Abstract
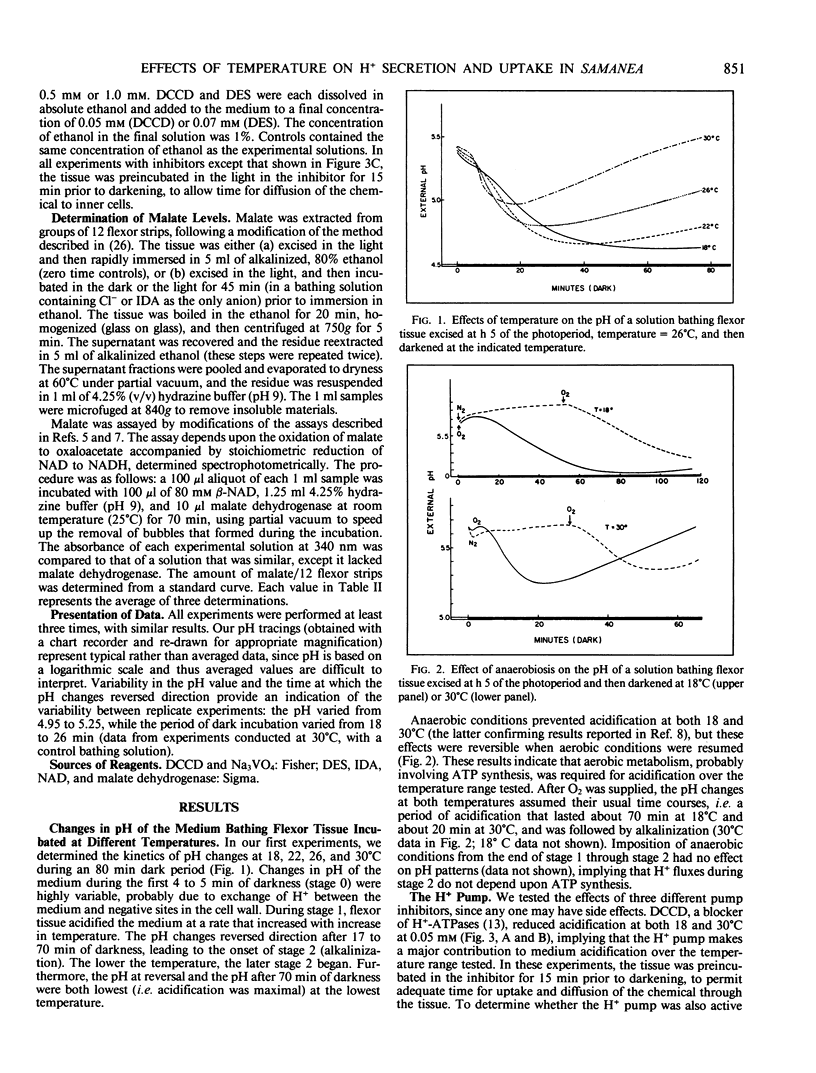
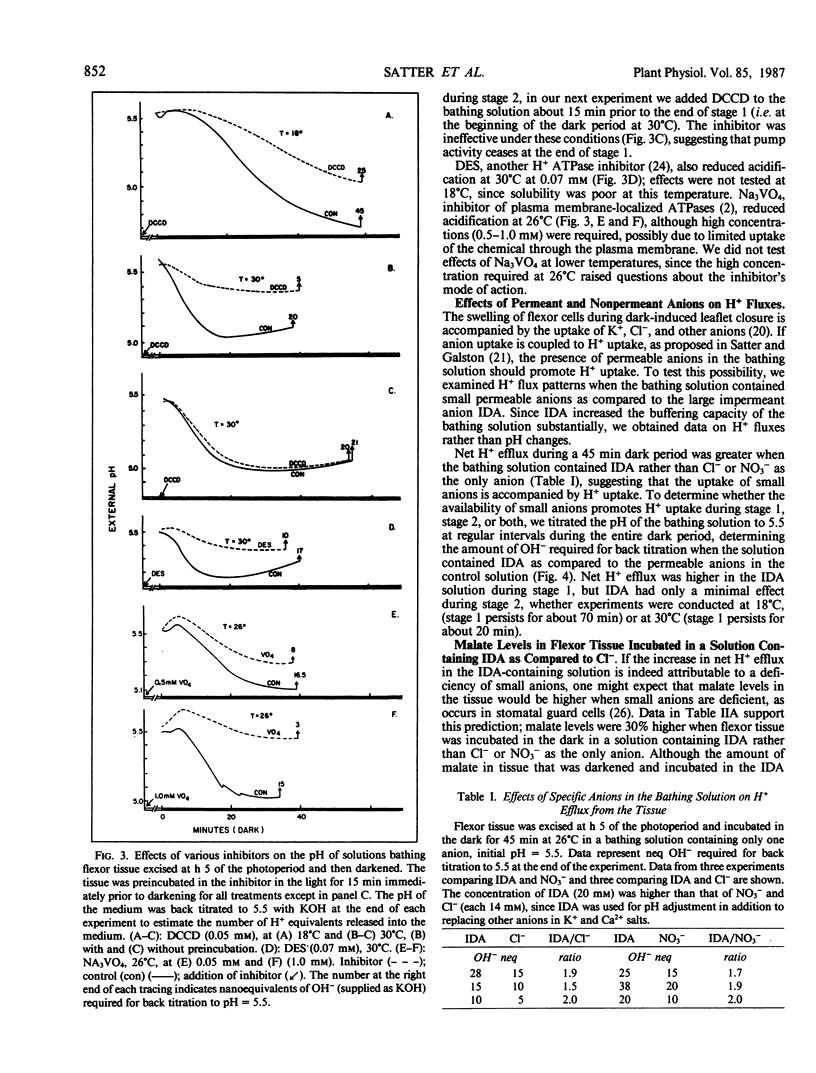
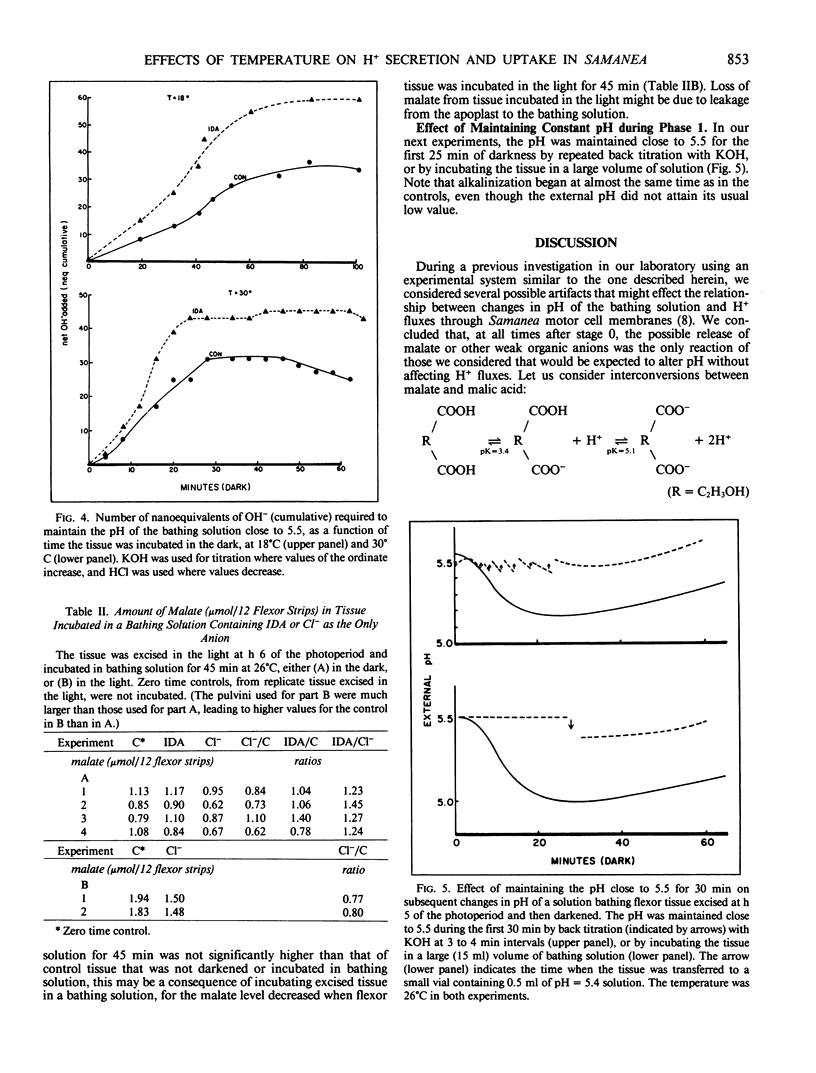
Previous studies reveal that dark-induced closure of Samanea leaflets is accompanied by H+ secretion from flexor motor cells. We now report that flexor tissue excised in the light, incubated in a weakly buffered bathing solution, and then darkened at different temperatures (18°C-30°C) acidified the medium (indicating net H+ efflux) at all temperatures tested, but most rapidly at the highest temperature. However, pH changes reversed direction after 20 to 70 minutes; the lower the temperature, the later pH reversal occurred, and the lower the pH at reversal and after 45 minutes. These data provide a basis for the previously reported promotive effect of low temperature on dark-induced leaflet closure, assuming net H+ and K+ fluxes are opposite in direction. Net H+ efflux at all temperatures tested was greater when the impermeant molecule iminodiacetate replaced small permeant anions in the bathing solution, suggesting that H+ uptake is coupled to anion