Abstract
The renal handling of ofloxacin in rats which were given ofloxacin either alone or in combination with probenecid or cimetidine was studied. In the presence of cimetidine or probenecid, ofloxacin’s total and renal clearances were reduced and its half-life was prolonged. This suggests that ofloxacin is secreted by both the anionic and cationic transport systems.
Ofloxacin is a quinolone antibacterial agent which possesses broad activity against both gram-positive and gram-negative organisms. The exact mechanism of renal excretion of ofloxacin has not been extensively studied in either animals or humans. Ofloxacin is a zwitterion at physiological pHs and thus may be secreted in the renal tubules through either the anionic or cationic system, or possibly both. An in vitro investigation, using isolated brush border membranes, suggested that ofloxacin is secreted in the renal tubules via only the organic cation transport system (16). Other quinolones have been reported to be transported by both the anionic (7, 8, 19) and cationic (20) transport systems in humans. The objective of this three-arm, parallel-design study was to identify the active transport system of renal tubular secretion of ofloxacin in rats by using inhibitors of the anionic (probenecid) (3, 5, 15) and cationic (cimetidine) (3, 23) transport systems.
Twelve male Sprague-Dawley rats (303 to 413 g) were anesthetized by intraperitoneal (i.p.) injection of pentobarbital (50 mg/kg of body weight). The left femoral vein and artery and the bladder were cannulated with polyethylene tubing. Mean arterial blood pressure (MAP) was monitored throughout the experiment. As needed, rats were redosed with pentobarbital (5 mg) to maintain anesthesia throughout the clearance experiment. After the surgical procedure, rats were hydrated intravenously (i.v.) with 3 ml of normal saline (infused over approximately 5 to 10 min) followed by a continuous infusion of normal saline at 3.7 ml/h for 1 h (via a calibrated syringe pump). A predose urine sample was obtained during this period. Afterward, a 50-mg load of inulin was administered, followed by a 3-mg/min infusion. The inulin infusion was continued for 30 min to achieve near-steady-state plasma inulin concentrations of approximately 100 mg/dl (documented in preliminary experiments [data not shown]). Urine was then collected over a 30-min period for determination of the baseline glomerular filtration rate (GFR).
Blood (150 μl), for analysis of inulin and ofloxacin concentrations, was collected in minimally heparinized microcentrifuge tubes via the femoral artery. The blood was immediately centrifuged, and the resultant plasma was saved. Resultant erythrocytes were combined with an equal amount of allogeneic rat plasma and returned to the experimental rat. Urine volume and pH were measured. All plasma and urine samples were frozen in polyethylene tubes at −70°C until analysis.
Rats were divided into three groups (n = 4 per group): ofloxacin alone (group O), ofloxacin plus cimetidine (group O+C), and ofloxacin plus probenecid (group O+P). At time zero, all rats received ofloxacin (15 mg/kg) as an i.v. bolus through the femoral vein. Blood was collected from group O rats prior to and at 0.25, 0.5, 1, 2.5, and 4 h after drug administration. Prior to dosing with ofloxacin, group O+C and O+P rats were pretreated with cimetidine and probenecid, respectively. Group O+C rats received cimetidine at 200 mg/kg (100 mg/kg given i.p. and 100 mg/kg given i.v.) followed by an infusion of 1.85 mg/kg/min. The bolus dose of cimetidine was split between the i.p. and i.v. routes to minimize hypotension. Group O+P rats received an i.v. bolus of probenecid (15 mg/kg) followed by an infusion of 0.60 mg/kg/min. Infusions of inulin and the inhibitor of secretion (probenecid or cimetidine) were prepared in a normal saline solution at a concentration such that 3.7 ml of solution was administered per h to all rats. The blood collection protocol for these two groups was the same as for group O with the exception of an additional sample taken at 5.5 h. Urine was collected throughout the experiment from rats of all three groups.
Commercially available i.v. ofloxacin (Floxin; Ortho Pharmaceuticals, Raritan, N.J.), cimetidine (Tagamet; SmithKline-Beecham, Philadelphia, Pa.), and inulin (Iso-Tex Diagnostics, Friendswood, Tex.) were used. Ofloxacin powder was obtained from Ortho Pharmaceuticals, and difloxacin (used as an internal standard) was donated by Abbott Laboratories (Abbott Park, Ill.). For i.v. administration, probenecid powder (200 mg) was diluted in approximately 1 ml of 0.5 M NaOH, further diluted with physiological saline, and then adjusted to a pH of approximately 9.0 with a 0.05 M HCl solution. The final concentration of the solution was approximately 30 mg/ml. A fresh probenecid solution was prepared daily. All other reagents were of analytical grade and were commercially available.
