Abstract
Assessing factors that influence chronic stress biomarkers like hair cortisol concentrations (HCCs) in pregnancy is critical to prevent adverse pregnancy outcomes. Thus, we aimed to identify correlates of HCC preconception and during pregnancy. 2,581 pregnant women participated in the study. HCC was available at four time periods: pre-pregnancy (0–3 months preconception, n = 1,023), and in the first (1–12 weeks, n = 1,734), second (13–24 weeks, n = 1,534), and third (25–36 weeks, n = 835) trimesters. HCC was assessed using liquid chromatography tandem mass spectrometry (LC-MS/MS). Sociodemographic, pregnancy- and hair-related characteristics, and measures of psychosocial stress, were interrogated as potential correlates of HCC. Spearman correlations, paired t-tests, and ANOVA were used to assess differences in log-transformed values of HCC (logHCC) across maternal characteristics. Multivariable linear regressions were used to identify the correlates of HCCs after adjusting for confounders. Mean logHCC values increased across the four prenatal periods (P < 0.001). In multivariable analyses, pre-pregnancy BMI was consistently associated with all HCCs, while gestational age, economic hardship, hair dyeing, and depression, showed time-specific associations with HCC. In conclusion, this study showed evidence of factors influencing HCC levels before and during pregnancy. The most consistent association was seen with pre-pregnancy BMI. Depression was also associated with HCC concentrations.
Keywords: hair cortisol concentrations, preconception, pregnancy, modifiable risk factors, correlates
INTRODUCTION
Psychological distress is one of the most pressing issues of public health worldwide. Globally, more than 250 million people are affected by depression 1 and more than 45 million people suffer from anxiety, making both disorders the leading contributors to global burden of disease and global disability 1–4. Women are disproportionally more exposed to stressors and factors that contribute to depression and anxiety than men 4,5, particularly in women of reproductive age and from low- and middle-income countries (LMICs) 5,6. In LMICs, symptoms of antenatal psychological distress, including depression and anxiety, are often unrecognized and untreated 7, and are associated with an elevated risk for preterm birth 8–12, low birth weight 13, postpartum depression 14–16, and impaired infant neurodevelopment 17,18 among others, implicating adverse health outcomes across generations 8.
The biological mechanisms linking antenatal distress to adverse health outcomes remain incompletely understood, partly because the specific etiology of antenatal distress, such as depression and anxiety itself is highly multifaceted 19–21. One facet that contributes to anxiety and depression etiology is the experience of severe or chronic stress 22. A well-understood biological stress response system is the hypothalamic-pituitary-adrenal (HPA) axis, which becomes increasingly activated during stressful experiences, leading to the secretion of glucocorticoids and catecholamines 23–25. The effector glucocorticoid of the HPA axis is cortisol, which can be used as an objective biomarker of stress 23–25. Due to situational and diurnal changes in cortisol secretion, cortisol measurement is challenging 26. Responding to these challenges, cortisol measurement in hair emerged as a promising biomarker, because it provides retrospective, aggregate measurement of cortisol secretion over long time periods (e.g. up to 9 months) 27,28.
Interestingly, HPA axis glucocorticoids also undergo massive alterations during the pregnancy course 29–32, indicating that HPA axis activity may be a decisive correlate to the elevated mental disorder prevalence of pregnant women. However, previous studies investigating the association between hair cortisol concentration (HCC) and antenatal psychological distress yielded inconclusive results showing both, positive associations 33,34 and null findings 35–37. Some of these studies have only poorly or highly heterogeneously addressed the influence of pregnancy-related and hair-related characteristics on HCC 26, despite the importance of characterizing HCC correlates to understand its influence on maternal mental health and pregnancy outcomes. Lastly, sample sizes in previous studies have been rather small (N range 23 to 768, median N = 108), and may not have had adequate statistical power for detecting the effects investigated 37–41. Given (i), the intergenerational impact on health outcomes associated with antenatal mental health disorders, particularly in LMICs, and (ii) inconclusive prior results about pregnancy-related, characteristics, and hair-related HCC correlates, we investigated (a) the influence of pregnancy and hair-related characteristics on HCC levels before and throughout pregnancy, and (b) the association between prenatal HCC and symptoms of antenatal depression, anxiety, and stress, using a large sample of pregnant Peruvian women (N = 2,581).
RESULTS
Population at baseline
We described the characteristics of the study population in Table 1. Briefly, the mean ± SD age of participants was 28 ± 6.3 years, most of them identified themselves as mestizo (84%), were married (83%), had normal (18.5–24.9 kg/m2) pre-pregnancy BMI (48%) and were multiparous (56%). Half of the participants reported having more than 12 years of education, almost the same proportion (47%) was employed in pregnancy, 39% reported having difficulty accessing basic foods, and 57% indicated that the index pregnancy was unplanned. Only 2% and 8% of participants reported tobacco smoking and using alcohol during pregnancy, respectively. Antenatal symptoms of depression and anxiety showed a prevalence of 23% and 48% in our study sample, respectively, and the mean PSS value was 19.9 ± 7.38.
Table 1.
Characteristics of narticinants at enrollment N = 2581)
| Characteristics | Mean or N | SD or % |
|---|---|---|
| Sociodemographics | ||
| Maternal Age (years) Mean ± SD | 28.4 | 6.27 |
| Maternal Age categorical | ||
| 18–19 | 128 | 5.0 |
| 20–29 | 1390 | 53.9 |
| 30–34 | 574 | 22.2 |
| ≥35 | 488 | 18.9 |
| Education (years) | ||
| ≤6 | 58 | 2.2 |
| 7–12 | 1203 | 46.6 |
| >12 | 1307 | 50.6 |
| Ethnicity Mestizo | 2169 | 84.0 |
| Employed during pregnancy, yes % | 1207 | 46.8 |
| Married or living with partner | 2146 | 83.1 |
| Planned pregnancy, yes % | 1090 | 42.2 |
| Difficulty accessing basic foods, yes % | 998 | 38.7 |
| Difficulty paying for medical care, yes % | 1390 | 53.9 |
| Smoking during pregnancy, yes % | 51 | 2.0 |
| Alcohol during pregnancy, yes % | 208 | 8.1 |
| Pre-pregnancy BMI (kg/m2) Mean ±SD | 25.6 | 4.06 |
| Pre-pregnancy BMI categorical | ||
| < 18.5 | 34 | 1.3 |
| 18.5–24.9 | 1240 | 48.0 |
| 25.0–29.9 | 938 | 36.3 |
| ≥ 30 | 341 | 13.2 |
| Gestational age at HCC collection (weeks) Mean ± SD | 16.7 | 8.09 |
| Gestational age at delivery (weeks) Mean ±SD | 38.7 | 1.89 |
| Parity | 1.13 | 1.22 |
| Nulliparous, yes % | 1118 | 43.3 |
| Infant sex, Female % | 1089 | 42.2 |
| Infant Birth Weight (g) Mean ± SD | 3400 | 599 |
| Stress measures | ||
| 9-item Patient Health Questionnaire-9 (PHQ-9) score Mean ±SD | 6.71 | 4.65 |
| PHQ-9 ≥ 10, yes % | 586 | 22.7 |
| 7-item Generalized Anxiety Disorder (GAD-7) score Mean ±SD | 6.58 | 4.56 |
| GAD ≥ 7, yes % | 1225 | 47.5 |
| Perceived Stress Scale Mean ± SD | 19.9 | 7.38 |
| PSS tertiles | ||
| Tertile 1 (low) | 844 | 32.7 |
| Tertile 2 (middle) | 844 | 32.7 |
| Tertile 3 (high) | 844 | 32.7 |
| Maternal HCC Mean ± SD | ||
| Pre-pregnancy (pg/mg) | 3.99 | 5.61 |
| First Trimester (pg/mg) | 4.16 | 4.49 |
| Second Trimester (pg/mg) | 4.59 | 5.75 |
| Third Trimester (pg/mg) | 5.99 | 7.75 |
| Hair Characteristics | ||
| Natural hair color | ||
| Black | 1522 | 59.0 |
| Brown | 191 | 7.4 |
| Other | 798 | 30.9 |
| Natural hair structure | ||
| Straight | 1909 | 74.0 |
| Curly | 595 | 23.1 |
| Hair washing frequency (in weeks) | ||
| 1–2 times | 148 | 5.7 |
| 3–5 times | 1717 | 66.5 |
| 6–7 times | 645 | 25.0 |
| Product used for hair washing | ||
| Shampoo only | 805 | 31.2 |
| Shampoo and Conditioner | 1690 | 65.5 |
| Other | 15 | 0.6 |
| Tinting, yes % | 1077 | 41.7 |
| Dyeing, yes % | 302 | 11.7 |
| Frequency haircuts | ||
| Every month | 134 | 5.2 |
| Every 3 months | 662 | 25.6 |
| Every 6 months | 745 | 28.9 |
| Once a year | 751 | 29.1 |
| Other | 201 | 7.8 |
Differences between HCCs
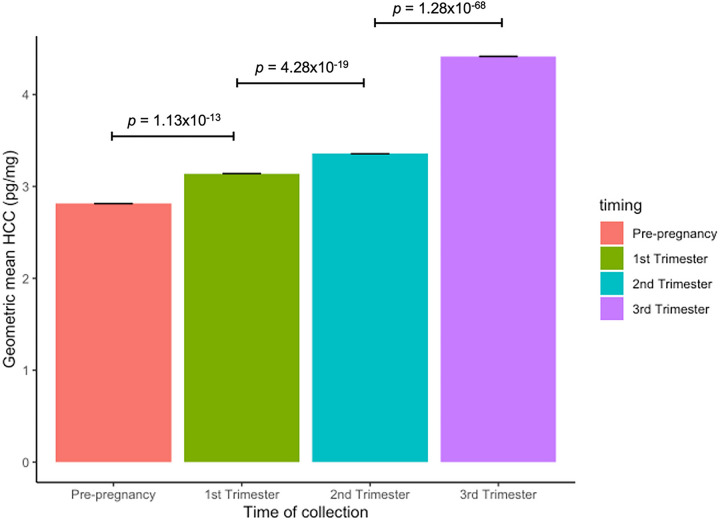
We observed increasing values of HCC from the pre-pregnancy period to the third trimester (Fig. 2). For instance, mean values of HCC pre-pregnancy (mean = 3.8 ± SD = 4.1 pg/mg) were 0.15 logHCC units lower (n = 1022, 95%CI = 0.11, 0.19) than mean values of HCC in the first trimester (4.1 ± 3.9 pg/mg). Mean values of HCC in the second trimester (4.5 ± 5.0 pg/mg) were 0.21 logHCC units higher (n = 694, 95%CI = 0.16, 0.25) than mean values of HCC in the first trimester. Lastly, mean values of HCC in the third trimester (5.6 ± 4.4 pg/mg) were 0.35 logHCC units higher (n = 832, 95%CI = 0.32, 039) than mean values of HCC in the second trimester. Using the Pearson’s method, strong positive correlations (r range 0.62–0.70, P < 0.001) were seen in the pairwise comparisons of logHCCs between the four consecutive periods (Supplementary Table 1).
