Summary
Conservative kidney management (CKM) has been increasingly accepted as a therapeutic option for seriously ill patients with advanced chronic kidney disease. CKM is active medical management of advanced chronic kidney disease without dialysis, with a focus on delaying the worsening of kidney disease and minimizing symptom burden. CKM may be considered a suitable option for kidney transplant recipients with poorly functioning and declining allografts, defined as patients with low estimated glomerular filtration rate (<20 mL/min per 1.73 m2) who are approaching allograft failure. CKM may be a fitting option for transplant patients facing high morbidity and mortality with or without dialysis resumption, and it should be offered as a choice for this patient population. In this review, we describe clinical considerations in caring for patients with poorly functioning and declining kidney allografts, especially the unique decision-making process around kidney replacement therapies. We discuss ways to incorporate CKM as an option for these patients. We also discuss financial and policy considerations in providing CKM for this population. Patients with poorly functioning and declining kidney allografts should be supported throughout transitions of care by an interprofessional and multidisciplinary team attuned to their unique challenges. Further research on when, who, and how to integrate CKM into existing care structures for patients with poorly functioning and declining kidney allografts is needed.
Keywords: Allograft failure, conservative kidney management, end-stage kidney disease, kidney transplant, palliative care, shared decision making
Conservative kidney management (CKM) is medical management of advanced chronic kidney disease (CKD) without kidney replacement therapy (KRT). It has been used increasingly as a valid and sometimes optimal therapeutic option for seriously ill patients with advanced CKD, especially in care systems outside of the United States, such as Canada, the United Kingdom, and Australia. CKM includes optimization of treatments to delay further decline of kidney function and a focus on treating symptoms and maximizing quality of life.1
However, the use of CKM in kidney transplant recipients is not well established. In this review, we describe clinical considerations in caring for patients with poorly functioning and declining kidney allografts, especially the limitations of current terminology in outcomes data registries for transplant patients and the unique decision-making process around treatment options for allograft failure. We discuss opportunities to incorporate CKM into the care of kidney transplant patients, as well as financial and policy considerations in the provision of CKM in this population.
ALLOGRAFT FUNCTION TRAJECTORY AND BARRIERS TO CKM FOR TRANSPLANT PATIENTS
Kidney transplantation improves survival and quality of life for patients with end-stage kidney disease (ESKD). Although allograft and patient survival have increased continuously over the past several decades,2 the 10-year, death-censored, kidney allograft survival, defined as the time from transplant to allograft failure, censoring for death with a functioning allograft, still is suboptimal: currently 49.5% for deceased donor kidney transplants and 65.5% for living donor kidney transplants. Consequently, allograft failure is common; 40% of patients experience allograft failure within 10 years.3
As with native kidney function, kidney allograft function declines over time, with a variable tempo and severity. Mayrdorfer et al4 recently described the complexity of death-censored kidney allograft failure. By analyzing 303 allograft losses among 1,642 patients in a single center in Germany, they identified that 51% of allograft loss was attributed to more than one cause, including inter-current medical events, acute rejection episodes, and chronic rejections. A multicenter cohort analysis by Raynaud et al5 showed eight different patterns of estimated glomerular filtration rate (eGFR) trajectories after transplant (Fig. 1). In examining patients with advanced allograft dysfunction, some experienced a steady decline (trajectories 3 and 5), some experienced an accelerated decline (latent class 8), and others experienced a low steady eGFR (latent classes 6 and 7).
Figure 1.
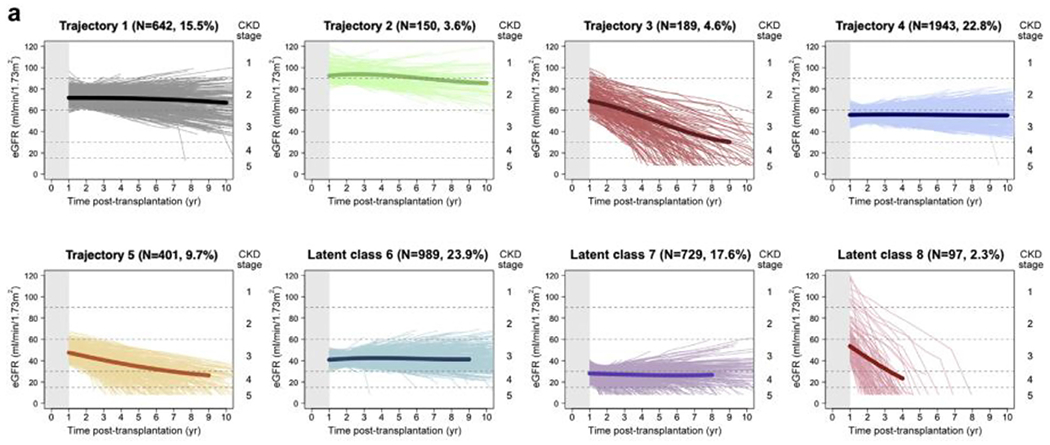
Trajectory of glomerular filtration rate after kidney transplantation. The main profiles of kidney function identified using latent class mixed models. Estimated glomerular filtration rate (eGFR) trajectory 1: patients with high baseline renal function (~70 mL/min per 1.73 m2) that remained stable; eGFR trajectory 2: patients with very high baseline kidney function (~90 mL/min per 1.73 m2) with slightly decreasing function over time; eGFR trajectory 3: patients with high baseline kidney function (~70 mL/min per 1.73 m2) and fast decline over time; eGFR trajectory 4: a pattern of intermediate baseline eGFR (~55 mL/min per 1.73 m2) and stability over time; eGFR trajectory 5: intermediate/low baseline eGFR (48 mL/min per 1.73 m2) with decreasing function over time; eGFR trajectory 6: patients with low baseline eGFR (~40 mL/min per 1.73 m2) with stability over time; eGFR trajectory 7: patients with very low baseline eGFR (28 mL/min per 1.73 m2) and mildly declining function over time; and eGFR trajectory 8: patients with intermediate eGFR (58 mL/min per 1.73 m2) but a very fast subsequent decline in eGFR trajectory. Abbreviation: CKD, chronic kidney disease. Recreated with permission from Raynaud et al.5
Kidney allograft failure is defined as initiation of KRT, through either dialysis or retransplantation, and is a metric reported to the Scientific Registry of Transplant Recipients. However, a comprehensive consensus definition of “failing” allograft remains under discussion and is difficult to define,6 especially when considering the right time to discuss KRT and CKM with kidney transplant patients. The Kidney Disease Improving Global Outcomes (KDIGO) Controversies Conference on Challenges in Management of Kidney Allograft: From Decline to Failure (KDIGO Controversies Conferences, hereafter), held in March 10-13, 2022, gathered stake-holders (transplant nephrologists, transplant surgeons, social workers, patients, and caregivers) to explore issues in managing patients with poorly functioning and declining allografts.7 They discussed what definitions and terminology are needed to accurately describe the nuances of allograft failure. One key consideration was the term failure because it can sound accusatory for both patients and clinicians and thus it may be better to use terms such as poorly functioning and declining allograft to capture the longitudinal trends of allograft dysfunction. We use this term to describe patients with a low eGFR (<20 mL/min per 1.73 m2) who are nearing the decision about KRT or CKM. A suggestion was made to introduce an eGFR cut-off value to define the term failing allograft, such as an eGFR less than 20 mL/min per 1.73 m2, which is used routinely as a threshold to list patients for transplantation. However, as evidenced by the trajectory study,5 it may not be accurate to categorize low stable kidney function as a failing allograft, and thus a definition based on an eGFR cut-off value has limitations. For now, the term allograft failure remains defined by the initiation of dialysis or preemptive repeat transplantation, which functionally excludes CKM from being viewed as a treatment option for this population.