The assay for ofloxacin in plasma was adapted from previously published high-performance liquid chromatography methods (2, 9). The mobile phase was a solution consisting of 14% (vol/vol) methanol, 5% (vol/vol) acetonitrile, and 0.61% (wt/vol) tetrabutylammonium hydroxide in 0.02 M potassium dihydrogen phosphate, with a flow rate of 1 ml/min. The stationary phase was a Suplex PKB-100 (5-μm particle size) packed in a 25-cm-long stainless steel column (outer diameter, 0.25 in.; inner diameter, 4.6 mm) (Supelco Inc., Bellefonte, Pa.). The eluate was monitored at an excitation wavelength of 280 nm and an emission wavelength of 500 nm. Ofloxacin calibration graphs obtained with control human plasma were found to be linear for the concentrations ranging from 0.1 to 25 μg/ml. The limit of detection was 0.25 μg/ml. The within-day and between-day coefficients of variation were less than 11%. The within-day and between-day percentages of error (a measure of accuracy) were less than 14%. The ofloxacin calibration graph obtained for the urine assay was linear for concentrations ranging from 25 to 1,000 μg/ml. The within-day precision and accuracy of the urine method were less than 4% and 7%, respectively. The protein binding of ofloxacin was determined at various plasma concentrations in both the presence and the absence of the inhibitors. The concentration of unbound ofloxacin in plasma was determined by the centrifugal ultrafiltration method. Standard calorimetric methods were used to determine inulin concentrations in urine and plasma samples (6). The standard curve for the inulin assay was linear between 0.25 and 10 mg/dl. Quality control specimens (75 and 325 mg/dl) and test samples of both serum and urine were diluted to be within this concentration range. The within-day and between-day coefficients of variation for both serum and urine were less than 10%.
Based on visual inspection of the individual plasma concentration-time curves, pharmacokinetic parameters were determined by fitting individual plasma concentrations of ofloxacin to an i.v. bolus, two-compartment model, using PCNONLIN (version 4.2; Statistical Consultants, Inc.) (14). Differences in pharmacokinetic parameters were evaluated by analysis of variance with a Tukey post hoc test when appropriate (Systat; Systat Inc.). Statistical significance was assessed at the P < 0.05 level. Data are presented as means ± standard deviations (SD).
There were no statistically significant differences in weight, GFR, urine flow rate, or MAP between groups (Table 1). The O+C group rats did tend to become hypotensive after receiving the loading dose of cimetidine; the extent of these episodes varied, and they were short-lived (3 to 5 min). As a group, the hypotensive episodes appeared to have a small but nonsignificant effect on urine output and GFR. However, there was significant intragroup variability in GFR determinations, and in individual animals there did not appear to be any correlation between GFR or urine flow rate and blood pressure. The mean urinary pH at the beginning of the experiment was 6.3 ± 0.3, and at the end it was 7.0 ± 0.5, but these changes were similar for all groups.
TABLE 1.
Pharmacokinetic parameters of ofloxacin in rats
| Group | Weight (kg) | GFR (ml/min/kg) | Mean value ± SD fora:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| V (ml/min) | MAP (mm Hg) | V1 (ml/kg) | t1/2βb,d (h) | AUCb,d (μg · h/ml) | CLb,c,d (ml/min/kg) | feb (%) | CLRb,c,d (ml/min/kg) | CLNRb,d (ml/min/kg) | |||
| O | 0.362 ± 0.034 | 10.9 ± 2.5 | 0.08 ± 0.04 | 130 ± 2 | 1,095.9 ± 318.6 | 1.84 ± 1.00 | 9.22 ± 0.79 | 27.2 ± 2.8 | 45.9 ± 6.9 | 15.3 ± 1.6 | 12.0 ± 2.1 |
| O+C | 0.351 ± 0.014 | 9.1 ± 0.4 | 0.07 ± 0.01 | 110 ± 5 | 1,222.6 ± 238.7 | 3.74 ± 0.84 | 25.36 ± 4.07 | 10.0 ± 1.9 | 28.6 ± 2.4 | 4.6 ± 0.7 | 5.4 ± 1.3 |
| O+P | 0.342 ± 0.037 | 10.4 ± 2.9 | 0.05 ± 0.01 | 119 ± 3 | 1,376.7 ± 451.4 | 2.21 ± 0.31 | 12.88 ± 1.54 | 19.5 ± 2.8 | 39.2 ± 6.7 | 8.4 ± 2.2 | 11.1 ± 1.8 |
Each value represents the mean ± SD for four rats. V, urinary flow rate; t1/2β, half-life in plasma of the terminal (beta) phase; AUC, area under the plasma concentration-time curve; CL, total body clearance; fe, fraction of drug excreted in the urine.
