Abstract
Purpose
To retrospectively review locally advanced cervical cancer (CC) cases treated with three-dimensional image-guided brachytherapy (3D-IGBT) and two-dimensional (2D)-IGBT.
Materials and Methods
Patients with Stage IB–IVa CC who underwent intracavitary irradiation between 2007 and 2021 were divided into the 3D-IGBT and 2D-IGBT groups. Local control (LC), distant metastasis-free survival (DMFS), progression-free survival (PFS), overall survival (OS), and gastrointestinal toxicity (G3 or more) were investigated at 2/3 years post-treatment.
Results
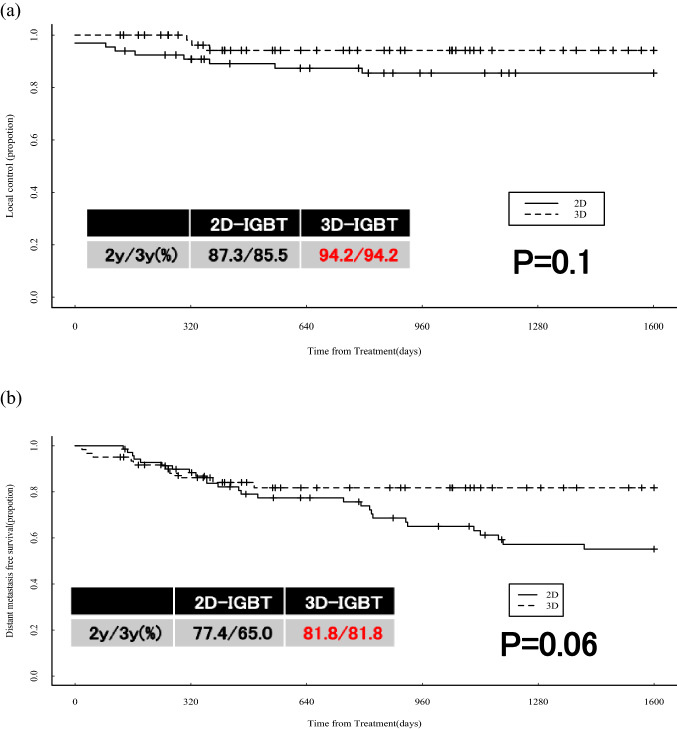
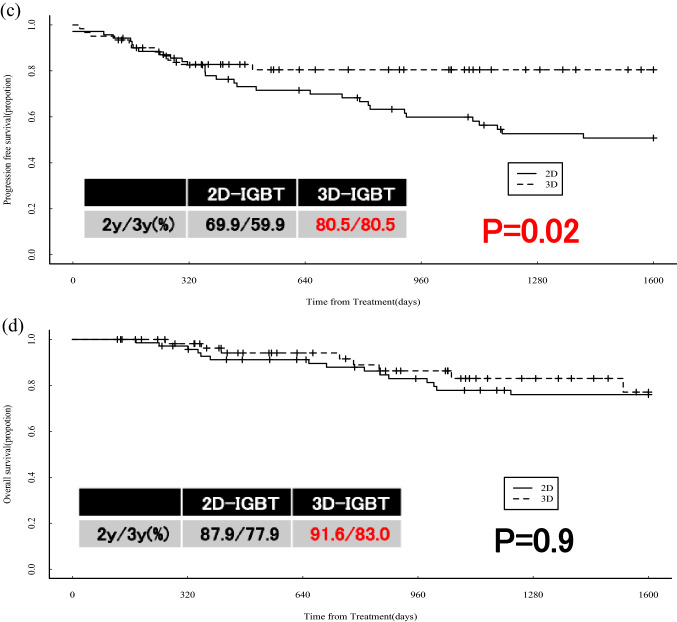
Seventy-one patients in the 2D-IGBT group from 2007 to 2016 and 61 patients in the 3D-IGBT group from 2016–2021 were included in the study. The median follow-up period was 72.7 (4.6–183.9) months in the 2D-IGBT group and 30.0 (4.2–70.5) months in the 3D-IGBT group. The median age was 65.0 (40–93) years in the 2D-IGBT group and 60.0 (28–87) years in the 3D-IGBT group, but there was no difference in FIGO stage, histology, or tumor size between the groups. In treatment, the median A point dose was 56.1 (40.0–74.0) Gy in the 2D-IGBT group and 64.0 (52.0–76.8) Gy in the 3D-IGBT group (P < 0.0001), and the proportion of patients who underwent chemotherapy more than five times was 54.3% in the 2D-IGBT group and 80.8% in the 3D-IGBT group (P = 0.0004). The 2/3-year LC, DMFS, PFS, and OS rates were 87.3%/85.5%, 77.4%/65.0%, 69.9%/59.9%, and 87.9%/77.9% in the 2D-IGBT group, and 94.2%/94.2%, 81.8%/81.8%, 80.5%/80.5%, and 91.6%/83.0% in the 3D-IGBT group, respectively. A significant difference was observed in PFS (P = 0.02). There was no difference in gastrointestinal toxicity, but there were four intestinal perforations in the patients from the 3D-IGBT group, three of whom had a history of bevacizumab treatment.