Another structural barrier to offering and tracking CKM in the care of transplant patients is the use of the term death with function to describe any kidney transplant recipient who dies without initiating dialysis or undergoing retransplantation.8 The Scientific Registry of Transplant Recipients outcome metrics do not capture patients who experience severe allograft dysfunction and opt not to pursue dialysis. Death with function is a broad category that includes both those who never had an indication to re-initiate KRT (those with a functioning graft, either with normal or stable/low function) and those who may have opted against KRT when they developed a conventional indication for dialysis initiation. The latter includes two distinct clinical scenarios: gradual dysfunction leading to progressive allograft CKD or acute decline in allograft function from acute kidney injury (AKI). This distinction is important because the former group may be best supported by CKM, whereas those with severe AKI who choose not to pursue dialysis, if oliguric or anuric, consequently will die within days to weeks. These AKI patients usually receive short-term, comfort-focused, end-of-life care in a hospital or in hospice care, which is different from CKM, a longitudinal proactive multidisciplinary approach with a heavy focus on CKD management and symptom reduction. Ideally, the transplant community will develop more nuanced terminology in clinical practice and in data registries to describe these nuanced trajectories, outcomes, choices, and care options.
TREATMENT OPTIONS FOR KIDNEY TRANSPLANT PATIENTS WITH POORLY FUNCTIONING AND DECLINING ALLOGRAFTS
Patients with chronic poorly functioning and declining allografts have several different therapeutic options: initiating dialysis, relisting for another kidney transplantation, and CKM. The decision around KRT after allograft failure is nuanced and complex. Currently, according to the US Renal Data System (USRDS) annual report, more than 20% of kidney transplant recipients are age 65 years or older.3 By the time they develop poorly functioning and declining allografts, they inevitably are older and often sicker than at the time of their first transplant. Patients with allograft failure who resume dialysis suffer from two to three times higher mortality9,10 when compared with those who initiate dialysis without previous kidney transplantation, with 1-, 2-, and 3-year mortality rates of 16%, 25%, and 33%, respectively.11 It is critical to understand this prognostic landscape for patients with allograft failure because prognosis can inform patients’ care preferences as well as clinicians’ approach to shared decision making, which should aim to align the treatment choice with the patient’s goals and values.
Dialysis
Hemodialysis (HD) is the most common treatment choice after allograft failure worldwide. In a study by the Catalan Renal Registry in Spain, 89.4% of patients with allograft failure initiated HD, 8.2% initiated peritoneal dialysis (PD), and 2.4% received a preemptive kidney transplantation.12 In the United States, according to the USRDS annual report, approximately 82% of patients with allograft failure initiated HD (including 1.1%-1.5% who initiated home HD), 14% initiated PD, and 3% to 4% received a preemptive kidney transplantation. These trends have remained steady over the past 10 years.3 For nontransplant patients with advanced CKD, current National Kidney Foundation–Kidney Disease Outcomes Quality Initiatives13 and KDIGO14 guidelines recommend starting dialysis based on signs and patient-reported symptoms rather than relying on eGFR estimation alone (previously, initiation was recommended at an approximate eGFR of 10 to 15 mL/min per 1.73 m2, respectively), based on the seminal Initiating Dialysis Early and Late (IDEAL) study,15 which indicated no benefit in early dialysis initiation compared with late or symptom-driven initiation. Patients with poorly functioning and declining allografts tend to start dialysis with a lower eGFR (mean eGFR, 8.4 mL/min per 1.73 m2)11 than nontransplant patients with advanced CKD (mean eGFR, 9.5 mL/min per 1.73 m2).3 Delayed initiation of KRT initially was thought to be associated with worse survival in transplant patients with allograft failure.16 However, more recent cohort studies have shown that an earlier start for HD for transplant patients with allograft failure (at >10.5 mL/min per 1.73 m2) is associated with worse overall survival compared with a late start (eGFR <10.5 mL/min per 1.73 m2).11,17 In addition, patients initiating dialysis after allograft failure frequently use a dialysis catheter (65.4%), when compared with an arteriovenous fistula (27.7%) or arteriovenous graft (6.9%),18,19 which contributes to infection rates,20 especially in those who are maintained on immunosuppression. When considering other dialysis modalities, registry studies from the Canadian Organ Replacement Registry and the USRDS have reported similar overall survival rates between patients who initiated PD compared with those who initiated HD.21,22
Retransplantation
