Abstract
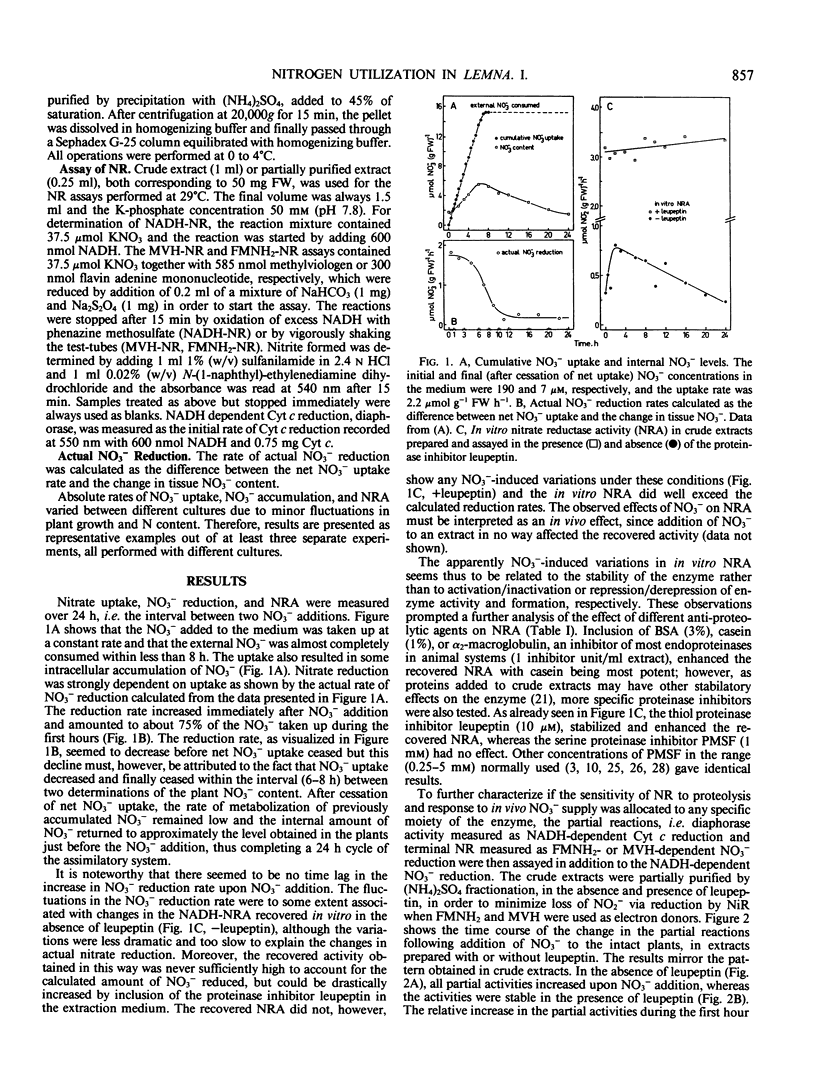
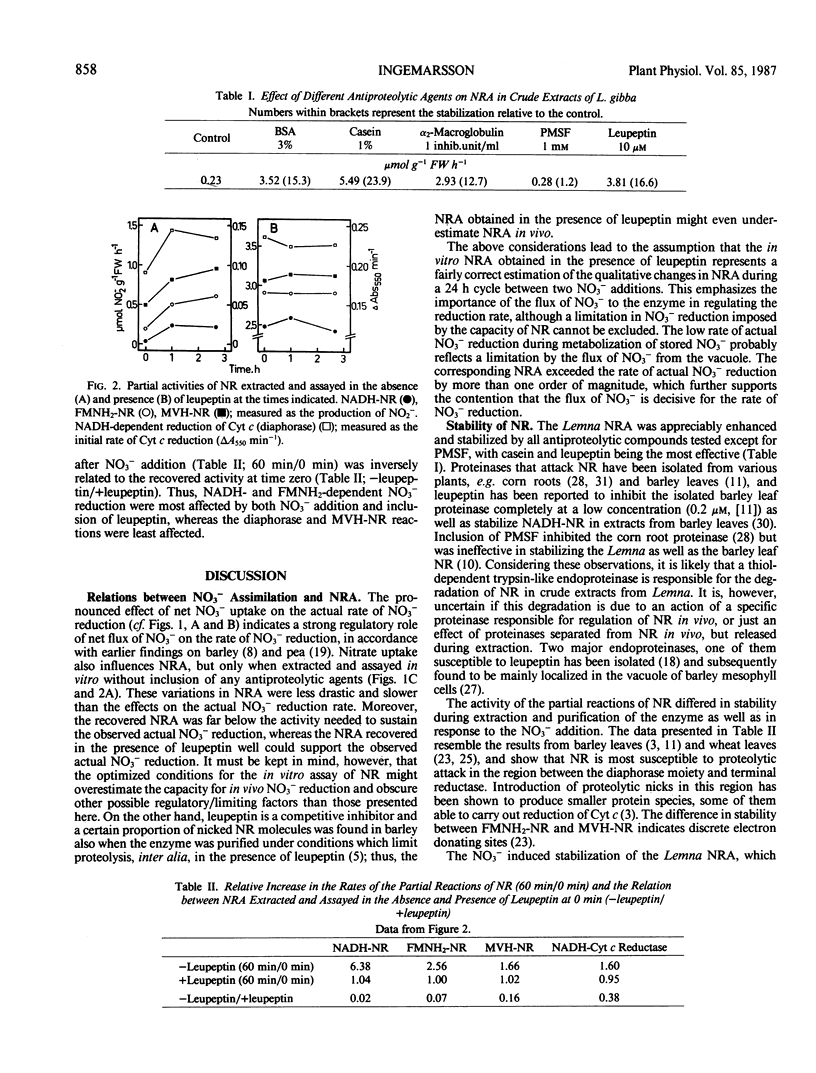
Cultures of Lemna gibba L. G3 were maintained at a constant, N-limited growth rate by adding nitrate daily in amounts calculated to sustain a rate of culture N increment of 0.20 day−1. Nitrate added to the culture was consumed within 8 to 10 hours and the partitioning to reduction and accumulation during this phase corresponded to, on the average, 75 and 25% of net uptake, respectively. The calculated rate of nitrate reduction was stimulated by onset of net uptake without delay and decreased when net uptake ceased. NADH-nitrate reductase (NR) activity measured in vitro without inclusion of antiproteolytic agents more than doubled during the first hour after nitrate addition and then gradually fell to its original level over the rest of the 24 hour interval. In the presence of the proteinase inhibitor leupeptin during extraction, however, NR activity was in general much higher and without any apparent cycles. The relative stabilizing effect of leupeptin was greatest on NADH-NR and reduced flavin adenine mononucleotide-NR activities whereas the effect was less on NADH-cytochrome c reductase activity (diaphorase) and reduced methylviologen-NR activity. The constant nitrate reductase activity measured in the presence of proteinase inhibitors is assumed to reflect the physiological situation. It thus appeares that short-term changes in nitrate assimilation by N-limited Lemna is related to the flux of nitrate to the reducing site and not to changes in nitrate reductase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aryan A. P., Batt R. G., Wallace W. Reversible Inactivation of Nitrate Reductase by NADH and the Occurrence of Partially Inactive Enzyme in the Wheat Leaf. Plant Physiol. 1983 Mar;71(3):582–587. doi: 10.1104/pp.71.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. E., Ryan C. A. Isolation and characterization of a wound-induced trypsin inhibitor from alfalfa leaves. Biochemistry. 1984 Jul 17;23(15):3418–3422. doi: 10.1021/bi00310a006. [DOI] [PubMed] [Google Scholar]
