Abstract
Background:
National Blood Collection and Utilization Surveys (NBCUS) have reported decreases in U.S. blood collections and transfusions since 2008. The declines began to stabilize in 2015–2017, with a subsequent increase in transfusions in 2019. Data from the 2021 NBCUS were analyzed to understand the current dynamics of blood collection and use in the United States.
Methods:
In March 2022, all community-based (53) and hospital-based (83) blood collection centers, a randomly selected 40% of transfusing hospitals performing 100–999 annual inpatient surgeries, and all transfusing hospitals performing ≥1000 annual inpatient surgeries were sent a 2021 NBCUS survey to ascertain blood collection and transfusion data. Responses were compiled, and national estimates were calculated for the number of units of blood and blood components collected, distributed, transfused, and outdated in 2021. Weighting and imputation were applied to account for non-responses and missing data, respectively.
Results:
Survey response rates were 92.5% (49/53) for community-based blood centers, 74.7% (62/83) for hospital-based blood centers, and 76.3% (2102/2754) for transfusing hospitals. Overall, 11,784,000 (95% confidence interval [CI], 11,392,000–12,177,000) whole blood and apheresis red blood cell (RBC) units were collected in 2021, a 1.7% increase from 2019; 10,764,000 (95% CI, 10,357,000–11,171,000) whole blood-derived and apheresis RBC units were transfused, a 0.8% decrease. Total platelet units distributed increased by 0.8%; platelet units transfused decreased by 3.0%; plasma units distributed increased by 16.2%; and plasma units transfused increased by 1.4%.
Discussion:
The 2021 NBCUS findings demonstrate a stabilization in U.S. blood collections and transfusions, suggesting a plateau has been reached for both.
Keywords: blood donation, blood transfusion, survey
1 |. INTRODUCTION
Although the percentage of hospitalized patients who receive a red blood cell (RBC) transfusion in the United States has decreased since 2014, blood transfusion remains one of the most commonly performed hospital procedures.1 As of 2018, an estimated 3.8% of hospitalizations in the United States included an RBC transfusion.1 National surveys of U.S. blood collection centers and transfusing facilities have been conducted intermittently since 1971 to understand national trends in blood collection and use and whether the national blood supply is adequately meeting the demand for blood. Since 2013, the Centers for Disease Control and Prevention (CDC) and the Office of the Assistant Secretary for Health (OASH) have administered the National Blood Collection and Utilization Survey (NBCUS), which has been the main source of data on blood collection and use in the United States since it was launched in 1997.2–5 NBCUS continues to be an important source of generalizable and nationally representative data on blood collection and use.
Results from NBCUS have highlighted decreasing trends in blood and blood component collections and transfusions in the United States since 2008.2–5 These declines in blood collection and use are likely due to several factors, including evidence-based updates to transfusion guidelines that recommend a more restrictive transfusion threshold, at a lower hemoglobin level; advanced minimally invasive surgeries; use of pharmacologic agents that decrease the need for transfusion; and adoption of patient blood management programs.1,5–11 Observed decreases in blood collection and transfusion in the United States were most pronounced from 2008 to 2013, with a stabilization of the rate of decline first observed in 2015.3 Results from the 2019 NBCUS were the first national data on blood collection and use since 2008 that suggested the declining trend in transfusions had stopped, as no year-to-year decrease was observed.5
To understand the current dynamics of blood collection and use in the United States, we present both the data and analysis on blood and blood component collection, distribution, and transfusion in the United States during 2021.
2 |. METHODS
Methods for survey development, management, and analysis were like those used for previous NBCUS surveys.2–5
The sampling frame for acute care hospitals was determined using the American Hospital Association (AHA) 2019 annual survey database.12 The sampling frame was enhanced in June 2021 by comparison with AHA’s monthly updates of facilities that had opened or closed since 2019. Hospitals were excluded if they: (a) performed fewer than 100 inpatient surgeries a year; (b) were in a U.S. territory or freely associated state; (c) were operated by the military or Department of Justice; or (d) were classified as a rehabilitation, long-term acute care, or psychiatric hospital. Overall, 3767 hospitals were included in the sampling frame (Figure 1). Among these, 40% of hospitals that performed 100–999 inpatient surgeries a year (n = 677/1690) were selected at random to participate, and 100% of hospitals performing 1000 or more inpatient surgeries a year (n = 2077) were selected to participate. The sampling frame for blood collection centers was determined using the U.S. Food and Drug Administration’s (FDA) Blood Establishment Registration database. Overall, 53 community-based (i.e., non-hospital-based) blood collection centers and 83 hospital-based blood collection centers were identified and included in the 2021 NBCUS (Figure 1).
FIGURE 1.