P < 0.05, group O versus group O+C.
P < 0.05, group O versus group O+P.
P < 0.05, group O+C versus group O+P.
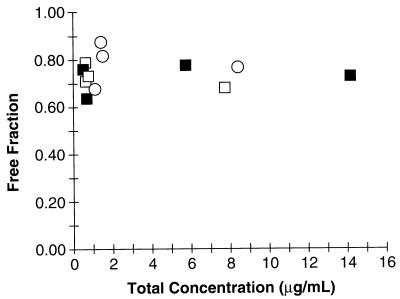
Ofloxacin’s free fraction was determined to be 74% ± 0.06%. The presence of probenecid or cimetidine did not alter protein binding, nor did protein binding appear to be concentration dependent (Fig. 1). There was significant binding of ofloxacin to the filter (26%) when the drug was diluted in water and subjected to ultrafiltration.
FIG. 1.
Relationship between total concentration of ofloxacin and ofloxacin’s free fraction for rats treated with ofloxacin alone (group O [▪]), with ofloxacin plus cimetidine (group O+C [○]), or with ofloxacin plus probenecid (group O+P [□]).
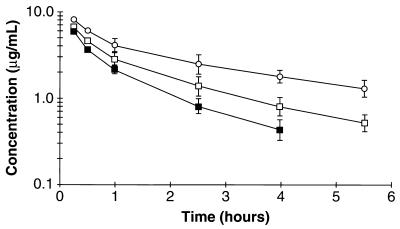
Figure 2 depicts mean ofloxacin concentrations (± SD) after a single 15-mg/kg dose alone or in the presence of cimetidine or probenecid. In general, individual plasma ofloxacin concentration curves demonstrated a biexponential decay in all three groups. Mean plasma ofloxacin concentrations in the O+C and O+P groups were higher than that in the O group. Mean pharmacokinetic parameters of ofloxacin for the three treatment groups are presented in Table 1. Cimetidine and probenecid both reduced the renal clearance (CLR) of ofloxacin, presumably due to inhibition of renal tubular secretion. Secretion clearance values were 7.2, −2.2, and 0.7 ml/min/kg in the O, O+C, and O+P groups, respectively (P < 0.05 between all three groups). Although the relative differences are plausible, the absolute values for secretion clearance may be suspect due to significant filter binding. The lack of differences between groups in the volume of distribution in the central compartment (V1) and GFR rule out other pharmacokinetic and physiological causes for the differences noted. Nonrenal clearance (CLNR) was also reduced in the presence of cimetidine and probenecid; this was most evident in the cimetidine-treated rats. The area under the plasma ofloxacin concentration-time curve for the O+C group was significantly higher than that for either the O or O+P group, probably as a result of the combined alterations in CLR and CLNR.
FIG. 2.
Mean plasma ofloxacin concentration-time curves for rats treated with ofloxacin alone (group O [▪]), with ofloxacin plus cimetidine (group O+C [○]), or with ofloxacin plus probenecid (group O+P [□]) after a 15-mg/kg i.v. dose. Each plot represents the mean ± SD for four rats. Error bars overlap between groups for the 0.25- and 0.5-h time points and thus are not depicted.
Based on the results of our study, it appears that both the anionic and cationic transport systems participate in the CLR of ofloxacin in rats. We did not measure the concentrations of the inhibitors, but the doses used were similar to those employed in other published studies (3, 4, 11, 12, 15, 21). Although it appears that cimetidine may be a more potent inhibitor of tubular secretion than probenecid, this effect may be more a function of the plasma concentrations of the inhibitors obtained than a true difference.
