Abstract
Macrophages function as tissue immune sentinels and mediate key antimicrobial responses against bacterial pathogens. Yet they can also act as a cellular niche for intracellular bacteria, such as Salmonella enterica, to persist in infected tissues. Macrophages exhibit heterogenous activation, or polarization, states that are linked to differential antibacterial responses and bacteria permissiveness. Remarkably, recent studies demonstrate that Salmonella and other intracellular bacteria inject virulence effectors into the cellular cytoplasm to skew the macrophage polarization state and reprogram these immune cells into a permissive niche. Here, we review mechanisms of macrophage reprogramming by Salmonella and highlight manipulation of macrophage polarization as a shared bacterial pathogenesis strategy. In addition, we discuss how the interplay of bacterial effector mechanisms, microenvironmental signals, and ontogeny may shape macrophage cell states and functions. Finally, we propose ideas of how further research will advance our understanding of macrophage functional diversity and immunobiology.
Keywords: Salmonella, intracellular bacteria, macrophages, polarization, SteE, STAT3, granulomas, heterogeneity, effector, alternatively activated
Introduction:
Macrophages are mononuclear phagocytes that act as tissue immune sentinels and perform critical homeostatic functions, including phagocytosing spent cells, recycling nutrients, remodeling tissues, and resolving inflammation(1, 2). Armed with arsenals of cell surface and cytosolic pattern recognition receptors (PRRs) that sense pathogen associated molecular patterns (PAMPS), during bacterial infection macrophages readily phagocytose invading pathogens, release inflammatory mediators to facilitate innate and adaptive immune responses, and kill bacteria via production of reactive metabolites and multistep inflammasome activation(3, 4). Paradoxically macrophages can also serve as a cellular niche for intracellular bacterial pathogens, such as Salmonella enterica, Bartonella henselae, and Mycobacterium tuberculosis (Mtb), to survive within infected tissues(5-7). Macrophages exhibit differential polarization, or activation states, and these functional states have distinct cellular phenotypes and functions(8-10). Macrophage polarization has been described using a conceptually simplified M1 and M2 framework(11-13). The classical, or M1, macrophage activation paradigm occurs in microenvironments upon recognition of PAMPs, such as LPS, and inflammatory immune signaling, such as IFN-γ. These stimuli activate downstream transcriptional regulators, including NFκB and STAT1, to generate inflammatory and antibacterial functional states(10, 11). On the other hand, the alternatively activated, M2 polarized state prototypically entails macrophage activation from the type-2 immunity associated cytokines such as IL-4 and IL-13 or IL-10 that trigger STAT6, STAT3, and Peroxisome Proliferator-Activated Receptors (PPARs) among other regulators, to elicit macrophage activities involved in resolving inflammation and tissue repair. The dichotomous M1 and M2 framework does not, however, fully capture the heterogeneous functional states of tissue macrophages in vivo. Increasingly macrophage polarization and heterogenous phenotypes have been recognized as a spectrum of functional states, with overlapping cellular features that are shaped by a multitude of factors and dependent on pathophysiological contexts(10, 14, 15).
Accumulating studies over the past decade have linked macrophage polarization states to differential antibacterial capacity and permissiveness, with an M2-like functional state being more permissive for intracellular bacterial replication and survival(6, 16-18).
Macrophage ontogeny and microenvironmental factors influence their functional phenotypes, such as antibacterial responses(19). Intracellular bacteria that exploit macrophages as a cellular niche to establish persistent infection, such as Salmonella enterica serovar Typhimurium (STm), express macromolecular secretion systems to inject virulence effector proteins into the host cell cytoplasm to co-opt cellular activities(5, 20, 21). Thus, in addition to evading antibacterial innate immune responses and exploiting existing favorable macrophage functional states, a fascinating question for some time is whether intracellular bacteria have specific mechanisms to actively skew macrophage polarization toward more permissive, M2-like states. Herein, we review recent research that uncover mechanisms by which Salmonella and other intracellular bacteria employ injected virulence effectors to manipulate macrophage polarization and reprogram macrophage functional states. We highlight how the STAT3 pathway has emerged as a critical cellular target for intracellular bacteria to reprogram macrophages and may represent a convergent bacterial pathogenesis strategy. These fascinating findings from studies of intracellular bacterial infections have broadened our perspectives on macrophage heterogeneity and generate exciting questions for future research that will further our understanding of macrophage immunobiology.
