Abstract
Prior studies have compared neural connectivity during mentalizing tasks in autism (ASD) to non-autistic individuals and found reduced connectivity between the inferior frontal gyrus (IFG) and mentalizing regions. However, given that the IFG is involved in motor processing, and about 80% of autistic individuals have motor-related difficulties, it is necessary to explore if these differences are specific to ASD or instead similar across other developmental motor disorders, such as developmental coordination disorder (DCD). Participants (29 ASD, 20 DCD, 31 typically developing [TD]; ages 8-17) completed a mentalizing task in the fMRI scanner, where they were asked to think about why someone was performing an action. Results indicated that the ASD group, as compared to both TD and DCD groups, showed significant functional connectivity differences when mentalizing about other’s actions. The left IFG seed revealed ASD connectivity differences with the: bilateral TPJ, left insular cortex, and bilateral DLPFC. Connectivity differences using the right IFG seed revealed ASD differences in the: left insula, and right DLPFC. These results indicate that connectivity differences between the IFG, mentalizing regions, emotion and motor processing regions are specific to ASD and not a result of potentially co-occurring motor differences.
Keywords: Autism, developmental coordination disorder, mentalizing, mirror neuron, theory of mind, neurodevelopmental conditions
1. Introduction
Autistic individuals commonly display difficulties with understanding others’ intentions (Colombi et al., 2009; Koster-Hale et al., 2012; Margoni & Surian, 2016; Moran et al., 2011; Rosenblau et al., 2015; Schuwerk et al., 2016; Zalla & Leboyer, 2011). Previous studies in typically developing (TD) individuals indicate that understanding why a person makes a particular action relies on communication between the putative mentalizing network, important for cognitively deducing other people’s intentions (for a review, see Frith & Frith, 2006) and the putative mirror neuron system (MNS), important for using one’s own motor regions to process other’s actions (Arioli et al., 2021; Ciaramidaro et al., 2015; Gallese, 2009; Sperduti et al., 2014; Spunt & Lieberman, 2013). Core regions of the mentalizing system include the medial prefrontal cortex (mPFC), and temporoparietal junction (TPJ; Frith & Frith, 2006; Kliemann et al., 2018; Schurz et al., 2014), with the dorsomedial prefrontal cortex (dmPFC) involved in processing other’s internal states from their actions (Amodio & Frith, 2006; Frith & Frith, 2006; Sallet et al., 2013). Core regions of the MNS include the pars opercularis of the inferior frontal gyrus (IFGop) and the inferior parietal lobe (IPL; Rizzolatti & Craighero, 2004; Rizzolatti & Sinigaglia, 2010).
When processing someone’s intentions from their actions, both these networks appear to be involved (de Lange et al., 2008; Jacob & Jeannerod, 2005; Liew et al., 2011; Mainieri et al., 2013), with increased connectivity between the MNS and mentalizing systems (e.g., dmPFC and right IFG; Spunt & Lieberman, 2013). In addition, when trying to understand others’ emotional states from other’s actions (including facial actions), emotion-related networks, including the insula, may be involved alongside mentalizing and MNS networks (for a review, see Aziz-Zadeh et al., 2018). Thus, the mentalizing, MNS, and emotion-related brain regions may be involved in deciphering intent from other people’s actions, depending on the stimuli and the task.
Autistic children commonly show atypical processing within regions of these networks (insula: Di Martino et al., 2009; MNS: Dapretto et al., 2006; Kilroy et al., 2021; Mentalizing: Baron-Cohen et al., 1999; Happé et al., 1996; Holt et al., 2014; Kana et al., 2009; note, developmental age has been found to correlate with these findings [Bastiaansen et al., 2011], which may explain null findings in studies comparing adult populations). In particular, in autism (ASD), the right anterior insula consistently shows reduced activity in social vs. nonsocial processing (Di Martino et al., 2009) and altered resting state functional connectivity with numerous regions (Francis et al., 2019; Safar et al., 2018). Further, Cole and colleagues (2019) specifically showed that when asked to infer other’s intentions from their hand actions, autistic individuals, compared to a TD group, had reduced connectivity between the MNS and mentalizing network (dfMPC and IFG). Such aberrant connectivity between MNS and mentalizing regions in autism has also been found at rest (e.g., posterior cingulate cortex/precuneus overconnected with left IFG), with connectivity patterns related to ASD social symptomatology (Fishman et al., 2014).
Behaviorally, several studies show that autistic individuals may have difficulty with theory of mind (ToM)/mentalizing tasks (e.g., Strange Stories test and Eyes test [Brent et al., 2004]; Deception task [Pilowsky et al., 2000]; Free-sorting task of representational objects [Shulman et al., 1995]; also Bos & Stokes, 2019; De Coster et al., 2018; Mazza et al., 2014; Mul et al., 2018; Rueda et al., 2015; Senland & Higgins-D’Alessandro, 2016; Vyas et al., 2017). Additionally, they show difficulties with imitation tasks that may rely, in part, on MNS processing (Heiser et al., 2003), including implicit imitation (Dimberg, 1982; McIntosh et al., 2006) and imitation of meaningful gestures (Abrams et al., 2022; Kilroy et al., 2022). Such imitation difficulties have been associated with many core autism social symptomologies, including affect recognition ability (Abrams et al., 2022), joint attention (Bottema-Beutel et al., 2019; Carpenter et al., 2002; Dadgar et al., 2017; Ingersoll & Schreibman, 2006; Rogers et al., 2003), empathy skills (Adornetti et al., 2019; Dowell et al., 2009; McAuliffe et al., 2017; Mostofsky et al., 2006), theory of mind (Bottema-Beutel et al., 2019; Mainieri et al., 2013; Perra et al., 2008), and social skills (Colombi et al., 2009; Dziuk et al., 2007; Libby et al., 1998; McDuffie et al., 2007; Stone et al., 1997; Vivanti, 2013; Young et al., 2011). In addition, imitation skills have been found to significantly correlate with autism severity (Gizzonio et al., 2015; Ingersoll & Meyer, 2011; Pittet et al., 2022; Rogers et al., 2003; Zachor et al., 2010). Finally, autistic individuals may have difficulty interpreting other people’s facial expressions (Uljarevic & Hamilton, 2013; A. T. Wang et al., 2004; Yeung, 2022; Yeung et al., 2020). Thus, there is behavioral evidence to expect differences in processing in ASD among neural regions within the MNS, mentalizing networks, and the insula, as well as the interplay between them.
However, the MNS is, by definition, composed of motor brain regions (Rizzolatti & Sinigaglia, 2016). Given that about 80% of individuals with ASD also have motor deficits and meet criteria for developmental coordination disorder (DCD; Bhat, 2020; Green et al., 2009; Hannant et al., 2018; Hilton et al., 2012; Kangarani-Farahani et al., 2023; Miller et al., 2021; Williams et al., 2004), it is difficult to ascertain whether the connectivity differences described above are due to potential co-occurring motor deficits, rather than related to social deficits, which are the core symptomology of ASD.
To address this possibility, here we compare ASD, DCD, and TD groups in functional connectivity during a mentalizing task where they are asked to think about the intentions of other people’s actions. These included both bimanual hand actions as well as emotional and non-emotional facial expressions, as both stimuli have been found to yield differences in ASD as compared to TD groups (Dapretto et al., 2006; Kilroy et al. 2022). We focus on the IFGop since it has a prominent role in motor function and thus connectivity with this region may be impacted by motor disturbances. Indeed, the IFGop has been found to be hypoactive in ASD (Dapretto et al., 2006; Kilroy et al., 2021), and, in some cases, also in DCD (Kilroy et al., 2022). Here we compare connectivity between groups (ASD, DCD, TD) between the IFGop and: 1) other regions within the MNS; 2) regions within the mentalizing network; and 3) the insula. We predict that the ASD group will show aberrant connectivity between the MNS and mentalizing networks and the insula, compared to both the DCD and TD groups. By contrast, as we do not expect motor deficits to impact connectivity between these networks, we do not expect functional connectivity differences between or within these networks for the DCD group compared to TD. These findings will help better understand if prior studies reporting connectivity differences between MNS, mentalizing networks, and insula in ASD are indeed specific to ASD, rather than potential DCD co-occurrence.
2. Methods
2.1. Participants
Eight-six participants participated in the study; however six participants were excluded from data analyses due to excessive head motion or framewise displacement (absolute FD>1.5mm; Jenkinson et al., 2002). Thus, 80 right-handed youth ages 8-17 were included in the the study, in one of three groups: ASD (N = 29; mean age=12.11 ± 2.3), DCD (N = 20; mean age=12.49 ± 2.16), or TD (N = 31; mean age=12.08 ± 2.29). Participants were recruited from clinics in the greater Los Angeles healthcare system, word-of-mouth, through local schools, and advertising on social media. Across groups, participants were included only if they: (a) had IQ≥80 (2 SD below the mean) on Full Scale Intelligence Quotient (FSIQ-4) of the Wechsler Abbreviated Scale of Intelligence 2nd edition (WASI-II; Wechsler, 2011); and (b) were right-handed as assessed by an adaptation of the Edinburgh handedness questionnaire (Crovitz & Zener, 1962; Oldfield, 1971). They were excluded for: (a) a history of head injury with loss of consciousness greater than five minutes; (b) insufficient fluency in English or parent(s) without English proficiency; (c) premature birth before 36 weeks of gestation; (d) contraindications to MRI procedures. All participants and their parents were evaluated for their ability to provide informed assent/consent and then gave their child’s written assent and parental consent as required by the study protocols approved by the University of Southern California’s Institutional Review Board. We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
2.1.1. Participants with ASD (N=29, 7 female)
Participants were included in the ASD group if they had previously received a diagnosis either through an ASD diagnostic assessment, a clinical ASD diagnostic interview, or both. Autism diagnosis was verified by research-reliable staff using the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al., 2000, 2012), and the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994). Two females had scores below the ADOS-2 threshold but qualified through the ADI-R and clinician review. Additional exclusion criteria included: any other neurological or psychological disorder diagnoses except for attention deficit disorders or generalized anxiety disorder (both have high co-occurrence with ASD; Avni et al., 2018). Ten ASD participants were on psychotropic medication prescriptions at the time of data collection. We note that 23 out of 29 of our participants met criteria for probable DCD as based on the Movement Assessment Battery for Children (MABC-2; Brown, 2013; Brown & Lalor, 2009; Henderson et al., 1992) standard total score, and 27 out of 29 of our participants met criteria for potential ADHD based on the Conners 3AI-Parent report measure (Conners, 2008). Thus, it should be noted that most of this group was likely to have not only ASD, but also DCD (79%) and ADHD (93%).