Patients with poorly functioning and declining allografts also may be candidates for retransplantation. The proportion of waitlist candidates with prior transplant and transplant recipients with previous allograft failure has declined over the past decade (2008-2018) from 15% to 12% and 12% to 10%, respectively.23,24 According to Eurotransplant data, the proportion of patients with failed allografts on the waitlist declined from 14.4% in 2012 to 12.8% in 2021.25 Schold et al26 showed that the proportion of patients with declining and poorly functioning allografts who were relisted for transplant was 25% within 12 months of dialysis re-initiation, and eventually the proportion increased up to 45% within 5 to 6 years on dialysis, with a preemptive relisting rate of 15.3%. National registry studies have shown that retransplantation is associated with significantly reduced mortality compared with remaining on dialysis without relisting.10,27 Notably, however, a longer wait time between allograft failure to retransplantation is associated with an increased risk of rejection and worse overall survival of a subsequent allograft.28 It is estimated that retransplantation within 1 year after allograft failure was associated with a gain of 8 months of life, compared with approximately 3 days for those who waited for 8 years after allograft failure.29
Conservative kidney management
CKM has been used increasingly for patients with advanced native CKD, but has not been offered routinely to patients with poorly functioning and declining allografts. CKM is an interprofessional model of care that aims to maximize the palliative and nephrology care of patients with advanced CKD who choose to forgo dialysis. CKM encompasses careful attention to treatments to delay progression of kidney disease, management of physical and psychological symptoms, caregiver support, planning for clinical crises, and emphasis on serious illness communication and shared decision making.30,31 According to a meta-analysis examining the outcomes of 5,102 patients with advanced CKD who refrained from maintenance dialysis, the median survival of varied cohorts ranged from 1 to 41 months and overall quality of life, including physical and mental well-being, was stable during the observation period, although none of these cohorts were, to our knowledge, specific to patients with prior transplantation.32 However, it seems from existing evidence that older adults with poorly functioning and declining allografts can expect to have a life expectancy in the range of months to 1 to 2 years on CKM and those who undergo dialysis usually live a bit longer.33 However, for patients older than age 80 years with poor functional status and a high comorbidity burden, particularly cardiovascular disease, there may be no difference in the expected lifespan between CKM and dialysis.33 In addition, for those CKD patients who are older with significant comorbidities who do pursue dialysis, studies naturally indicate that a longer lifespan includes more time in dialysis treatments and in the hospital with health setbacks.34 Furthermore, data also suggest that, unlike older adults who start dialysis, some cohorts who pursue CKM maintain functional status until the final month of life.35 As such, similar to CKD, for older transplant patients with comorbidities or functional impairments who value life extension over time at home and comfort, dialysis or retransplantation may be the best means of meeting their goals. However, for those who value being at home and avoiding procedures and are willing to trade this for the possibility of a shorter lifespan, CKM may be their best option. We discuss further details on the discussions and decisions around CKM and the practice models for patients with poorly functioning and declining allograft later.
PROVIDING CKM FOR PATIENTS WITH POORLY FUNCTIONING AND DECLINING ALLOGRAFTS
In considering the provision of CKM to patients with poorly functioning and declining allografts, some unique needs complicate the secondary decision around KRT and the practice of CKM. Patients with declining allograft function carry a notable burden of their kidney disease, with increased mortality, infectious complications, poorer psychological outcomes, complex grief and coping, and reduced quality of life when compared with transplant-naïve patients on the transplant waitlist.36–38 In addition, people with poorly functioning and declining allografts have additional elements in their care that create more complexity in applying CKM, including considerations around immunosuppression and removal of the allograft itself23 for those relatively few who need it removed because of symptoms or a systemic reaction.39 To address this, the typical team-based approach to CKM should consider adding additional layers of support unique to the population of kidney transplant patients with poorly functioning and declining allografts. These can include intentionally crafted partnerships between palliative care and the transplant team, embedded within transplant care. This integrated team pays special attention to transplant-specific issues, such as immunosuppression, and close involvement of palliative care for team-based management of the unique decision making (Fig. 2).40–42 Immunosuppression regimens in kidney transplant patients receiving CKM should be tailored based on the individual clinical context. Similar to patients with allograft failure who resume dialysis, it is reasonable to minimize immunosuppression to reduce infection and cancer risk. However, stopping immunosuppression completely might lead to acute rejection and associated pain in the allograft, and should be avoided if possible.6 We are not aware at this time of any specific models of CKM specifically designed for transplant recipients with poorly functioning and declining allografts, and data are notably lacking in this space.
Figure 2.
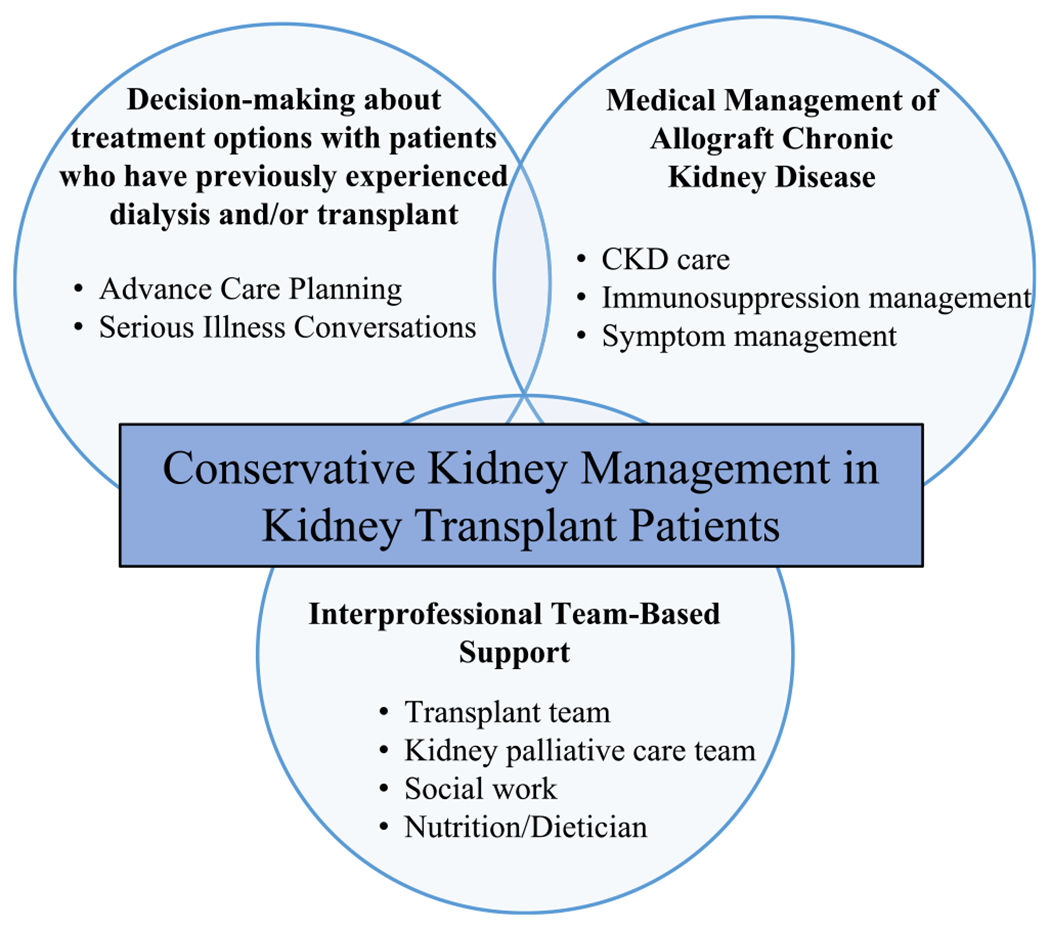
Conceptual framework of conservative kidney management in kidney transplant. CKD; chronic kidney disease.