Macrophage heterogenous polarization states and antibacterial capacities
The mechanisms involved in early steps of macrophage recognition of and activation by PAMPs through PRRs during bacterial infections that lead to macrophage inflammasome activation, antibacterial immune responses, and bacterial killing have been active areas of investigations for many years and are extensively reviewed(4, 22). Although macrophage polarization states have been more widely used to describe differential phenotypes and functional properties associated with inflammatory immune responses, metabolic activities, tissue repair, and anti-tumor responses, they have also been linked with differential antibacterial capacities. Early experiments examining the functional impact of polarization states showed pre-treatment with either IL-4 or IFN-γ differentially affected the antimicrobial response of macrophages to Mtb infection, such as production of reactive nitrogen species(23). Using single-cell RNA-sequencing (scRNA-seq) and bulk population analyses, the expression levels of surface macrophage IL-4Rα, which is a canonical functional marker of alternatively activated or M2-like macrophages, have been shown to tightly correlate with macrophage capacity to support replicating and persistent intracellular STm in murine bone marrow-derived macrophages (BMDMs) in vitro and tissue macrophages in vivo(17, 24-26). IL-4Rα is the shared subunit of IL-4 and IL-13 receptors that transduce signals activating STAT6. In macrophages, IL-4 stimulated DNA binding of the transcriptional regulator STAT6 turns on an anti-inflammatory, M2-like functional program and also represses inflammatory responses(27, 28). IL-4 treatment results in significantly higher intracellular bacterial levels in STm-infected BMDMs, compared to IFN-γ treatment(29). Furthermore, conditional knockout mice in which Il4ra is deleted in myeloid cells, including monocytes and macrophages, by the LysM Cre recombinase activity have reduced bacterial levels in infected tissues during persistent STm infection(29). In addition to IL-4 and IL-13/IL-4R/STAT6 axis, the IL-10/IL-10R/STAT3 pathway is a key cytokine signaling axis that promotes M2-like macrophage polarization states and anti-inflammatory programming(11). During mycobacterial infection, the IL-10 mediated, anti-inflammatory response of macrophages is thought to limit excessive tissue damage from inflammation, but this host response may be exploited by bacteria for survival. IL-10−/− mice had been shown to have enhanced protection from Mtb infection, which was associated with more robust Th1 response(30). However, the protective effect from disabling IL-10 signaling may vary with the ability to induce IL-10 production among Mtb strains, host genetic differences, and other factors(30, 31). In addition, a recent study found that induced Pluripotent Stem Cells (iPSC)-derived human macrophages lacking IL-10R have higher intracellular bacterial levels when infected with STm and this phenotype is prostaglandin E-dependent, suggesting additional factors controlling intracellular bacteria survival within macrophages are independent of IL-10 signaling capacity(32).
Increasing evidence suggests macrophage polarization and functional states are interrelated with their cellular metabolic activities(10, 11). During intracellular bacterial infections, M2-like metabolic states have been associated with increased macrophage capacity to promote bacterial replication and survival. PPARα, PPARβ/δ, and PPARγ are a family of nuclear receptor proteins that regulate carbohydrate and lipid metabolism, energy balance, and inflammation. Their expression and activities can be induced by upstream IL-4, IL-13, and IL-10 signals and have been recognized as a metabolic signature of M2-like polarization states(10, 11). In BMDMs, PPARβ/δ and PPARγ enhance intracellular STm and Brucella abortus bacterial levels, respectively(29, 42). PPAR-driven permissiveness of macrophages for intracellular STm and B. abortus is dependent on macrophage glucose availability and the abilities of bacteria to utilize glucose, linking macrophage metabolic activities with intracellular bacteria metabolism and survival. Furthermore, PPARδ−/− and PPARγ−/− mice infected with STm and B. abortus, respectively, have lower abundances of M2-like macrophages and reduced bacterial levels in infected tissues, such as spleens and mesenteric lymph nodes, during persistent infection(29, 42). Similarly, macrophages also exhibit differential metabolic activities and bacteria permissiveness during Mtb infection, with PPAR expression and function correlating with higher capacity to support intracellular bacterial growth and persistence(43-45). The growing body of studies on macrophage metabolism and its functional impacts is being reviewed by Avraham R. et. at. in this issue. A summary of key host factors influencing macrophage polarization during Salmonella infection is provided in table 1.
Table 1.
Key cellular regulators of macrophage polarization in Salmonella infection
| Protein (gene) | Function | Gene/protein manipulation |
Macrophage polarization effect |
References |
|---|---|---|---|---|
| PAMPS/PRR pathways(various) | various | various | promoting M1-like polarization | (4, 10, 22) |
| IFNκ | cytokine | cytokine stimulation | promoting M1-like polarization | (29, 33) |
| IL-10 | cytokine | complete knockout, cytokine stimulation | promoting M2-like polarization | (25, 34, 35) |
| IL-4 and IL-13 | cytokines | cytokine stimulation | Promoting M2-like polarization | (29, 33) |
| IL-4Rα | IL-4 and IL-13 signaling | conditional knockout (LysM-Cre) | Promoting M2-like polarization | (17, 29) |
| TNF | cytokine | complete knockout, cytokine neutralization | promoting M1-like phenotypes and restraining M2-like polarization | (26, 36) |
| PPARδ | transcription factor | complete knockout | Promoting M2-like polarization | (29) |
| STAT1 | transcription factor | complete knockout | promoting M1-like polarization | (37, 38) |
| STAT3 | transcription factor | siRNA, chemical inhibitors | Promoting M2-like polarization | (25, 26, 39) |
| GSK3 | protein kinase | complete knockout, chemical inhibitors | Promoting M2-like polarization | (25, 39) |
| IRF5 | Transcription factor | siRNA | Promoting M1-like polarization | (40) |
| KDM6B | epigenetic regulator | siRNA, chemical inhibitors | Promoting M2-like polarization | (41) |
Macrophage polarization has functionally been tied to infection outcomes. In infected tissues, intracellular bacterial pathogens are contained and persist within granulomas, which are immunological microstructures comprised of macrophages and diverse types of immune and non-immune cells(26, 46-49). In silico, computational modeling studies suggest that the relative M1 and M2 activities may predict infection containment or dissemination in Mtb-infected lungs of nonhuman primates(16). Transcriptomics analyses of STm-infected BMDMs have identified an inflammatory, M1-like transcriptional signature associated with bystander macrophages, which were exposed to bacteria but not infected, and macrophages containing intracellular, host-killed bacteria. In contrast, an M2-like transcriptional signature was associated with macrophages containing persistent and growing bacteria(17, 24). During persistent infection in mice, the levels of STm tissue persistence were linked to relative abundances of different macrophage polarization and functional states, which were dependent on TNF, a pleotropic cytokine that retrains M2-like polarization among its many pathophysiologic activities(26, 27). Additionally, STAT6, which is a potent transcriptional regulator of M2-like polarization state, was recently found to control granuloma macrophage epithelial reprogramming and bacterial levels during Mycobacterium marinum infection in zebrafish(50). Inhibition of IL-4R had a lesser effect(50). Although examination of Il4ra−/− mice infected with Mtb reported no major bacterial control and histopathological defects, in vitro experiments with primary macrophages infected with Mtb showed IL-4 impaired bacterial restriction(51, 52).