2.1.2. Participants with probable DCD (N=20, 10 female)
Participants were included if they had: (a) a performance ≤16th percentile on the MABC-2; and (b) no first-degree autistic relatives and no current or previous concerns about an autism diagnosis. The Conners 3AI-Parent report measure was used to discern attention deficit and hyperactivity disorder (ADHD) symptoms but was not part of the exclusion criteria due to high co-occurrence of ADHD in DCD (Martin et al., 2006). Four children in the DCD group had Social Responsiveness Scale, Second Edition (SRS-2; Constantino & Gruber, 2012) T-scores ranging from 65-74, in line with previous studies indicating that some children with DCD have elevated scores on the SRS (Kilroy et al., 2022; Sumner et al., 2016). For those children, we administered the ADOS-2, but they did not meet criteria for ASD and thus were included in the DCD group. Four children were on psychotropic medication prescriptions at the time of data collection. Twelve out of 20 participants met criteria for potential ADHD based on the Conners 3AI-Parent report measure (Conners, 2008).
2.1.3. Typically developing (TD) participants (N= 31, 11 female)
TD participants were excluded if they had: (a) any psychological or neurological disorder diagnosis, including attention deficit disorders or generalized anxiety disorder; (b) a first-degree autistic relative; (c) T-score>65 on the Conners 3AI-Parent report which indicates a risk of a ADHD; (d) a MABC-2 score<25th percentile or “probable DCD” determined by the Developmental Coordination Disorder Questionnaire (DCDQ; Wilson et al., 2000, 2009); and (e) SRS-2 T-score>60, indicating a risk of autistic traits.
2.2. Experimental Design
2.2.1. Measures
All participants completed a battery of behavioral surveys within the two weeks prior to scanning. All participants also completed: the WASI-II (Wechsler, 2011), an full-scale IQ measure; the SRS-2 (Constantino & Gruber, 2012), a measure of social skills in DCD and those affected by autism; and, the Conners (Conners, 2008), a measure of ADHD symptoms. For both SRS-2 and Conners, T-scores ranged from 0-100, with higher scores on SRS-2 (60 T-score cutoff) and Conners (65 T-score cutoff) indicating more severe social and attention-deficit symptoms respectively. Autistic participants completed the ADOS-2 (Lord et al., 2000, 2012) during their first day of participation, and parents of autistic participants completed the ADI-R (Lord et al., 1994) during the participant fMRI sessions. Motor ability of all participants was assessed first using the DCDQ screener for motor difficulties (Wilson et al., 2000, 2009), then with the standard scores of the MABC-2 observation measure (Brown, 2013; Brown & Lalor, 2009; Henderson et al., 1992) consisting of the total standard score and three subtest standard scores: Manual Dexterity, Aiming and Catching skills, and Balance. The MABC-2 standard scores corresponded to percentiles, with scores below 25th percentile indicating probable motor difficulties. Theory of Mind (ToM or mentalizing) skills were assessed using the Developmental NEuroPSYchological Assessment (NEPSY-II; Korkman et al., 2007). ToM verbal scores and contextual scores were used to calculate a ToM total score within the Social Perception domain of the NEPSY-II. Lower scores on theWASI, MABC-2, and NEPSY-IIcorrespond to lower IQ, lower motor ability, and lower ToM skills, respectively.
Demographic measures were analyzed with IBM SPSS Statistics (Version 28). Outliers were determined by noting scores >2.2 times the interquartile range from both the first and third quartiles (Hoaglin & Iglewicz, 1987), and were omitted from the final behavioral dataset. No participants were omitted due to outliers in behavioral scores. Groups were compared for differences in behavioral measures using one-way ANOVAs with the Scheffé post-hoc correction for groupwise comparisons.
2.2.2. fMRI Procedure
Functional MRI procedure, task stimuli, fMRI acquisition, and data preprocessing were completed following the protocol previously published in Kilroy et al., 2021. Prior to scanning, all participants completed a practice session in a mock scanner to prepare for the scanning environment and to minimize head motion. There were no significant differences in absolute head motion between the three groups (F=1.553, p=0.218) and we utilized a head-motion cut-off of absolute FD>1.5 mm (Kilroy et al., 2021).
Stimuli were presented using the Psychophysics Toolbox (Brainard, 1997) on MATLAB. In a single 9-minute run, there were 5 blocks of video-stimuli per condition. As Figure 1 shows, blocks consisted of emotional facial expressions (EMO; e.g., happy expression), non-emotional facial expressions (NEMO; e.g., tongue to lip), or bimanual hand actions (HANDS; e.g., playing the xylophone; see Figure 1). Each video was presented for 3.75 seconds followed by a 1.25 second black screen between each stimulus. There were 3 videos per block, and both male and female actors were included in each block. For further details on stimuli, please see Kilroy et al., 2021. Participants were instructed to silently think about why the actor was performing the facial expressions and hand actions presented. For example, if they saw someone cutting paper, they might think that the actor intends to create an art project; if they saw someone smile, they might think they were happy because they received a present. The task was practiced outside the scanner prior to scanning, so that if participants labeled actions (i.e., “they are happy”) instead of stating “why” an actor was performing an action (i.e., “they received a present”) they were instructed to try again focusing on why the actor was making the action. Outside the MRI, a post-task mentalizing behavioral task was performed to measure the accuracy and quality of participant’s mentalization responses (Kilroy et al., 2021).
Figure 1. Mentalizing task stimuli.

Still image examples of video stimuli presented during fMRI scanning. Stimuli were divided into three categories: emotion facial expressions, non-emotional facial expressions, and bimanual hand actions. For all videos, participants were instructed to think about why the actor was performing the facial or hand actions presented.
2.2.3. MRI data acquisition
Functional and structural data were acquired on a 3 Tesla MAGNETOM Prisma (Siemens, Erlangen, Germany) with a 20-channel head coil. Functional scans used echo-planar imaging (EPI; 150 volumes) with the following parameters: TR = 2 sec, TE = 30 msec, flip angle = 90°, in-plane resolution 2.5x2.5mm, 64x64 matrix, 41 transverse slices (each 2.5mm thick), and a multiband factor of 3. Structural T1-weighted MPRAGE was acquired for each participant using a 5-minute scan with parameters: TR = 1950 msec, TE = 3.09 msec, flip angle = 10°, 176 sagittal slices, 256 x 256 matrix, 1mm isotropic resolution. Field map data, for off-resonance distortion correction, was also acquired using spin echo EPI in AP and PA directions with identical geometry to the functional data with parameters: TR = 1020 msec, TE1 = 10 msec, TE2 = 12.46 msec, flip angle = 90°, voxel size = 2.5mm isotropic, FOV = 224 × 224 × 191 mm3.
2.3. MRI Data analysis
2.3.1. Within-subject analyses
First-level (subject-level) functional data analysis was completed using FSL 6.0 (Jenkinson et al., 2012). In order, the preprocessing pipeline included: (a) brain extraction for non-brain removal; (b) B0 unwarping along the y-direction; (c) realignment of functional volumes using MCFLIRT; (d) high pass filtering at a 100 sec (0.01 Hz) cutoff period; (e) spatial smoothing using a Gaussian kernel of FWHM 5mm; (f) ICA-AROMA (Pruim et al., 2015), used to remove motion and physiology-related spurious noisy components.
Registration of functional images to the high-resolution T1 anatomical image was performed using a 7-degrees of freedom (dof) boundary-based transformation. Anatomical and functional images were registered to MNI-152 template using a 12-dof affine transformation, and then this transformation was further refined using nonlinear registration (FNIRT; Jenkinson et al., 2002; Jenkinson & Smith, 2001). Experimental stimuli were modeled using separate regressors for each condition, by convolving the task design with a double gamma function to represent the hemodynamic BOLD response. The temporal derivative of each condition regressor was also modeled as an additional regressor. Subject-specific framewise head motion were entered as nuisance regressors. The first block of stimuli was always modeled out as a junk block and discarded to adjust for the initial stabilization effects of the gradient field and the excitation time of the brain tissue (Soares et al., 2016).
2.3.2. Functional Connectivity Analysis
To investigate group differences in connectivity of brain regions with the IFG during facial expression mentalizing trials, psycho-physiological interaction (PPI; McLaren et al., 2012) analyses were performed. The PPI analysis was used to compare the functional correlation of a core region for action observation (left and right inferior frontal gyrus pars opercularis or IFGop) to the rest of the brain during the task. Data for the seed region was extracted from individual time series from the anatomically defined region of interest for the left and right IFGop (see Figure 2) and modeled as the physiological regressor. Correlated activity between the seed region and the other regions in the brain were modeled as interaction effects of the physiological regressor with the condition regressor defined during first-level analysis. For each of the three experimental conditions, a separate regressor was modeled in the general linear model to capture their respective PPI effect. Thus, we had four unique PPI effects (one PPI each for EMO, NEMO, HANDS and all the stimuli [ALL]) as main effects for each participant during first-level analysis. Significantly different activations between task and rest resulted in the PPI effect, or measure of functional connectivity with the IFGop. Second-level analysis compared the groups on each of these four PPI main effects. It should be noted that we refrained here from running correlational analyses between our behavioral data and functional connectivity differences, as some studies indicate the need for large samples for such analyses (Marek et al., 2022).
Figure 2. Psycho-physiological interaction (PPI) seed region.
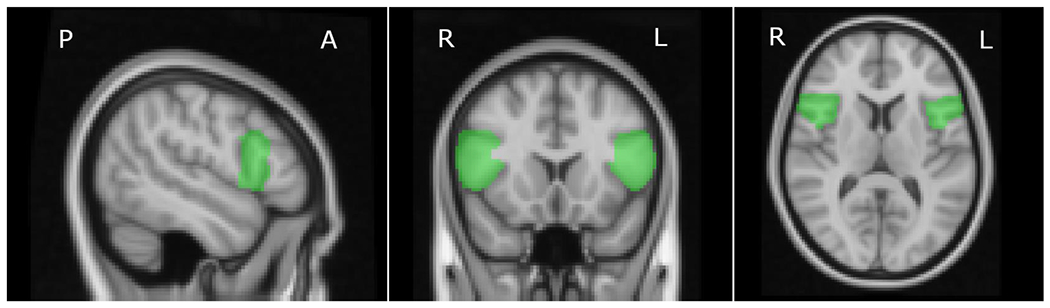
Anatomically defined seed region of the left and right inferior frontal gyrus, pars opercularis (IFGop) used in the extraction of the physiological regressor.