The pharmacokinetic parameters determined here are consistent with those of other studies (10, 18). Of note, although we found significant binding to the membrane during our protein binding experiments, the binding values obtained here were similar to those determined in other studies (69% [10] and 77% [18] free versus 74% in our study). Filter binding is a well-recognized disadvantage of the use of ultrafiltration for estimating protein binding (17, 24). Although the absolute values of protein binding may be suspect, the relative differences between groups are probably sound. This study was also somewhat limited by the small numbers of animals in each group. In spite of this, however, we were able to detect a statistically significant difference between treatment groups for the primary study endpoints. We should note, however, that there was insufficient power (<80%) to detect a difference of 25% in GFR, urinary flow rate, or V1 between groups.
The CLNR of ofloxacin was significantly reduced in the presence of cimetidine. Probenecid also reduced the CLNR of ofloxacin, but the effect was of a smaller magnitude and did not reach statistical significance. In contrast to humans, in which renal elimination is the major route of clearance, rats metabolize and renally excrete ofloxacin to equal degrees. Cimetidine is a well-documented inhibitor of the cytochrome P-450 oxidative systems, while probenecid inhibits glucuronidation (1, 13, 22). Given that ofloxacin’s metabolism occurs primarily via glucuronidation, it is unclear why the effect on CLNR was greater in the cimetidine group than in the probenecid group.
In conclusion, the results of this trial suggest that ofloxacin is transported by both the cationic and anionic transport systems in the renal tubules. Probenecid and cimetidine both decrease the CLR of ofloxacin. A clinical study in humans to document potential drug interactions is warranted.
Acknowledgments
We thank Mike O’Donnell and Ronald P. Cody for their advice on this project. The technical assistance of Denny Wheeler, Lane Bushman, and Mark Hilegas is appreciated.
REFERENCES
- 1.Abernethy D R, Greenblatt D J, Ameer B, Shader R I. Probenecid impairment of acetaminophen and lorazepam clearance: direct inhibition of ether glucuronide formation. J Pharmacol Exp Ther. 1985;234:345–349. [PubMed] [Google Scholar]
- 2.Awni W M, Clarkson J, Guay D R P. Determination of ciprofloxacin and its 7-ethylenediamine metabolite in human serum and urine by high-performance liquid chromatography. J Chromatogr. 1987;419:414–420. doi: 10.1016/0378-4347(87)80309-2. [DOI] [PubMed] [Google Scholar]
- 3.Chatton J, Odone M, Besseghir K, Roch-Ramel R. Renal secretion of 3′-azido-3′-deoxythymidine by the rat. J Pharmacol Exp Ther. 1990;255:140–155. [PubMed] [Google Scholar]
- 4.Darling I M, Morris M E. Evaluation of “true” creatinine clearance in rats reveals extensive renal secretion. Pharm Res. 1991;8:1318–1322. doi: 10.1023/a:1015820316660. [DOI] [PubMed] [Google Scholar]
- 5.Drummer O H, Thompson J, Hooper R, Jarrott B. Effect of probenecid on the disposition of captopril and captopril dimer in the rat. Biochem Pharmacol. 1985;34:3347–3351. doi: 10.1016/0006-2952(85)90356-9. [DOI] [PubMed] [Google Scholar]
- 6.Fjeldbo W, Stamey T A. Adapted method for determination of inulin in serum and urine with an autoanalyzer. J Lab Clin Med. 1968;72:353–358. [PubMed] [Google Scholar]
- 7.Gemba M, Komamura T, Matsushima Y, Itoh T, Miyata K, Nakamura M. Cinoxacin: competitive inhibitory effect on p-aminohippurate transport and its uptake in renal cortical slices. Arch Int Pharmacodyn Ther. 1983;261:308–315. [PubMed] [Google Scholar]