Intracellular bacteria effector mechanisms that reprogram macrophage polarization states and antibacterial capacities
Salmonella, mycobacteria, and other intracellular bacteria that exploit host cells as a niche to survive and establish persistent infection have membrane-spanning, multiprotein secretion systems to inject virulence effector proteins into the host cytoplasm to modulate cellular activities. STm, which is commonly utilized as model Salmonella enterica serovar for experimental infections, encodes two type 3 secretion systems (T3SSs) on the Salmonella Pathogenicity Islands (SPI)1 and 2 in its genome. These T3SS-1 and 2 are required to inject various effectors that facilitate cellular invasion, immune evasion, and intra-macrophage replication and persistence(20, 53). For example, STm deploys multiple injected effectors, to inhibit NFκB signaling, including SpvD, which interferes with nuclear import of the NFκB subunit p65 and SteA, which suppresses ubiquitination and degradation of the NFκB inhibitor IκB(20, 54, 55). Similarly, B. abortus and Mtb employ various secreted effectors to co-opt host pathways for forming bacteria-containing vacuoles, interfering with antigen presentation, inhibiting NFκB, and impairing reactive oxygen species production(5, 56).
Recent studies demonstrate that intracellular bacterial pathogens have specific effector mechanisms to actively skew macrophage polarization and reprogram macrophages into a permissive niche. It had been known that exposure to viable STm induces STAT3 phosphorylation in BMDMs, though how much of this induction is driven by host signaling and/or directly mediated by bacterial mechanisms was not defined(34). In macrophages, STAT3 can be activated by IL-6 and IL-10 signaling and regulates expression of a number of target genes, including Il4ra, which encode gene products that collectively trigger and reinforce anti-inflammatory responses to limit inflammation during mycobacterial infection and other pathophysiologic settings(57-61). Using gene deleted BMDMs, Lin et. al. found deletion of Il6 or Il10 upstream cytokine signaling did not abolish STm-induced STAT3 phosphorylation(34). Clues that STm may inject effector(s) to manipulate macrophage polarization emerged when the SPI2 T3SS effector SteE (also known as SarA) was shown to be necessary and sufficient for STAT3 phosphorylation in lymphoblastoid cell lines(62). Subsequently studies demonstrated that SteE drives STAT3 phosphorylation and IL-4Rα expression to promote M2-like polarization and bacteria permissiveness of primary and tissue macrophages(24-26). Intriguingly, Panagi et. al. showed that SteE binding to the host serine/threonine Glycogen Synthase Kinase 3 (GSK3) changes its substrate binding pocket and specificity, enabling it to phosphorylate and activate STAT3 at the residue Tyr-705(25). In addition, Gibbs et. al. found that SteE/SarA C-terminus biochemically and functionally mimics the cytoplasmic domain of the common glycoprotein 130 (gp130) subunit of type I cytokine receptors such as IL-6R(39, 63). SteE contains a YXXQ motif that when phosphorylated binds to STAT3 with greater affinity than the YXXQ motif of gp130(39). SteE-driven polarization of macrophages to M2-like, permissive states counteracts TNF-mediated restriction and promotes STm persistence in granulomas and infected spleens in mice, demonstrating how manipulation of macrophage polarization is an important bacterial pathogenesis mechanism facilitating tissue persistence despite host immune responses(26).