Each group was entered into multivariate linear regression models for the task for exploring main effects and between-group comparisons of connectivity. In all whole-brain analyses, mean-centered age, sex assigned at birth and IQ were entered as covariates in the model. Individual participants’ statistical images were entered into the higher level mixed-effects analyses using FSL’s FLAME algorithm. Resulting group level images for all models were thresholded using FSL’s cluster probability algorithm, with a cluster-forming threshold of Z > 3.1, and a cluster size probability threshold of p < 0.05. These analyses were performed for each individual stimulus condition (EMO, NEMO and HANDS) as well as for across all stimuli (ALL). For regions of interest, an additional small volume correction (SVC) analysis was performed with a significance threshold of p < 0.05 using a predefined mask. The small volume mask comprised of regions often associated with mentalizing , like the TPJ, dorsolateral prefrontal cortex (dlPFC), dmPFC and insula, and all ROIs were defined utilizing the Neurosynth database (which performs automated large-scale meta-analyses of fMRI data). For parcellating the insula, we additionally used structural definitions from previous research (Deen et al., 2011). All data is archived and can be found at: https://nda.nih.gov/edit_collection.html?id=2254.
3. Results
3.1. Behavioral Results
Table 1 lists significant differences between the three groups in demographic and behavioral measure. Groups did not differ significantly in age, IQ, or sex assigned at birth. The ASD group significantly differed from the TD and DCD group in mentalizing ability, as measured by the NEPSY ToM total. As expected, both ASD and DCD groups significantly differed from the TD group in motor skills as measured by the MABC-2. Additionally, there were no significant between group differences in the post-fMRI mentalizing task (F = 1.967, p = 0.147; all post-hoc groupwise comparisons ps>0.05; see Table 1), suggesting all participants were capable of performing it at rudimentary levels. Nevertheless, more nuanced analyses of the responses by two-raters indicated that the autistic group had near significantly more poor responses than the TD group (t = −1.998, p = 0.05) and showed an overall trend toward lower mentalizing quality in their responses (t = 1.761, p = 0.084) compared to the TD group, consistent with the NEPSY ToM results.
Table 1.
Descriptive statistics and groupwise comparisons for all participants
| Descriptive Statistics | Scheffe’s test value | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Group | Variable | N | Mean | SD | TD | ASD | DCD |
| TD, F=11 | Age | 31 | 12.08 | 2.289 | 0.998 | 0.822 | |
| IQ | 31 | 114.26 | 12.514 | 0.501 | 0.773 | ||
| SRS total T-score | 31 | 45.00 | 5.092 | <0.001 | <0.001 | ||
| Conners T-score | 31 | 45.48 | 3.086 | <0.001 | <0.001 | ||
| DCDQ total | 31 | 74.94 | 8.691 | <0.001 | <0.001 | ||
| MABC Manual dexterity | 31 | 9.94 | 2.190 | <0.001 | <0.001 | ||
| MABC Aiming & Catching | 31 | 10.71 | 3.485 | <0.001 | <0.001 | ||
| MABC Balance | 31 | 10.65 | 2.416 | <0.001 | <0.001 | ||
| MABC total Standard Score | 31 | 10.45 | 1.67 | <0.001 | <0.001 | ||
| NEPSY ToM total | 31 | 25.00 | 1.966 | 0.011 | 0.551 | ||
| Mentalizing behavioral score | 31 | 0.02 | 0.068 | 0.164 | 0.925 | ||
|
| |||||||
| ASD, F=7 | Age | 29 | 12.11 | 2.300 | 0.998 | 0.851 | |
| IQ | 29 | 109.21 | 18.956 | 0.501 | 0.943 | ||
| SRS total T-score | 29 | 75.48 | 9.764 | <0.001 | <0.001 | ||
| Conners T-score | 29 | 83.03 | 10.217 | <0.001 | 0.01 | ||
| DCDQ total | 29 | 46.38 | 10.608 | <0.001 | 0.787 | ||
| MABC Manual dexterity | 28 | 5.71 | 2.853 | <0.001 | 0.784 | ||
| MABC Aiming & Catching | 29 | 6.45 | 3.480 | <0.001 | 0.995 | ||
| MABC Balance | 29 | 6.55 | 2.995 | <0.001 | 0.141 | ||
| MABC total Standard score | 28 | 5.29 | 2.522 | <0.001 | 0.259 | ||
| NEPSY ToM total | 26 | 23.04 | 3.143 | 0.011 | 0.001 | ||
| Mentalizing behavioral score | 28 | −0.03 | 0.130 | 0.164 | 0.42 | ||
|
| |||||||
| DCD, F=10 | Age | 20 | 12.49 | 2.163 | 0.822 | 0.851 | |
| IQ | 20 | 110.85 | 18.259 | 0.773 | 0.943 | ||
| SRS total T-score | 20 | 56.70 | 8.548 | <0.001 | <0.001 | ||
| Conners T-score | 20 | 74.15 | 16.775 | <0.001 | 0.01 | ||
| DCDQ total | 20 | 44.40 | 10.323 | <0.001 | 0.787 | ||
| MABC Manual dexterity | 20 | 5.20 | 2.484 | <0.001 | 0.784 | ||
| MABC Aiming & Catching | 20 | 6.35 | 3.100 | <0.001 | 0.995 | ||
| MABC Balance | 20 | 5.00 | 2.513 | <0.001 | 0.141 | ||
| MABC total Standard score | 20 | 4.3 | 1.75 | <0.001 | 0.259 | ||
| NEPSY ToM total | 20 | 25.75 | 1.743 | 0.551 | 0.001 | ||
| Mentalizing behavioral score | 20 | 0.01 | 0.084 | 0.925 | 0.42 | ||
3.2. Head Motion.
Six participants showed significantly high head movement beyond the FD threshold and were excluded from our sample. After exclusion, the remaining participants showed no significant differences in the head motion and motion correction algorithm employed during first-level analysis (ICA-AROMA) adequately removed noise components related to participant movement. Further details on head motion in this sample can be found in Kilroy et al. (2021).
3.3. Functional Connectivity Results.
No significant results were found in the whole-brain analysis (Z>3.1, cluster corrected; see Supplementary Table 1 for uncorrected results), and thus, we focus on significant results found in hypothesized regions using small-volume correction (Z>2.3, SVC). Table 2 lists significant connectivity differences when comparing TD vs. ASD, TD vs. DCD, and ASD vs. DCD for the left and right IFGop seeds. We outline a few patterns for ASD compared to both comparison groups (TD and DCD) below.
Table 2.
Group comparisons of bilateral IFGop connectivity, after small volume correction (SVC p<0.05), during the mentalizing fMRI task.
| Seed | Condition | Contrast | Max Z score | X | Y | Z | Region |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Left IFGop | All Stimuli | TD>ASD | 2.61 | −54 | −60 | 26 | Left TPJa |
| 2.38 | 62 | −44 | 11 | Right TPJ | |||
| 2.79 | −46 | 24 | 32 | Left dlPFC | |||
| DCD>ASD | 3.22 | −54 | −63 | 24 | Left TPJa | ||
| ASD>TD | 3.17 | −39 | −5 | 5 | Left insulab | ||
| 3.34 | 43 | 50 | 26 | Right dlPFCb | |||
| 3.25 | −30 | 54 | 15 | Left dlPFCb | |||
| 2.67 | −39 | 16 | −6 | Left ventroanterior insula | |||
| ASD>DCD | 3.01 | −39 | −2 | 6 | Left insulab | ||
| 3.26 | 42 | 50 | 26 | Right dlPFCb | |||
| 2.63 | −39 | 40 | 31 | Left dlPFCb | |||
| 2.96 | 34 | 16 | 6 | Right dorsoanterior insula | |||
|
|
|||||||
| Emotional Faces | TD>ASD | 2.46 | −56 | −50 | 13 | Left TPJa | |
| 2.91 | 37 | 22 | 2 | Right anterior insula | |||
| DCD>ASD | 3.03 | −49 | 48 | 20 | Left TPJa | ||
| ASD>TD | 2.77 | −42 | −8 | 6 | Left insulab | ||
| 2.85 | 30 | 48 | 31 | Right dlPFCb | |||
| 2.67 | −21 | 50 | 29 | Left dlPFCb | |||
| ASD>DCD | 3.82 | −41 | −2 | 6 | Left insulab | ||
| 3.03 | 41 | 50 | 25 | Right dlPFCb | |||
| 2.8 | −49 | 35 | 29 | Left dlPFCb | |||
| 3 | 40 | −10 | 7 | Right posterior insula | |||
|
|
|||||||
| Hand actions | TD>ASD | 2.68 | 48 | −47 | 24 | Right TPJa | |
| 3.03 | 40 | 6 | −6 | Right ventroanterior insula | |||
| DCD>ASD | 3.41 | 47 | −48 | 24 | Right TPJa | ||
| ASD>TD | 2.63 | 42 | 42 | 31 | Right dlPFCb | ||
| 2.6 | −37 | 38 | 38 | Left dlPFCb | |||
| 2.98 | −34 | 18 | 5 | Left dorsoanterior insula | |||
| 2.81 | −38 | −6 | 8 | Left insula | |||
| ASD>DCD | 2.85 | 38 | 40 | 20 | Right dlPFCb | ||
| 2.93 | −28 | 52 | 12 | Left dlPFCb | |||
| 2.86 | 34 | 19 | 6 | Right dorsoanterior insula | |||
|
| |||||||
| Right IFGop | All Stimuli | TD>ASD | 2.38 | −43 | 28 | 30 | Left caudal dlPFC (frontal eye fields) |
| DCD>ASD | 3.03 | −58 | −62 | 19 | Left TPJ | ||
| ASD>TD | 2.48 | −40 | 14 | −7 | Left ventroanterior insulab | ||
| 3.48 | −39 | −4 | 10 | Left insulab | |||
| 3.8 | 44 | 42 | 33 | Right dlPFCb | |||
| 2.48 | −36 | 40 | 37 | Left dlPFCb | |||
| 3.02 | 49 | −60 | 24 | Right TPJ | |||
| ASD>DCD | 2.72 | −41 | 14 | −6 | Left ventroanterior insulab | ||
| 2.61 | −40 | −3 | 4 | Left insulab | |||
| 2.88 | 42 | 40 | 34 | Right dlPFCb | |||
| 3.01 | 44 | 26 | 28 | Right dlPFCb | |||
| 2.36 | −36 | 26 | 40 | Left dlPFCb | |||
|
|
|||||||
| Emotional Faces | TD>ASD | 2.54 | 18 | 46 | 45 | Right dlPFC | |
| DCD>ASD | 2.79 | −55 | −62 | 19 | Left TPJ | ||
| ASD>TD | 3.42 | 43 | 40 | 35 | Right dlPFCb | ||
| 3.28 | −29 | 34 | 34 | Left dlPFCb | |||
| 4.06 | 62 | −52 | 28 | Right TPJ | |||
| 2.97 | −44 | −2 | 3 | Left insula | |||
| ASD>DCD | 3.06 | 32 | 50 | 38 | Right dlPFCb | ||
| 2.95 | −35 | 26 | 36 | Left dlPFCb | |||
| 2.84 | −40 | 14 | −8 | Left ventroanterior insula | |||
| 2.48 | 39 | 11 | −3 | Right dorsoanterior insula | |||
Note that the one’s labeled here as “left insula’’ fall on the anterior/posterior border of insular subregions as demarcated by Uddin et al., 2017; please see Figures below for visual depictions of some of these activations.