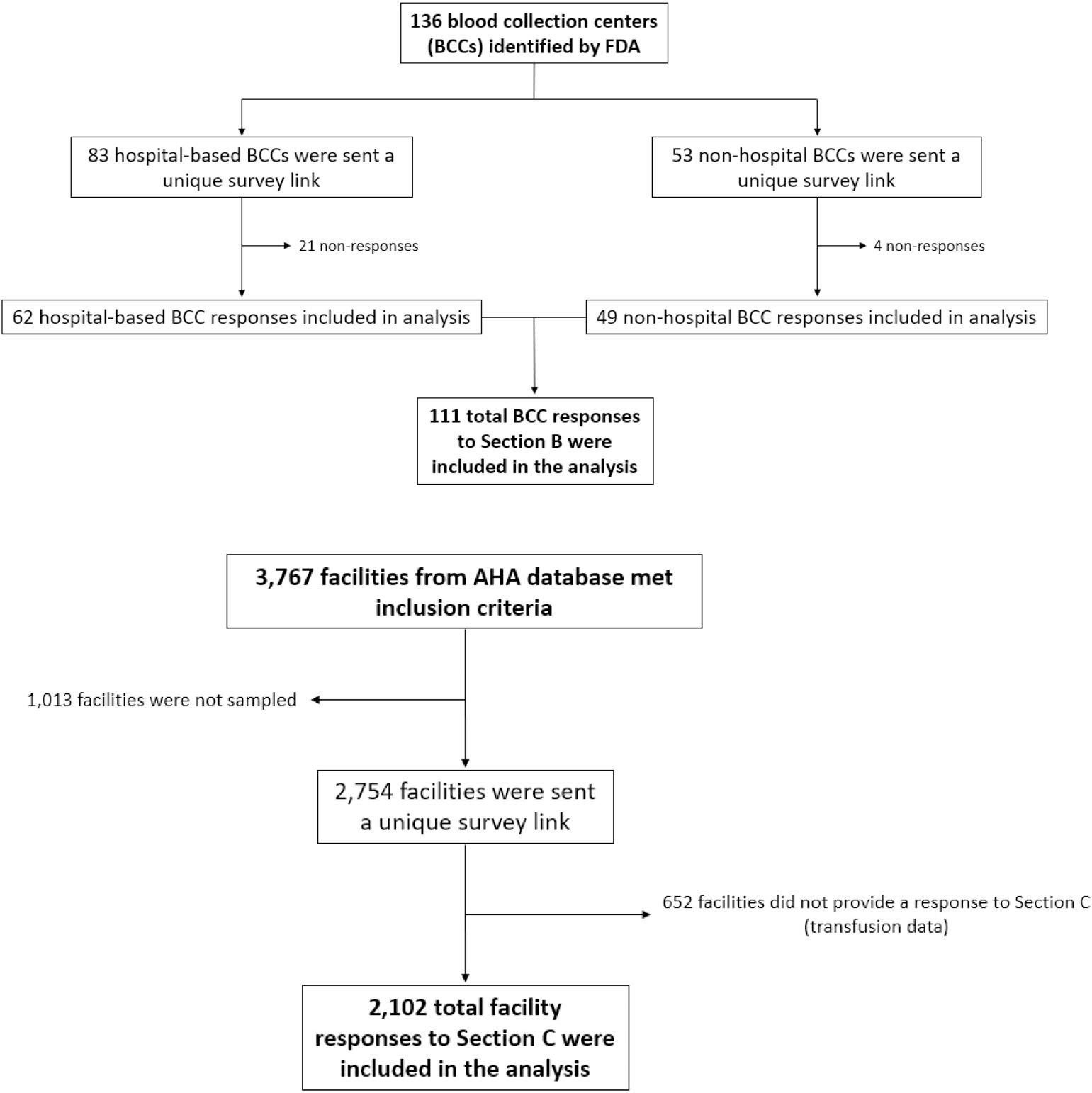
Flow diagram depicting identification, sampling, non-responses, and total responses analyzed of 2021 National Blood Collection and Utilization Survey respondents. AHA, American Hospital Association.
Hospitals selected to participate in the 2021 NBCUS were emailed a web-based REDCap (Research Electronic Data Capture, Nashville, Tennessee) survey to confirm the correct contact information for each facility. Non-respondents were subsequently contacted via email, telephone, and/or U.S. mail. Community-based blood collection centers were contacted to confirm contact information by email, with telephone follow-up where necessary. These data were obtained and confirmed from October 2021 to March 2022. The NBCUS was launched in March 2022 with 49 questions in total grouped into four sections: Blood Collection, Processing, and Testing during 2021; Blood Transfusion during 2021; a brief supplemental section on Blood Collection during 2020; and a brief supplemental section on Blood Transfusion during 2020. The two supplemental sections were added to understand the impact of the COVID-19 pandemic on blood collection and use during 2020. A unique survey link was sent electronically to the confirmed points of contact at all sampled hospitals and blood collection centers. Facilities were given two months to complete the survey, with a three-week extension. After survey dissemination, reminders were sent, via email and U.S. mail, to facilities that had an incomplete or unopened survey. Additional follow-up was conducted by telephone.
National estimates, rounded to the nearest 1000, were calculated for the number of units of blood and blood components collected, distributed (i.e., units collected minus units rejected and outdated at the blood center), rejected, outdated, and transfused in 2021. Community-based blood collection centers were stratified based on the number of whole blood or RBC units collected in 2019 into the following strata: fewer than 50,000; 50,000–199,999; 200,000–399,999, and 400,000 or more units. Hospital-based blood collection centers were stratified for blood collection data based on the number of inpatient surgical operations in 2019 from the AHA annual survey into the following strata: fewer than 1000; 1000–7999; and 8000 or more inpatient surgical operations. Hospitals were stratified separately for transfusion data based on the number of inpatient surgical operations in 2019 from the AHA annual survey into the following categories: 100–999, 1000–1399, 1400–2399, 2400–4999, 5000–7999, and 8000 or more inpatient surgical operations.
Survey weighting was used to account for non-responses using weights calculated as the ratio of the total number of eligible participants to the total number of respondents. Community-based blood collection centers in the strata with 400,000 or more RBC collections were designated a weight of 1.0. The Taylor Series method was used to calculate confidence intervals (CIs) for national collection and transfusion estimates.13 Multiple imputation was used to account for missing data elements among respondents. Imputed variables were all continuous and non-normally distributed. A two-step imputation process was used to accommodate distributions skewed toward zero using established imputation factors from previous surveys.14–16
Multiple imputation and survey weighting were applied to whole blood and apheresis RBCs collected, distributed, rejected, outdated, and transfused, and to whole blood-derived and apheresis platelets, plasma, and cryoprecipitated antihemophilic factor (AHF) units distributed and transfused. Whole blood-derived platelets were expressed as apheresis equivalents based on the reported pool size. An additional analysis of the unweighted mean and median number of RBCs transfused in facilities with data reported in both 2021 and 2019 stratified by inpatient surgical procedure category is presented as a sensitivity analysis to understand trends.