Previous studies with the STm effector SteE raises the possibility that other intracellular bacteria may similarly polarize and reprogram macrophages to permissive states using injected virulence effectors. Recent studies with the intracellular bacterium Bartonella henselae have identified an effector BepD, which is translocated into host cell through a Type 4 Secretion System (T4SS), plays a role in reprogramming macrophages to M2-like, permissive states(64). Using mononuclear phagocyte cell lines and macrophages, Sorg et. al. showed that when injected into the cellular cytoplasm, BepD serves as a scaffold to bring together the c-ABL tyrosine kinase and STAT3 and facilitates phosphorylation of STAT3 at the Tyr-705 residue in a c-ABL dependent manner(64). This BepD-mediated activation of STAT3 promotes cellular IL-10 production and impairs TNF secretion, skewing macrophages toward an M2-like functional state. Additionally, Mtb infection and the Mtb T7SS effector ESAT-6 have been found to induce STAT3 phosphorylation in BMDMs(65, 66). However, whether Mtb virulence effectors directly mediate STAT3 phosphorylation remain to be defined. Interestingly, orthologous coding DNA sequence of SteE is absent in Salmonella enterica serovar Typhi(20), which is a human-restricted pathogen that has predilection for causing persistent infection(53). However, it is possible that S. Typhi employs a different virulence effector other than SteE to skew macrophage polarization or dampens the antibacterial responses of macrophages via mechanisms that are not dependent on active manipulation of polarization. Collectively, these studies suggest that the ability of intracellular bacterial pathogens to manipulate macrophage polarization and functional states represents a convergent evolution strategy to persist in the mammalian hosts (Table 2) (Figure 1).
Table 2.
Intracellular bacterial effector mechanisms and macrophage polarization
| Bacterium | Injected effector |
Host target | Macrophage polarization effect | References |
|---|---|---|---|---|
| S. Typhimurium | SteE | GSK3/STAT3 | promoting M2-like polarization by mediating STAT3 phosphorylation | (24, 25, 26, 39, 62) |
| S. Typhimurium | SteA | CUL-1/IκB | dampening M1-like polarization by interfering with IκB degradation | (55) |
| S. Typhimurium | SseK1 | FADD/TRADD | dampening M1-like polarization by inhibiting NFκB signaling | (20, 67) |
| S. Typhimurium | SseK3 | TRIM32/TRADD | dampening M1-like polarization by inhibiting NFκB signaling | (20, 67) |
| S. Typhimurium | SpvD | XP02/NFκB | dampening M1-like polarization by interfering with NFκB subunit nuclear localization | (20, 54) |
| S. Typhimurium | GogB | SKP1/FBXO22 | dampening M1-like polarization by inhibiting NFκB signaling | (20, 68) |
| B. henselae | BepD | STAT3/c-ABL | promoting M2-like polarization by mediating STAT3 phosphorylation | (64) |
| M. tuberculosis | ESAT-6? | unknown | may modulate macrophage polarization by targeting STAT3 phosphorylation | (65, 66) |
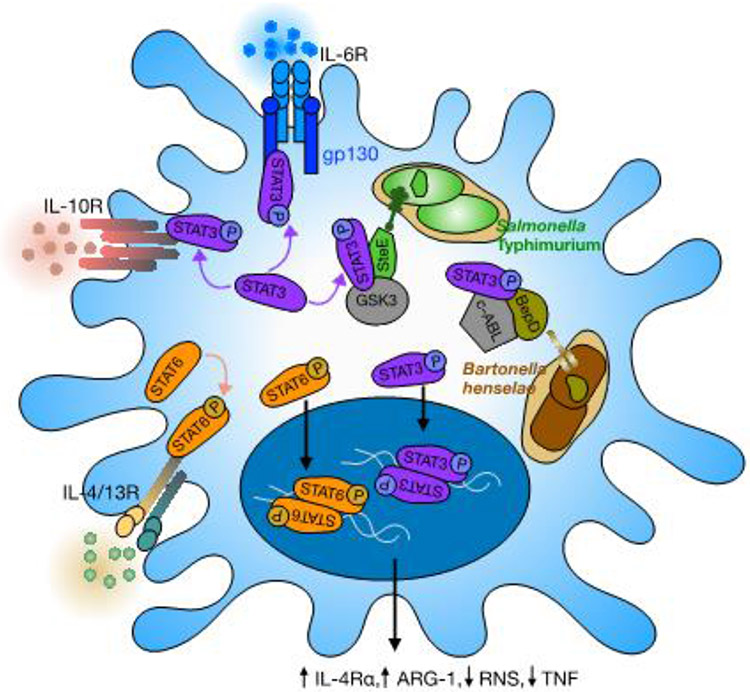
Figure 1: Intracellular bacteria manipulate macrophage polarization via STAT3 signaling.
Inside macrophages, S. Typhimurium reside within modified, Salmonella-containing vacuoles and inject the virulence effector SteE through T3SS into cellular cytoplasm. SteE promotes phosphorylation and activation of STAT3 by GSK3. Host IL-6 and IL-10 signaling also induce STAT3 phosphorylation and activation. Phosphorylated STAT3 translocates into the cellular nucleus and regulates expression of target genes, including Il4ra and Arg1, culminating in M2-like, anti-inflammatory responses that are more bacteria permissive, such as enhanced IL-4R/STAT6 signaling and reduced production of Reactive Nitrogen Species (RNS). Similarly, during B. henselae infection, vacuole-residing intracellular bacteria inject the effector BepD through T4SS to mediate phosphorylation of macrophage STAT3 by c-ABL. Whether a macrophage ultimately acting as and antibacterial cell or a permissive niche for bacteria may vary depending on the summative strengths of microenvironmental signals such as of IL-10 and IL-6, other macrophage antimicrobial properties, and the robustness of bacterial effector mechanisms to skew macrophage polarization.