= hypoconnectivity in the ASD group compared to BOTH the DCD and TD groups (TD & DCD>ASD);
= hyperconnectivity in the ASD group compared to BOTH the DCD and TD groups (ASD>TD & DCD).
Hypoconnectivity in the ASD group (see Figure 3 and Table 2 listings with a superscript “a”), as compared to both TD and DCD groups, during mentalizing was significantly found between: LEFT IFGop: left TPJ for all stimuli blocks (mentalizing about other’s facial and hand actions); right TPJ for bimanual hand actions.
Figure 3. Reduced connectivity in ASD between IFG and TPJ.
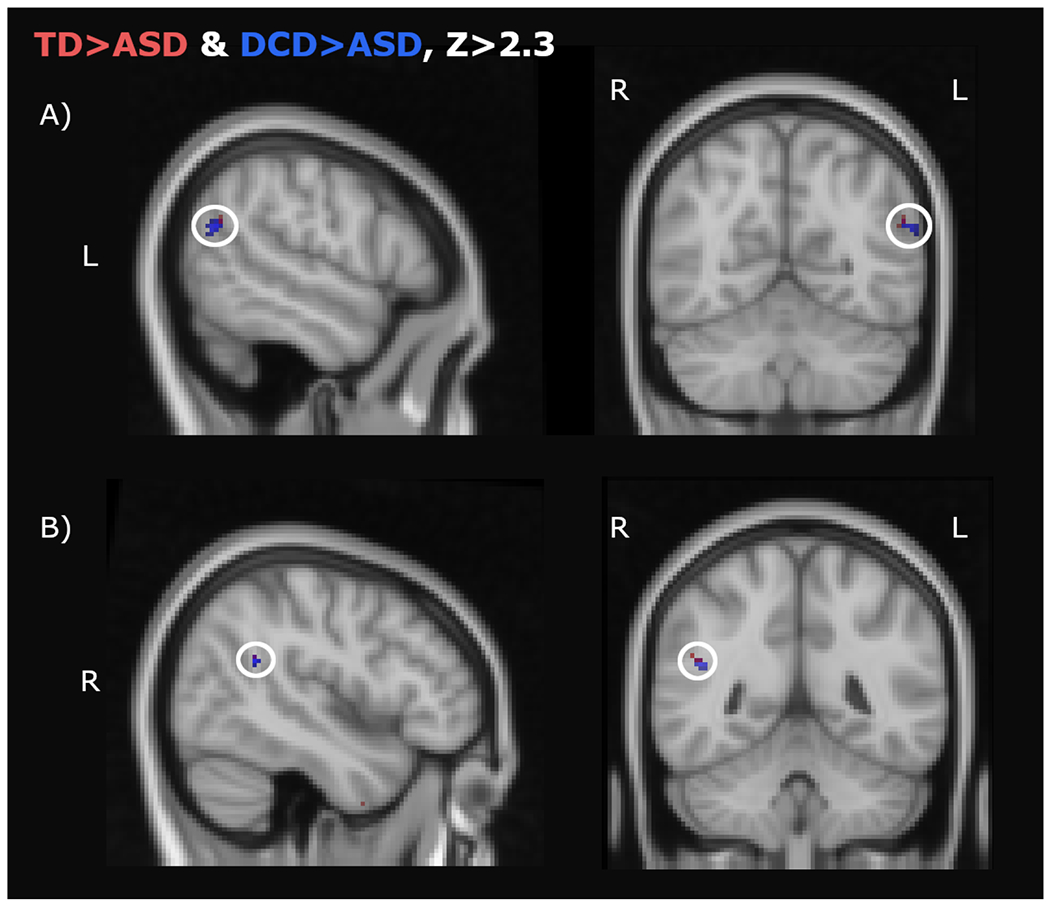
Hypoconnectivity with the left IFGop in the ASD group as compared to the TD (red clusters) and DCD (blue clusters) groups during the mentalizing task. Overlap is depicted in purple. A) Left temporoparietal junction (TPJ) during the mentalizing of all stimuli (hands and faces); B) Right TPJ during mentalizing about other’s hand actions(Z>2.3, SVC).
Hyperconnectivity in the ASD group (see Figure 4 and Table 2 listings with a superscript “b”), as compared to both TD and DCD groups, during mentalizing was significantly found between: 1) LEFT IFGop: left insula (along the central insular sulcus, as seen in Uddin et al., 2017) and bilateral dlPFC for all stimuli blocks; bilateral dlPFC during bimanual hand actions; 2) RIGHT IFGop: left insula (along the central insular sulcus), left ventroanterior insula (vAI), bilateral dlPFC for mentalizing of all stimuli blocks; bilateral dlPFC for mentalizing of emotional facial expressions.
Figure 4. Increased connectivity in ASD.

Hyperconnectivity with the right (A-C) and left (D-F) IFGop in the ASD group as compared to the TD (red clusters) and DCD (blue clusters) groups during the mentalizing task, thresholded at Z>2.3, SVC. Hyperconnectivity with right IFGop: A) Left ventroanterior and posterior insula during mentalizing of all stimuli ; B) Right dorsolateral prefrontal cortex (dlPFC) during mentalizing of all stimuli; C) Left dlPFC during during mentalizing of all stimuli . Hyperconnectivity with left IFGop: D) Right dlPFC during mentalizing of all action stimuli; E) Left posterior insula during mentalizing of all action stimuli; F) Left dlPFC during mentalizing of all action stimuli.
4. Discussion
Previous literature indicates that autistic individuals may have difficulty interpreting other’s intentions by viewing their actions (Adornetti et al., 2019; Ciaramidaro et al., 2015; Cole et al., 2019; Colombi et al., 2009; Mainieri et al., 2013). There has been prior work indicating that the neural differences in connectivity between the MNS and mentalizing system may underlie some of these differences (Cole et al., 2019; Fishman et al., 2014), as well as connectivity between the MNS and the insula (Di Martino et al., 2009). However, given that, by definition, MNS regions are critical for motor processing (Rizzolatti & Sinigaglia, 2010), and that about 80% of autistic individuals have motor difficulties with probable DCD (Bhat, 2020; Green et al., 2009; Hannant et al., 2018; Hilton et al., 2012; Miller et al., 2021; Williams et al., 2004), it is unclear if prior results indicating IFGop connectivity differences in autism are due to motor issues, rather than the social issues that are core to the diagnosis. To better disentangle social impairment from motor impairment and to find connectivity patterns potentially unique to autism, we compared autistic individuals to both DCD and TD groups. Initially, one might have predicted that, given socio-emotional differences being a prominent hallmark of autism, we would mainly find between group differences predominately for emotional face processing. Contrary to this assumption, we find differences between ASD and TD/DCD groups during mentalizing of others’ bimanual hand actions, a non-emotional stimuli set, just as we do for others’ emotional facial expressions, though different networks may be activated.
Our results indicate that when mentalizing about other’s intentions from their actions, two unique connectivity patterns are found in the ASD group compared to DCD and TD groups. First, across tasks, in autism, we find less connectivity between the left IFGop and bilateral TPJ. This may indicate decreased cross-talk between core regions of the MNS and mentalizing system when inferring other’s intentions from their actions. Second, in autism, we find increased connectivity between the IFGop and: 1) the left insula and; 2) the dlPFC. Specifically, the IFGop in both hemispheres has differential connectivity with the left insula, especially when thinking about others’ facial expressions. Lastly, the IFGop in both hemispheres has increased connectivity with the bilateral dlPFC when thinking about both others’ hand and facial actions. These patterns may indicate differential emotional and executive functioning in autism when inferring other’s intents from their actions. Here we discuss these three findings in greater detail.
4.1. Autism compared to TD/DCD: Decreased Connectivity Between the MNS and Mentalizing Network (left IFGop and bilateral TPJ).
When thinking about why someone is making an action, there is typically increased functional connectivity between regions within the mentalizing system and MNS (e.g., dmPFC and right IFG [Spunt & Lieberman, 2013]; dmPFC and IFG/IPL [Cole et al., 2019]). This indicates increased cross-talk between these two systems may underlie action-based intent processing. However, prior data indicates that autistic individuals may have aberrant communication between these networks. In a study by Cole et al. (2019), task-based functional connectivity was assessed in autistic adults and non-autistic adults when trying to understand the intentions of others from their hand actions. Their results indicate that during this task, unlike the TD group, autistic individuals show no increase in connectivity between the two networks (dmPFC and IFG/IPL) during mentalizing tasks as compared to non-mentalizing tasks. Further, when looking across groups, reduced functional connectivity between the dmPFC and the IFG during the mentalizing task was predicted by increased scores on autistic traits and lower ability to infer other’s intents (Cole et al., 2019).
In the current study, we also find reduced connectivity between IFGop and mentalizing network as compared to the TD group during action-based intent processing, and we find this pattern even when compared to a DCD group which largely shares motor impairment with our ASD group (Kilroy et al., 2019, 2021; McAuliffe et al., 2020; Mostofsky & Ewen, 2011). Thus, although the IFGop is involved in motor processing, these aberrant connectivity patterns can not be explained by motor impairment in the ASD group.
However, while the study by Cole et al. (2019) found in ASD hypoconnectivity between the IFG and the dmPFC, we find no connectivity differences with dmPFC, and instead hypoconnectvity between the left IFG with the bilateral TPJ when mentalizing. One discrepancy between the current study and prior studies (Cole et al., 2019; Spunt & Lieberman, 2013) is that while prior studies used adult populations, here we focus on youth aged 8-18. Prior studies indicate that age strongly impacts connectivity differences in ASD (Nomi & Uddin, 2015a). Indeed in a prior study in adolescents, resting state connectivity differences between the TPJ and the IFGop were found in autistic individuals compared to a TD group (Fishman et al., 2014), supporting the current results.