Figure 2.
Geometric means of HCC across four prenatal time periods (pre-pregnancy, first trimester, second trimester, and third trimester) in pregnant women in Lima, Perú (N= 2,581). HCC is represented using the geometric mean (pg/mg). P-values comparing the statistical difference in the mean of logHCCs across consecutive time periods, were calculated using paired t-tests.
Bivariate analysis
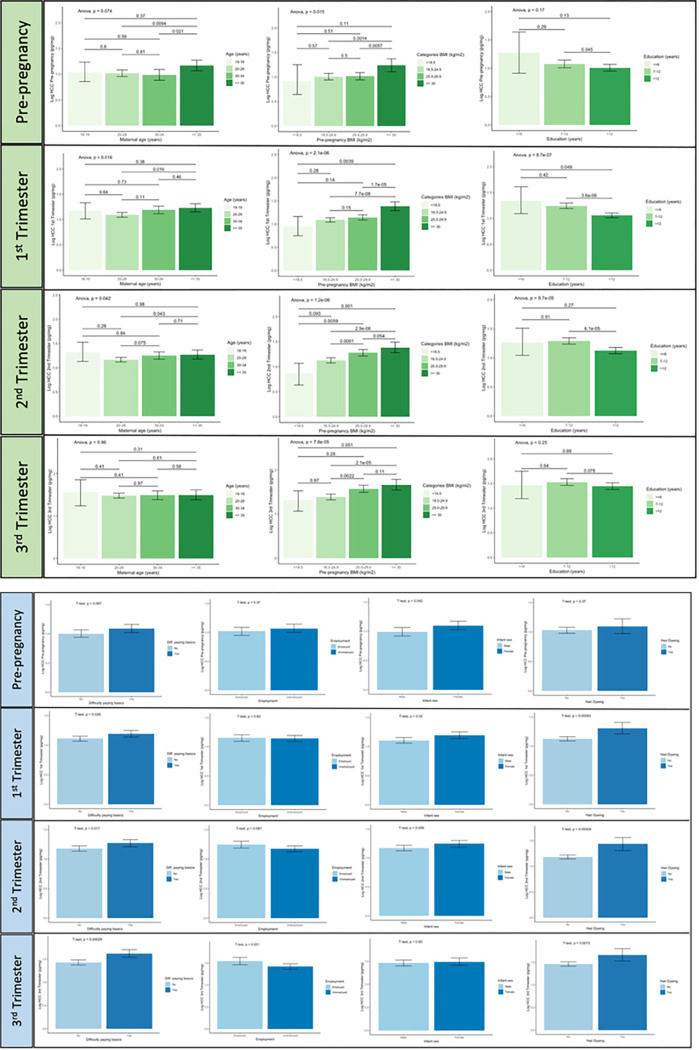
In the bivariate analysis, pre-pregnancy BMI was positively associated with logHCCs in all time periods (r range 0.11 to 0.18, P < 0.001). Gestational age at HCC collection was negatively associated with logHCC in the first and second trimesters (r range − 0.064 to −0.065, P < 0.01), while a positive association was seen with logHCC in the third trimester (r = 0.15, P < 0.001) (Table 2). Parity was correlated with logHCC values pre-pregnancy (r = 0.10, P < 0.001) and in the third trimester (r = −0.09, P < 0.01), and the PSS was inversely correlated with logHCC in the second trimester (r = −0.02, P < 0.01). GAD-7 and the PHQ-9 scores showed a tendency towards an association with logHCC in the second (r = −0.05) and third trimester (r = 0.07) (P ≤ 0.05). Including adjustment variables (maternal age, gestational age, pre-pregnancy BMI, and parity) did not change the magnitude of these correlations substantially (Table 2). Statistically significant differences in the geometric mean of HCCs were also seen with respect to categories of maternal age, pre-pregnancy BMI, parity, education, employment, difficulty accessing basic foods, infant sex, hair dyeing, frequency of hair cutting, and GAD-7 in at least two time periods (Table 3). As shown in Fig. 3, a trend towards increasing values of logHCCs was seen with increasing values of pre-pregnancy BMI in all time periods (PANOVA < 0.01). LogHCCs in the first and second trimesters decreased with increasing years of education (PANOVA < 0.001), and a U-shape association was seen with maternal age (PANOVA < 0.05) in the same trimesters. Having difficulty accessing basic foods, dyed hair, and a female infant, were commonly associated with higher logHCC in the pre-pregnancy and pregnancy periods (Fig. 3). Participants with symptoms of antenatal anxiety (GAD-7 score ≥ 7) had lower logHCCs in the second and third trimesters compared to women without symptoms of anxiety (Supplementary Fig. 2). No differences in the mean of logHCCs were observed with respect to symptoms of antenatal depression and perceived stress (PSS) in the four periods evaluated (Supplementary Fig. 2).
Table 2.
Pairwise correlations between log-transformed values of HCCs (logHCCs) and continuous covariates in pregnant women in Lima, Peru (N = 2,581). Correlations were adjusted for maternal age, gestational age at HCC collection (GA), pre-pregnancy BMI and parity
| Model | Maternal age (years) † | GA (weeks) | Pre-pregnancy BMI (kg/m2) † | Parity | PSS scale | PHQ-9 score | GAD-7 score |
|---|---|---|---|---|---|---|---|
| HCC Pre-pregnancy | |||||||
| Unadjusted | 0.047 | 0.031 | 0.105*** | 0.102*** | −0.036 | −0.049 | −0.035 |
| Adj. Age | – | 0.032 | 0.094*** | 0.078* | −0.033 | −0.051 | −0.036 |
| Adj. Age and GA | – | – | 0.091*** | 0.076* | −0.035 | −0.052 | −0.035 |
| Adj. Age, GA, and pre-pregnancy BMI | – | – | – | 0.057 | −0.035 | −0.051 | −0.034 |
| Adj. Age, GA, pre-pregnancy BMI and Parity | – | – | – | – | −0.042 | −0.064. | −0.046 |
| HCC First Trimester | |||||||
| Unadjusted | 0.038 | −0.064* | 0.120*** | 0.022 | −0.053. | −0.034 | −0.032 |
| Adj. Age | – | −0.063* | 0.108*** | 0.002 | −0.051. | −0.034 | −0.033 |
| Adj. Age and GA | – | – | 0.110*** | 0.018 | −0.048. | −0.041 | −0.021 |
| Adj. Age, GA, and pre-pregnancy BMI | – | – | – | 4.0E-05 | −0.049. | −0.046 | −0.026 |
| Adj. Age, GA, pre-pregnancy BMI and Parity | – | – | – | – | −0.051. | −0.047 | −0.03 |
| HCC Second Trimester | |||||||
| Unadjusted | 0.034 | −0.065* | 0.133*** | −0.019 | −0.015* | −0.001 | −0.050. |
| Adj. Age | – | −0.067* | 0.122*** | −0.037 | −0.014* | 0.001 | −0.051. |
| Adj. Age and GA | – | – | 0.123*** | −0.029 | −0.020 | −0.005* | −0.039 |
| Adj. Age, GA, and pre-pregnancy BMI | – | – | – | −0.045 | −0.020 | −0.005* | −0.044 |
| Adj. Age, GA, pre-pregnancy BMI and Parity | – | – | – | – | −0.010 | −0.008 | −0.027 |
| HCC Third Trimester | |||||||
| Unadjusted | 0.006 | 0.152*** | 0.177*** | −0.099* | −0.020 | 0.067. | −0.065 |
| Adj. Age | – | 0.152*** | 0.175*** | −0.102** | −0.020 | 0.068. | −0.066 |
| Adj. Age and GA | – | – | 0.182*** | −0.075* | −0.005 | 0.052 | −0.029 |
| Adj. Age, GA, and pre-pregnancy BMI | – | – | – | −0.102** | 0.001 | 0.053 | −0.031 |
| Adj. Age, GA, pre-pregnancy BMI and Parity | – | – | – | – | 0.034 | 0.046 | 0.008 |
Note. GA, gestational age at HCC collection (weeks); PSS, Perceived stress scale; GAD-7, 7-item generalized anxiety score; PHQ-9, 9-item Patient Health questionnaire score.