Impacts of ontogeny and microenvironment on macrophage functional states and antibacterial capacities
The functional phenotypes and programing of macrophages are shaped by lineage determining transcriptional factors and signal dependent transcriptional factors that are driven by their microenvironment milieu(69). In mammalian tissues, a fraction of macrophages originate from embryonic precursors and others share a hematopoietic developmental origin with monocytes(1,2, 15). A recent sc-RNAseq study of acute STm infection in mice showed that a non-classical monocyte-derived macrophage population harbors more intracellular bacilli than other types of macrophages in the infected spleens, including red pulp macrophages, which have been known to originate from embryonic precursors(70, 71). Others have found that localization to microenvironments with varying surrounding cell-cell interactions and signaling milieu in infected tissues influences the antibacterial capacities of macrophages. In the spleens of mice infected with STm, CXCL9/10+ macrophages preferentially localize to the periphery of granulomas, closer to T-cell rich areas during persistent infection(47). These macrophages are less likely to harbor intracellular bacteria compared to macrophages that occupy the center of granulomas and express high levels of the inducible Nitric Oxide Synthase (iNOS), which is involved in production of Reactive Nitrogen Species (RNS) and commonly associated with M1-like, antibacterial macrophage functions(47, 72). However, many iNOS+ granuloma macrophages are not infected and many macrophages harboring bacteria have undetectable iNOS levels, underscoring the spectrum and functional state heterogeneity of macrophages in infected tissues.
The link between ontogeny and tissue microenvironments to macrophage functional states and phenotypes has also been observed in mycobacterial infections. In Mtb-infected nonhuman primate lungs, macrophages that distribute in different regions of granulomas exhibit varying functional features, such as iNOS and arginase expression(73). In mice, tissue-resident alveolar macrophages, which originate from embryonic precursor cells, have been shown to be a more favorable replicative niche for Mtb bacilli than monocyte-derived interstitial macrophages and this functional difference may be due in part to distinct metabolic programming of the two macrophage populations(45, 74). However, alveolar macrophages can re-localize to the lung interstitial region, which is dependent on IL-1R/MyD88 signaling(74). Furthermore, during and after an inflammatory state, depleted tissue alveolar macrophages can be replaced by hematopoietic, monocyte-derived macrophages(75, 76). Thus, ontogeny, microenvironmental signals, and pathogen factors may dynamically interact to shape a spectrum of macrophage functional states and antibacterial capacities in infected tissues (Figure 1). Consistent with this notion, recent scRNA-seq studies showed that both alveolar macrophages and interstitial macrophages are comprised of multiple distinct subsets with varying capacities to restrict intracellular bacteria(77). Approaches to defining macrophage heterogenous polarization and functional states in infected tissues often depend on biased cellular markers and bulk population analysis. Emerging scRNA-seq studies will be critical for identifying key cellular features of macrophage heterogenous functional states in an unbiased manner that capture the full heterogeneous spectrum and interplay of many host and pathogen factors influencing macrophage diverse phenotypes and functions(72, 77).
Perspective:
Defining the factors and mechanisms that shape tissue macrophage capacities to produce antibacterial responses and eliminate invading pathogens or act as a cellular niche for diverse intracellular bacteria to persist infected tissues is critical for the understanding of microbial pathogenesis and macrophage biology. Although studies over the years have provided insights and expanded the concept of macrophage cell states and functional diversity substantially from an early M1 and M2 framework, findings on how intracellular bacteria employ virulence effectors to actively reprogram macrophage functional states and turn these immune cells into permissive cellular niches have advanced the mechanistic and conceptual principles underlying macrophage heterogeneity and diverse functions. A number of intriguing questions remains that will be exciting subjects of further inquiries. Firstly, do other intracellular bacteria, such as S. Typhi, use injected virulence factors to reprogram macrophage cell states? What are the host pathways that these bacterial factors co-opt? We propose that actively reprogramming macrophages into a permissive cellular niche represents evolutionarily convergent microbial pathogenesis strategy for diverse intracellular bacteria to establish persistent infection. Another interesting question is how macrophages integrate bacteria-driven effects and host signaling effects on the same pathway, such as activation of STAT3 by SteE and upstream IL-6 or IL-10 cytokine signals? Are there differences in downstream transcriptional and functional outcomes when STAT3 is activated by a bacteria-driven mechanism versus host signaling? Since the bacterial levels and tissue microenvironmental milieu dynamically change over the course of infection, bacterial effects on macrophage polarization states and reprogramming likely vary throughout the course of infection. Reprograming of macrophages by intracellular bacteria may also vary significantly across different macrophage populations in infected tissues. For example, SteE may differentially affect red pulp macrophages versus monocyte-derived macrophages in STm-infected spleens. Finally, how stable are the effects of bacterial reprogramming on macrophages and what are the long-term fates of the macrophages that have been reprogrammed to be a permissive niche? Elucidating these outstanding questions will further our understanding of macrophage biology and bacterial pathogenesis, as well as enable us to more precisely target and harness macrophage pathways for therapeutic purposes.