To summarize, our findings are consistent with the notion that there is underconnectivity between the MNS and a core region of the mentalizing system in autistic individuals when trying to understand the mental states of others by viewing their actions. Nevertheless, which nodes across these networks are hyperconnected may differ based on age of the participants, and/or potential task differences. These connectivity differences appear specific to ASD compared to both DCD and TD groups, and thus can not be attributed to difficulties with motor ability in the ASD group.
4.2. Autism compared to TD/DCD: Increased Connectivity Between MNS and Emotion Processing (IFGop and left insula).
Our data indicate that, when inferring mental states from other people’s hand and facial actions, there is hyperconnectivity between the IFGop and the left insula in the ASD group compared to both DCD and TD groups. The regions of the left insula that were hyperconnected with the bilateral IFGop include a region along the anterior-posterior insular boundary, or along the central insular sulcus (Benarroch, 2019; Uddin et al., 2017; with bilateral IFGop), as well as a second region in the left ventroanterior insula (with the right IFGop; Figure 4). Generally, these insular regions are known to be involved in sensorimotor integration, integration of interoceptive processing with emotion processing, salience, and socioemotional processing (Benarroch, 2019; Chang et al., 2013). While there is no strong consensus of laterality differences in the insula, clinical studies indicate that stimulation of the left insula altered ability to recognize emotional expressions (for a review, see Uddin et al., 2017), while another study indicated that the left insula was more involved in speech, emotional and affective-cognitive deficits in patients with fronto-temporal dementia (Fathy et al., 2020). Connectivity of these insular regions with the IFGop, which is commonly active when viewing others hand or facial actions (Dapretto et al., 2006; Kilroy et al., 2021), may thus indicate the interplay of processing socio-emotional content from other people’s actions and facial expressions. Autistic individuals typically show reduced activity in both regions when processing other’s emotional facial expressions (Dapretto et al., 2006; Di Martino et al., 2009; Kilroy et al., 2021). Further, behavioral data indicate that autistic individuals commonly feel increased personal distress when thinking about other people’s emotional experiences (Butera et al., 2022).
In addition, prior studies indicate abnormal connectivity between the insula and other neural regions during social tasks may be a prominent feature of ASD (Goodwill et al., 2023; Uddin et al., 2017). A prior study from our group found that in autistic individuals, interoceptive ability was related to increased connectivity between the insula and the IFGop when passively processing other’s emotional facial expressions (Butera et al., 2023). These may indicate ASD differences in interoceptive ability, salience or emotion processing when processing other’s socio-emotional states. Taken together, increased connectivity between the insula and the IFGop may reflect increased emotional reactions when thinking about others’ experiences from their facial expressions, which in turn, behaviorally, may lead to socio-emotional differences, including increased personal distress in autism.
4.3. Autism compared to TD/DCD: Increased Connectivity Between IFGop and dlPFC
The dlPFC is involved in a number of cognitive processes, such as executive function skills of planning, goal-directed behavior, attentional control, working memory, and top-down control (Benarroch, 2019; Hertrich et al., 2021; Richey et al., 2022; Y. Wang et al., 2020; Zemestani et al., 2022). Prior large scale meta-analyses indicate broad executive dysfunction in autism that remains relatively stable with development (Demetriou et al., 2018). Given these executive function differences in autism, increased task-based connectivity between the dlPFC and IFGop may be related to increased recruitment of executive functioning for this mentalizing task. Additionally, the dlPFC is also involved in gaze processing, and previous research has shown that processing of direct gaze of others may recruit significantly more activation of the dlPFC in autistic individuals than the comparison group (Pitskel et al., 2011). Given differences in gaze processing, especially of facial stimuli, in autistic individuals (Cañigueral & Hamilton, 2019; Davies et al., 2011; Nomi & Uddin, 2015b; Pelphrey et al., 2005; Stuart et al., 2022), such activity differences between the dlPFC and the IFGop may reflect gaze differences during this mentalizing task. Finally, prior research has shown increased connectivity between the MNS (including the IFGop) and the dlPFC when observing familiar actions, suggesting that autistic individuals may rely more strongly on executive functions based on memory of familiar actions (Shih et al., 2010). Here, we also find increased connectivity between these regions for stimuli that are highly familiar (emotional facial actions, common bimanual hand actions), but do not see the same effect for novel actions (non-emotional facial expressions), consistent with Shih et al. (2010). Thus, in autistic individuals, increased dlPFC-IFGop connectivity may be found for mentalizing about other people’s actions that they are more familiar with.
4.4. Limitations
In this study, about half of our DCD sample was female. Studies indicate that DCD is more common in males, with recent estimations of prevalence ratios of 1.5 males to 1 female (Lee et al., 2019; Sujatha B et al., 2020). In the current study, to account for any potential sex effects, we adjusted our fMRI models to account for effects stemming from sex in all groups. Nevertheless, future studies may benefit from considering sex, age, and more heterogeneous samples to better understand the impact of development and factors such as IQ, handedness, and biological sex on these connectivity patterns. We note that no correlation or regression analyses were performed to evaluate the effect of demographic variables because of the low sample size required for robust statistical analysis (Marek et al., 2022), which was another limitation of our study. Future studies in larger samples are necessary, which may reveal connectivity patterns in other regions, outside of those focused on here. Further, we note that the majority of the participants in our autism group had probable DCD (79%) and ADHD (93%), and the majority of people in our DCD group had probable ADHD (60%). While these patterns are inline with common co-occuring diagnoses in autism (Hours et al., 2022) and DCD (Goulardins et al., 2017), future studies considering autism populations with and without DCD/ADHD may help better understand neurological patterns specific to ASD.
5. Conclusion
Here, we aimed to understand how differences in connectivity between mentalizing and mirror neuron regions may impact differences in inferring mental states from other’s actions in autistic youth, as compared to DCD and TD peers. We found hypoconnectivity between the IFGop and the TPJ, supporting the hypothesis that in autism, differences with inferring mental states from other’s actions may be due to hypoactivity between key mentalizing and mirror regions. By contrast, findings of increased connectivity between the IFGop and the left insula and bilateral dlPFC indicate that increased recruitment of socio-emotional processing and executive functioning may also impact mentalizing tasks in autism. Given the IFGop is involved in motor processing, one important question is whether these results are due to motor differences in ASD. By utilizing a DCD comparison group in addition to a TD comparison group, we show, for the first time, that these connectivity differences are unlikely to be due to motor impairment, but most likely related to socio-emotional differences in autism.
Supplementary Material
Acknowledgments:
We thank all our participants and research assistants for contributing to this study. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD079432. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was additionally supported by the Department of Defense through the Idea Development Award under award number AR170062. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. The study hypotheses, methodology, and analyses as proposed to NICHD can be found at https://reporter.nih.gov/project-details/8818269.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability statement:
Individual raw behavioral and imaging data have been publicly shared on the National Institute of Mental Health (NIMH) Data Archive (NDA) and can be accessed using the Collection ID #2254 (https://nda.nih.gov/edit_collection.html?id=2254). Data structures that have been defined in the NDA Data Dictionary are available at https://nda.nih.gov/general-query.html?q=query=data-structure. We have publicly archived the analysis code used in this study on GitHub and it can be accessed at https://github.com/CeNEC/Ment_PPI.git. Readers seeking access to fMRI task stimuli will find it publicly available at https://dornsife.usc.edu/cenec/data-sharing/. Legal copyright restrictions prevent public archiving of WASI-II, SRS-2, Conners, NEPSY-II, DCDQ, MABC-2, ADOS-2, and ADI-R which can be obtained from the copyright holders in the cited references. No part of the study procedures or analysis plans was preregistered prior to the research being conducted.