Variables log-transformed. GA, GAD-7, PHQ-9, and parity were analyzed using the Spearman method. For other covariates, Pearson's correlations were used. Adjusted correlations were obtained after retrieving logHCC residuals from stepwise adjusted linear regression models using as covariates maternal age, GA, pre-pregnancy BMI, and parity.
p < 0.01
p < 0.01
p < 0.001.
Table 3.
Geometric means of HCC (pg/mg) at four time periods (pre-pregnancy, first trimester, second trimester, and third trimester) across categories of maternal ba characteristics (N = 2,581).
| HCC Pre-pregnancy (N=1023) | HCC First Trimester (N=1734) | HCC Second Trimester (N=1534) | HCC Third Trimester (N=835) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Geometric Mean | SD | p | N | Geometric Mean | SD | p | N | Geometric Mean | SD | p | N | Geometric Mean | SD |
| Maternal Age (years) | |||||||||||||||
| 18–19 | 52 | 2.82 | 1.91 | <.05 | 101 | 3.33 | 2.20 | <.01 | 75 | 3.84 | 2.35 | .04 | 27 | 4.66 | 2.08 |
| 20–29 | 548 | 2.75 | 2.12 | 945 | 2.98 | 2.01 | 826 | 3.21 | 1.97 | 437 | 4.41 | 1.91 | |||
| 30–34 | 235 | 2.66 | 2.22 | 369 | 3.28 | 2.08 | 335 | 3.50 | 2.00 | 203 | 4.48 | 1.94 | |||
| ≥35 | 188 | 3.22 | 2.03 | 318 | 3.43 | 2.00 | 297 | 3.51 | 2.19 | 168 | 4.33 | 2.03 | |||
| Education (years) | |||||||||||||||
| ≤6 | 18 | 3.69 | 2.18 | .07 | 31 | 3.84 | 1.98 | <.01 | 40 | 3.76 | 2.07 | <.01 | 27 | 4.59 | 2.08 |
| 7–12 | 424 | 2.93 | 2.02 | 753 | 3.46 | 1.95 | 767 | 3.58 | 2.03 | 445 | 4.52 | 1.97 | |||
| >12 | 573 | 2.70 | 2.18 | 938 | 2.88 | 2.09 | 722 | 3.11 | 2.04 | 362 | 4.28 | 1.90 | |||
| Ethnicity | |||||||||||||||
| Mestizo | 829 | 2.80 | 2.12 | 0.65 | 1417 | 3.10 | 2.04 | .13 | 1319 | 3.29 | 2.04 | <.01 | 740 | 4.34 | 1.91 |
| Not Mestizo | 192 | 2.87 | 2.13 | 314 | 3.32 | 2.03 | 213 | 3.78 | 2.02 | 94 | 5.03 | 2.16 | |||
| Employment | |||||||||||||||
| Employed | 534 | 2.77 | 2.13 | .55 | 857 | 3.14 | 2.04 | .91 | 662 | 3.5 | 2.08 | .04 | 346 | 4.77 | 2.04 |
| Unemployed | 487 | 2.85 | 2.10 | 874 | 3.13 | 2.04 | 871 | 3.25 | 2.01 | 489 | 4.18 | 1.86 | |||
| Marital Status | |||||||||||||||
| Married | 849 | 2.84 | 2.16 | .20 | 1444 | 3.14 | 2.05 | .93 | 1277 | 3.33 | 2.05 | .36 | 691 | 4.40 | 1.96 |
| Not Married | 171 | 2.65 | 1.91 | 284 | 3.13 | 1.99 | 252 | 3.48 | 2.04 | 141 | 4.47 | 1.82 | |||
| Planned Pregnancy | |||||||||||||||
| Planned | 433 | 2.94 | 2.07 | .08 | 712 | 3.22 | 2.02 | .19 | 647 | 3.39 | 2.09 | .70 | 373 | 4.34 | 2.00 |
| Unplanned | 585 | 2.71 | 2.15 | 1016 | 3.08 | 2.05 | 879 | 3.34 | 2.01 | 455 | 4.47 | 1.90 | |||
| Difficulty accessing basic foods | |||||||||||||||
| Yes | 446 | 2.92 | 2.12 | .13 | 735 | 3.30 | 2.08 | .01 | 542 | 3.56 | 2.06 | .02 | 259 | 4.96 | 1.67 |
| No | 573 | 2.72 | 2.12 | 989 | 3.03 | 2.01 | 986 | 3.25 | 2.03 | 5.76 | 4.19 | 1.92 | |||
| Difficulty paying for medical care | |||||||||||||||
| Yes | 421 | 3.01 | 2.09 | <.01 | 774 | 3.26 | 2.04 | .03 | 953 | 3.30 | 2.01 | .30 | 610 | 4.31 | 1.93 |
| No | 589 | 2.66 | 2.13 | 939 | 3.03 | 2.04 | 568 | 3.44 | 2.10 | 220 | 4.70 | 1.99 | |||
| Smoking during pregnancy | |||||||||||||||
| Yes | 22 | 2.30 | 1.85 | .27 | 34 | 2.87 | 1.97 | .45 | 28 | 3.48 | 1.90 | .77 | 17 | 5.52 | 1.66 |
| No | 999 | 2.82 | 2.12 | 1601 | 3.15 | 2.04 | 1502 | 3.35 | 2.05 | 816 | 4.40 | 1.95 | |||
| Alcohol during pregnancy | |||||||||||||||
| Yes | 77 | 3.11 | 2.31 | .27 | 127 | 3.34 | 2.19 | .35 | 130 | 3.60 | 2.25 | .30 | 78 | 4.59 | 1.83 |
| No | 941 | 2.78 | 2.10 | 1601 | 3.12 | 2.03 | 1402 | 3.33 | 2.02 | 756 | 4.39 | 1.96 | |||
| Pre-pregnancy BMI (kg/m2) | |||||||||||||||
| <18.5 | 10 | 2.5 | 1.7 | .03 | 20 | 2.64 | 1.64 | <.01 | 24 | 2.61 | 1.79 | <.01 | 14 | 3.67 | 1.52 |
| 18.5–24.9 | 492 | 2.70 | 2.13 | 833 | 2.98 | 2.00 | 736 | 3.11 | 2.02 | 401 | 4.02 | 1.91 | |||
| 25.0–29.9 | 384 | 2.78 | 2.09 | 650 | 3.13 | 2.09 | 545 | 3.58 | 2.06 | 122 | 5.23 | 1.91 | |||
| ≥30 | 131 | 3.35 | 2.12 | 219 | 3.91 | 2.03 | 207 | 3.96 | 2.06 | 122 | 5.23 | 1.91 | |||
| Parity categorical | |||||||||||||||
| 0 | 480 | 2.68 | 2.04 | <.01 | 753 | 3.17 | 2.03 | <.01 | 464 | 3.47 | 2.06 | .26 | 195 | 4.93 | 1.95 |
| 1 | 360 | 2.72 | 2.21 | 580 | 2.91 | 2.03 | 484 | 3.24 | 2.03 | 270 | 4.47 | 2.02 | |||
| 2 | 131 | 3.26 | 2.08 | 264 | 3.39 | 2.05 | 301 | 3.49 | 2.13 | 168 | 4.21 | 1.96 | |||
| ≥3 | 49 | 3.59 | 1.94 | 132 | 3.51 | 2.08 | 280 | 3.23 | 1.96 | 199 | 4.04 | 1.81 | |||
| Nulliparous | |||||||||||||||
| Yes | 480 | 2.68 | 2.04 | .06 | 782 | 3.16 | 2.02 | .77 | 627 | 3.31 | 2.01 | .52 | 329 | 4.51 | 1.91 |
| No | 540 | 2.92 | 2.16 | 947 | 3.12 | 2.06 | 902 | 3.39 | 2.07 | 503 | 4.34 | 1.97 | |||
| Infant sex | |||||||||||||||
| Female | 442 | 2.98 | 2.11 | .02 | 726 | 3.30 | 2.12 | .01 | 638 | 3.48 | 2.08 | .06 | 355 | 4.43 | 2.03 |
| Male | 426 | 2.66 | 2.10 | 782 | 3.00 | 1.99 | 747 | 3.24 | 2.00 | 399 | 4.33 | 1.88 | |||
| Depression | |||||||||||||||
| PHQ-9 < 10 | 755 | 2.86 | 2.16 | .23 | 1290 | 3.19 | 2.07 | .07 | 1215 | 3.35 | 2.05 | .98 | 688 | 4.38 | 1.93 |
| PHQ-9 ≥ 10 | 263 | 2.69 | 1.99 | 437 | 2.98 | 1.94 | 316 | 3.35 | 2.2 | 146 | 4.56 | 2.04 | |||
| Anxiety | |||||||||||||||
| GAD-7 <7 | 681 | 2.84 | 2.10 | .48 | 1075 | 3.18 | 2.04 | .33 | 652 | 3.54 | 2.11 | .01 | 264 | 4.93 | 2.01 |
| GAD-7 ≥7 | 337 | 2.74 | 2.15 | 650 | 3.07 | 2.05 | 8.76 | 3.22 | 2.00 | 569 | 4.19 | 1.90 | |||
| PSS tertiles | |||||||||||||||
| Tertile 1 (low) | 339 | 2.92 | 2.08 | .84 | 554 | 3.24 | 1.98 | .27 | 498 | 3.46 | 2.12 | .27 | 288 | 4.51 | 2.02 |
| Tertile 2 (middle) | 342 | 2,88 | 2.25 | 547 | 3.17 | 2.10 | 493 | 3.23 | 2.01 | 292 | 4.35 | 1.91 | |||
| Tertile 3 (high) | 318 | 2.78 | 2.00 | 594 | 3.03 | 2.03 | 518 | 3.40 | 1.99 | 245 | 4.35 | 1.91 | |||
| Hair structure | |||||||||||||||
| Straight | 706 | 2.82 | 2.12 | .81 | 1197 | 3.16 | 2.06 | .58 | 1188 | 3.30 | 2.02 | .50 | 702 | 4.37 | 1.94 |
| Curly | 306 | 2.78 | 2.12 | 508 | 3.10 | 2.00 | 281 | 3.42 | 2.12 | 85 | 4.67 | 2.03 | |||
| Hair color | |||||||||||||||
| Black | 538 | 2.81 | 2.13 | .27 | 956 | 3.17 | 2.03 | .32 | 970 | 3.27 | 2.00 | .11 | 558 | 4.19 | 1.91 |
| Brown | 61 | 2.43 | 2.19 | 108 | 2.84 | 1.92 | 128 | 3.23 | 2.13 | 82 | 4.27 | 1.99 | |||
| Other | 412 | 2.87 | 2.10 | 642 | 3.15 | 2.08 | 379 | 3.56 | 2.13 | 153 | 5.51 | 2.03 | |||
| Tinting | |||||||||||||||
| Yes | 448 | 2.69 | 2.05 | .10 | 744 | 3.25 | 1.98 | .07 | 619 | 3.55 | 2.09 | <.01 | 327 | 4.59 | 1.96 |
