two immune cells. Pain. 2016;157 Suppl 1:S2–S6. [DOI] [PubMed] [Google Scholar]
- 50.Ray PR, Shiers S, Caruso JP, Tavares-Ferreira D, Sankaranarayanan I, Uhelski ML, et al. RNA profiling of human dorsal root ganglia reveals sex-differences in mechanisms promoting neuropathic pain. Brain. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