Acknowledgements:
The authors thank members of the Monack laboratory for valuable discussions. This work is supported in part by grant K08-AI143796 from the NIAID (TP), the Stanford Maternal and Child Health Research Institute (TP), and grant R01-AI116059 from the NIAID (DM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statements:
Nothing declared
Declaration of Competing Interest
Nothing declared
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Cox N, Pokrovskii M, Vicario R, Geissmann F. Origins, Biology, and Diseases of Tissue Macrophages. Annu Rev Immunol. 2021;39:313–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park MD, Silvin A, Ginhoux F, Merad M. Macrophages in health and disease. Cell. 2022;185(23):4259–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264(1): 182–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storek KM, Monack DM. Bacterial recognition pathways that lead to inflammasome activation. Immunol Rev. 2015;265(1):112–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar Y, Valdivia RH. Leading a sheltered life: intracellular pathogens and maintenance of vacuolar compartments. Cell Host Microbe. 2009;5(6):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marakalala MJ, Martinez FO, Pluddemann A, Gordon S. Macrophage Heterogeneity in the Immunopathogenesis of Tuberculosis. Front Microbiol. 2018;9:1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price JV, Vance RE. The macrophage paradox. Immunity. 2014;41(5):685–93. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. [DOI] [PubMed] [Google Scholar]
- 9.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541–66. • This is a comprehensive review of the evolution of macrophage polarization concept and the molecular pathways that have been implicated in macrophage polarization.
- 11.Porta C, Riboldi E, Ippolito A, Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. 2015;27(4):237–48. [DOI] [PubMed] [Google Scholar]
- 12.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–73. [DOI] [PubMed] [Google Scholar]
- 14.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleriot C, Chakarov S, Ginhoux F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity. 2020;52(6):957–70. [DOI] [PubMed] [Google Scholar]
- 16.Marino S, Cilfone NA, Mattila JT, Linderman JJ, Flynn JL, Kirschner DE. Macrophage polarization drives granuloma outcome during Mycobacterium tuberculosis infection. Infect Immun. 2015;83(1):324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saliba AE, Li L, Westermann AJ, Appenzeller S, Stapels DA, Schulte LN, et al. Single-cell RNA-seq ties macrophage polarization to growth rate of intracellular Salmonella. Nat Microbiol. 2016;2:16206. • This sc-RNAseq study links macrophage M1-like and M2-like transcriptional signatures to the capacities of macrophages to support intracellular Salmonella growth and persistence.
- 18.Byndloss MX, Tsolis RM. Chronic Bacterial Pathogens: Mechanisms of Persistence. Microbiol Spectr. 2016;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheu KM, Hoffmann A. Functional Hallmarks of Healthy Macrophage Responses: Their Regulatory Basis and Disease Relevance. Annu Rev Immunol. 2022;40:295–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennings E, Thurston TLM, Holden DW. Salmonella SPI-2 Type III Secretion System Effectors: Molecular Mechanisms And Physiological Consequences. Cell Host Microbe. 2017;22(2):217–31. [DOI] [PubMed] [Google Scholar]
- 21.Panagi I, Thurston TL. Ready, STAT3, Go! Bacteria in the race for M2 macrophage polarisation. Curr Opin Microbiol. 2023;73:102285. [DOI] [PubMed] [Google Scholar]
- 22.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K, Mollenkopf H, et al. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36(3):631–47. [DOI] [PubMed] [Google Scholar]
- 24. Stapels DAC, Hill PWS, Westermann AJ, Fisher RA, Thurston TL, Saliba AE, et al. Salmonella persisters undermine host immune defenses during antibiotic treatment. Science. 2018;362(6419):1156–60. •• This study identifies the SPI-2 T3SS effector SteE promotes M2-like programming in S. Typhimurium-infected macrophages.
- 25. Panagi I, Jennings E, Zeng J, Gunster RA, Stones CD, Mak H, et al. Salmonella Effector SteE Converts the Mammalian Serine/Threonine Kinase GSK3 into a Tyrosine Kinase to Direct Macrophage Polarization. Cell Host Microbe. 2020;27(1):41–53 e6. •• This study demonstrates that the S. Typhimurium effector SteE mediates phosphorylation of macrophage STAT3 by GSK3.
- 26. Pham THM, Brewer SM, Thurston T, Massis LM, Honeycutt J, Lugo K, et al. Salmonella-Driven Polarization of Granuloma Macrophages Antagonizes TNF-Mediated Pathogen Restriction during Persistent Infection. Cell Host Microbe. 2020;27(1):54–67 e5. •• This study shows that the S. Typhimurium effector SteE drives M2-like polarization and counteracts TNF-mediated restriction to promote bacterial persistence in infected spleens in mice.