| No | 564 | 2.90 | 2.17 | 963 | 3.06 | 2.09 | 857 | 3.19 | 2.00 | 465 | 4.32 | 1.95 | |||
| Dyeing | |||||||||||||||
| Yes | 127 | 2.98 | 2.07 | .32 | 190 | 3.67 | 1.98 | <.01 | 172 | 4.16 | 2.32 | <.01 | 110 | 5.37 | 2.04 |
| No | 885 | 2.78 | 2.13 | 190 | 3.67 | 1.98 | 1302 | 3.24 | 2.00 | 681 | 4.29 | 1.93 | |||
| Product used for hair washing | |||||||||||||||
| Shampoo only | 263 | 3.13 | 2.21 | .02 | 478 | 3.32 | 1.95 | .11 | 531 | 3.47 | 2.01 | .30 | 322 | 4.56 | 1.98 |
| Shampoo and Conditioner | 740 | 2.71 | 2.08 | 1219 | 3.07 | 2.07 | 938 | 3.27 | 2.06 | 464 | 4.34 | 1.94 | |||
| Other | 8 | 2.31 | 2.14 | 9 | 3.66 | 2.53 | 7 | 3.23 | 1.64 | 6 | 3.77 | 1.86 | |||
| Hair washing frequency (in weeks) | |||||||||||||||
| 1–2 times | 37 | 3.43 | 1.92 | .17 | 72 | 3.51 | 1.79 | .14 | 109 | 3.77 | 2.13 | .16 | 73 | 3.78 | 2.06 |
| 3–5 times | 753 | 2.75 | 2.12 | 1246 | 3.08 | 2.08 | 949 | 3.28 | 2.02 | 462 | 4.42 | 1.93 | |||
| 6–7 times | 221 | 2.89 | 2.13 | 388 | 3.27 | 1.96 | 418 | 3.36 | 2.06 | 257 | 4.63 | 1.96 | |||
| Frequency haircut | |||||||||||||||
| Every month | 45 | 2.83 | 2.09 | .01 | 86 | 3.23 | 1.96 | .01 | 89 | 3.16 | 2.00 | <.01 | 48 | 3.58 | 1.65 |
| Every 3 months | 272 | 2.55 | 2.25 | 461 | 2.86 | 2.22 | 384 | 3.04 | 2.08 | 195 | 4.07 | 1.90 | |||
| Every 6 months | 314 | 2.79 | 2.10 | 516 | 3.14 | 1.95 | 425 | 3.31 | 2.00 | 227 | 4.59 | 1.97 | |||
| Once a year | 295 | 2.86 | 2.01 | 484 | 3.30 | 1.97 | 446 | 3.63 | 2.02 | 264 | 4.60 | 2.02 | |||
| Other | 83 | 3.62 | 2.06 | 155 | 3.49 | 2.00 | 117 | 3.81 | 2.09 | 45 | 5.72 | 1.92 | |||
Mean (SD) corresponds to geometric mean values and standard deviation of geometric means of HCC in pg/mg. PHQ-9, 9-item Patient Health Questionnaire. 7-item Generalized Anxiety Disorder. PSS, Perceived Stress Scale.
Figure 3.
Distribution of Log-transformed values of HCC (logHCC) across categorical variables (N range 835–1734). P-values assessing the mean difference in logHCC between groups were calculated using unpaired t-tests for binary variables, and one-way Analysis of Variance (ANOVA) for ordinal variables. The post-hoc Tukey-Kramer test was implemented to report the p-value for the pairwise comparison of means across categories of maternal age (years), pre-pregnancy BMI (kg/m2), and education (years). Results were considered significant at p < 0.05.
Multivariable regressions
Parity was excluded from the multivariable analysis as it was strongly correlated with maternal age. Implementing fully adjusted models, we observed that pre-pregnancy BMI, gestational age at HCC, and hair dyeing, were associated with logHCC in different time periods, while difficulty accessing basic foods and symptoms of antenatal depression were associated with logHCC at a single time in pregnancy (Table 4). For instance, a 1 kg/m2 increase in pre-pregnancy BMI was on average associated with a 0.02 to 0.03 unit increase in logHCCs (P < 0.001) across the four prenatal periods (Table 4). Every 1-week increase in gestational age was associated with a 0.01 unit decrease in logHCC in the first and second trimesters (Table 4), but this association was positive with third trimester logHCC (β= 0.04 logHCC units, 95%CI = 0.02, 0.06). Likewise, women who dyed their hair had 0.12 and 0.20 higher logHCC in the first and second trimesters, respectively. Participants with symptoms of antenatal depression (PHQ-9 score ≥ 10) had 0.11 (95%CI= −0.20, −0.02) lower logHCC in the first trimester compared to women with no/mild antenatal depression. Having difficulty accessing basic foods was associated with higher logHCC in the third trimester alone (β= 0.18, 95%CI= 0.08, 0.29). Similar associations were obtained in the stepwise adjusted models (Supplementary Table 2).
Table 4.
Multivariable linear regressions assessing the association of logHCC with maternal predictors. HCC was measured in four time periods (pre-pregnancy, first trimester second trimester and third trimester) (N range 766–1 636)
| LogHCC Pre-pregnancya | LogHCC First Trimestera | LogHCC Second Trimestera | LogHCC Third Trimestera | |||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Maternal age (years) | 0.003 | −0.006, 0.012 | −0.001 | −0.007, 0.006 | −0.002 | −0.008, 0.004 | −0.007 | −0.015, 0.001 |
| Gestational age at HCC collection (weeks) | 0.011 | −0.013, 0.035 | −0.008* | −0.016, 0.000 | −0.009** | −0.016, −0.003 | 0.039*** | 0.016, 0.062 |
| Pre-pregnant BMI (kg/m2) | 0.015** | 0.002, 0.029 | 0.019*** | 0.009, 0.029 | 0.023*** | 0.0140 0.0325 | 0.033*** | 0.021, 0.045 |
| Maternal education (years) | ||||||||
| ≤6 | Ref | – | Ref | – | Ref | – | Ref | – |
| 7–12 | −0.115 | −0.503, 0.272 | −0.081 | −0.372, 0.210 | −0.017 | −0.261, 0.226 | 0.007 | −0.264, 0.279 |
| >12 | −0.161 | −0.548, 0.226 | −0.241 | −0.533, 0.050 | −0.169 | −0.414, 0.075 | −0.057 | −0.329, 0.216 |
| Maternal Employment | ||||||||
| Employed | Ref | – | Ref | – | Ref | – | Ref | – |
| Unemployed | 0.052 | −0.051, 0.155 | −0.007 | −0.082, 0.068 | −0.062 | −0.140, 0.015 | −0.090 | −0.190, 0.010 |
| Infant sex | ||||||||
| Male | Ref | – | Ref | – | Ref | – | Ref | – |
| Female | 0.097 | −0.162, 0.142 | 0.065 | −0.009, 0.140 | 0.057 | −0.020, 0.134 | −0.002 | −0.100, 0.096 |
| Difficulty accessing basic foods | ||||||||
| No | Ref | – | Ref | – | Ref | – | Ref | – |
| Yes | 0.093 | −0.010, 0.197 | 0.066 | −0.010, 0.142 | 0.066 | −0.015, 0.147 | 0.185*** | 0.079, 0.291 |
| Hair Dyeing | ||||||||
| No | Ref | – | Ref | – | Ref | – | Ref | – |
| Yes | −0.010 | −0.162, 0.142 | 0.123* | 0.005, 0.241 | 0.203*** | 0.081, 0.325 | 0.094 | −0.051, 0.239 |
| PSS tertiles | ||||||||
| Tertile 1 (low) | Ref | – | Ref | – | Ref | – | Ref | – |
| Tertile 2 (middle) | −0.019 | −0.142, 0.199 | −0.028 | −0.120, 0.064 | −0.068 | −0.161, 0.026 | −0.004 | −0.121, 0.112 |
| Tertile 3 (high) | −0.033 | −0.158, 0.092 | −0.073 | −0.164, 0.018 | −0.029 | −0.123, 0.066 | 0.004 | −0.120, 0.127 |
| GAD-7 ≥ 7 | ||||||||
| No anxiety | Ref | – | Ref | – | Ref | – | Ref | – |
| Anxiety | −0.017 | −0.092, 0.126 | −0.030 | −0.108, 0.048 | −0.074 | −0.155, 0.008 | −0.054 | −0.167, 0.059 |
| PHQ-9 ≥ 10 | ||||||||
| No depression | Ref. | – | Ref | – | Ref | – | Ref | – |
| Depression | −0.064 | −0.181, 0.053 | −0.114* | −0.200, −0.027 | −0.041 | −0.138, 0.055 | 0.029 | −0.100, 0.157 |
Note. PHQ-9, 9-item Patient Health Questionnaire. GAD-7, 7-item Generalized Anxiety Disorder. PSS, Perceived Stress Scale.