National rates of whole blood and RBC units collected per 1000 population were calculated by dividing the total estimated number of units collected, before the removal of rejected units, by the 2019 U.S. population aged 16–64 years. National rates of whole blood and RBC units transfused per 1000 population were calculated by dividing the total estimated number of units transfused by the entire 2019 U.S. population. Population estimates were determined using state-specific and age-specific estimates from the U.S. Census Bureau for 2021.17
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3 |. RESULTS
3.1 |. Survey response rates
The response rates for the 2021 NBCUS were 92.5% (49/53) for community-based blood collection facilities, 74.7% (62/83) for hospital-based blood collection facilities, and 76.3% (2102/2754) for transfusing hospitals (Figure 1). Based on the FDA’s Blood Establishment Registration database and America’s Blood Centers Members List, the number of community-based blood collection facilities was stable between 2019 and 2021 (53), following a decrease from 65 in 2017 to 53 in 2019. During 2019–2021, the only changes observed were a decrease in the number of community-based blood collection facilities reporting between 50,000 and 199,999 whole-blood and RBC collections per year (from 19 in 2019 to 17 in 2021) and a corresponding increase in the number of facilities reporting <50,000 RBC collections (25 in 2019 to 26 in 2021) and between 200,000 and 399,999 RBC collections (4 in 2019 to 5 in 2021). The number of hospital-based blood collection facilities was also stable between 2019 and 2021, with 90 facilities meeting eligibility criteria in 2019 and 83 meeting eligibility criteria in 2021.
3.2 |. Whole blood and RBC collections and transfusions
During 2021, 11,784,000 whole blood and apheresis RBC units (95% confidence interval [CI], 11,392,000–12,177,000 units) were collected in the United States, a 1.7% increase from 2019, when 11,590,000 units of whole blood and apheresis RBCs were collected (Table 1). Among the whole blood units collected in the United States during 2021, 99.9% were collected for allogeneic, nondirected transfusions. The total number of whole blood units collected for allogeneic, nondirected transfusions during 2021 was 9,842,000 units (95% CI, 9,491,000–10,193,000 units), a 0.7% increase from 2019; the number of whole blood units collected for directed transfusions was unchanged; and the number of whole blood units collected for autologous transfusions decreased by 40.0%, from 5000 units in 2019 to 3000 units (95% CI, 2000–3000 units) in 2021. During the same time period, the number of apheresis RBC units collected increased by 7.3%, from 1,800,000 units in 2019 to 1,931,000 units (95% CI, 1,771,000–2,090,000 units) in 2021. Reversing the trend seen in 2019, the number of whole blood-derived and apheresis RBC units distributed (i.e., units collected minus units rejected and outdated at the blood center) from blood collection facilities to hospitals increased by 1.4% between 2019 and 2021, from 10,879,000 units in 2019 to 11,033,000 units (95% CI, 10,647,000–11,420,000 units) in 2021 (Figure 2, Table 1).
TABLE 1.
Estimated numbers of whole blood and RBC units collected, transfused, and outdated in 2021 and 2019 (expressed in thousands) in the United States, as reported to the National Blood Collection and Utilization Survey.
| Blood centers |
Hospitals | Combined totals |
95% CI | 2019 totalsa |
% change 2021–2019 |
|
|---|---|---|---|---|---|---|
| Collections | ||||||
| Whole blood units | ||||||
| Allogeneic, nondirected | 9490 | 351 | 9842 | (9491–10,193) | 9777 | 0.7% |
| Autologous | 2 | 1 | 3b | (2–3)c | 5 | −40.0% |
| Directed | 4 | 5 | 9 | (6–13) | 9 | 0.0% |
| Apheresis RBC unitsd | 1922 | 9 | 1931 | (1771–2090) | 1800 | 7.3% |
| Total supply | 11,419 | 366 | 11,784 | (11,392–12,177) | 11,590 | 1.7% |
| Rejected on testing | 117 | 4 | 121 | (108–133) | 103 | 17.5% |
| Rejected for other reasonse | 610 | 20 | 630 | (551–709) | 608 | 3.6% |
| Total available supply | 10,692 | 342 | 11,033 | (10,647–11,420) | 10,879 | 1.4% |
| Transfusions | ||||||
| Allogeneic, nondirected | 10,752 | (10,346–11,159) | 10,834 | −0.8% | ||
| Autologous | 2f | (1–2)g | 9 | −77.8% | ||
| Directed | 10 | (2–18) | 9 | 11.1% | ||
| Total transfusions | 10,764 | (10,357–11,171) | 10,852 | −0.8% | ||
| Outdated whole blood or RBCs | 159 | 146 | 305 | (254–356) | 349 | −12.6% |
2019 totals were obtained from the 2019 NBCUS report.5
Estimate is rounded from 2649 to 3000.
Lower bound of the 95% CI is rounded from 2219 to 2000; upper bound of the 95% CI is rounded from 3080 to 3000.
Apheresis RBC units include allogeneic, autologous, directed, and concurrent collections.
Units rejected for other reasons do not include outdated units.
Estimate is rounded from 1615 to 2000.
Lower bound of the 95% CI is rounded from 810 to 1000; upper bound of the 95% CI is rounded from 2420 to 2000.
FIGURE 2.