Models for dedicated delivery of palliative care for patients with kidney transplants, including but not limited to CKM, are emerging but remain limited in scope and geography in the United States.43–46 Although the current model of palliative care delivery for transplant patients is predominantly inpatient-focused in acute settings, future models should provide transplant teams with collaborative longitudinal relationships and ease of outpatient consultation. This may help to familiarize clinicians, patients, and their caregivers with the possibility of high-quality CKM across the continuum of transplant care, especially with a focus on outpatient and home-based provision, for those patients with allograft dysfunction who are not eligible for retransplantation and not interested in dialysis initiation.47
CONSIDERING TRANSITIONS FOR PATIENTS WITH POORLY FUNCTIONING AND DECLINING ALLOGRAFTS
The transition period as an allograft begins to function poorly is a key moment when considering the option of CKM in transplant populations. Kidney transplant patients have key unique characteristics that complicate this time period when compared with other CKD and ESKD patients, including prior personal lived experiences with KRT, established donor/recipient/clinician relationships around a now poorly functioning organ, treatment with immunosuppressive medications, and complexities with allograft nephrectomy and what it may mean for peritoneal dialysis access (see Table 1 for case examples). Notably, key knowledge gaps related to transitions of care among patients with poorly functioning allografts include timing of counseling regarding treatment options, appropriate dialysis access placement, optimal strategies for preemptive relisting, timing of retransplantation, transition of care from transplant to general CKD clinics, and consideration of palliative care referrals.23 Each of these potential transitional issues unique to allograft decline involves a diversity of clinical providers and settings and thus depends on collaboration for successful management.
Table 1.
Clinical Vignettes Highlighting Examples of Unique Transplant Patient Issues and Needs Addressed by a Team-Based Approach for the Care of Poorly Functioning Allografts With Declining Function
| Patient Case | Unique Transplant Issues | Goals and Values | Hopes for Kidney Treatment | Examples of Current Needs for Team-Based Approach to Allograft Care Moving Forward |
|---|---|---|---|---|
| 62-year-old man with a history of polycystic kidney disease, received a preemptive living kidney transplant from his wife 22 years ago. His allograft function has been slowly declining to a Cr level of 3.8 mg/dL (eGFR, 18 mL/min per 1.73 m2) most recently. He reports fatigue and not able flank pain at the site of his graft. He owns his business and lives independently. He is married with his wife of 40 years, and has two daughters and one son living faraway. He has no known living donor options. | Prior major abdominal surgeries Allograft from current wife of 40 years is now functioning poorly Prolonged allograft function of 22 years |
Continue his business and active life Continue to travel readily to see his children as they build their families Not to burden his wife with his illness and treatments |
He is thinking about having a transplant again, but is worried about long wait time (average, 5-6 y) and wants to hear other available options After education on retransplantation, HD, PD, and CKM, given his desire to travel, he would prefer PD as a bridge to retransplantation if needed and has residual renal function |
Surgery and transplant team consideration of PD given prior surgeries and possible allograft nephrectomy Transplant team evaluation for retransplantation Pain and fatigue are greatly disrupting his quality of life. Kidney palliative care team to join nephrology for management of this as well as grief counseling for him and his wife around the loss of her graft donation |
| 72-year-old woman with a history of systemic lupus erythematosus who received a deceased kidney transplant 16 years ago. She was on hemodialysis for 8 years before undergoing transplantation and this was a hard experience for her. She was diagnosed with ischemic heart failure a year ago and her allograft function has been declining for the past several months now with a Cr level of 4.2 mg/dL (eGFR, 14 mL/min per 1.73 m2). She was admitted to the hospital three times in the past 6 months for fluid overload, requiring intravenous diuresis. She is retired and lives with her husband. She has no children. She feels her energy level is down and is bothered by lower-leg edema. | Prior experience with KRT Previously had significant difficulties with HD and is now more chronically ill and frail |
Retired, hoping to spend time at home, with her husband, quietly, reading books and enjoying the garden at their home Has really struggled with admissions for her heart failure and would like to minimize them if possible |
She is not sure if she would go through another kidney transplant again, and she also had a discussion with her transplant nephrologist that having another transplant would be difficult because of her age and comorbidity She is not sure if she would try dialysis either, based on her previous experience of hemodialysis before transplantation, and is interested in learning more about CKM |
Longitudinal follow-up evaluation with transplant team, nephrology, and kidney palliative care for ongoing decision making and delivery of CKM Careful consideration of how to balance management of her heart failure, fatigue, and edema with the kidney palliative care team, nephrology, and her cardiologist |
Abbreviations: Cr, creatinine; eGFR, estimated glomerular filtration rate; KRT, kidney replacement therapy; HD, hemodialysis; PD, peritoneal dialysis; CKM, conservative kidney management.
Currently, the transition of care at the time of allograft failure varies under different care models and may happen rather abruptly at the time of initiation of dialysis, when the patient with allograft failure transitions from the primary longitudinal follow-up evaluation by a known team of transplant or CKD clinicians to care in a dialysis unit.48 Studies have suggested that patients with a poorly functioning and declining allograft may receive suboptimal CKD care when compared with those without a history of transplantation.49,50 Huml and Sehgal51 examined dialysis quality metrics in the first year after allograft failure and found that patients have lower hemoglobin, higher phosphorus, and lower albumin levels compared with patients with advanced CKD without previous allograft failure. The transplant field currently is seeking to address these issues and they are relevant as one considers the transition to CKM for this patient population, which is marked by keen attention to medical management of kidney disease.