Conclusion
The 2/3-year LC of the 3D-IGBT group was excellent and PFS also tended to improve. Care should be taken with concomitant use of bevacizumab after radiotherapy.
Keywords: Locally advanced cervical cancer, CT-based 3D-IGBT, Outcome, Bavazcizumab
Introduction
Cervical cancer (CC) is one of the most common malignant diseases affecting female individuals, remaining a major cause of cancer death among female adolescents and young adults [1, 2]. The standard treatment for locally advanced cervical cancer (LACC) is concurrent chemoradiotherapy (CCRT) [3–7]. The radiotherapy (RT) aspect of the treatment often comprises external beam radiotherapy (EBRT) and brachytherapy (BT). Recently, the Retro-EMBRACE cohort study reported excellent local control (LC) and pelvic control (91% and 87% at 3 years, respectively) as well as overall survival (OS) and cancer-specific survival rates (74% and 79% at 3 years, respectively) with limited severe morbidity [8]. However, regardless of excellent LC, the high proportion of distant metastasis (DM) remains an important issue related to this disease.
The purpose of this retrospective study was to examine LC, distant metastasis-free survival (DMFS), progression-free survival (PFS), and OS among patients with CC who received definitive RT with BT from two institutions. Moreover, we aimed to compare cases treated with three-dimensional image-guided brachytherapy (3D-IGBT) and two-dimensional (2D)-IGBT. Moreover, we searched for current status and problems related to this treatment.
Materials and methods
Patients
The data from consecutive CC patients who received treatment from two institutions between 2007 and 2021 were retrospectively analyzed. This study was approved by the Institutional Review Boards of the involved institutions. We included patients pathologically diagnosed with International Federation of Gynecology and Obstetrics (FIGO) 2008 Stages Ib–IVa who were treated with EBRT followed by high dose–rate intracavitary BT. BT was performed at the same facility. We excluded patients treated with RT without BT. Moreover, the patient cohort included those with para-aortic lymph node metastasis.