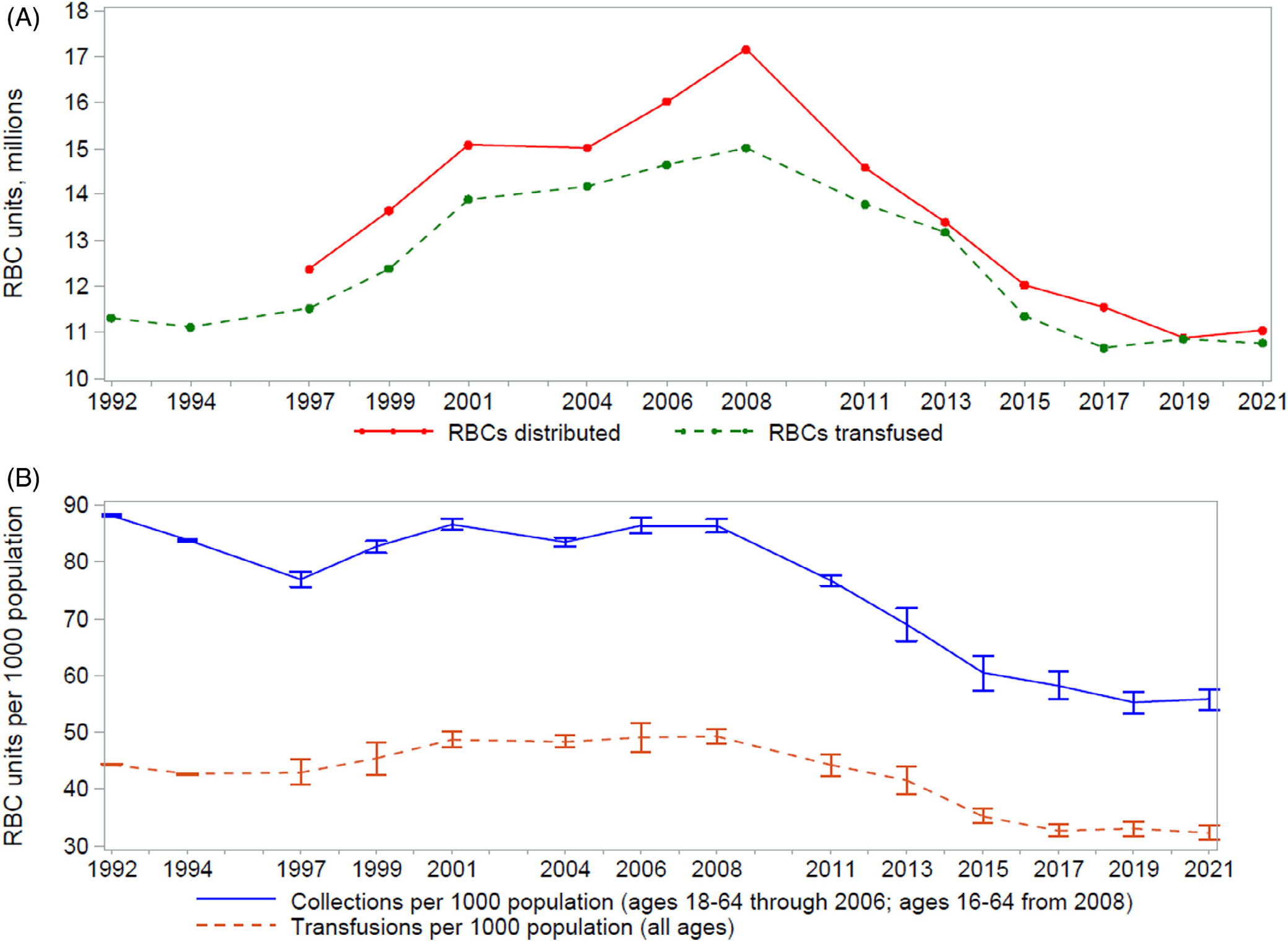
Trends in (A) RBC distributions (i.e., units collected minus units rejected and outdated at the blood center) and transfusions and (B) RBC collections and transfusions per 1000 population, United States, from the National Blood Collection and Utilization Survey, 1992–2021. The U.S. population aged 16–64 years (18–64 years prior to 2008) was used to calculate the rate of RBC units collected per 1000 population, while the entire U.S. population was used to calculate the rate of RBC units transfused per 1000 population. This difference in denominator populations leads to an apparent wider gap observed between the lines denoting RBC collections and transfusions in (B) when compared with (A).
During 2021, 10,764,000 units of whole blood-derived and apheresis RBCs were transfused in the United States (95% CI, 10,357,000–11,171,000 units), a 0.8% decrease from 2019, when 10,852,000 units were transfused, although this is not a statistically significant difference (Table 1). Of the 10,764,000 units transfused in the United States during 2021, 99.9% were allogeneic, nondirected transfusions; 10,000 units (95% CI, 2000–18,−000 units) were for directed transfusions, representing an increase of 11.1% from 2019; and 2000 units (95% CI, 1000–2000 units) were for autologous transfusions.
No significant differences were seen between 2019 and 2021 for whole blood-derived and apheresis RBC units collected and transfused per 1000 population (Figure 2). In 2021, the number of whole blood-derived and apheresis RBC units collected per 1000 population was 55.8 (compared to 55.3 in 2019), and the number transfused per 1000 population was 32.4 (compared to 33.1 in 2019).
During 2021, 121,000 whole blood-derived and apheresis RBC units (95% CI, 108,000–133,000 units) were rejected based on abnormal disease marker results after collection, a 17.5% increase from 2019 (when 103,000 units were rejected based on testing) (Table 1). An additional 630,000 units (95% CI, 551,000–709,000 units) were rejected for other reasons after collection, a 3.6% increase from 2019. The total available supply (collections minus rejections) of whole blood-derived and apheresis RBC units in 2021 was 11,033,000 units (95% CI, 10,647,000–11,420,000 units), which represents a 1.4% increase from 2019. There were 305,000 whole blood-derived and apheresis RBC units (95% CI, 254,000–356,000 units) outdated during 2021, a 12.6% decrease from 2019, when 349,000 units were outdated (Table 1).
3.3 |. RBC transfusion by hospital size and by location within a healthcare facility
From sub-analyses using matched data from 1453 hospitals that provided allogeneic RBC transfusion data for both the 2019 and 2021 NBCUS surveys, the median percent difference in the number of allogeneic RBC units transfused between 2019 and 2021 was a 1.5% increase (Table 2). Although the median percent difference differed within each surgical volume category, the differences were not pronounced, with the only negative change from 2019 to 2021 seen within the facilities in the lowest surgical volume category (those performing between 100 and 999 inpatient surgeries per year), at −0.9%. For facilities performing between 1000 and 1399 surgeries per year, transfusions increased by 6.0% between 2019 and 2021. For the remaining facilities (those performing ≥1400 inpatient surgeries per year), the median percent change in allogeneic RBC units transfused ranged from an increase of 0.5% to an increase of 1.8% from 2019 to 2021.