p < 0.01
p < 0.01
p < 0.001
Regression estimates for depression, anxiety and perceived stress were obtained from independent fully adjusted models.
DISCUSSION
Pregnancy is one of the most critical periods of human life, requiring massive changes in extremely complex physiological circuits. Interferences in these circuits can have adverse effects across generations. Hence, understanding these changes is key to preventing adverse effects and promoting a healthy life for children. Our study investigated HCC, a biomarker of HPA axis activity, with several covariates, including psychosocial stress measures (stress, anxiety, and depression) during the pre-pregnancy and pregnancy periods in a large sample of pregnant Peruvian women. Overall, we observed increased levels of HCC before and throughout pregnancy, peaking in the third trimester. Furthermore, we found that HCC was associated with pre-pregnancy BMI in all four prenatal periods, but time-specific associations were seen between HCC and gestational age at HCC collection, hair treatment, difficulty accessing basic foods, and symptoms of antenatal depression, after controlling for well-known confounders. We found no statistically significant associations between HCC and symptoms of anxiety or perceived stress neither before nor during pregnancy.
The mean HCC values observed in our sample were, on average, lower compared to other studies that investigated HCC during pregnancy, as shown in a recent systematic review of 56 studies by Marceau et al. 51. Nevertheless, the observed mean values in our sample ranging between 3.38 and 5.59 pg/mg, lie within most mean levels observed by Marceau et al. (2020). We are aware of only one study investigating HCC in a pregnant Peruvian sample 52 (not overlapping with samples in this study) 41,44,45,53,54. Interestingly, Dobernecker et al. 52 observed considerably higher HCC levels in 39 participants, which may be due to the small sample size used and the characteristics of the participants, given that more than 50% reported previous experiences of trauma, implicating a higher chronic cortisol secretion. Furthermore, we observed an increase in HCC across pregnancy, peaking in the third trimester. This increase in HCC across pregnancy was also found in previous studies, although not consistently. For example, Marceau et al. 51 did not find this increase in half of the reviewed studies, questioning this often-referenced assumption. Recently, this questioning was mitigated, as qualitatively more precise studies examining individual HCC trajectories 55 or more fine-grained time intervals 56 obtained HCC increases across pregnancy, although in a non-linear way, with interindividual differences and massive within-person variations. This non-linear cortisol increase is in accordance with established biological findings. Beginning with gestational week seven, cortisol secretion is increasingly stimulated by the temporary growth of the pituitary gland and by the placenta 57–61, due to an isolated excitatory influence of the placental cortisol-releasing hormone on cortisol secretion, leading to cortisol elevations up to five-fold in certain tissues 61–64.
For providing a methodological foundation for further investigations of HCC in pregnant samples, we investigated the bivariate influence of twenty-one pregnancy-related, sociodemographic, and hair-related covariates on HCC. The only covariates that showed a significant bivariate influence on HCC in at least two time points and in a consistent manner were maternal BMI, maternal education, difficulty accessing basic foods, and infant sex, which is partly consistent with previous studies 38–40,45,65. For instance, we showed a significant difference in prenatal HCC by infant sex, whereby women giving birth to a female infant had significantly higher HCC preconception and in the first trimester, compared to women giving birth to a male infant. The vast majority of previous studies did not find such an effect 33,38,39,44,61,66–73. However, Romero-Gonzalez et al.65 recently reported significant differences in prenatal HCC by infant sex. Similar to our findings, they found a higher first-trimester HCC in women carrying a female compared to a male baby 65. These findings indicate that prenatal experiences of stress may influence the survival of the fetus and the secondary sex ratio distribution in the population; however, further results from Romero-Gonzalez et al. 65 found no significant impact on infant sex by perceived stress.
The correlation observed between educational level and HCC is similarly inconsistent based on results from previous pregnancy studies. While some studies found a statistically significant influence of educational level on HCC 38–40,74,75, others found no such effect 61,68,76–78. For example, in a subsample of N = 62 pregnant women from Spain, HCC differed significantly regarding educational level across the three trimesters, with participants of higher education showing lower HCC in the first and second, but not in the third trimester 40. Our results were directionally and time-consistently related to the associations identified by Garcia-Leon et al. 40. Given the close relationship between educational attainment and income 79, it is possible that our finding of the correlation between education and HCC corresponds with the association identified between difficulty accessing basic foods and HCC in our study. This notion is supported by the fact that we found both variables, education and difficulty accessing basic foods, to be correlated, although this correlation was weak. We did not find other studies except our own 45,53 investigating difficulty accessing basic foods among pregnant women, which might be explained by the uniqueness of our LMIC sample, as most studies examining HCC among pregnant women have been conducted in high-income countries in North America and Europe 51 where including difficulty accessing basic foods might be less important.
In bivariate and multivariable analyses, we found that pre-pregnancy BMI was the strongest predictor of HCC before and during pregnancy. This finding is in accordance with some 39,44,55,66,74,75,80,81 but not all 38,45,67,77,82,83 previous studies. To our knowledge, only two studies have investigated the association between HCC and maternal BMI across all three trimesters 40,74 with partly conflicting findings. While Bosquet Enlow et al. 74 found a significant positive correlation between BMI and HCC in the second but not in the first and third trimesters in a sample of n = 93 U.S.-American women, Garcia-Leon et al. 40 failed to find such an association in their Spanish sample of n = 62 women. As we investigated HCC in a pregnant sample three to ten-fold larger than in previous studies, our findings of an unbiased and consistent association between pre-pregnancy BMI and HCC in all four prenatal periods (i.e., from preconception to the third trimester), add to this existing evidence. An association between BMI and HCC is likely, as associations between measures of obesity and both, HPA-axis activity 84 and stress 85, are well established in non-pregnant samples.
From the different psychosocial stress measures considered in our study, maternal symptoms of antenatal depression was the only measure associated exclusively with first-trimester HCC after controlling for relevant covariates. While such findings have been reported in previous studies, evidence is still conflicting about the temporal aspect of this association. For instance, there have been studies that report a positive association of HCC with antenatal depression in every trimester of pregnancy 33,34, except for the pre-pregnancy period 41. However, an equal number of studies support a null association of antenatal depression with HCC in late pregnancy 35–37, or throughout pregnancy 41. Identifying an association of depression with HCC restricted to the first trimester is a plausible finding. One explanation for this finding is the fact that levels of HCC in early pregnancy are lower than in subsequent trimesters, where they tend to be more influenced by physiological factors, which implies that the associations between psychosocial stressors like depression and HCC are more likely to be detected in early versus in late pregnancy 86. This observation agrees with the concept of a “ceiling effect” for stressors measured in pregnancy, which suggests that their influence is only apparent when the physiological response measured is low to moderate 41. The fact that in our study we assessed antenatal depression at the time of the interview in early and mid-pregnancy and HCC from preconception to the third trimester, indicates that our results are less likely to be influenced by the time in which the stressor was measured and more related to the time in which the psychoendocrine association is interrogated.
Our study has different strengths. First, this is the largest study conducted to date assessing the correlates of HCC in the prenatal period, affording greater power to identify true associations with HCC compared to previous smaller studies. In addition, we were able to assess HCC from preconception through to the third trimester, which allowed us to better characterize HCC’s relative response in relation to multiple factors acting during pregnancy. Lastly, we included a comprehensive list of sociodemographic, pregnancy-related, hair-related, and psychosocial covariates in the characterization of HCC correlates in pregnancy, and this helped us to confirm existing evidence and provide foundational evidence about the role of multiple stressors on relative changes in HCC during pregnancy.