The KDIGO Controversies Conference aimed to identify the optimal model of care for managing patients with poorly functioning and declining allografts. Possibilities included general nephrology multidisciplinary clinics, ongoing routine transplant care, and creation of special dedicated multidisciplinary transplant clinics for patients with poorly functioning and declining allografts. Such dedicated multidisciplinary models may create an optimal environment for educating patients and families on the option of CKM. A study in the United Kingdom tested a model of a dedicated clinic, termed low-clearance transplant clinics (LCTCs), for advanced CKD after transplant. LCTCs provided multidisciplinary care by nephrologists, renal dieticians, and specialty pharmacists. LCTCs improved KRT counseling and evaluation for retransplantation, but the discussion of CKM was not studied, and there was no difference in the rate of preemptive retransplantation.52 In addition, the Kidney Recipients with Allograft Failure, Transition of Care workgroup, under the American Society of Transplantation, put effort into better understanding patients’ needs during the transitions of care around poorly functioning and declining allografts. Their survey of transplant clinicians found a heterogeneity of care among patients with poorly functioning allografts and a need for clinical guidance specifically related to transitions of care.53 Existing research acknowledges the limited data available to guide clinical practice for patients with poorly functioning allografts, and the guidance that is available tends to focus on risks and benefits of various therapies, but not coordination between care teams or identification of who guides the transition from conventional transplant care to either KRT or CKM.54
Nephrology clinicians who have described their experience providing CKM report that health systems are not always supportive of CKM.55 Nephrologists in this study who championed the principles of CKM among colleagues also noted the need to assume a range of different health care roles to prepare patients to navigate systems in which the initiation of dialysis is a powerful default.32,55 The findings of this study likely would hold true for clinicians caring for transplant patients with poorly functioning and declining allografts who desire CKM, and additional complexities also may surface that are unique to transplant patients. In the context of variable management strategies across clinicians, a shared-care model has been proposed to improve coordination between transplant providers and general nephrologists.6 Notably, this approach focuses on preparation for return to dialysis or retransplantation, but no mention of CKM. We propose that the best current approach should be an interprofessional team, akin to the LCTC model, integrating transplant nephrologists, general nephrologists, and specialty kidney palliative care clinicians, that explores retransplantation, dialysis, and CKM options depending on patients’ particular clinical situation, goals, and preferences (see Table 1 for case examples).
DECISION MAKING DURING DECLINE OF POORLY FUNCTIONING ALLOGRAFTS
Care decisions for patients with poorly functioning and declining allografts should be driven fundamentally by patient goals and preferences. As discussed earlier, the population of transplant patients with poorly functioning and declining allografts is unique in that they often already have experienced dialysis before and are facing this situation when they are older and more medically complex. In addition, patients, caregivers, and clinicians in this context have collectively experienced a focus on life extension during prior transplant evaluation, selection, and postoperative periods,56 and this likely affects decision making in unique and complex ways. Naming and acknowledging these factors may be important during shared decision making about treatment options for poorly functioning and declining allografts.
Discerning patients’ goals and preferences can occur throughout their clinical course through different types of conversations: early in the treatment course through advance care planning (ACP) discussions, and later, as patients become seriously ill, through serious illness conversations. ACP discussions can happen early in the post-transplant treatment course during which care teams ask in advance of allograft decline about patients’ goals and preferences for hypothetical future medical care to prepare for future decision making.57,58 As patients become sicker, when their lifespan may be limited and a patient’s collection of illnesses are impairing their functional status and quality of life, or significantly impacting their caregivers, clinicians may conduct serious illness conversations to build shared prognostic awareness and explore what matters most to patients in the context of advancing serious illness. An example structure for serious illness conversations with suggested patient-tested language is available in Figure 3.59–61 Although there is a lack of research assessing ACP and serious illness conversations specifically among transplant patients with poorly functioning and declining allografts, there are studies of ACP and decision making among people with advanced CKD from which some extrapolations can be made. A qualitative study of patients and caregivers about perspectives on planning for ESKD62 highlights some key communication needs. The researchers identified several themes, including the importance to patients of articulation of personal values and autonomy. The researchers also described a theme around decisional disempowerment, with patients reporting feeling angry because they believed that key information had been withheld by clinicians and disappointed that clinicians had not engaged in planning for the future. Reinforcing these findings, patients surveyed about their perspectives on dialysis decision making and end-of-life care have expressed a desire for more frequent discussions about their disease, prognosis, and planning.63 Preparing for care transitions is key to smoothing the tumultuous time around the decline of poorly functioning allografts and preventing initiation of dialysis by default, instead facilitating an individualized patient choice guided by clinicians closely involved in transplant care.
Figure 3.
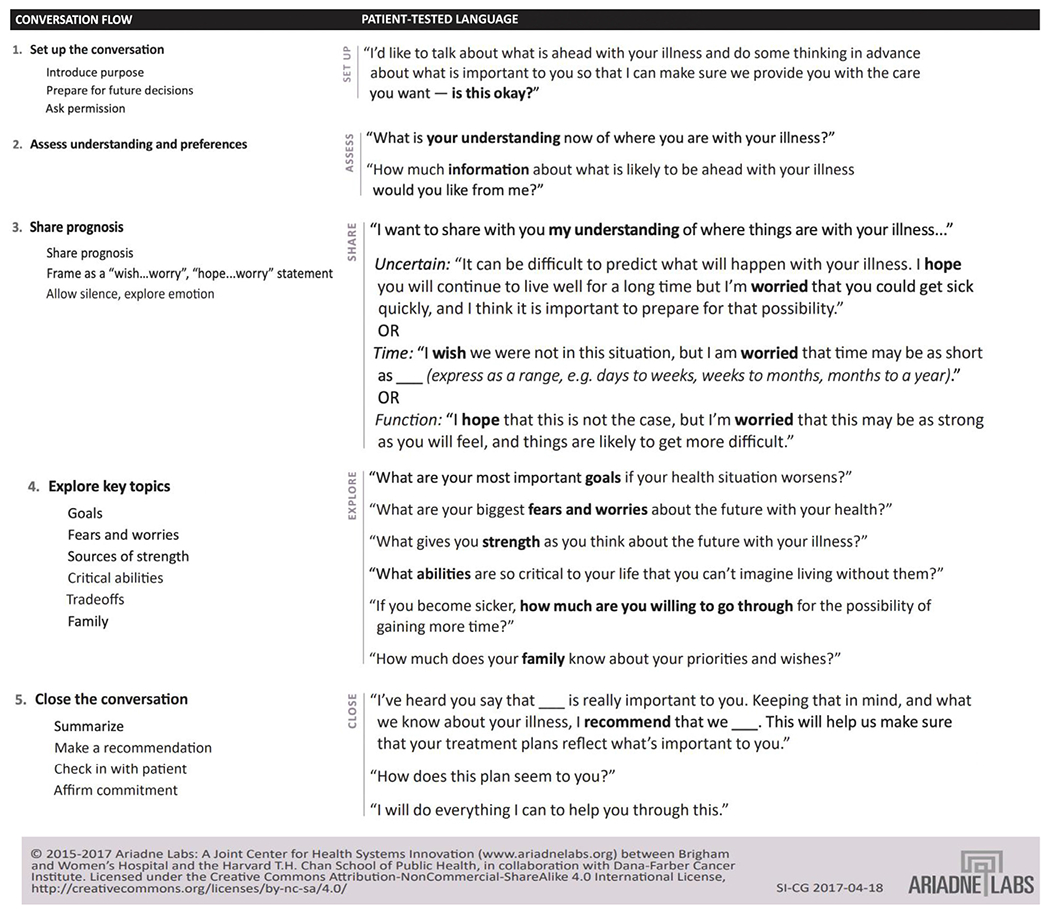
Serious illness conversation guide for decision making with patients with poorly functioning and declining allografts.