TABLE 2.
Percent difference in allogeneic RBC units transfused in 2021 and 2019 from matched hospitals participating in the National Blood Collection and Utilization Survey.
| Surgical volume category | Na | Median (mean) 2021 allogeneic RBCsa |
Median % differenceb |
IQRc of % differenceb |
|---|---|---|---|---|
| 100–999 surgeries per year | 153 | 489 (616) | −0.9% | 37.2% |
| 1000–1399 surgeries per year | 182 | 1302 (1398) | 6.0% | 34.0% |
| 1400–2399 surgeries per year | 346 | 2021 (2252) | 1.5% | 24.7% |
| 2400–4999 surgeries per year | 430 | 3524 (3967) | 0.5% | 19.9% |
| 5000–7999 surgeries per year | 184 | 6379 (7339) | 1.8% | 22.7% |
| 8000 or more surgeries per year | 158 | 13,210 (16,185) | 1.5% | 13.3% |
| Total | 1453 | 2809 (4640) | 1.5% | 23.4% |
Based on matched facilities reporting allogeneic RBC transfusions in both 2019 and 2021 NBCUS surveys.
% difference calculated as 100 × (2021–2019)/2019.
Interquartile range (75th–25th percentile).
Results of analyses looking at locations within healthcare facilities where transfusions were performed during 2021 were similar to prior NBCUS results (Table 3). During 2021, the most RBC units were transfused in inpatient medicine settings (including hematology/oncology), with 3,703,000 RBC units (95% CI, 3,404,000–4,002,000 units) transfused, followed by critical care settings, with 1,776,000 RBC units (95% CI, 1,586,000–1,966,000 units) transfused, outpatient and non-acute inpatient settings, with 1,388,000 RBC units (95% CI, 1,176,000–1,600,000 units) transfused, emergency departments, with 1,380,000 RBC units (95% CI, 1,269,000–1,491,000 units) transfused, and surgery settings (including transplant), with 1,181,000 RBC units (95% CI, 995,000–1,366,000 units) transfused. Overall, 205,000 RBC units (95% CI, 181,000–229,000 units) were transfused in obstetrics/gynecology settings; 164,000 RBC units (95% CI, 103,000–224,000 units) were transfused in pediatric settings; and 137,000 RBC units (95% CI, 98,000–175,000 units) were transfused in neonatal settings.
TABLE 3.
Red blood cell units transfused by location in 2021 and 2019 (expressed in thousands) as reported to the National Blood Collection and Utilization Survey.
| 2021 (95% CI) | 2019 | % difference | Matched median % difference |
|
|---|---|---|---|---|
| All surgery (including transplant) | 1181 (995–1366, n = 781) | 1380 | −16.9% | −4.6% (n = 357) |
| Emergency department | 1380 (1269–1491, n = 797) | 1277 | 7.5% | 26.0% (n = 358) |
| Inpatient medicine (including hematology/oncology) | 3703 (3404–4002, n = 784) | 3909 | −5.6% | −0.7% (n = 353) |
| Obstetrics/Gynecology | 205 (181–229, n = 772) | 219 | −6.4% | 0.0% (n = 268) |
| Pediatrics | 164 (103–224, n = 755) | 159 | 3.0% | 3.0% (n = 67) |
| Neonatal | 137 (98–175, n = 786) | 103 | 24.6% | 0.0% (n = 141) |
| Critical care | 1776 (1586–1966, n = 705) | 1810 | −1.9% | 0.3% (n = 268) |
| Outpatient and non-acute inpatient settingsa | 1388 (1176–1600, n = 769) | 1512 | −9.0% | −5.8% (n = 277) |
Includes outpatient dialysis, rehabilitation, and long-term care.
Trends in RBC transfusions differed by healthcare facility location (Table 3). The largest increase from 2019 to 2021 occurred in neonatal settings, with a 24.6% increase. Increases in RBC transfusions between 2019 and 2021 were also observed in emergency departments (7.5% increase) and pediatric settings (3.0%). The largest decrease from 2019 to 2021 occurred in surgery settings, with a difference of −16.9%. Decreases in RBC transfusions between 2019 and 2021 were also observed in outpatient and non-acute inpatient settings (−9.0%), obstetrics/gynecology settings (−6.4%), inpatient medicine settings (−5.6%), and critical care settings (−1.9%). Among transfusing hospitals reporting data on RBC units transfused by location in both 2019 and 2021, the largest increase in matched median percent difference of RBC units transfused was in emergency departments (26.0%). Non-significant differences were seen in inpatient medicine, obstetrics/gynecology, pediatric, neonatal, and critical care settings. Outpatient and non-acute inpatient settings and surgery settings both saw decreases in the matched median percent difference in RBC units transfused, with a difference of −5.8% in outpatient and non-acute inpatient settings and − 4.6% in surgery settings.
3.4 |. Platelet, plasma, and cryoprecipitate distributions and transfusions
During 2021, 2,528,000 total platelet units (95% CI, 2,372,000–2,684,000 platelets) were distributed in the United States, an increase of 0.8% compared to 2019, when 2,508,000 platelets were distributed (Table 4). Among the total platelets distributed in 2021, 2,422,000 units (95% CI, 2,298,000–2,545,000 units) were apheresis platelets, an increase of 2.7% compared to 2019, when 2,359,000 apheresis platelet units were distributed. The proportion of total platelets distributed in 2021 that were apheresis platelets was 95.8%, an increase from 2019 when 94.1% of all platelet units distributed were apheresis platelets. Of all apheresis platelets distributed in 2021, 951,000 units (95% CI, 884,000–1,017,000 units), or 39.3%, were treated with pathogen reduction technology. During 2021, 107,000 units (in apheresis equivalents; 95% CI, 38,000–175,000 units) of whole blood-derived platelets were distributed, a 28.2% decrease compared to 2019, when 149,000 units were distributed.