- 27.Kratochvill F, Neale G, Haverkamp JM, Van de Velde LA, Smith AM, Kawauchi D, et al. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015;12(11):1902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czimmerer Z, Daniel B, Horvath A, Ruckerl D, Nagy G, Kiss M, et al. The Transcription Factor STAT6 Mediates Direct Repression of Inflammatory Enhancers and Limits Activation of Alternatively Polarized Macrophages. Immunity. 2018;48(1):75–90 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisele NA, Ruby T, Jacobson A, Manzanillo PS, Cox JS, Lam L, et al. Salmonella require the fatty acid regulator PPARdelta for the establishment of a metabolic environment essential for long-term persistence. Cell Host Microbe. 2013;14(2):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, et al. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol. 2010;40(8):2200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redford PS, Murray PJ, O'Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4(3):261–70. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay S, Heinz E, Porreca I, Alasoo K, Yeung A, Yang HT, et al. Loss of IL-10 signaling in macrophages limits bacterial killing driven by prostaglandin E2. J Exp Med. 2020;217(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jungi TW, Brcic M, Sager H, Dobbelaere DA, Furger A, Roditi I. Antagonistic effects of IL-4 and interferon-gamma (IFN-gamma) on inducible nitric oxide synthase expression in bovine macrophages exposed to gram-positive bacteria. Clin Exp Immunol. 1997;109(3):431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin T, Bost KL. STAT3 activation in macrophages following infection with Salmonella. Biochem Biophys Res Commun. 2004;321(4):828–34. [DOI] [PubMed] [Google Scholar]
- 35.Lee KS, Jeong ES, Heo SH, Seo JH, Jeong DG, Choi YK. IL-10 suppresses bactericidal response of macrophages against Salmonella Typhimurium. J Microbiol. 2011;49(6):1050–3. [DOI] [PubMed] [Google Scholar]
- 36.Abies GP, Takamatsu D, Noma H, El-Shazly S, Jin HK, Taniguchi T, et al. The roles of Nramp1 and Tnfa genes in nitric oxide production and their effect on the growth of Salmonella typhimurium in macrophages from Nramp1 congenic and tumor necrosis factor-alpha−/− mice. J Interferon Cytokine Res. 2001;21(1):53–62. [DOI] [PubMed] [Google Scholar]
- 37.Ohmori Y, Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. J Leukoc Biol. 2001;69(4):598–604. [PubMed] [Google Scholar]
- 38.Kovarik P, Stoiber D, Novy M, Decker T. Stat1 combines signals derived from IFN-gamma and LPS receptors during macrophage activation. EMBO J. 1998;17(13):3660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gibbs KD, Washington EJ, Jaslow SL, Bourgeois JS, Foster MW, Guo R, et al. The Salmonella Secreted Effector SarA/SteE Mimics Cytokine Receptor Signaling to Activate STAT3. Cell Host Microbe. 2020;27(1):129–39 e4. •• This study shows that the S. Typhimurium effector SarA/SteE promotes STAT3 phosphorylation by mimicking a binding and signaling motif of the cytokine receptor subunit gp130.
- 40.Hedl M, Yan J, Witt H, Abraham C. IRF5 Is Required for Bacterial Clearance in Human M1-Polarized Macrophages, and IRF5 Immune-Mediated Disease Risk Variants Modulate This Outcome. J Immunol. 2019;202(3):920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rana S, Maurya S, Mohapatra G, Singh S, Babar R, Chandrasekhar H, et al. Activation of epigenetic regulator KDM6B by Salmonella Typhimurium enables chronic infections. Gut Microbes. 2021;13(1):1986665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xavier MN, Winter MG, Spees AM, den Hartigh AB, Nguyen K, Roux CM, et al. PPARgamma-mediated increase in glucose availability sustains chronic Brucella abortus infection in alternatively activated macrophages. Cell Host Microbe. 2013;14(2):159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnett E, Weaver AM, Woodyard KC, Montoya MJ, Li M, Hoang KV, et al. PPARgamma is critical for Mycobacterium tuberculosis induction of Mcl-1 and limitation of human macrophage apoptosis. PLoS Pathog. 2018;14(6):e1007100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guirado E, Rajaram MV, Chawla A, Daigle J, La Perle KM, Arnett E, et al. Deletion of PPARgamma in lung macrophages provides an immunoprotective response against M. tuberculosis infection in mice. Tuberculosis (Edinb). 2018;111:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J Exp Med. 2018;215(4):1135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen SB, Gern BH, Urdahl KB. The Tuberculous Granuloma and Preexisting Immunity. Annu Rev Immunol. 2022;40:589–614. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg MF, Roeske EK, Ward LN, Pengo T, Dileepan T, Kotov DI, et al. Salmonella Persist in Activated Macrophages in T Cell-Sparse Granulomas but Are Contained by Surrounding CXCR3 Ligand-Positioned Th1 Cells. Immunity. 2018;49(6):1090–102 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gideon HP, Hughes TK, Tzouanas CN, Wadsworth MH 2nd, Tu AA, Gierahn TM, et al. Multimodal profiling of lung granulomas in macaques reveals cellular correlates of tuberculosis control. Immunity. 2022;55(5):827–46 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma F, Hughes TK, Teles RMB, Andrade PR, de Andrade Silva BJ, Plazyo O, et al. The cellular architecture of the antimicrobial response network in human leprosy granulomas. Nat Immunol. 2021;22(7):839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cronan MR, Hughes EJ, Brewer WJ, Viswanathan G, Hunt EG, Singh B, et al. A non-canonical type 2 immune response coordinates tuberculous granuloma formation and epithelialization. Cell. 2021;184(7):1757–74 e14. • This study shows STAT6 mediates epithelial reprogramming of macrophages in zebrafish during M. marinum infection.