ACP and serious illness conversations may be especially complex for transplant patients who already have experienced a range of treatment modalities and are revisiting prior experiences that may have been initially traumatic and about which they may have gained new perspective over time. A multidisciplinary team working to integrate palliative care practices into routine clinical care for patients with CKD noted that formalizing ACP processes and making discussions more frequent are key factors in aligning patient preferences with clinical care across health professionals64; it is reasonable to assume this would hold true in the transplant population as well. Key tensions remain, however, in clinician and patient/caregiver perspectives on planning and communication of options, particularly regarding the option to choose CKM. One qualitative analysis65 of nephrologists, primary care physicians, and patients/caregivers found that even when clinicians aligned regarding responsibility for ACP and how to integrate CKM options, patients and caregivers reported a lack of clarity regarding several aspects of their care and a desire for earlier conversations. Again, this seems likely to hold true in the population of patients with poorly functioning and declining allografts, but further research specific to this population is required.
FINANCIAL AND POLICY CONSIDERATIONS IN PROVIDING CKM FOR PATIENTS WITH POORLY FUNCTIONING AND DECLINING ALLOGRAFTS
The financial burden of allograft failure among patients is substantial and estimated to include $78,079 in additional medical costs per patient and a loss of 1.66 quality-adjusted life-years.66 In addition, insurance and policy factors around poorly functioning and declining allografts are complex and vary by payer and stage of illness and treatment. For example, patients who do not have contraindications for retransplantation should be referred for evaluation in early allograft dysfunction when possible, but not all insurance carriers cover evaluation at an early stage (eGFR, >20 mL/min per 1.73 m2),6 creating insurance-driven disparities in care.
New payment models attempt to address some of this inconsistency. Hippen and Maddux67 recommend an expanded Comprehensive ESKD Care (CEC) model that focused on quality care for transplant patients with poorly functioning and declining allografts. Specifically, they proposed a shared-care model with general and transplant nephrology teams to maximize quality and safety for these patients, while minimizing unnecessary costs and avoidable hospitalizations. The CEC model arose from Center for Medicare and Medicaid Innovation pilot projects to encourage general nephrology practices and dialysis organizations to collaborate in providing shared care for these patients while meeting quality metrics. Models similar to this, if implemented properly, could provide pathways for more equitable and comprehensive CKM for transplant patients.
The CEC program ended in 2021. The Kidney Care Choices model builds on its structure68 and is funded from January 2022 until the end of 2026. The Kidney Care Choices model aims to center the patient in its design and allow for multiple kidney disease treatment paths that are not the current default of in-center HD. The goal is to better integrate and plan care, using payment levels and education to drive engagement in decision making and an increase in home treatment modalities. If CKM could be considered a home modality, it would create an incentive for the development of comprehensive CKM care infrastructure in the United States69 and also make CKM more available for patients with declining and poorly functioning allografts.
From a policy perspective, two key factors may function as disincentives to offering CKM to transplant patients. At the health organization level, 1-year survival metrics collected by the Centers for Medicare and Medicaid Services are used for program evaluation and reimbursement. These metrics may have the unintended consequence of overprioritizing quantity over quality of life for all patients early after transplant, even if that does not align with an individual patient’s outlook. Because patient-centered quality-of-life metrics are not integrated into algorithms for organ allocation or program assessment, patient priorities may receive insufficient emphasis when compared with mortality statistics.70 An additional factor that serves as a barrier to initiation of CKM is the current Medicare payment structure. Under the ESKD Prospective Payment System,69,71 Medicare will reimburse comprehensively for costs associated with dialysis, including home dialysis, but CKM is not included and instead is billed individually by provider (ie, nephrologist visit, palliative care visit). Categorizing CKM as a home modality would help to package it as a valid team-based treatment modality in the care of transplant patients. Despite stated efforts to offer benefits with potential positive impacts on patients’ quality of life, home-based care modalities still are limited and do not include the resources needed to deliver high-quality CKM to transplant patients.
CONCLUSIONS
In the kidney transplant population, seriously ill patients with poorly functioning and declining allografts would benefit from consideration of CKM. This group of patients has a notable burden of morbidity and mortality and faces a transition period that can be more complex and nuanced than that in the advanced CKD population without previous transplantation. Decision making around dialysis, retransplantation, and CKM is an important process that should be initiated early and focus on helping patients and their families envision a future with declining allograft function while learning about their goals and values to prepare for that potential future course. For patients who choose CKM, the decision should be supported by an interprofessional and multidisciplinary team attuned to the specific challenges of patients with poorly functioning and declining allografts. Each of these aspects is poorly understood in the transplant population and early work is underway, but more research is needed.
Footnotes
Financial disclosure and conflict of interest statements: none.