TABLE 4.
Estimated numbers of platelets, plasma, and cryoprecipitated AHF units distributed, transfused, and outdated in 2021 and 2019 (expressed in thousands) in the United States, as reported to the National Blood Collection and Utilization Survey.
| Blood centers |
Hospitals | Combined totals |
95% CI | 2019 totalsa |
% change 2021–2019 |
|
|---|---|---|---|---|---|---|
| Distributed | ||||||
| Apheresis platelets | 2265 | 157 | 2422 | (2298–2545) | 2359 | 2.7% |
| Whole blood-derived plateletsb | 62 | 45 | 107 | (38–175) | 149 | −28.2% |
| Total platelets | 2327 | 202 | 2528 | (2372–2684) | 2508 | 0.8% |
| Pathogen-reduced apheresis platelets | 916 | 35 | 951 | (884–1017) | n/ac | |
| Total plasmad | 2985 | 129 | 3114 | (2929–3298) | 2679 | 16.2% |
| Cryoprecipitated AHFe | 2306 | 143 | 2449 | (2234–2664) | 2304 | 6.3% |
| Blood center outdatesf | 179 | 35 | 214 | (161–267) | 229 | −6.6% |
| Transfused | ||||||
| Apheresis platelets | 2091 | (1919–2264) | 1996 | 4.8% | ||
| Whole blood-derived plateletsb | 80 | (47–112) | 243 | −67.1% | ||
| Total platelets (includes directed units) | 2175 | (1997–2353) | 2243 | −3.0% | ||
| Pathogen-reduced apheresis platelets | 843 | (712–974) | n/ac | |||
| Total plasmad | 2215 | (2084–2347) | 2185 | 1.4% | ||
| Cryoprecipitated AHFe | 1248 | (1075–1421) | 1184 | 5.4% | ||
| Hospital outdatesg | 523 | (468–578) | 500 | 4.6% | ||
2019 totals were obtained from the 2019 NBCUS report.5
Whole blood-derived platelets are expressed as apheresis equivalents.
Estimate not available for 2019.
Excludes COVID-19 convalescent plasma.
Cryoprecipitated AHF is expressed as individual unit equivalents.
Blood center outdates are units that were outdated at non-hospital and hospital-based blood centers.
Hospital outdates are units that were outdated at transfusing hospitals.
During 2021, 2,175,000 total platelet units (95% CI, 1,997,000–2,353,000 units) were transfused in the United States, a decrease of 3.0% from 2019, when 2,243,000 total platelet units were transfused (Table 4). Among the total platelets transfused in 2021, 2,091,000 units (95% CI, 1,919,000–2,264,000 units) were apheresis platelets, an increase of 4.8% from 2019, when 1,996,000 apheresis platelet units were transfused. The proportion of total platelets transfused in 2021 that were apheresis platelets was 96.1%, an increase from 2019 when 89.0% of all platelet units transfused were apheresis platelets. Of all apheresis platelets transfused in 2021, 843,000 units (95% CI, 712,000–974,000 units), or 40.3%, were subjected to pathogen-reduction technology. Overall, 80,000 units (in apheresis equivalents; 95% CI, 47,000–112,000 units) of whole blood-derived platelets were transfused in 2021, a 67.1% decrease compared to 2019, when 243,000 units were transfused. For locations within healthcare facilities where platelet transfusions were performed in 2021, the highest volume was transfused in inpatient medicine settings (including hematology/oncology), where 815,000 platelet units (95% CI, 653,000–977,000 units) were transfused, followed by critical care settings (398,000 units; 95% CI, 329,000–466,000 units), outpatient and non-acute inpatient settings (322,000 units; 95% CI, 252,000–392,000 units), surgery settings (281,000 units; 95% CI, 220,000–342,000 units), and emergency departments (105,000 units; 95% CI, 92,000–119,000 units) (Table 5). From 2019 to 2021, the largest increases in platelet transfusions occurred in obstetrics/gynecology settings (18.6%), neonatal settings (14.8%), and pediatric settings (14.1%). The largest observed decreases in platelet transfusions from 2019 to 2021 were in outpatient and non-acute inpatient settings (−43.6%), inpatient medicine settings (−28.9%), and critical care settings (−12.7%).
TABLE 5.
Platelet units transfused by location in 2021 and 2019 (expressed in thousands) as reported to the National Blood Collection and Utilization Survey.
| 2021 (95% CI) | 2019 | % difference | Matched median % difference |
|
|---|---|---|---|---|
| All surgery (including transplant) | 281 (220–342, n = 697) | 280 | 0.4% | −4.4% (n = 254) |
| Emergency department | 105 (92–119, n = 706) | 105 | 0.1% | 14.3% (n = 237) |
| Inpatient medicine (including hematology/oncology) | 815 (653–977, n = 707) | 1050 | −28.9% | 0.0% (n = 276) |
| Obstetrics/Gynecology | 14 (11–16, n = 686) | 11 | 18.6% | 0.0% (n = 121) |
| Pediatrics | 92 (55–129, n = 692) | 79 | 14.1% | 0.0% (n = 40) |
| Neonatal | 40 (26–54, n = 702) | 34 | 14.8% | −10.5% (n = 74) |
| Critical care | 398 (329–466, n = 658) | 448 | −12.7% | 0.2% (n = 221) |
| Outpatient and non-acute inpatient settingsa | 322 (252–392, n = 697) | 462 | −43.6% | −5.4% (n = 189) |
Includes outpatient dialysis, rehabilitation, and long-term care.
In total, 3,114,000 plasma units (including fresh-frozen plasma, plasma frozen within 24 h of collection, cryoprecipitate-reduced plasma, and liquid plasma; 95% CI, 2,929,000–3,298,000 units) were distributed in the United States in 2021 (Table 4). This represents a 16.2% increase from 2019 when 2,679,000 units of plasma were distributed. There were 2,215,000 units (95% CI, 2,084,000–2,347,000 units) of plasma transfused in the United States in 2021, an increase of 1.4% from 2019 when 2,185,000 plasma units were transfused.