References
- Abrams G, Jayashankar A, Kilroy E, Butera C, Harrison L, Ring P, Houssain A, Nalbach A, Cermak SA, & Aziz-Zadeh L (2022). Differences in Praxis Errors in Autism Spectrum Disorder Compared to Developmental Coordination Disorder. Journal of Autism and Developmental Disorders. 10.1007/s10803-022-05858-8 [DOI] [PubMed] [Google Scholar]
- Adornetti I, Ferretti F, Chiera A, Wacewicz S, Żywiczyński P, Deriu V, Marini A, Magni R, Casula L, Vicari S, & Valeri G (2019). Do Children With Autism Spectrum Disorders Understand Pantomimic Events? Frontiers in Psychology, 10. 10.3389/fpsyg.2019.01382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, & Frith CD (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews. Neuroscience, 7(4), 268–277. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Arioli M, Basso G, Poggi P, & Canessa N (2021). Fronto-temporal brain activity and connectivity track implicit attention to positive and negative social words in a novel socio-emotional Stroop task. NeuroImage, 226, 117580. 10.1016/j.neuroimage.2020.117580 [DOI] [PubMed] [Google Scholar]
- Avni E, Ben-Itzchak E, & Zachor DA (2018). The Presence of Comorbid ADHD and Anxiety Symptoms in Autism Spectrum Disorder: Clinical Presentation and Predictors. Frontiers in Psychiatry, 9, 717. 10.3389/fpsyt.2018.00717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Kilroy E, & Corcelli G (2018). Understanding activation patterns in shared circuits: Toward a value driven model. Frontiers in Human Neuroscience, 12. 10.3389/fnhum.2018.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, & Williams SC (1999). Social intelligence in the normal and autistic brain: An fMRI study. The European Journal of Neuroscience, 11(6), 1891–1898. 10.1046/j.1460-9568.1999.00621.x [DOI] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Nanetti L, van der Gaag C, Ketelaars C, Minderaa R, & Keysers C (2011). Age-Related Increase in Inferior Frontal Gyrus Activity and Social Functioning in Autism Spectrum Disorder. Biological Psychiatry, 69(9), 832–838. 10.1016/j.biopsych.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Benarroch EE (2019). Insular cortex: Functional complexity and clinical correlations. Neurology, 93(21), 932–938. 10.1212/WNL.0000000000008525 [DOI] [PubMed] [Google Scholar]
- Bhat AN (2020). Is Motor Impairment in Autism Spectrum Disorder Distinct From Developmental Coordination Disorder? A Report From the SPARK Study. Physical Therapy, 100(4), 633–644. 10.1093/ptj/pzz190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biotteau M, Albaret J-M, & Chaix Y (2020). Chapter 1—Developmental coordination disorder. In Gallagher A, Bulteau C, Cohen D, & Michaud JL (Eds.), Handbook of Clinical Neurology (Vol. 174, pp. 3–20). Elsevier. 10.1016/B978-0-444-64148-9.00001-6 [DOI] [PubMed] [Google Scholar]
- Bos J, & Stokes MA (2019). Cognitive empathy moderates the relationship between affective empathy and wellbeing in adolescents with autism spectrum disorder. European Journal of Developmental Psychology, 16(4), 433–446. 10.1080/17405629.2018.1444987 [DOI] [Google Scholar]
- Bottema-Beutel K, Kim SY, & Crowley S (2019). A systematic review and meta-regression analysis of social functioning correlates in autism and typical development. Autism Research, 12(2), 152–175. 10.1002/aur.2055 [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997). The Psychophysics Toolbox. Spatial Vision, 10, 433–436. 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Brent E, Rios P, Happé F, & Charman T (2004). Performance of Children with Autism Spectrum Disorder on Advanced Theory of Mind Tasks. Autism, 8(3), 283–299. 10.1177/1362361304045217 [DOI] [PubMed] [Google Scholar]
- Brown T. (2013). Movement Assessment Battery for Children: 2nd Edition (MABC-2). In Volkmar FR (Ed.), Encyclopedia of Autism Spectrum Disorders (pp. 1925–1939). Springer. 10.1007/978-1-4419-1698-3_1922 [DOI] [Google Scholar]
- Brown T, & Lalor A (2009). The movement assessment battery for children—second edition (MABC-2): A review and critique. Physical & Occupational Therapy in Pediatrics, 29(1), 86–103. [DOI] [PubMed] [Google Scholar]
- Butera CD, Harrison L, Kilroy E, Jayashankar A, Shipkova M, Pruyser A, & Aziz-Zadeh L (2022). Relationships between alexithymia, interoception, and emotional empathy in autism spectrum disorder. Autism, 13623613221111310. 10.1177/13623613221111310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera C, Kaplan J, Kilroy E, Harrison L, Jayashankar A, Loureiro F, & Aziz-Zadeh L (2023). The relationship between alexithymia, interoception, and neural functional connectivity during facial expression processing in autism spectrum disorder. Neuropsychologia, 180, 108469. 10.1016/j.neuropsychologia.2023.108469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañigueral R, & Hamilton A. F. de C., (2019). The Role of Eye Gaze During Natural Social Interactions in Typical and Autistic People. Frontiers in Psychology, 10. 10.3389/fpsyg.2019.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M, Pennington BF, & Rogers SJ (2002). Interrelations Among Social-Cognitive Skills in Young Children with Autism. Journal of Autism and Developmental Disorders, 32(2), 91–106. 10.1023/A:1014836521114 [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, & Sanfey AG (2013). Decoding the Role of the Insula in Human Cognition: Functional Parcellation and Large-Scale Reverse Inference. Cerebral Cortex, 23(3), 739–749. 10.1093/cercor/bhs065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramidaro A, Bölte S, Schlitt S, Hainz D, Poustka F, Weber B, Bara BG, Freitag C, & Walter H (2015). Schizophrenia and autism as contrasting minds: Neural evidence for the hypo-hyper-intentionality hypothesis. Schizophrenia Bulletin, 41(1), 171–179. 10.1093/schbul/sbu124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole EJ, Barraclough NE, & Andrews TJ (2019). Reduced connectivity between mentalizing and mirror systems in autism spectrum condition. Neuropsychologia, 122, 88–97. 10.1016/j.neuropsychologia.2018.11.008 [DOI] [PubMed] [Google Scholar]
- Colombi C, Liebal K, Tomasello M, Young G, Warneken F, & Rogers SJ (2009). Examining correlates of cooperation in autism: Imitation, joint attention, and understanding intentions. Autism: The International Journal of Research and Practice, 13(2), 143–163. 10.1177/1362361308098514 [DOI] [PubMed] [Google Scholar]
- Conners CK (2008). Conners 3.
- Constantino JN, & Gruber CP (2012). Social responsiveness scale: SRS-2. Western psychological services Torrance, CA. [Google Scholar]
- Crovitz HF, & Zener K (1962). A Group-Test for Assessing Hand- and Eye-Dominance. The American Journal of Psychology, 75(2), 271–276. 10.2307/1419611 [DOI] [PubMed] [Google Scholar]
- Dadgar H, Alaghband Rad J, Soleymani Z, Khorammi A, McCleery J, & Maroufizadeh S (2017). The Relationship between Motor, Imitation, and Early Social Communication Skills in Children with Autism. Iranian Journal of Psychiatry, 12(4), 236–240. [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, & Iacoboni M (2006). Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience, 9(1), 28–30. 10.1038/nn1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MS, Dapretto M, Sigman M, Sepeta L, & Bookheimer SY (2011). Neural bases of gaze and emotion processing in children with autism spectrum disorders. Brain and Behavior, 1(1), 1–11. 10.1002/brb3.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster L, Wiersema JR, Deschrijver E, & Brass M (2018). The effect of being imitated on empathy for pain in adults with high-functioning autism: Disturbed self-other distinction leads to altered empathic responding. Autism: The International Journal of Research and Practice, 22(6), 712–727. 10.1177/1362361317701268 [DOI] [PubMed] [Google Scholar]
- de Lange AH, De Witte H, & Notelaers G (2008). Should I stay or should I go? Examining longitudinal relations among job resources and work engagement for stayers versus movers. Work & Stress, 22(3), 201–223. 10.1080/02678370802390132 [DOI] [Google Scholar]
- Deen B, Pitskel NB, & Pelphrey KA (2011). Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex (New York, N.Y.: 1991), 21(7), 1498–1506. 10.1093/cercor/bhq186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou EA, Lampit A, Quintana DS, Naismith SL, Song YJC, Pye JE, Hickie I, & Guastella AJ (2018). Autism spectrum disorders: A meta-analysis of executive function. Molecular Psychiatry, 23(5), Article 5. 10.1038/mp.2017.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, & Milham MP (2009). Functional brain correlates of social and nonsocial processes in autism spectrum disorders: An activation likelihood estimation meta-analysis. Biological Psychiatry, 65(1), 63–74. 10.1016/j.biopsych.2008.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimberg U. (1982). Facial reactions to facial expressions. Psychophysiology, 19, 643–647. 10.1111/j.1469-8986.1982.tb02516.x [DOI] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, & Mostofsky SH (2009). Associations of postural knowledge and basic motor skill with dyspraxia in autism: Implication for abnormalities in distributed connectivity and motor learning. Neuropsychology, 23, 563–570. 10.1037/a0015640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziuk MA, Gidley Larson JC, Apostu A, Mahone EM, Denckla MB, & Mostofsky SH (2007). Dyspraxia in autism: Association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology, 49(10), 734–739. 10.1111/j.1469-8749.2007.00734.X [DOI] [PubMed] [Google Scholar]
- Fathy YY, Hoogers SE, Berendse HW, van der Werf YD, Visser PJ, de Jong FJ, & van de Berg WDJ (2020). Differential insular cortex sub-regional atrophy in neurodegenerative diseases: A systematic review and meta-analysis. Brain Imaging and Behavior, 14(6), 2799–2816. 10.1007/s11682-019-00099-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman I, Keown CL, Lincoln AJ, Pineda JA, & Müller R-A (2014). Atypical Cross Talk Between Mentalizing and Mirror Neuron Networks in Autism Spectrum Disorder. JAMA Psychiatry, 71(7), 751–760. 10.1001/jamapsychiatry.2014.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SM, Camchong J, Brickman L, Goelkel-Garcia L, Mueller BA, Tseng A, Lim KO, & Jacob S (2019). Hypoconnectivity of insular resting-state networks in adolescents with Autism Spectrum Disorder. Psychiatry Research. Neuroimaging, 283, 104–112. 10.1016/j.pscychresns.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, & Frith U (2006). The Neural Basis of Mentalizing. Neuron, 50(4), 531–534. 10.1016/j.neuron.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Gallese V. (2009). Mirror Neurons, Embodied Simulation, and the Neural Basis of Social Identification. Psychoanalytic Dialogues, 19(5), 519–536. 10.1080/10481880903231910 [DOI] [Google Scholar]
- Gizzonio V, Avanzini P, Campi C, Orivoli S, Piccolo B, Cantalupo G, Tassinari CA, Rizzolatti G, & Fabbri-Destro M (2015). Failure in Pantomime Action Execution Correlates with the Severity of Social Behavior Deficits in Children with Autism: A Praxis Study. Journal of Autism and Developmental Disorders, 45(10), 3085–3097. 10.1007/s10803-015-2461-2 [DOI] [PubMed] [Google Scholar]