REFERENCES
- 1.Gelfand SL, Scherer JS, Koncicki HM. Kidney supportive care: core curriculum 2020. Am J Kidney Dis. 2020;75(5):793–806. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan S, Israni AK, Danovitch G. Long-term survival after kidney transplantation. N Engl J Med. 2021;385(8):729–43. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health. United States Renal Data System. 2021 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2021. [Google Scholar]
- 4.Mayrdorfer M, Liefeldt L, Wu K, et al. Exploring the complexity of death-censored kidney allograft failure. J Am Soc Nephrol. 2021;32(6):1513–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raynaud M, Aubert O, Reese PP, et al. Trajectories of glomerular filtration rate and progression to end stage kidney disease after kidney transplantation. Kidney Int. 2021;99(1):186–97. [DOI] [PubMed] [Google Scholar]
- 6.Lubetzky M, Tantisattamo E, Molnar MZ, et al. The failing kidney allograft: a review and recommendations for the care and management of a complex group of patients. Am J Transplant. 2021;21(9):2937–49. [DOI] [PubMed] [Google Scholar]
- 7.Controversies conference on challenges in management of the kidney allograft: from decline to failure – KDIGO. Accessed September 12, 2022 https://kdigo.org/conferences/challenging-allograft. [DOI] [PubMed]
- 8.Gaston RS, Fieberg A, Helgeson ES, et al. Late graft loss after kidney transplantation: is “death with function” really death with a functioning allograft? Transplantation. 2020;104(7): 1483–90. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant. 2002;2(10):970–4. [DOI] [PubMed] [Google Scholar]
- 10.Rao PS, Schaubel DE, Jia X, et al. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis. 2007;49(2):294–300. [DOI] [PubMed] [Google Scholar]
- 11.Gill JS, Abichandani R, Kausz AT, et al. Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int. 2002;62(5):1875–83. [DOI] [PubMed] [Google Scholar]
- 12.Couceiro C, Rama I, Comas J, et al. Effect of kidney replacement therapy modality after first kidney graft failure on second kidney transplantation outcomes. Clin Kidney J. 2022;15 (11):2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slinin Y, Greer N, Ishani A, et al. Timing of dialysis initiation, duration and frequency of hemodialysis sessions, and membrane flux: a systematic review for a KDOQI clinical practice guideline. Am J Kidney Dis. 2015;66(5):823–36. [DOI] [PubMed] [Google Scholar]
- 14.Chan CT, Blankestijn PJ, Dember LM, et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2019;96(1):37–47. [DOI] [PubMed] [Google Scholar]
- 15.Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–19. [DOI] [PubMed] [Google Scholar]
- 16.Ojo A, Wolfe RA, Agodoa LY, et al. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation. 1998;66(12):1651–9. [DOI] [PubMed] [Google Scholar]
- 17.Molnar MZ, Streja E, Kovesdy CP, et al. Estimated glomerular filtration rate at reinitiation of dialysis and mortality in failed kidney transplant recipients. Nephrol Dial Transplant. 2012;27(7):2913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan MR, Oza-Gajera B, Chapla K, et al. Initial vascular access type in patients with a failed renal transplant. Clin J Am Soc Nephrol. 2014;9(7):1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JC, Al-Jaishi A, Perl J, et al. Hemodialysis arteriovenous vascular access creation after kidney transplant failure. Am J Kidney Dis. 2015;66(4):646–54. [DOI] [PubMed] [Google Scholar]
- 20.Woodside KJ, Schirm ZW, Noon KA, et al. Fever, infection, and rejection after kidney transplant failure. Transplantation. 2014;97 (6):648–53. [DOI] [PubMed] [Google Scholar]
- 21.Perl J, Hasan O, Bargman JM, et al. Impact of dialysis modality on survival after kidney transplant failure. Clin J Am Soc Nephrol. 2011;6(3):582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perl J, Dong J, Rose C, et al. Is dialysis modality a factor in the survival of patients initiating dialysis after kidney transplant failure? Perit Dial Int. 2013;33(6):618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis S, Mohan S. Managing patients with failing kidney allograft: many questions remain. Clin J Am Soc Nephrol. 2022;17 (3):444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark S, Kadatz M, Gill J, et al. Access to kidney transplantation after a failed first kidney transplant and associations with patient and allograft survival: an analysis of national data to inform allocation policy. Clin J Am Soc Nephrol. 2019;14 (8):1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eurotransplant Annual Report. Eurotransplant. Accessed September 12, 2022. https://www.eurotransplant.org/statistics/annual-report
- 26.Schold JD, Augustine JJ, Huml AM, et al. Modest rates and wide variation in timely access to repeat kidney transplantation in the United States. Am J Transplant. 2020;20(3):769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao PS, Schaubel DE, Wei G, et al. Evaluating the survival benefit of kidney retransplantation. Transplantation. 2006;82(5):669–74. [DOI] [PubMed] [Google Scholar]
- 28.Wong G, Chua S, Chadban SJ, et al. Waiting time between failure of first graft and second kidney transplant and graft and patient survival. Transplantation. 2016;100(8):1767–75. [DOI] [PubMed] [Google Scholar]
- 29.Kainz A, Kammer M, Reindl-Schwaighofer R, et al. Waiting time for second kidney transplantation and mortality. Clin J Am Soc Nephrol. 2022;17(1):90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO controversies conference on supportive care in chronic kidney disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447–59. [DOI] [PubMed] [Google Scholar]
- 31.Davison SN, Tupala B, Wasylynuk BA, et al. Recommendations for the care of patients receiving conservative kidney management: focus on management of CKD and symptoms. Clin J Am Soc Nephrol. 2019;14(4):626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong SPY, Rubenzik T, Zelnick L, et al. Long-term outcomes among patients with advanced kidney disease who forgo maintenance dialysis: a systematic review. JAMA Netw Open. 2022;5 (3):e222255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou A, Li KC, Brown MA. Survival of older patients with advanced CKD managed without dialysis: a narrative review. Kidney Med. 2022;4(5):100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carson RC, Juszczak M, Davenport A, et al. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4(10):1611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murtagh FEM, Addington-Hall JM, Higginson IJ. End-stage renal disease: a new trajectory of functional decline in the last year of life. J Am Geriatr Soc. 2011;59(2):304–8. [DOI] [PubMed] [Google Scholar]
- 36.Perl J, Zhang J, Gillespie B, et al. Reduced survival and quality of life following return to dialysis after transplant failure: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2012;27(12):4464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gill P, Lowes L. Renal transplant failure and disenfranchised grief: participants’ experiences in the first year post-graft failure–a qualitative longitudinal study. Int J Nurs Stud. 2014;51 (9):1271–80. [DOI] [PubMed] [Google Scholar]