Our study comes with some limitations. Despite our large sample size, not all individuals in our sample had longitudinal measures of HCC from preconception through to the third trimester, reason why comparisons of HCCs were only possible between consecutive time periods, where paired measures were available. Despite this limitation, groups of samples in our study with at least two consecutive measures of HCC in pregnancy (n from 60–1,022) were larger than the number of samples included in a recent study addressing longitudinal changes of HCC in pregnancy (n = 98) 41. The lack of a full longitudinal assessment of HCC in our study prevented us from interrogating individual patterns of HCC change across pregnancy, and from identifying interindividual trajectories of HCC change, and their association with multiple stressors measured in pregnancy. Future studies addressing this gap are required in larger samples from longitudinal pre-birth cohort studies. Lastly, our study included pregnant women from Perú, and the specific socioeconomic and cultural context of this population means that our findings may not be generalizable to other populations from different socioeconomic backgrounds.
CONCLUSIONS
The need for valid biomarkers of psychological distress is high, but their identification remains a challenge. Facing this challenge, we investigated the novel biomarker HCC and its association with pregnancy-related, sociodemographic, and hair-related covariates in the pre-pregnancy and pregnancy periods. We found that maternal HCC was significantly and consistently associated with pre-pregnancy BMI across four prenatal periods, while other correlates such as gestational age at HCC collection, hair treatment, difficulty accessing basic foods, and symptoms of antenatal depression, showed time-specific associations with HCC after adjustment for relevant covariates. The results of our study set a foundation for a better understanding of the biological factors involved in the regulation of maternal HPA-axis functioning and its influence on psychosocial distress measures in pregnancy. This understanding is urgently needed to eventually reduce the global burden of prenatal psychological distress on adverse health outcomes for the next generation.
METHODS
Study population
Participants in this study were drawn from two cohort studies of the same underlying source population. The first study is the Pregnancy Outcomes, Maternal, and Infant Study (PrOMIS), a prospective cohort study designed to assess the maternal social and behavioral determinants of adverse pregnancy outcomes 42. The second study is the Screening Treatment and Effective Management of Gestational Diabetes Mellitus Study (STEM-GDM), a cross-sectional study designed to assess the prevalence of gestational diabetes among pregnant Peruvian women, with the goal of improving GDM screening and management 43. Women recruited in both studies attended prenatal care clinics at the Instituto Nacional Materno Perinatal (INMP) in Lima, Perú. The INMP is Perú’s national reference center for perinatal and neonatal care, operated by the Ministry of Health. Details of each study have been provided before 42,44. Briefly, for PrOMIS, recruitment started in February 2012 and ended in November 2015. Participants were enrolled in PrOMIS if they initiated prenatal care in early pregnancy (< 16 gestational weeks) and were followed up until delivery. For the STEM-GDM study, recruitment started in February 2013 and ended in June 2014. Participants were invited to participate in the study if they initiated prenatal care prior to 28 weeks (mean gestational age 26 ± 1.3 weeks). For both studies, eligible participants were > 18 years of age, had singletons, were able to speak, read and write in Spanish, and were planning to deliver at the INMP; otherwise, women were excluded. In addition, for STEM-GDM, women were excluded if they had pre-existing diabetes, took glucose-lowering medication, or had a chronic condition 43. Human subjects research was conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants in PrOMIS and STEM-GDM, and review boards from the INMP and the Harvard T.H. Chan School of Public Health approved all study procedures.
Analytic sample
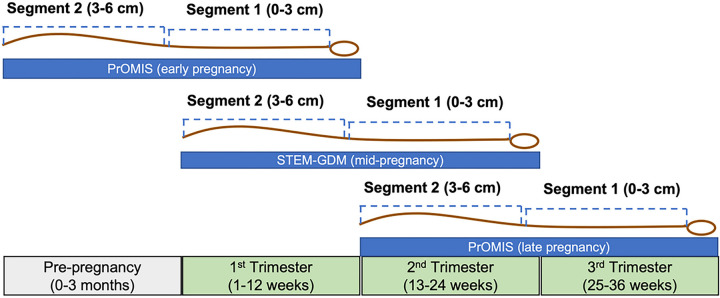
Participants included in the analysis were a subset of the total sample in PrOMIS and STEM-GDM who provided a 6 cm scalp hair sample at the time of enrollment. In PrOMIS, 1,623 participants had HCC measured from a hair sample collected at enrollment in early pregnancy (4–19 gestational weeks), while 427 (29%) participants had HCC from a hair sample collected in late pregnancy (24–36 gestational weeks). For STEM-GDM, HCC was available in 530 participants who contributed a hair sample at the time of enrollment in mid-pregnancy (24–28 gestational weeks). Each hair sample was cut into two 3 cm hair segments to assess HCC at two time periods. The hair segment closest to the scalp (0–3 cm) measured HCC around the time of the visit, which occurred either in the first, second or third trimester (Fig. 1). The second hair segment farther from the scalp (3–6 cm) in the same participant, measured HCC retrospectively 3 months prior to the time of hair collection (i.e., HCC pre-conception, in the first trimester or the second trimester, respectively) (Fig. 1). Before analyses, we set as missing values of HCC for 7 participants whose measurements were > 60 pg/mg pre-pregnancy or in the first trimester, > 80 pg/mg in the second trimester, or > 40 pg/mg in the third trimester. These cut-offs corresponded to HCC values that fell more than 3 times outside the interquartile range value of HCC from above (Quartile 3 + 3*IQR) or below (Quartile 1–3*IQR). In total, 2,581 participants were included in the analysis considering their availability of HCC in at least one time period: pre-pregnancy (0–3 months preconception), first trimester (1–12 gestational weeks), second trimester (13–24 gestational weeks), or third trimester (25–36 gestational weeks).
Figure 1.
Diagram showing the segmental analysis of HCC from scalp hair samples collected across pregnancy from two pre-birth cohorts in Lima, Perú (N=2,581). Each hair sample collected in early, mid-, and late pregnancy, was segmented into two hair segments (3 cm each) to assess current HCC measures from segment 1 (0–3 cm from the scalp), and HCC in the 3 months prior to the hair sampling from segment 2 (3–6 cm from the scalp).
Hair collection and HCC assessment
Detail of the procedures implemented for hair collection and HCC assessment have been previously described 41,44,45. Briefly, trained staff collected a lock of hair (~ 100 strands of hair) from the back of the head (posterior vertex) as close to the scalp as possible. Hair samples were stored in foil paper at room temperature until their analysis. For each participant, each 6 cm of hair was segmented into two 3 cm hair segments and analyzed in the same batch to avoid within-subject variability due to batch effects. Cortisol concentrations were measured in pg/mg and obtained using a standardized liquid chromatography tandem mass spectrometry (LC-MS/MS) assay as previously described 41,45, with a lower limit of detection of 0.1 pg/mg. Six blinded control samples were randomly allocated to assess variability 45. The inter-assay coefficient of variation was 8.1%, which is within acceptable limits46.
Sociodemographic, pregnancy-related, and hair-characteristic measures
We recorded information about the participant’s sociodemographic and lifestyle variables, anthropometrics, reproductive health, and hair characteristics using structured questionnaires conducted by research staff at enrollment. Sociodemographic and lifestyle variables included age (years), years of education (< 6 years, 7–12 years, > 12 years), ethnicity (mestizo/not mestizo), employment status during pregnancy, marital status, infant sex, difficulty accessing basic foods (yes vs no), smoking, and alcohol use during pregnancy. Anthropometric variables included self-reported pre-pregnancy BMI (kg/m2) and infant birth weight (g) abstracted from medical records. Pre-pregnancy BMI was also used categorically (< 18.5, 18.5–24.9, 25–29.9, ≥ 30). Questions about the women’s reproductive health assessed the number of previous pregnancies (parity), if the pregnancy was planned, gestational age at the time of hair collection (weeks), and gestational age at delivery (weeks). Gestational age at visit was calculated using the last menstrual period. Hair characteristics included the natural hair structure (straight, curly), hair color (black, brown, other), frequency of hair washing (1–2, 3–5, or 6–7 times per week), and cutting (every month, every 3 months, 6 months or once a year), the product used to wash hair (shampoo, and conditioner, other), and the use of chemical products for hair tinting and dyeing.
Antenatal symptoms of Depression, Generalized Anxiety, and Perceived Stress
Different psychosocial stress measures were assessed at the time of the interview in early pregnancy and in mid-pregnancy from participants of the PrOMIS and STEM-GDM studies, respectively, using the same instruments. These scales have been previously translated and validated to use in Spanish-speaking populations 41. Symptoms of antenatal depression, perceived stress, and generalized anxiety were assessed using the 9-item Patient Health Questionnaire (PHQ-9) 47, the 14-item Perceived Stress Scale (PSS) 48, and the 7-item Generalized Anxiety Disorder Assessment (GAD-7) 49, respectively. The PHQ-9 measured symptoms of antenatal depression during the last 14 days from the time of the interview, it ranges from 0–27, and a score ≥ 10 was deemed positive for antenatal depressive symptoms 45,50. The PSS assessed perceived feelings and thoughts of stress for situations happening during the last month. PSS ranges from 0–56, and was categorized using tertiles, each tertile representing increasing values of the score (Tertile 1 = low, Tertile 2 = middle, and Tertile 3 = high PSS). The GAD-7 scale measured symptoms of antenatal anxiety during the last 14 days, it ranges from 0–21, and a cut-off of 7 was used to define anxiety symptoms.