- Chantarotwong W., Huffaker R. C., Miller B. L., Granstedt R. C. In vivo nitrate reduction in relation to nitrate uptake, nitrate content, and in vitro nitrate reductase activity in intact barley seedlings. Plant Physiol. 1976 Apr;57(4):519–522. doi: 10.1104/pp.57.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnelli M., Cioni P., Romagnoli A., Gabellieri E., Balestreri E., Felicioli R. Purification and characterization of two leaf polypeptide inhibitors of leaf protease from alfalfa (Medicago sativa). Arch Biochem Biophys. 1985 Apr;238(1):206–212. doi: 10.1016/0003-9861(85)90157-2. [DOI] [PubMed] [Google Scholar]
- Jolly S. O., Tolbert N. E. NADH-Nitrate Reductase Inhibitor from Soybean Leaves. Plant Physiol. 1978 Aug;62(2):197–203. doi: 10.1104/pp.62.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. M., Pearce G., Ryan C. A. Isolation and characterization of proteinase inhibitor I from etiolated tobacco leaves. Arch Biochem Biophys. 1984 May 1;230(2):504–510. doi: 10.1016/0003-9861(84)90430-2. [DOI] [PubMed] [Google Scholar]
- Miller B. L., Huffaker R. C. Partial purification and characterization of endoproteinases from senescing barley leaves. Plant Physiol. 1981 Oct;68(4):930–936. doi: 10.1104/pp.68.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmler J. L., Campbell W. H. Regulation of Corn Leaf Nitrate Reductase : II. Synthesis and Turnover of the Enzyme's Activity and Protein. Plant Physiol. 1986 Feb;80(2):442–447. doi: 10.1104/pp.80.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Cataldo D. A., Peterson D. M. Use of protein in extraction and stabilization of nitrate reductase. Plant Physiol. 1974 May;53(5):688–690. doi: 10.1104/pp.53.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner D. L., Boyer J. S. Nitrate Reductase Activity in Maize (Zea mays L.) Leaves: I. Regulation by Nitrate Flux. Plant Physiol. 