Approximately 2,449,000 units (95% CI, 2,234,000–2,664,000 units) of cryoprecipitated AHF were distributed in the United States in 2021, an increase of 6.3% from 2019 when 2,304,000 units were distributed (Table 4). Over the same time period, 1,248,000 units (95% CI, 1,075,000–1,421,000 units) of cryoprecipitated AHF were transfused in the United States, an increase of 5.4% from 2019, when 1,184,000 units were transfused.
Overall, 214,000 units of platelets, plasma, and cryoprecipitated AHF (95% CI, 161,000–267,000 units) were outdated in community- and hospital-based blood collection facilities in 2021, a decrease of 6.6% from 2019, when 229,000 units were outdated in blood centers (Table 4). Over the same time period, a total of 523,000 units of platelets, plasma, and cryoprecipitated AHF (95% CI, 468,000–578,000 units) were outdated in transfusing hospitals, an increase of 4.6% from 2019, when 500,000 units were outdated in hospitals.
4 |. DISCUSSION
The 2021 NBCUS shows a continued stabilization in transfusions in the United States and marks the first time since 2008 that blood collections in the United States have not decreased year-to-year, suggesting a plateau has been reached for both blood collection and use. This is in contrast to the trends observed since 2008. Between 2008 and 2017, results from NBCUS identified a persistent decline in both blood collections and transfusions in the United States.2–5 Subsequently, the 2017 NBCUS results suggested the rate of decline in blood collections was starting to slow, as was the rate of decline in transfusions, although to a lesser extent.4 Data from the 2019 NBCUS were the first to show a reversal of the decreasing annual trend in transfusions in the United States but continued to show a decrease in collections.5 Results from the 2021 NBCUS continue to support a reversal of the declining trends historically observed in U.S. blood collections and transfusions.
Similar to previous years, 2021 NBCUS results show trends in RBC use differed by location within healthcare facilities where transfusions were performed, with the largest decrease in RBC use between 2019 and 2021 seen in surgery settings. Although increases in RBC use were seen in neonatal settings, emergency departments, and pediatric settings, relative stability was observed (or small to insignificant decreases were observed) in all other healthcare facility locations. This notable decrease in RBC use in surgery settings is a continuation of the trend seen over the past few NBCUS cycles, although the decrease in use in surgery settings in 2021 is slightly more pronounced. This result is interesting considering the stabilization observed in blood use overall. While the recent decline in blood use observed nationally appeared to be driven at least in part by improvements in technology and surgical technique and less reliance on transfusions during surgical procedures, it is interesting that blood use in surgical settings continued to decrease in 2021 while the overall trend in blood use plateaued. This could reflect a general stabilization in blood use in the broader healthcare system, with a continued trend toward less-invasive surgeries but a nadir in the effect of increased adoption of patient blood management programs in non-surgical clinical settings. Furthermore, the slightly more pronounced decrease in transfusions in surgery settings in 2021 compared to prior years could also reflect a continued impact the COVID-19 pandemic had on surgery cancelations, as the surgical volume had not fully rebounded to pre-pandemic levels by the beginning of 2021.18 Additionally, unlike NBCUS results from 2017 and 2019, in 2021 there was less variability seen in differences in RBC use by hospital size. In 2021, a small decrease (0.9%) was seen within the smallest size category of hospitals (facilities performing between 100 and 999 inpatient surgeries per year). Small corresponding increases in RBC use were concurrently seen in larger hospitals in 2021. From a statistical standpoint, this represents a relatively stable picture.
A predictive model from 2015, which supported the continued use of annual inpatient surgical volume as the variable by which to stratify hospitals in NBCUS, showed a projected decrease in RBC use from 2015 to 2020 of between 7.6% and 23.2%.19 This contrasted with other predictions that posited a reduction of up to 40% in blood use in the United States by 2020 if current trends at the time continued.3,6,20 From 2015 to 2021, the actually observed decrease in blood use in the United States was 5.2%.3–5 Based on the 2021 NBCUS results, instead of a continuation of the decline observed between 2008 and 2015, a plateau seems to have been reached. A stabilization in blood collection and use is further supported by the results of the 2020 data collected in the 2021 NBCUS Supplemental sections, which were added to ascertain blood collection and use trends in the United States during the COVID-19 pandemic.21 Data from the Supplemental sections suggest that annualized blood collection and use during 2020 were also stable.21
The stabilization of blood collection and use seen in 2021 suggests blood supply is currently meeting demand. Furthermore, it appears the recent declining trends may have been driven mostly by a decline in demand. However, the demand for blood depends on a clinical need. Although patient blood management programs and restrictive use of transfusions in surgical settings have likely contributed historically to a decline in blood use,1,5–10 there is a lower limit to this trend. In fact, looking at the epidemiology of common conditions that require blood transfusions, such as cancer, sickle cell disease, trauma, and complications of maternal medicine, as the U.S. population increases and gets older, the incidence and overall burden of these conditions are expected to increase, which suggests that transfusion demand may also grow in the coming years.22–25
The current findings are subject to the following limitations. First, NBCUS is a time- and labor-intensive survey that relies on an increasingly burdened industry. The healthcare industry is facing increasing staff shortages and burnout and increasing demands on staff time.26 Consequently, although every effort is made to encourage participation in NBCUS, approximately 24% of sampled hospitals did not respond to the survey. Second, NBCUS data are self-reported and only manually verified in rare circumstances, which can limit the accuracy. Third, excluding certain types of hospitals (e.g., smaller facilities, those located in U.S. territories, and military, rehabilitation, long-term acute care, and psychiatric hospitals) and outpatient facilities can lead to underestimates of national utilization measures, although the impact on these findings is likely minimal. Lastly, because imputation and weighting are used to calculate national estimates, comparisons to previous NBCUS estimates can be affected by changes in sampling and response rates.