- 38.Streltzer J, Moe M, Yanagida E, et al. Coping with transplant failure: grief vs. denial. Int J Psychiatry Med. 1983;13(2):97–106. [DOI] [PubMed] [Google Scholar]
- 39.Ayus JC, Achinger SG, Lee S, et al. Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol. 2010;21(2):374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wentlandt K, Weiss A, O’Connor E, et al. Palliative and end of life care in solid organ transplantation. Am J Transplant. 2017;17 (12):3008–19. [DOI] [PubMed] [Google Scholar]
- 41.Murakami N, Baggett ND, Schwarze ML, et al. Top ten tips palliative care clinicians should know about solid organ transplantation. J Palliat Med. 2022;25(7):1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mejia C Failing kidney transplants require a multidisciplinary approach, including palliative care. Kidney News. 2022;14(3):34. [Google Scholar]
- 43.Murakami N, Gelfand SL, Sciacca KR, et al. Inpatient kidney palliative care for kidney transplant recipients with failing allografts. Kidney Med. 2022;4(2):100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherer JS, Rau ME, Krieger A, et al. A pilot randomized controlled trial of integrated palliative care and nephrology care. Kidney 360. 2022;3(10):1720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lakin JR, Sciacca K, Leiter R, et al. Creating KidneyPal: a specialty-aligned palliative care service for people with kidney disease. J Pain Symptom Manage. 2022;64(6):e331–9. [DOI] [PubMed] [Google Scholar]
- 46.Gelfand SL, Lakin JR, Sciacca KR, et al. Specialty-aligned palliative care: responding to the needs of a tertiary care health system. J Pain Symptom Manage. 2022;64(6):e341–6. [DOI] [PubMed] [Google Scholar]
- 47.Reich AJ, Lakin JR, He K, et al. Kidney transplant clinician perceptions and experiences of palliative care for patients with failing allograft. AcademyHealth annual research meeting abstract. Accessed June 6, 2022. https://academyhealth.confex.com/academyhealth/2022arm/meetingapp.cgi/Paper/54092 [Google Scholar]
- 48.The perspective of general nephrologists toward transitions of care and management of patients with failing kidney allografts. ATC Abstracts. 2022. Accessed September 12, 2022 https://atc-meetingabstracts.com/abstract/the-perspective-of-general-nephrologists-toward-transitions-of-care-and-management-of-patients-with-failing-kidney-allografts. [Google Scholar]
- 49.Almond MK, Tailor D, Marsh FP, et al. Increased erythropoietin requirements in patients with failed renal transplants returning to a dialysis programme. Nephrol Dial Transplant. 1994;9(3):270–3. [PubMed] [Google Scholar]
- 50.Gill JS, Abichandani R, Khan S, et al. Opportunities to improve the care of patients with kidney transplant failure. Kidney Int. 2002;61(6):2193–200. [DOI] [PubMed] [Google Scholar]
- 51.Huml AM, Sehgal AR. Hemodialysis quality metrics in the first year following a failed kidney transplant. Am J Nephrol. 2019;50 (3):161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans RDR, Bekele S, Campbell SM, et al. Assessment of a dedicated transplant low clearance clinic and patient outcomes on dialysis after renal allograft loss at 2 UK transplant centers. Transplant Direct. 2018;4(6):e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alhamad T, Lubetzky M, Lentine KL, et al. Kidney recipients with allograft failure, transition of kidney care (KRAFT): a survey of contemporary practices of transplant providers. Am J Transplant. 2021;21(9):3034–42. [DOI] [PubMed] [Google Scholar]
- 54.Lea-Henry T, Chacko B. Management considerations in the failing renal allograft. Nephrology (Carlton). 2018;23(1):12–9. [DOI] [PubMed] [Google Scholar]
- 55.Wong SPY, Boyapati S, Engelberg RA, et al. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis. 2020;75(2):167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butler CR, Reese PP, Perkins JD, et al. End-of-life care among US adults with ESKD who were waitlisted or received a kidney transplant, 2005-2014. J Am Soc Nephrol. 2020;31(10):2424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153(4):256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage. 2017;53(5):821–32. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernacki RE, Block SD. American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003. [DOI] [PubMed] [Google Scholar]
- 61.Lakin JR, Block SD, Billings JA, et al. Improving communication about serious illness in primary care: a review. JAMA Intern Med. 2016;176(9):1380–7. [DOI] [PubMed] [Google Scholar]
- 62.Sellars M, Clayton JM, Morton RL, et al. An interview study of patient and caregiver perspectives on advance care planning in ESRD. Am J Kidney Dis. 2018;71(2):216–24. [DOI] [PubMed] [Google Scholar]
- 63.Saeed F, Sardar MA, Davison SN, et al. Patients’ perspectives on dialysis decision-making and end-of-life care. Clin Nephrol. 2019;91(5):294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiu HHL, Murphy-Burke DM, Thomas SA, et al. Advancing palliative care in patients with CKD: from ideas to practice. Am J Kidney Dis. 2021;77(3):420–6. [DOI] [PubMed] [Google Scholar]
- 65.Eneanya ND, Labbe AK, Stallings TL, et al. Caring for older patients with advanced chronic kidney disease and considering their needs: a qualitative study. BMC Nephrol. 2020;21(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sussell J, Silverstein AR, Goutam P, et al. The economic burden of kidney graft failure in the United States. Am J Transplant. 2020;20(5):1323–33. [DOI] [PubMed] [Google Scholar]
- 67.Hippen BE, Maddux FW. Integrating kidney transplantation into value-based care for people with renal failure. Am J Transplant. 2018;18(1):43–52. [DOI] [PubMed] [Google Scholar]
- 68.Comprehensive ESRD care model. Accessed September 4, 2022. https://innovation.cms.gov/innovation-models/comprehensive-esrd-care
- 69.Gelfand SL, Mandel EI, Mendu ML, et al. Palliative care in the advancing American kidney health initiative: a call for inclusion in kidney care delivery models. Am J Kidney Dis. 2020;76(6):877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lamas DJ, Lakin JR, Trindade AJ, et al. Looking beyond mortality in transplantation outcomes. N Engl J Med. 2018;379 (20):1889–91. [DOI] [PubMed] [Google Scholar]
- 71.Medicare Program; end-stage renal disease prospective payment system, payment for renal dialysis services furnished to individuals with acute kidney injury, end-stage renal disease quality incentive program, and end-stage renal disease treatment choices model. A rule by the Centers for Medicare & Medicaid Services; on November 8, 2021. Accessed September 12, 2022 https://www.federalregister.gov/documents/2021/11/08/2021-23907/medicare-program-end-stage-renal-disease-prospective-payment-system-payment-for-renal-dialysis [Google Scholar]