Statistical Analysis
Continuous variables were visually inspected for normality using histograms and quantile (Q-Q) plots. Baseline characteristics of participants were described using mean ± standard deviation (SD), or frequency and percent. Due to the right-skewed distribution of HCC,, we used geometric means and SD to compare HCCs concentrations across categories of maternal covariates. Values of HCC were log-transformed (logHCC) for further analyses (Supplementary Fig. 1). We used paired t-tests to compare mean differences in logHCCs for consecutive time periods (i.e., pre-pregnancy vs first trimester, first trimester vs second trimester, and second trimester vs third trimester). Unpaired t-tests and one-way analysis of variance (ANOVA) were used to assess mean differences in logHCCs across categorical binary and ordinal covariates, respectively. Post-hoc Tukey Kramer tests were used in the pairwise comparison of means of logHCCs across categories of ordinal covariates. Spearman correlations were used to evaluate the unadjusted and adjusted correlations of logHCCs with most of the continuous covariates, except for maternal age, pre-pregnancy BMI (log-transformed), and PSS, for which the Pearson method was used. Adjusted correlations were obtained using the residuals from stepwise fitted regressions of logHCCs with the covariates. Multivariable linear regressions were used to estimate the independent associations of logHCCs with multiple covariates and with measures of maternal psychosocial stress using a fully adjusted model. We also used stepwise fitted models to establish the covariates that were more correlated with logHCCs. Covariates included in the stepwise-adjusted and in the fully-adjusted models, were selected based on their association with HCC in at least two time periods from bivariate analyses, and based on previous literature 11,12. Thus, adjustment was applied for maternal age, gestational age at HCC collection, pre-pregnancy BMI, difficulty accessing basic foods, education, hair dyeing, and infant sex. Parity was strongly correlated with maternal age (r = 0.5, P < 0.01); thus, it was excluded from the final list of covariates to avoid collinearity issues. We reported the coefficients of association (β) and the 95% confidence intervals in logHCC units. To ease interpretation, we also presented association estimates in pg/mg of HCC. For all the analyses, a two-tailed P < 0.05 was considered statistically significant. All analyses were conducted in R version 4.1.2.
ACKNOWLEDGEMENTS
The authors thank the dedicated staff members of Asociación Civil Proyectos en Salud (PROESA), Perú and Instituto Materno Perinatal, Perú for their expert technical assistance with this research.
FUNDING DISCLOSURE
The PrOMIS study was supported by awards from the National Institutes of Health (NIH) (R01HD102342; R21HD102822; R21MH28985, R01HD059835). The STEM-GDM study was supported by awards from Roche Diagnostic Operations Inc (project number 208617-5074547). The funders had no further involvement in the study design, analysis, and interpretation of evidence, not in the manuscript preparation and decision to submit the paper for publication.
Footnotes
Declarations
ADDITIONAL INFORMATION
No potential conflicts of interest were reported by the authors.
Contributor Information
Diana L. Juvinao-Quintero, Department of Epidemiology, Harvard T.H. Chan School of Public Health
Richard G. Künzel, Department of Epidemiology, Harvard T.H. Chan School of Public Health
Gloria Larabure-Torrealva, Instituto Nacional Materno Perinatal.
Laramie Duncan, Department of Psychiatry and Behavioral Sciences, Stanford University.
Clemens Kirschbaum, Technische Universität Dresden.
Sixto E. Sanchez, Universidad de San Martin de Porres, Facultad de Medicina Humana, Instituto de Investigacion
Bizu Gelaye, Department of Epidemiology, Harvard T.H. Chan School of Public Health.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not openly available due to reasons of sensitivity. It will be available from the corresponding author upon reasonable request.
References
- 1.Liu Q. et al. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 126, 134–140 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Murray C. J. L. et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet Lond. Engl. 380, 2197–2223 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Smith K. Mental health: A world of depression. Nature 515, 180–181 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Yang X. et al. Global, regional and national burden of anxiety disorders from 1990 to 2019: results from the Global Burden of Disease Study 2019. Epidemiol. Psychiatr. Sci. 30, e36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faravelli C., Alessandra Scarpato M., Castellini G. & Lo Sauro C. Gender differences in depression and anxiety: The role of age. Psychiatry Res. 210, 1301–1303 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Woody C. A., Ferrari A. J., Siskind D. J., Whiteford H. A. & Harris M. G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 219, 86–92 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Fisher J. et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull. World Health Organ. 90, 139G–149G (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davalos D. B., Yadon C. A. & Tregellas H. C. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch. Womens Ment. Health 15, 1–14 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Eke A., Saccone G. & Berghella V. Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and risk of preterm birth: a systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 123, 1900–1907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez S. E. et al. Risk of Spontaneous Preterm Birth in Relation to Maternal Depressive, Anxiety and Stress Symptoms. (2014). [PMC free article] [PubMed] [Google Scholar]
- 11.Staneva A., Bogossian F., Pritchard M. & Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women Birth 28, 179–193 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Wisner K. L. et al. Major Depression and Antidepressant Treatment: Impact on Pregnancy and Neonatal Outcomes. Am. J. Psychiatry 166, 557–566 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman S. & Hatch M. C. Depressive symptomatology during pregnancy: Evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol. 19, 535–543 (2000). [PubMed] [Google Scholar]
- 14.Norhayati M. N., Nik Hazlina N. H., Asrenee A. R. & Wan Emilin W. M. A. Magnitude and risk factors for postpartum symptoms: A literature review. J. Affect. Disord. 175, 34–52 (2015). [DOI] [PubMed] [Google Scholar]
- 15.O’Hara M. W. Postpartum depression: what we know. J. Clin. Psychol. 65, 1258–1269 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Robertson E., Grace S., Wallington T. & Stewart D. E. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen. Hosp. Psychiatry 26, 289–295 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Barros M. C., de M., Mitsuhiro S. S., Chalem E., Laranjeira R. R. & Guinsburg R. Depression during gestation in adolescent mothers interferes with neonatal neurobehavior. Rev. Bras. Psiquiatr. Sao Paulo Braz. 1999 35, 353–359 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Piallini G. et al. How Do Maternal Subclinical Symptoms Influence Infant Motor Development during the First Year of Life? Front. Psychol. 7, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brummelte S. & Galea L. A. M. Postpartum depression: Etiology, treatment and consequences for maternal care. Horm. Behav. 77, 153–166 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Newman M. G., Llera S. J., Erickson T. M., Przeworski A. & Castonguay L. G. Worry and Generalized Anxiety Disorder: A Review and Theoretical Synthesis of Evidence on Nature, Etiology, Mechanisms, and Treatment. Annu. Rev. Clin. Psychol. 9, 275–297 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saveanu R. V. & Nemeroff C. B. Etiology of Depression: Genetic and Environmental Factors. Psychiatr. Clin. 35, 51–71 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Biaggi A., Conroy S., Pawlby S. & Pariante C. M. Identifying the women at risk of antenatal anxiety and depression: A systematic review. J. Affect. Disord. 191, 62–77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller W. L. The Hypothalamic-Pituitary-Adrenal Axis: A Brief History. Horm. Res. Paediatr. 89, 212–223 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Sheng J. A. et al. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 14, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer R. L. & Deak T. A users guide to HPA axis research. Physiol. Behav. 178, 43–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stalder T. & Kirschbaum C. Analysis of cortisol in hair – State of the art and future directions. Brain. Behav. Immun. 26, 1019–1029 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Kirschbaum C., Tietze A., Skoluda N. & Dettenborn L. Hair as a retrospective calendar of cortisol production—Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34, 32–37 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Raul J.-S., Cirimele V., Ludes B. & Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin. Biochem. 37, 1105–1111 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Carr B. R., Parker C. R., Madden J. D., MacDonald P. C. & Porter J. C. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am. J. Obstet. Gynecol. 139, 416–422 (1981). [DOI] [PubMed] [Google Scholar]
- 30.Cousins L. et al. Qualitative and quantitative assessment of the circadian rhythm of cortisol in pregnancy. Am. J. Obstet. Gynecol. 145, 411–416 (1983). [DOI] [PubMed] [Google Scholar]
- 31.Demey-Ponsart E., Foidart J. M., Sulon J. & Sodoyez J. C. Serum CBG, free and total cortisol and circadian patterns of adrenal function in normal pregnancy. J. Steroid Biochem. 16, 165–169 (1982). [DOI] [PubMed] [Google Scholar]