NBCUS most accurately estimates RBC collection and transfusion. Results from the 2021 NBCUS suggest that blood collection and use in the United States has reached a plateau. NBCUS will continue to be an important source of data on national blood collection and utilization estimates in the United States. CDC and OASH will continue to monitor NBCUS data on national blood collection and use, to support policy and planning efforts that ensure a safe and available blood supply.
Abbreviations:
- AHA
American Hospital Association
- AHF
antihemophilic factor
- BCC
blood collection center
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- FDA
U.S. Food and Drug Administration
- IQR
interquartile range
- NBCUS
National Blood Collection and Utilization Survey
- OASH
Office of the Assistant Secretary for Health
- RBC
red blood cell
- REDCap
Research Electronic Data Capture
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have disclosed no conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Goel R, Zhu X, Patel EU, Crowe EP, Ness PM, Katz LM, et al. Blood transfusion trends in the United States: national inpatient sample, 2015 to 2018. Blood Adv. 2021;5(20):4179–84. 10.1182/bloodadvances.2021005361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung KW, Basavaraju SV, Mu Y, van Santen KL, Haass KA, Henry R, et al. Declining blood collection and utilization in the United States. Transfusion. 2016;56:2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellingson KD, Sapiano MRP, Haass KA, Savinkina AA, Baker ML, Chung KW, et al. Continued decline in blood collection and transfusion in the United States–2015. Transfusion. 2017;57(Suppl 2):1588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones JM, Sapiano MRP, Savinkina AA, Haass KA, Baker ML, Henry RA, et al. Slowing decline in blood collection and transfusion in the United States - 2017. Transfusion. 2020;60(Suppl 2):S1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones JM, Sapiano MRP, Mowla S, Bota D, Berger JJ, Basavaraju SV. Has the trend of declining blood transfusions in the United States ended? Findings of the 2019 National Blood Collection and utilization survey. Transfusion. 2021;61:1–10. 10.1111/trf.16449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulcahy AW, Kapinos KA, Briscombe B, et al. Chapter 4: “Trends affecting blood system sustainability”. Toward a sustainable blood supply in the United States: an analysis of the current system and alternatives for the future. Santa Monica, CA: RAND Corporation; 2016. p. 29–44 Available: https://www.rand.org/pubs/research_reports/RR1575.html [Google Scholar]
- 7.Fredrick J, Berger JJ, Menitove JE. Strategic issues currently facing the US blood system. Transfusion. 2020;60:1093–6. 10.1111/trf.15769 [DOI] [PubMed] [Google Scholar]
- 8.Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10:CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016; 316(19):2025–35. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman RM, Djulbegovic B, Gernsheimer T, Gernsheimer T, Kleinman S, Tinmouth AT, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162(3):205–13. [DOI] [PubMed] [Google Scholar]
- 11.AuBuchon JP, Puca K, Saxena S, Shulman IA, Waters JH. Getting started in patient blood management. AABB, Bethesda, MD; 2011. Accessed 5 December 2022. https://www.aabb.org/docs/default-source/default-document-library/resources/112024db.pdf [Google Scholar]
- 12.AHA. American Hospital Association Annual Survey 2019. Available from: https://ahasurvey.org/taker/asindex.do
- 13.Woodruff R A simple method for approximating the variance of a complicated estimate. J Am Stat Assoc. 1971;66:411–4. [Google Scholar]
- 14.Sapiano MRP, Jones JM, Savinkina AA, Haass KA, Berger JJ, Basavaraju SV. Supplemental findings of the 2017 National Blood Collection and utilization survey. Transfusion. 2020;60-(Suppl 2):S17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He YRT. Tukey’s gh distribution for multiple imputation. Am Stat. 2006;60:251–6. [Google Scholar]
- 16.Rubin D Multiple imputation for nonresponse in surveys. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 17.Census Bureau US. Population estimates by state and age. Suitland, MD: US Census Bureau; 2021. [Google Scholar]
- 18.Ghoshal S, Rigney G, Cheng D, Brumit R, Gee MS, Hodin RA, et al. Institutional surgical response and associated volume trends throughout the COVID-19 pandemic and postvaccination recovery period. JAMA Netw Open. 2022; 5(8):e2227443. 10.1001/jamanetworkopen.2022.27443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savinkina A, Sapiano MRP, Berger J, Basavaraju SV. Is surgical volume still the most accurate indicator of blood usage in the United States? Transfusion. 2019;59(3):1125–31. 10.1111/trf.15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein HG, Hrouda JC, Epstein JS. Crisis in the sustainability of the U.S. blood system. N Engl J Med. 2018;378(3):305–6. 10.1056/NEJMc1714807 [DOI] [PubMed] [Google Scholar]
- 21.Basavaraju SV, Free RJ, Chavez Ortiz JL, Stewart P, Berger J, Sapiano MRP. Impact of the COVID-19 pandemic on blood donation and transfusions in the United States in 2020. Transfusiond. 2023. 10.1111/trf.17359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2021 submission data (1999–2019): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released in November 2022. https://www.cdc.gov/cancer/dataviz
- 23.Sedrak A, Kondamudi NP. Sickle cell disease. [updated 2022 Aug 29]. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482384/ [PubMed] [Google Scholar]
- 24.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, et al. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. 10.1097/SLA.0000000000000600 [DOI] [PubMed] [Google Scholar]
- 25.Roberts N, James SL, Delaney M, et al. Blood transfusion trends by disease category in the United States, 2000 to 2014. Transfus Apher Sci. 2021;60(1):103012. 10.1016/j.transci.2020.103012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad K, McLoughlin C, Stillman M, Poplau S, Goelz E, Taylor S, et al. Prevalence and correlates of stress and burnout among U.S. healthcare workers during the COVID-19 pandemic: a national cross-sectional survey study. EClinicalMedicine. 2021;35:100879–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