- Goodwill AM, Low LT, Fox PT, Fox PM, Poon KK, Bhowmick SS, & Chen SHA (2023). Meta-analytic connectivity modelling of functional magnetic resonance imaging studies in autism spectrum disorders. Brain Imaging and Behavior. 10.1007/s11682-022-00754-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulardins JB, Marques JCB, & De Oliveira JA (2017). Attention Deficit Hyperactivity Disorder and Motor Impairment: A Critical Review. Perceptual and Motor Skills, 124(2), 425–440. 10.1177/0031512517690607 [DOI] [PubMed] [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, & Baird G (2009). Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine and Child Neurology, 51(4), 311–316. 10.1111/j.1469-8749.2008.03242.X [DOI] [PubMed] [Google Scholar]
- Hannant P, Cassidy S, Van de Weyer R, & Mooncey S (2018). Sensory and motor differences in Autism Spectrum Conditions and developmental coordination disorder in children: A cross-syndrome study. Human Movement Science, 58, 108–118. 10.1016/j.humov.2018.01.010 [DOI] [PubMed] [Google Scholar]
- Happé F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, Dolan R, Frackowiak R, & Frith C (1996). “Theory of mind” in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport, 8(1), 197–201. 10.1097/00001756-199612200-00040 [DOI] [PubMed] [Google Scholar]
- Heiser M, Iacoboni M, Maeda F, Marcus J, & Mazziotta JC (2003). The essential role of Broca’s area in imitation. European Journal of Neuroscience, 17(5), 1123–1128. 10.1046/j.1460-9568.2003.02530.x [DOI] [PubMed] [Google Scholar]
- Henderson SE, Sugden D, & Barnett AL (1992). Movement assessment battery for children-2. Research in Developmental Disabilities. [Google Scholar]
- Hertrich I, Dietrich S, Blum C, & Ackermann H (2021). The Role of the Dorsolateral Prefrontal Cortex for Speech and Language Processing. Frontiers in Human Neuroscience, 15, 645209. 10.3389/fnhum.2021.645209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton CL, Zhang Y, Whilte MR, Klohr CL, & Constantino J (2012). Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism: The International Journal of Research and Practice, 16(4), 430–441. 10.1177/1362361311423018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoaglin DC, & Iglewicz B (1987). Fine-Tuning Some Resistant Rules for Outlier Labeling. Journal of the American Statistical Association, 82(400), 1147–1149. 10.1080/01621459.1987.10478551 [DOI] [Google Scholar]
- Holt RJ, Chura LR, Lai M-C, Suckling J, von dem Hagen E, Calder AJ, Bullmore ET, Baron-Cohen S, & Spencer MD (2014). “Reading the Mind in the Eyes”: An fMRI study of adolescents with autism and their siblings. Psychological Medicine, 44(15), 3215–3227. 10.1017/S0033291714000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hours C, Recasens C, & Baleyte J-M (2022). ASD and ADHD Comorbidity: What Are We Talking About? Frontiers in Psychiatry, 13. 10.3389/fpsyt.2022.837424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll B, & Meyer K (2011). Examination of correlates of different imitative functions in young children with autism spectrum disorders. Research in Autism Spectrum Disorders, 5(3), 1078–1085. 10.1016/j.rasd.2010.12.001 [DOI] [Google Scholar]
- Ingersoll B, & Schreibman L (2006). Teaching reciprocal imitation skills to young children with autism using a naturalistic behavioral approach: Effects on language, pretend play, and joint attention. Journal of Autism and Developmental Disorders, 36(4), 487–505. 10.1007/s10803-006-0089-y [DOI] [PubMed] [Google Scholar]
- Jacob P, & Jeannerod M (2005). The motor theory of social cognition: A critique. Trends in Cognitive Sciences, 9(1), 21–25. 10.1016/j.tics.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, & Smith SM (2012). FSL. NeuroImage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156. 10.1016/s1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, & Just MA (2009). Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social Neuroscience, 4(2), 135–152. 10.1080/17470910802198510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangarani-Farahani M, Malik MA, & Zwicker JG (2023). Motor Impairments in Children with Autism Spectrum Disorder: A Systematic Review and Meta-analysis. Journal of Autism and Developmental Disorders. 10.1007/s10803-023-05948-1 [DOI] [PubMed] [Google Scholar]
- Kilroy E, Cermak SA, & Aziz-Zadeh L (2019). A Review of Functional and Structural Neurobiology of the Action Observation Network in Autism Spectrum Disorder and Developmental Coordination Disorder. Brain Sciences, 9(4), 75. 10.3390/brainsci9040075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilroy E, Harrison L, Butera C, Jayashankar A, Cermak S, Kaplan J, Williams M, Haranin E, Bookheimer S, Dapretto M, & Aziz-Zadeh L (2021). Unique deficit in embodied simulation in autism: An fMRI study comparing autism and developmental coordination disorder. Human Brain Mapping, 42(5), 1532–1546. 10.1002/hbm.25312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilroy E, Ring P, Hossain A, Nalbach A, Butera C, Harrison L, Jayashankar A, Vigen C, Aziz-Zadeh L, & Cermak SA (2022). Motor performance, praxis, and social skills in autism spectrum disorder and developmental coordination disorder. Autism Research, 15(9), 1649–1664. 10.1002/aur.2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliemann N, Croker H, Johnson F, & Beeken RJ (2018). Starting university with high eating self-regulatory skills protects students against unhealthy dietary intake and substantial weight gain over 6 months. Eating Behaviors, 31, 105–112. 10.1016/j.eatbeh.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, & Kemp S (2007). NEPSY - Second Edition. 10.1037/t15125-000 [DOI] [Google Scholar]
- Koster-Hale J, Dungan J, Saxe R, & Young L (2012). Thinking in Patterns: Using multi-voxel pattern analyses to find neural correlates of moral judgment in neurotypical and ASD populations. Proceedings of the Annual Meeting of the Cognitive Science Society, 34(34). https://escholarship.org/uc/item/0km5x647 [Google Scholar]
- Lee K, Jung T, Lee DK, Lim J-C, Lee E, Jung Y, & Lee Y (2019). A comparison of using the DSM-5 and MABC-2 for estimating the developmental coordination disorder prevalence in Korean children. Research in Developmental Disabilities, 94, 103459. 10.1016/j.ridd.2019.103459 [DOI] [PubMed] [Google Scholar]
- Libby S, Powell S, Messer D, & Jordan R (1998). Spontaneous Play in Children with Autism: A Reappraisal. Journal of Autism and Developmental Disorders, 28(6), 487–497. 10.1023/A:1026095910558 [DOI] [PubMed] [Google Scholar]
- Liew S-L, Ma Y, Han S, & Aziz-Zadeh L (2011). Who’s Afraid of the Boss: Cultural Differences in Social Hierarchies Modulate Self-Face Recognition in Chinese and Americans. PLOS ONE, 6(2), e16901. 10.1371/journal.pone.0016901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, & Rutter M (2000). The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Corporation, 284. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Mainieri AG, Heim S, Straube B, Binkofski F, & Kircher T (2013). Differential role of the Mentalizing and the Mirror Neuron system in the imitation of communicative gestures. NeuroImage, 81, 294–305. 10.1016/j.neuroimage.2013.05.021 [DOI] [PubMed] [Google Scholar]
- Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, Donohue MR, Foran W, Miller RL, Hendrickson TJ, Malone SM, Kandala S, Feczko E, Miranda-Dominguez O, Graham AM, Earl EA, Perrone AJ, Cordova M, Doyle O, … Dosenbach NUF (2022). Reproducible brain-wide association studies require thousands of individuals. Nature, 603(7902), Article 7902. 10.1038/s41586-022-04492-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoni F, & Surian L (2016). Mental State Understanding and Moral Judgment in Children with Autistic Spectrum Disorder. Frontiers in Psychology, 7. 10.3389/fpsyg.2016.01478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NC, Piek JP, & Hay D (2006). DCD and ADHD: A genetic study of their shared aetiology. Human Movement Science, 25, 110–124. 10.1016/j.humov.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Mazza M, Pino MC, Mariano M, Tempesta D, Ferrara M, De Berardis D, Masedu F, & Valenti M (2014). Affective and cognitive empathy in adolescents with autism spectrum disorder. Frontiers in Human Neuroscience, 8. 10.3389/fnhum.2014.00791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe D, Pillai AS, Tiedemann A, Mostofsky SH, & Ewen JB (2017). Dyspraxia in ASD: Impaired coordination of movement elements. Autism Research, 10(4), 648–652. 10.1002/aur.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe D, Zhao Y, Pillai AS, Ament K, Adamek J, Caffo BS, Mostofsky SH, & Ewen JB (2020). Learning of skilled movements via imitation in ASD. Autism Research: Official Journal of the International Society for Autism Research, 13(5), 777–784. 10.1002/aur.2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Turner L, Stone W, Yoder P, Wolery M, & Ulman T (2007). Developmental Correlates of Different Types of Motor Imitation in Young Children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 37(3), 401–412. 10.1007/s10803-006-0175-1 [DOI] [PubMed] [Google Scholar]
- McIntosh DN, Reichmann-Decker A, Winkielman P, & Wilbarger JL (2006). When the social mirror breaks: Deficits in automatic, but not voluntary, mimicry of emotional facial expressions in autism. Developmental Science, 9(3), 295–302. 10.1111/j.1467-7687.2006.00492.x [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61(4), 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HL, Sherrod GM, Mauk JE, Fears NE, Hynan LS, & Tamplain PM (2021). Shared Features or Co-occurrence? Evaluating Symptoms of Developmental Coordination Disorder in Children and Adolescents with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 51(10), 3443–3455. 10.1007/s10803-020-04766-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Young LL, Saxe R, Lee SM, O’Young D, Mavros PL, & Gabrieli JD (2011). Impaired theory of mind for moral judgment in high-functioning autism. Proceedings of the National Academy of Sciences, 108(7), 2688–2692. 10.1073/pnas.1011734108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, & Denckla MB (2006). Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society: JINS, 12(3), 314–326. 10.1017/s1355617706060437 [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, & Ewen JB (2011). Altered connectivity and action model formation in autism is autism. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 17(4), 437–448. 10.1177/1073858410392381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul C, Stagg SD, Herbelin B, & Aspell JE (2018). The Feeling of Me Feeling for You: Interoception, Alexithymia and Empathy in Autism. Journal of Autism and Developmental Disorders, 48(9), 2953–2967. 10.1007/s10803-018-3564-3 [DOI] [PubMed] [Google Scholar]
- Nomi JS, & Uddin LQ (2015a). Developmental changes in large-scale network connectivity in autism. NeuroImage. Clinical, 7, 732–741. 10.1016/j.nicl.2015.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, & Uddin LQ (2015b). Face processing in autism spectrum disorders: From brain regions to brain networks. Neuropsychologia, 71, 201–216. 10.1016/j.neuropsychologia.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, & McCarthy G (2005). Neural basis of eye gaze processing deficits in autism. Brain, 128(5), 1038–1048. 10.1093/brain/awh404 [DOI] [PubMed] [Google Scholar]
- Perra O, Williams JHG, Whiten A, Fraser L, Benzie H, & Perrett DI (2008). Imitation and ‘theory of mind’ competencies in discrimination of autism from other neurodevelopmental disorders. Research in Autism Spectrum Disorders, 2(3), 456–468. 10.1016/j.rasd.2007.09.007 [DOI] [Google Scholar]