1976 Oct;58(4):499–504. doi: 10.1104/pp.58.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard J. H., Dalling M. J. In vitro stability of nitrate reductase from wheat leaves: I. Stability of highly purified enzyme and its component activities. Plant Physiol. 1979 Feb;63(2):346–353. doi: 10.1104/pp.63.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard J. H., Kennedy J. A., Dalling M. J. In Vitro Stability of Nitrate Reductase from Wheat Leaves: II. Isolation of Factors from Crude Extract Which Affect Stability of Highly Purified Nitrate Reductase. Plant Physiol. 1979 Sep;64(3):439–444. doi: 10.1104/pp.64.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard J. H., Kennedy J. A., Dalling M. J. In Vitro Stability of Nitrate Reductase from Wheat Leaves: III. Isolation and Partial Characterization of a Nitrate Reductase-inactivating Factor. Plant Physiol. 1979 Oct;64(4):640–645. doi: 10.1104/pp.64.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D. A., Kuo T. M., Kleinhofs A., Warner R. L., Oaks A. Synthesis and degradation of barley nitrate reductase. Plant Physiol. 1983 Aug;72(4):949–952. doi: 10.1104/pp.72.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. S., Huffaker R. C. Vacuolar Localization of Endoproteinases EP(1) and EP(2) in Barley Mesophyll Cells. Plant Physiol. 1984 May;75(1):70–73. doi: 10.1104/pp.75.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. Purification and properties of a nitrate reductase-inactivating enzyme. Biochim Biophys Acta. 1974 Mar 21;341(1):265–276. doi: 10.1016/0005-2744(74)90087-4. [DOI] [PubMed] [Google Scholar]
- Yamaya T., Oaks A., Boesel I. L. Characteristics of Nitrate Reductase-inactivating Proteins Obtained from Corn Roots and Rice Cell Cultures. Plant Physiol. 1980 Jan;65(1):141–145. doi: 10.1104/pp.65.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]