Treatment
In this study, the RT protocol was composed of a combination of EBRT and BT. The first course of whole-pelvic external irradiation was delivered at a dose of 1.8–2.0 Gy/fraction, five times/week, up to a total dose of 20–50.4 Gy. The second course of EBRT was performed with central shielding up to a total external dose of 50–50.4 Gy. Boost EBRT for nodal metastases was delivered using 4–10 Gy in 2–5 fractions. From 2020, EBRT was conducted with intensity-modulated radiotherapy (IMRT) or simultaneous integrated boost (SIB)-IMRT. The doses of SIB-IMRT were 45 Gy/25 fr for regional and 55 Gy/25 fr for lymph node metastasis. BT was performed with 2–4 cycles once or twice a week at a fractional dose of 5.5–6.5 Gy, for a total dose of 12–24 Gy at point A. From 2007 to 2015, BT with 2D-IGBT, was used, while after 2016, computed tomography (CT)-guided 3D-IGBT was used. In our institution, BT was conducted using tandem and ovoid. This therapy was performed through microSelectron v2 (Nucletron, Elekta Company, Stockholm, Sweden). For the methods of contouring of the organs at risk (OARs) and for the high-risk clinical target volume (HR-CTV), we referred to the recommendations from the gynecological (GYN) GEC ESTRO working group [9] and the Working Group of the Gynecological Tumor Committee of the Japanese Radiation Oncology Study Group [10]. The dose per fraction for HR-CTV D90 should be 5.8–6.0 Gy or higher. Those for the OARs were set at ≤ 6 Gy for rectum D2cc and sigmoid colon D2cc and at ≤ 7 Gy for bladder D2cc. The total doses of whole-pelvic irradiation and BT at point A were calculated using a biologically equivalent dose to 2 Gy/fraction (EQD2) through the linear-quadratic model, wherein α/β = 10 Gy. For OARs, the calculation was made with α/β = 3 Gy. The doses from whole-pelvic with central shielding were excluded in the total EQD2. The EQD2 total dose for HR-CTV was set to be ≥ 60 Gy. Those for rectum D2cc and sigmoid colon D2cc were set to be ≤ 75 Gy, while those for bladder D2cc were ≤ 85 Gy. Patients with para-aortic lymph node metastasis received extended radiation treatment.
For chemotherapy (CTx), cisplatin (40 mg/m2) or paclitaxel (60 mg/m2) plus a platinum antitumor agent (nedaplatin 25 mg/m2 or carboplatin with an area under the curve of 2 mg/mL/min) was administered weekly according to institutional policy.
Outcomes and statistical analyses
Statistical analyses were conducted using R version 3.3.4 (R Foundation for Statistical Computing, Vienna, Austria). Kaplan–Meier analysis was used to estimate LC, DMFS, PFS, and OS. We examined age, stage, histology, pelvic lymph node metastasis, para-aortic lymph node metastasis, tumor size, overall treatment time, point A dose (EQD2), CTx use, and the frequency of CTx. Fischer’s exact test was used to evaluate differences between the 2D-IGBT and 3D-IGBT groups. P values < 0.05 were considered to indicate statistical significance.
To evaluate late toxicity, the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) were used [11].
Results
Patient characteristics
A total of 132 patients with CC who received definitive RT were included in this study. A total of 92 cases came from one institution and the remaining 40 came from another. The patient, stage, tumor, and treatment characteristics are shown in Table 1. A total of 71 patients in the 2D-IGBT group from 2007 to 2016 and 61 patients in the 3D-IGBT group from 2016 to 2021 were included in the study. There were two patients treated with combined intracavitary and interstitial brachytherapy in 2021. There was no significant difference in the FIGO stage, histology, tumor size, and presence of lymph node metastasis between the groups. For treatment, there was no difference in the overall treatment time (OTT) between the two groups. The median EQD2 was 56.2 (40.0–74.0) Gy in the 2D-IGBT group and 64.1 (52.0–76.8) Gy in the 3D-IGBT group, and there was a significant difference between the two groups (P < 0.0001). The median dose of HR-CTV D90 was 70.9 (53.7–93.5) Gy in the 3D-IGBT group. The proportion of concurrent chemoradiotherapy (CCRT) was 64% in the 2D-IGBT group and 85% in the 3D-IGBT group. CCRT was common in the 3D-IGBT group. The percentage of patients who received five or more times of CTx was 54.3% in the 2D-IGBT group and 80.8% in the 3D-IGBT group (P = 0.0004).
Table 1.