uptake, probably via a H+/anion symport system. When permeant anions were deficient, the amount of malate in the tissue increased, presumably by new synthesis. Malate synthesis would substitute for H+/anion uptake in charge balance and in providing H+ for cytoplasmic pH regulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Gorton H. L. Water Relations in Pulvini from Samanea saman: I. Intact Pulvini. Plant Physiol. 1987 Apr;83(4):945–950. doi: 10.1104/pp.83.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias A., Satter R. L. H fluxes in excised samanea motor tissue : I. Promotion by light. Plant Physiol. 1983 Jun;72(2):564–569. doi: 10.1104/pp.72.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnett P. E., Beechey R. B. Inhibitors of the ATP synthethase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- Lowe A. G., Lambert A. Chloride-bicarbonate exchange and related transport processes. Biochim Biophys Acta. 1982 Dec;694(4):353–374. doi: 10.1016/0304-4157(82)90002-8. [DOI] [PubMed] [Google Scholar]
- O'neill S. D., Spanswick R. M. Effects of vanadate on the plasma membrane ATPase of red beet and corn. Plant Physiol. 1984 Jul;75(3):586–591. doi: 10.1104/pp.75.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro A., Blatt M. R., Slayman C. L. A potassium-proton symport in Neurospora crassa. J Gen Physiol. 1986 May;87(5):649–674. doi: 10.1085/jgp.87.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Geballe G. T., Applewhite P. B., Galston A. W. Potassium flux and leaf movement in Samanea saman. I. Rhythmic movement. J Gen Physiol. 1974 Oct;64(4):413–430. doi: 10.1085/jgp.64.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Schrempf M., Chaudhri J., Galston A. W. Phytochrome and Circadian Clocks in Samanea: Rhythmic Redistribution of Potassium and Chloride within the Pulvinus during Long Dark Periods. Plant Physiol. 1977 Feb;59(2):231–235. doi: 10.1104/pp.59.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kirk C. A., Raschke K. Presence of Chloride Reduces Malate Production in Epidermis during Stomatal Opening. Plant Physiol. 1978 Mar;61(3):361–364. doi: 10.1104/pp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]