- 51.Guler R, Parihar SP, Savvi S, Logan E, Schwegmann A, Roy S, et al. IL-4Ralpha-dependent alternative activation of macrophages is not decisive for Mycobacterium tuberculosis pathology and bacterial burden in mice. PLoS One. 2015;10(3):e0121070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pooran A, Davids M, Nel A, Shoko A, Blackburn J, Dheda K. IL-4 subverts mycobacterial containment in Mycobacterium tuberculosis-infected human macrophages. Eur Respir J. 2019;54(2). [DOI] [PubMed] [Google Scholar]
- 53.Wang BX, Butler DS, Hamblin M, Monack DM. One species, different diseases: the unique molecular mechanisms that underlie the pathogenesis of typhoidal Salmonella infections. Curr Opin Microbiol. 2023;72:102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rolhion N, Furniss RC, Grabe G, Ryan A, Liu M, Matthews SA, et al. Inhibition of Nuclear Transport of NF-κB p65 by the Salmonella Type III Secretion System Effector SpvD. PLoS Pathog. 2016;12(5):e1005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gulati A, Shukla R, Mukhopadhaya A. Salmonella Effector SteA Suppresses Proinflammatory Responses of the Host by Interfering With IkappaB Degradation. Front Immunol. 2019;10:2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pal R, Bisht MK, Mukhopadhyay S. Secretory proteins of Mycobacterium tuberculosis and their roles in modulation of host immune responses: focus on therapeutic targets. FEBS J. 2022;289(14):4146–71. [DOI] [PubMed] [Google Scholar]
- 57.Hutchins AP, Poulain S, Miranda-Saavedra D. Genome-wide analysis of STAT3 binding in vivo predicts effectors of the anti-inflammatory response in macrophages. Blood. 2012;119(13):e110–9. [DOI] [PubMed] [Google Scholar]
- 58.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169(5):2253–63. [DOI] [PubMed] [Google Scholar]
- 59.El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol. 2008;9(12):1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qualls JE, Neale G, Smith AM, Koo MS, DeFreitas AA, Zhang H, et al. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci Signal. 2010;3(135):ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15(5):423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jaslow SL, Gibbs KD, Fricke WF, Wang L, Pittman KJ, Mammel MK, et al. Salmonella Activation of STAT3 Signaling by SarA Effector Promotes Intracellular Replication and Production of IL-10. Cell Rep. 2018;23(12):3525–36. •• This study demonstrates that the S. Typhimurium effector SarA (also known as SteE) is necessary and sufficient to promote cellular STAT3 phosphorylation.
- 63.O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, Mclnnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sorg I, Schmutz C, Lu YY, Fromm K, Siewert LK, Bogli A, et al. A Bartonella Effector Acts as Signaling Hub for Intrinsic STAT3 Activation to Trigger Anti-inflammatory Responses. Cell Host Microbe. 2020;27(3):476–85 e7. •• This study demonstrates that the Bartonella T4SS effector BepD mediates STAT3 phosphorylation.
- 65.Jung BG, Wang X, Yi N, Ma J, Turner J, Samten B. Early Secreted Antigenic Target of 6-kDa of Mycobacterium tuberculosis Stimulates IL-6 Production by Macrophages through Activation of STAT3. Sci Rep. 2017;7:40984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Queval CJ, Song OR, Deboosere N, Delorme V, Debrie AS, lantomasi R, et al. STAT3 Represses Nitric Oxide Synthesis in Human Macrophages upon Mycobacterium tuberculosis Infection. Sci Rep. 2016;6:29297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunster RA, Matthews SA, Holden DW, Thurston TLM. SseK1 and SseK3 Type III Secretion System Effectors Inhibit NF-kappaB Signaling and Necroptotic Cell Death in Salmonella-Infected Macrophages. Infect Immun. 2017;85(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pilar AV, Reid-Yu SA, Cooper CA, Mulder DT, Coombes BK. GogB is an anti-inflammatory effector that limits tissue damage during Salmonella infection through interaction with human FBXO22 and Skp1. PLoS Pathog. 2012;8(6):e1002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gosselin D, Glass CK. Epigenomics of macrophages. Immunol Rev. 2014;262(1):96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffman D, Tevet Y, Trzebanski S, Rosenberg G, Vainman L, Solomon A, et al. A non-classical monocyte-derived macrophage subset provides a splenic replication niche for intracellular Salmonella. Immunity. 2021;54(12):2712–23 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurotaki D, Uede T, Tamura T. Functions and development of red pulp macrophages. Microbiol Immunol. 2015;59(2):55–62. [DOI] [PubMed] [Google Scholar]
- 72.Pham THM, Xue Y, Brewer SM, Bernstein KE, Quake SR, Monack DM. Single-cell profiling identifies ACE(+) granuloma macrophages as a nonpermissive niche for intracellular bacteria during persistent Salmonella infection. Sci Adv. 2023;9(1):eadd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 2013;191(2):773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen SB, Gern BH, Delahaye JL, Adams KN, Plumlee CR, Winkler JK, et al. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host Microbe. 2018;24(3):439–46 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, et al. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol. 2006;35(2):227–35. [DOI] [PubMed] [Google Scholar]
- 76.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter Jm, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214(8):2387–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pisu D, Huang L, Narang V, Theriault M, Le-Bury G, Lee B, et al. Single cell analysis of M. tuberculosis phenotype and macrophage lineages in the infected lung. J Exp Med. 2021;218(9). [DOI] [PMC free article] [PubMed] [Google Scholar]