Abstract
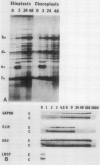
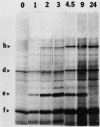
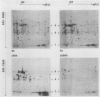
When isolated pea plastids are incubated with translation products of poly(A+) mRNA they specifically import precursor molecules of plastid polypeptides. Etioplasts and chloroplasts import the same polypeptides from identical translation products, and, the imported polypeptides can be well resolved by two-dimensional gel electrophoresis. Therefore, the posttranslational uptake system using isolated chloroplasts can monitor changes in the abundance of translatable plastid-targeted messages. Poly(A+) mRNA was isolated from peas at various times during greening and analyzed by this technique. (a) After 48 hours of illumination of dark-grown plants, the relative portion of nuclear encoded messages for plastid targeted proteins had increased by a factor of 2. The percentage of polypeptides recovered in the stroma fraction increased from about 50 to 65%. (b) More than 140 imported polypeptide species could be detected in fluorograms of two-dimensional gels, most of which could be identified throughout the time course of greening. At least 37 imported polypeptides decreased and 36 increased in relative abundance during greening of darkgreen plants. (c) In most cases, where differences in translatable messages were seen between dark- and light-grown plants, they were accompanied by parallel changes in polypeptide abundance.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Polypeptide turnover in darkness. Eur J Biochem. 1981 Aug;118(1):61–70. doi: 10.1111/j.1432-1033.1981.tb05486.x. [DOI] [PubMed] [Google Scholar]
- Bennett J., Jenkins G. I., Hartley M. R. Differential regulation of the accumulation of the light-harvesting chlorophyll a/b complex and ribulose bisphosphate carboxylase/oxygenase in greening pea leaves. J Cell Biochem. 1984;25(1):1–13. doi: 10.1002/jcb.240250102. [DOI] [PubMed] [Google Scholar]
- Berry J. O., Nikolau B. J., Carr J. P., Klessig D. F. Translational regulation of light-induced ribulose 1,5-bisphosphate carboxylase gene expression in amaranth. Mol Cell Biol. 1986 Jul;6(7):2347–2353. doi: 10.1128/mcb.6.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore A. R., Broadhurst M. K., Gray R. E. Cell-free synthesis of leaf protein: Identification of an apparent precursor of the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Feb;75(2):655–659. doi: 10.1073/pnas.75.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore A. R. Structure and expression of a pea nuclear gene encoding a chlorophyll a/b-binding polypeptide. Proc Natl Acad Sci U S A. 1984 May;81(10):2960–2964. doi: 10.1073/pnas.81.10.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Dietz K. J., Bogorad L. Plastid Development in Pisum sativum Leaves during Greening : I. A Comparison of Plastid Polypeptide Composition and in Organello Translation Characteristics. Plant Physiol. 1987 Nov;85(3):808–815. doi: 10.1104/pp.85.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986 Jul;81(3):802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. S., Roberts L. L., Briggs W. R., Thompson W. F. Phytochrome Control of Specific mRNA levels in Developing Pea Buds : Kinetics of Accumulation, Reciprocity, and Escape Kinetics of the Low Fluence Response. Plant Physiol. 1986 Aug;81(4):1033–1038. doi: 10.1104/pp.81.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G., Kloppstech K. A rapidly light-induced chloroplast protein with a high turnover coded for by pea nuclear DNA. Eur J Biochem. 1984 Jan 2;138(1):201–207. doi: 10.1111/j.1432-1033.1984.tb07900.x. [DOI] [PubMed] [Google Scholar]
- Mishkind M. L., Wessler S. R., Schmidt G. W. Functional determinants in transit sequences: import and partial maturation by vascular plant chloroplasts of the ribulose-1,5-bisphosphate carboxylase small subunit of Chlamydomonas. J Cell Biol. 1985 Jan;100(1):226–234. doi: 10.1083/jcb.100.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pfisterer J., Lachmann P., Kloppstech K. Transport of proteins into chloroplasts. Binding of nuclear-coded chloroplast proteins to the chloroplast envelope. Eur J Biochem. 1982 Aug;126(1):143–148. doi: 10.1111/j.1432-1033.1982.tb06758.x. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]
- Schindler C., Soll J. Protein transport in intact, purified pea etioplasts. Arch Biochem Biophys. 1986 May 15;247(1):211–220. doi: 10.1016/0003-9861(86)90550-3. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]