- 8.Jaehde U, Sorgel F, Reiter A, Sigl G, Naber K G, Schunack W. Effect of probenecid on the distribution and elimination of ciprofloxacin in humans. Clin Pharmacol Ther. 1995;58:532–541. doi: 10.1016/0009-9236(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 9.Katagiri Y, Naora K, Ichikawa N, Hayashibara M, Iwamoto K. Simultaneous determination of ofloxacin, fenbufen, and felbinac in rat plasma by high-performance liquid chromatography. J Chromatogr. 1988;431:135–142. doi: 10.1016/s0378-4347(00)83076-5. [DOI] [PubMed] [Google Scholar]
- 10.Katagiri Y, Naora K, Ichikawa N, Hayashibara M, Iwamoto K. Absence of pharmacokinetic interaction between ofloxacin and fenbufen in rats. J Pharm Pharmacol. 1989;41:717–719. doi: 10.1111/j.2042-7158.1989.tb06349.x. [DOI] [PubMed] [Google Scholar]
- 11.Lin J H, Chen I W, Ulm E H, Duggan D E. Differential renal handling of angiotensin-converting enzyme inhibitors enalaprilat and lisinopril in rats. Drug Metab Dispos. 1988;16:392–396. [PubMed] [Google Scholar]
- 12.Lin J H, Los L E, Ulm E H, Duggan D E. Kinetic studies on the competition between famotidine and cimetidine in rats. Evidence of multiple renal secretory systems for organic cations. Drug Metab Dispos. 1988;16:52–56. [PubMed] [Google Scholar]
- 13.Mays D C, Dixon K F, Balboa A, Pawluk L J, Bauer M R, Nawoot S, Gerber N. A nonprimate animal model applicable to zidovudine pharmacokinetics in humans: inhibition of glucuronidation and renal excretion of zidovudine by probenecid in rats. J Pharmacol Exp Ther. 1991;259:1261–1270. [PubMed] [Google Scholar]
- 14.Metzler C M, Elfring G L, McEwen A J. A package of computer programs for pharmacokinetic modeling. Biometrics. 1974;30:562–571. [Google Scholar]
- 15.Nadai M, Apichartpichean R, Hasegawa T, Nabeshima T. Pharmacokinetics and the effect of probenecid on the renal excretion mechanism of diprophylline. J Pharm Sci. 1992;81:1024–1027. doi: 10.1002/jps.2600811014. [DOI] [PubMed] [Google Scholar]
- 16.Okano T, Maegawa H, Inui K, Hori R. Interaction of ofloxacin with organic cation transport system in rat renal brush-border membranes. J Pharmacol Exp Ther. 1990;255:1033–1037. [PubMed] [Google Scholar]
- 17.Pacifici G M, Viani A. Methods of determining plasma and tissue binding of drugs. Pharmacokinetic consequences. Clin Pharmacokinet. 1992;23:449–468. doi: 10.2165/00003088-199223060-00005. . (Review.) [DOI] [PubMed] [Google Scholar]
- 18.Sato H, Okezaki E, Yamamoto S, Nagata O, Kato H, Tsuji A. Entry of the new quinolone antibacterial agents of ofloxacin and NY-198 into the central nervous system in rats. J Pharmacobio-Dyn. 1988;11:386–394. doi: 10.1248/bpb1978.11.386. [DOI] [PubMed] [Google Scholar]
- 19.Shiba K, Saito A, Shimada J, Hori S, Kaji M, Miyahara T, Kusajima H, Kaneko S, Saito S, Ooie T, Uchida H. Renal handling of fleroxacin in rabbits, dogs, and humans. Antimicrob Agents Chemother. 1990;34:58–64. doi: 10.1128/aac.34.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorgel, F., G. R. Granneman, U. Stephan, and C. Locke. 1992. Effect of cimetidine on the pharmacokinetics of temafloxacin. Clin. Pharmacokinet. 22(Suppl. 1):75–82. [DOI] [PubMed]
- 21.Viberti G, Morgensen C E, Groop L C, Pauls J F. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. JAMA. 1994;271:275–279. [PubMed] [Google Scholar]
- 22.von Moltke L L, Manis M, Harmatz J S, Poorman R, Greenblatt D J. Inhibition of acetaminophen and lorazepam glucuronidation in vitro by probenecid. Biopharm Drug Dispos. 1993;14:119–130. doi: 10.1002/bdd.2510140204. [DOI] [PubMed] [Google Scholar]
- 23.Weiner I M, Roth L. Renal excretion of cimetidine. J Pharmacol Exp Ther. 1981;216:516–520. [PubMed] [Google Scholar]
- 24.Wright J D, Boudinot F D, Ujhelyi M R. Measurement and analysis of unbound drug concentrations. Clin Pharmacokinet. 1996;30:445–462. doi: 10.2165/00003088-199630060-00003. . (Review.) [DOI] [PubMed] [Google Scholar]