- Pilowsky T, Yirmiya N, Arbelle S, & Mozes T (2000). Theory of mind abilities of children with schizophrenia, children with autism, and normally developing children. Schizophrenia Research, 42(2), 145–155. 10.1016/s0920-9964(99)00101-2 [DOI] [PubMed] [Google Scholar]
- Pitskel NB, Bolling DZ, Hudac CM, Lantz SD, Minshew NJ, Vander Wyk BC, & Pelphrey KA (2011). Brain Mechanisms for Processing Direct and Averted Gaze in Individuals with Autism. Journal of Autism and Developmental Disorders, 41(12), 1686–1693. 10.1007/s10803-011-1197-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet I, Kojovic N, Franchini M, & Schaer M (2022). Trajectories of imitation skills in preschoolers with autism spectrum disorders. Journal of Neurodevelopmental Disorders, 14(1), 2. 10.1186/s11689-021-09412-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, & Beckmann CF (2015). ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–277. 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- Richey JA, Gracanin D, LaConte S, Lisinski J, Kim I, Coffman M, Antezana L, Carlton CN, Garcia KM, & White SW (2022). Neural Mechanisms of Facial Emotion Recognition in Autism: Distinct Roles for Anterior Cingulate and dlPFC. Journal of Clinical Child & Adolescent Psychology, 51(3), 323–343. 10.1080/15374416.2022.2051528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, & Craighero L (2004). The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192. 10.1146/annurev.neuro.27.070203.144230 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, & Sinigaglia C (2010). The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nature Reviews Neuroscience, 11(4), Article 4. 10.1038/nrn2805 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, & Sinigaglia C (2016). The mirror mechanism: A basic principle of brain function. Nature Reviews Neuroscience, 17(12), Article 12. 10.1038/nrn.2016.135 [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, & Wehner E (2003a). Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44(5), 763–781. 10.1111/1469-7610.00162 [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, & Wehner E (2003b). Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44(5), 763–781. 10.1111/1469-7610.00162 [DOI] [PubMed] [Google Scholar]
- Rosenblau G, Kliemann D, Heekeren HR, & Dziobek I (2015). Approximating Implicit and Explicit Mentalizing with Two Naturalistic Video-Based Tasks in Typical Development and Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 45(4), 953–965. 10.1007/s10803-014-2249-9 [DOI] [PubMed] [Google Scholar]
- Rueda P, Fernández-Berrocal P, & Baron-Cohen S (2015). Dissociation between cognitive and affective empathy in youth with Asperger Syndrome. European Journal of Developmental Psychology, 12(1), 85–98. 10.1080/17405629.2014.950221 [DOI] [Google Scholar]
- Safar K, Wong SM, Leung RC, Dunkley BT, & Taylor MJ (2018). Increased Functional Connectivity During Emotional Face Processing in Children With Autism Spectrum Disorder. Frontiers in Human Neuroscience, 12. 10.3389/fnhum.2018.00408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Neubert F-X, Jbabdi S, O’Reilly JX, Filippini N, Thomas AG, & Rushworth MF (2013). The Organization of Dorsal Frontal Cortex in Humans and Macaques. Journal of Neuroscience, 33(30), 12255–12274. 10.1523/JNEUROSCI.5108-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, & Perner J (2014). Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Schuwerk T, Jarvers I, Vuori M, & Sodian B (2016). Implicit Mentalizing Persists beyond Early Childhood and Is Profoundly Impaired in Children with Autism Spectrum Condition. Frontiers in Psychology, 7. 10.3389/fpsyg.2016.01696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senland AK, & Higgins-D’Alessandro A (2016). Sociomoral Reasoning, Empathy, and Meeting Developmental Tasks During the Transition to Adulthood in Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 46(9), 3090–3105. 10.1007/s10803-016-2849-7 [DOI] [PubMed] [Google Scholar]
- Shih P, Shen M, Öttl B, Keehn B, Gaffrey MS, & Müller R-A (2010). Atypical network connectivity for imitation in autism spectrum disorder. Neuropsychologia, 48(10), 2931–2939. 10.1016/j.neuropsychologia.2010.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman C, Yirmiya N, & Greenbaum CW (1995). From categorization to classification: A comparison among individuals with autism, mental retardation, and normal development. Journal of Abnormal Psychology, 104, 601–609. 10.1037/0021-843X.104.4.601 [DOI] [PubMed] [Google Scholar]
- Soares JM, Magalhães R, Moreira PS, Sousa A, Ganz E, Sampaio A, Alves V, Marques P, & Sousa N (2016). A Hitchhiker’s Guide to Functional Magnetic Resonance Imaging. Frontiers in Neuroscience, 10. 10.3389/fnins.2016.00515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperduti M, Guionnet S, Fossati P, & Nadel J (2014). Mirror Neuron System and Mentalizing System connect during online social interaction. Cognitive Processing, 15(3), 307–316. 10.1007/s10339-014-0600-x [DOI] [PubMed] [Google Scholar]
- Spunt RP, & Lieberman MD (2013). The busy social brain: Evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychological Science, 24(1), 80–86. 10.1177/0956797612450884 [DOI] [PubMed] [Google Scholar]
- Stone J, Perry W, & Darley JM (1997). “White Men Can’t Jump”: Evidence for the Perceptual Confirmation of Racial Stereotypes Following a Basketball Game. Basic and Applied Social Psychology, 19(3), 291–306. 10.1207/s15324834basp1903_2 [DOI] [Google Scholar]
- Stuart N, Whitehouse A, Palermo R, Bothe E, & Badcock N (2022). Eye Gaze in Autism Spectrum Disorder: A Review of Neural Evidence for the Eye Avoidance Hypothesis. Journal of Autism and Developmental Disorders. 10.1007/s10803-022-05443-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujatha B, Alagesan J, Lai DV, & Rayna ABS (2020). Prevalence of Developmental Co-ordination Disorder in School Children. The Indian Journal of Pediatrics, 87(6), 454–456. 10.1007/s12098-020-03191-5 [DOI] [PubMed] [Google Scholar]
- Sumner E, Leonard HC, & Hill EL (2016). Overlapping Phenotypes in Autism Spectrum Disorder and Developmental Coordination Disorder: A Cross-Syndrome Comparison of Motor and Social Skills. Journal of Autism and Developmental Disorders, 46(8), 2609–2620. 10.1007/s10803-016-2794-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, & Boucher O (2017). Structure and function of the human insula. Journal of Clinical Neurophysiology : Official Publication of the American Electroencephalographic Society, 34(4), 300–306. 10.1097/WNP.0000000000000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljarevic M, & Hamilton A (2013). Recognition of Emotions in Autism: A Formal Meta-Analysis. Journal of Autism and Developmental Disorders, 43(7), 1517–1526. 10.1007/s10803-012-1695-5 [DOI] [PubMed] [Google Scholar]
- Vivanti G. (2013). Imitation in autism spectrum disorders: From research to treatment. Neurobiology, Diagnosis and Treatment in Autism. An Update, 161–165. [Google Scholar]
- Vyas K, Jameel L, Bellesi G, Crawford S, & Channon S (2017). Derailing the trolley: Everyday utilitarian judgments in groups high versus low in psychopathic traits or autistic traits. Psychiatry Research, 250, 84–91. 10.1016/j.psychres.2017.01.054 [DOI] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, & Bookheimer SY (2004). Neural Correlates of Facial Affect Processing in Children and Adolescents With Autism Spectrum Disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 43(4), 481–490. 10.1097/00004583-200404000-00015 [DOI] [PubMed] [Google Scholar]
- Wang Y, Cao N, Lin Y, Chen R, & Zhang J (2020). Hemispheric Differences in Functional Interactions Between the Dorsal Lateral Prefrontal Cortex and Ipsilateral Motor Cortex. Frontiers in Human Neuroscience, 14, 202. 10.3389/fnhum.2020.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (2011). WASI-II: Wechsler abbreviated scale of intelligence. PsychCorp. [Google Scholar]
- Williams JHG, Massaro DW, Peel NJ, Bosseler A, & Suddendorf T (2004). Visual–auditory integration during speech imitation in autism. Research in Developmental Disabilities, 25(6), 559–575. 10.1016/j.ridd.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Wilson BN, Crawford SG, Green D, Roberts G, Aylott A, & Kaplan BJ (2009). Psychometric Properties of the Revised Developmental Coordination Disorder Questionnaire. Physical & Occupational Therapy In Pediatrics, 29(2), 182–202. 10.1080/01942630902784761 [DOI] [PubMed] [Google Scholar]
- Wilson BN, Kaplan BJ, Crawford SG, Campbell A, & Dewey D (2000). Reliability and validity of a parent questionnaire on childhood motor skills. The American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association, 54(5), 484–493. 10.5014/ajot.54.5.484 [DOI] [PubMed] [Google Scholar]
- Yeung MK (2022). A systematic review and meta-analysis of facial emotion recognition in autism spectrum disorder: The specificity of deficits and the role of task characteristics. Neuroscience & Biobehaviorai Reviews, 133, 104518. 10.1016/j.neubiorev.2021.104518 [DOI] [PubMed] [Google Scholar]
- Yeung MK, Lee TL, & Chan AS (2020). Impaired Recognition of Negative Facial Expressions is Partly Related to Facial Perception Deficits in Adolescents with High-Functioning Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 50(5), 1596–1606. 10.1007/s10803-019-03915-3 [DOI] [PubMed] [Google Scholar]
- Young GS, Rogers SJ, Hutman T, Rozga A, Sigman M, & Ozonoff S (2011). Imitation from 12 to 24 months in autism and typical development: A longitudinal Rasch analysis. Developmental Psychology, 47, 1565–1578. 10.1037/a0025418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachor DA, Ilanit T, & Itzchak EB (2010). Autism severity and motor abilities correlates of imitation situations in children with autism spectrum disorders. Research in Autism Spectrum Disorders, 4(3), 438–443. 10.1016/j.rasd.2009.10.016 [DOI] [Google Scholar]
- Zalla T, & Leboyer M (2011). Judgment of intentionality and moral evaluation in individuals with high functioning autism. Review of Philosophy and Psychology, 2(4), 681–698. 10.1007/s13164-011-0048-1 [DOI] [Google Scholar]
- Zemestani M, Hoseinpanahi O, Salehinejad MA, & Nitsche MA (2022). The impact of prefrontal transcranial direct current stimulation (tDCS) on theory of mind, emotion regulation and emotional-behavioral functions in children with autism disorder: A randomized, sham-controlled, and parallel-group study. Autism Research, 15(10), 1985–2003. 10.1002/aur.2803 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual raw behavioral and imaging data have been publicly shared on the National Institute of Mental Health (NIMH) Data Archive (NDA) and can be accessed using the Collection ID #2254 (https://nda.nih.gov/edit_collection.html?id=2254). Data structures that have been defined in the NDA Data Dictionary are available at https://nda.nih.gov/general-query.html?q=query=data-structure. We have publicly archived the analysis code used in this study on GitHub and it can be accessed at https://github.com/CeNEC/Ment_PPI.git. Readers seeking access to fMRI task stimuli will find it publicly available at https://dornsife.usc.edu/cenec/data-sharing/. Legal copyright restrictions prevent public archiving of WASI-II, SRS-2, Conners, NEPSY-II, DCDQ, MABC-2, ADOS-2, and ADI-R which can be obtained from the copyright holders in the cited references. No part of the study procedures or analysis plans was preregistered prior to the research being conducted.