- 32.Fleming A. S., Steiner M. & Corter C. Cortisol, Hedonics, and Maternal Responsiveness in Human Mothers. Horm. Behav. 32, 85–98 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Caparros-Gonzalez R. A. et al. Hair cortisol levels, psychological stress and psychopathological symptoms as predictors of postpartum depression. PLOS ONE 12, e0182817 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman M. C., Mazzoni S. E., Wagner B. D., Laudenslager M. L. & Ross R. G. Measures of Maternal Stress and Mood in Relation to Preterm Birth. Obstet. Gynecol. 127, 545–552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wikenius E. et al. The Association between Hair Cortisol and Self-Reported Symptoms of Depression in Pregnant Women. PLOS ONE 11, e0161804 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braig S. et al. The Association of Hair Cortisol with Self-Reported Chronic Psychosocial Stress and Symptoms of Anxiety and Depression in Women Shortly after Delivery: Hair cortisol, self-reported chronic stress, and symptoms of anxiety and depression. Paediatr. Perinat. Epidemiol. 30, 97–104 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Scharlau F. et al. Evaluation of hair cortisol and cortisone change during pregnancy and the association with self-reported depression, somatization, and stress symptoms. Stress 21, 43–50 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Andhavarapu M., Orwa J., Temmerman M. & Musana J. W. Maternal Sociodemographic Factors and Antenatal Stress. Int. J. Environ. Res. Public. Health 18, 6812 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braig S. et al. Determinants of maternal hair cortisol concentrations at delivery reflecting the last trimester of pregnancy. Psychoneuroendocrinology 52, 289–296 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Leon M. A. et al. Hair cortisol concentrations in a Spanish sample of healthy adults. PLOS ONE 13, e0204807 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orta O. R. et al. Stress and hair cortisol concentrations from preconception to the third trimester. Stress 22, 60–69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrios Y. V. et al. Childhood Abuse and Early Menarche Among Peruvian Women. J. Adolesc. Health 56, 197–202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larrabure-Torrealva G. T. et al. Prevalence and risk factors of gestational diabetes mellitus: findings from a universal screening feasibility program in Lima, Peru. BMC Pregnancy Childbirth 18, 303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juvinao-Quintero D. L. et al. Maternal hair cortisol concentrations and its association with increased insulin resistance in midpregnancy. Ann. Epidemiol. S104727972300042X (2023) doi: 10.1016/j.annepidem.2023.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orta O. R. et al. An evaluation of distal hair cortisol concentrations collected at delivery. Stress 21, 355–365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tworoger S. S. & Hankinson S. E. Use of biomarkers in epidemiologic studies: minimizing the influence of measurement error in the study design and analysis. Cancer Causes Control CCC 17, 889–899 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Kroenke K., Spitzer R. L. & Williams J. B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen S., Kamarck T. & Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 24, (1983). [PubMed] [Google Scholar]
- 49.Spitzer R. L., Kroenke K., Williams J. B. W. & Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 166, 1092–1097 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Yang N. et al. Correlates of early pregnancy serum brain-derived neurotrophic factor in a Peruvian population. Arch. Womens Ment. Health 20, 777–785 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marceau K., Wang W., Robertson O. & Shirtcliff E. A. A systematic review of hair cortisol during pregnancy: Reference ranges and methodological considerations. Psychoneuroendocrinology 122, 104904 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dobernecker J. et al. Cumulative trauma predicts hair cortisol concentrations and symptoms of depression and anxiety in pregnant women—an investigation of community samples from Greece, Spain and Perú. Sci. Rep. 13, 1434 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gelaye B. et al. Chronic HPA activity in mothers with preterm delivery: A pilot nested case-control study. J. Neonatal-Perinat. Med. 13, 313–321 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Kelsall N. C. et al. Association between trauma exposure and glucocorticosteroid concentration in hair during pregnancy. Psychoneuroendocrinology 151, 106072 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marceau K., Rolan E., Robertson O. C., Wang W. & Shirtcliff E. A. Within-person changes of cortisol, dehydroepiandrosterone, testosterone, estradiol, and progesterone in hair across pregnancy, with comparison to a non-pregnant reference group. Compr. Psychoneuroendocrinology 5, 100024 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King L. S., Humphreys K. L., Cole D. A. & Gotlib I. H. Hair cortisol concentration across the peripartum period: Documenting changes and associations with depressive symptoms and recent adversity. Compr. Psychoneuroendocrinology 9, 100102 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Economides D. et al. Relationship between maternal and fetal corticotrophin-releasing hormone-41 and ACTH levels in human mid-trimester pregnancy. J. Endocrinol. 114, 497–501 (1987). [DOI] [PubMed] [Google Scholar]
- 58.Goland R. S., Wardlaw S. L., Blum M., Tropper P. J. & Stark R. I. Biologically active corticotropin-releasing hormone in maternal and fetal plasma during pregnancy. Am. J. Obstet. Gynecol. 159, 884–890 (1988). [DOI] [PubMed] [Google Scholar]
- 59.Jones S. A., Brookes A. N. & Challis J. R. G. Steroids Modulate Corticotropin-Releasing Hormone Production in Human Fetal Membranes and Placenta. J. Clin. Endocrinol. Metab. 68825–830, 825–830 (1989). [DOI] [PubMed] [Google Scholar]
- 60.Riley S. C., Walton J. C., Herlick J. M. & Challis J. R. G. The Localization and Distribution of Corticotropin-Releasing Hormone in the Human Placenta and Fetal Membranes throughout Gestation*. J. Clin. Endocrinol. Metab. 72, 1001–1007 (1991). [DOI] [PubMed] [Google Scholar]
- 61.Swales D. A. et al. Exposure to traumatic events in childhood predicts cortisol production among high risk pregnant women. Biol. Psychol. 139, 186–192 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allolio B. et al. Diurnal Salivary Cortisol Patterns During Pregnancy and After Delivery: Relationship to Plasma Corticotrophin-Releasing-Hormone. Clin. Endocrinol. (Oxf.) 33, 279–289 (1990). [DOI] [PubMed] [Google Scholar]
- 63.Glynn L. M., Davis E. P. & Sandman C. A. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides 47, 363–370 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Lowry P. J. Corticotropin-releasing factor and its binding protein in human plasma. Ciba Found. Symp. 172, 108–115; discussion 115–128 (1993). [PubMed] [Google Scholar]
- 65.Romero-Gonzalez B., Puertas-Gonzalez J. A., Gonzalez-Perez R., Davila M. & Peralta-Ramirez M. I. Hair cortisol levels in pregnancy as a possible determinant of fetal sex: a longitudinal study. J. Dev. Orig. Health Dis. 12, 902–907 (2021). [DOI] [PubMed] [Google Scholar]
- 66.Mustonen P. et al. Maternal prenatal hair cortisol is associated with prenatal depressive symptom trajectories. Psychoneuroendocrinology 109, 104383 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Schonblum A. et al. Can hair steroids predict pregnancy longevity? Reprod. Biol. 18, 410–415 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Galbally M. et al. Prenatal predictors of childhood anxiety disorders: An exploratory study of the role of attachment organization. Dev. Psychopathol. 1–12 (2021) doi: 10.1017/S0954579421001206. [DOI] [PubMed] [Google Scholar]
- 69.Conradt E. et al. Prenatal maternal hair cortisol concentrations are related to maternal prenatal emotion dysregulation but not neurodevelopmental or birth outcomes. Dev. Psychobiol. 62, 758–767 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galbally M., van Rossum E. F. C., Watson S. J., de Kloet E. R. & Lewis A. J. Trans-generational stress regulation: Mother-infant cortisol and maternal mental health across the perinatal period. Psychoneuroendocrinology 109, 104374 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Bruinhof N., Vacaru S. V., van den Heuvel M. I., de Weerth C. & Beijers R. Prenatal hair cortisol concentrations during the COVID-19 outbreak: Associations with maternal psychological stress and infant temperament. Psychoneuroendocrinology 144, 105863 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hunter S. K. et al. Maternal corticosteroids and depression during gestation and decreased fetal heart rate variability. NeuroReport 32, 1170–1174 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stoye D. Q. et al. Perinatal determinants of neonatal hair glucocorticoid concentrations. Psychoneuroendocrinology 128, 105223 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bosquet Enlow M. et al. Maternal cortisol output in pregnancy and newborn telomere length: Evidence for sex-specific effects. Psychoneuroendocrinology 102, 225–235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kajanoja J. et al. Alexithymic Traits and Hair Cortisol Concentrations in Pregnant Women. Front. Psychiatry 11, 421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howells M. et al. Maternal stress and hair cortisol among pregnant women following hurricane Florence. Am. J. Hum. Biol. 35, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Musana J. et al. Obstetric risk in pregnancy interacts with hair cortisone levels to reduce gestational length. Front. Glob. Womens Health 3, 878538 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Penner F., Khoury J. E., Bosquet Enlow M. & Lyons-Ruth K. Threat versus deprivation in mother’s childhood: Differential relations to hair cortisol and psychopathology in pregnancy. Child Abuse Negl. 139, 106107 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quispe-Mamani J. C. et al. Effect of Education on the Economic Income of Households in Peru, Application of the Mincer Theory in Times of Pandemic (COVID-19). Soc. Sci. 11, 300 (2022). [Google Scholar]
- 80.Nyström-Hansen M. et al. Hair cortisol in the perinatal period mediates associations between maternal adversity and disrupted maternal interaction in early infancy. Dev. Psychobiol. 61, 543–556 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bosquet Enlow M. et al. Maternal Lifetime Trauma Exposure, Prenatal Cortisol, and Infant Negative Affectivity. Infancy 22, 492–513 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Musana J. W. et al. Association of differential symptoms of stress to hair cortisol and cortisone concentrations among pregnant women in Kenya. Stress 23, 556–566 (2020). [DOI] [PubMed] [Google Scholar]
- 83.Verheyen V. J. et al. Residential exposure to air pollution and access to neighborhood greenspace in relation to hair cortisol concentrations during the second and third trimester of pregnancy. Environ. Health 20, 11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith S. R. THE ENDOCRINOLOGY OF OBESITY. Endocrinol. Metab. Clin. North Am. 25, 921–942 (1996). [DOI] [PubMed] [Google Scholar]
- 85.Tomiyama A. J. Stress and Obesity. (2018). [Google Scholar]
- 86.Entringer S. et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress Amst. Neth. 13, 258–268 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity. It will be available from the corresponding author upon reasonable request.