Patient, tumor, and treatment characteristics
| 2D-IGBTa (2007–2016) | 3D-IGBTb (2017–2021) | P value | |
|---|---|---|---|
| Number of cases | 71 | 61 | |
| Follow up period (months) | |||
| Median (range) | 72.7 (4.6–183.9) | 30.0 (4.2–70.5) | < 0.0001 |
| Age (years) | 65.0 (40–93) | 60.0 (28–87) | 0.0125 |
| Stage | |||
| Ib/II/III/IVa | 7/28/29/7 | 6/25/21/9 | 0.7998 |
| Histology | |||
| Squamous ca.c/Adeno.ca.d/Other | 59/10/2 | 59/6/0 | 0.4765 |
| Tumor diametere (cm) | 5.0 (1–10) | 5.7 (1–8.6) | 0.1468 |
| Pelvic lymph node involvementf | |||
| Yes/No | 35/36 | 41/20 | 0.0518 |
| Para-aortic lymph node involvement | |||
| Yes/No | 11/60 | 14/47 | 0.3732 |
| Pretreatment hemoglobin level(g/dL) | 11.1 (7.3–14.8) | 12.0 (6.4–15.0) | 0.1098 |
| Overall treatment time (days) | 48 (36–85) | 48 (38–63) | 0.3409 |
| Point A dose (Gyg) | 56.1 (40–74) | 64.0 (52–76.8) | < 0.0001 |
| HR-CTVh D90 (Gyg) | 70.9 (53.7–93.5) | ||
| Bladder D2cc (Gyg) | 77.6 (64.1–105.2) | ||
| Rectum D2cc (Gyg) | 58.5 (39.6–58.5) | ||
| Sigmoid colon D2cc (Gyg) | 58.2 (35.7–73.8) | ||
| Chemotherapy (CTx) | |||
| Yes/No | 46/25 | 52/9 | 0.0093 |
| Times of CTx | |||
| Less than five times/Five times or more | 21/25 | 10/42 | 0.0004 |
| Adjuvant CTx | |||
| Yes/No | 40/31 | 32/29 | 0.7268 |
| Local recurrence (cases) | 10 | 3 | |
| Regional recurrencei (cases) | 8 | 4 | |
| Distant metastasisj (cases) | 28 | 10 | |
| All recurrence (cases) | 32 | 11 | |
aBrachytherapy with two-dimensional imaging
bBrachytherapy with CT-guided three-dimensional imaging
cSquamous cell carcinoma
dAdenocarcinoma
eTumor diameter was measured using the maximum length meter
fLymph node involvement was defined as a diameter > 1 cm or positive FDG-PET
gEQD2 (biologically equivalent dose; reference dose per fraction = 2 Gy, linear quadratic model, α/β = 10 Gy point A and HR-CTV, α/β = 3 Gy Bladder, Rectum, and Sigmoid colon)
hHigh-risk clinical target volume
iRegional recurrence was defined as recurrence inside the radiation field except for the cervix of the uterus
jDistant metastasis was defined as recurrence outside the radiation field
Tumor response
There were 32 relapsed cases in the 2D-IGBT group and 11 in the 3D-IGBT group. Eight out of 11 cases in the 3D-IGBT group were treated with paclitaxel plus platinum-based antitumor agents, four with irinotecan, and five with bevacizumab. Bevacizumab was used in combination.
Local recurrence occurred in 13 patients, 10 of whom were in the 2D-IGBT group and three were in the 3D-IGBT group. One case had local recurrence only and all the other cases had metastases. The patient with local recurrence only refused treatment and was transferred to another hospital. Of the 12 patients with metastases, one patient underwent hysterectomy and CTx, and one patient received additional radiation therapy. Two patients with additional local treatment died of metastases in other sites. The other seven patients received CTx only, and three could not be treated due to unfavorable conditions. Regional recurrence occurred in 12 patients, eight of whom were in the 2D-IGBT group and four were in the 3D-IGBT group. Distant metastasis occurred in 38 patients, 28 of whom were in the 2D-IGBT group and 10 were in the 3D-IGBT group.
The results of the statistical analysis are shown in Fig. 1a–d. The 2/3 year LC, DMFS, PFS, and OS rates were 87.3%/85.5%, 77.4%/65.0%, 69.9%/59.9%, and 87.9%/77.9% in the 2D-IGBT group and 94.2%/94.2%, 81.8%/81.8%, 80.5%/80.5%, and 91.6%/83.0% in the 3D-IGBT group, respectively. LC and DMFS tended to improve in the 3D-IGBT group, and PFS was significantly better in the 3D-IGBT group than in the 2D-IGBT group (P = 0.02). There was no significant difference between the two groups for OS.
Fig. 1.
Patient outcomes: a local control, b distant-metastasis free survival, c progression-free survival, and d overall survival
Late toxicity
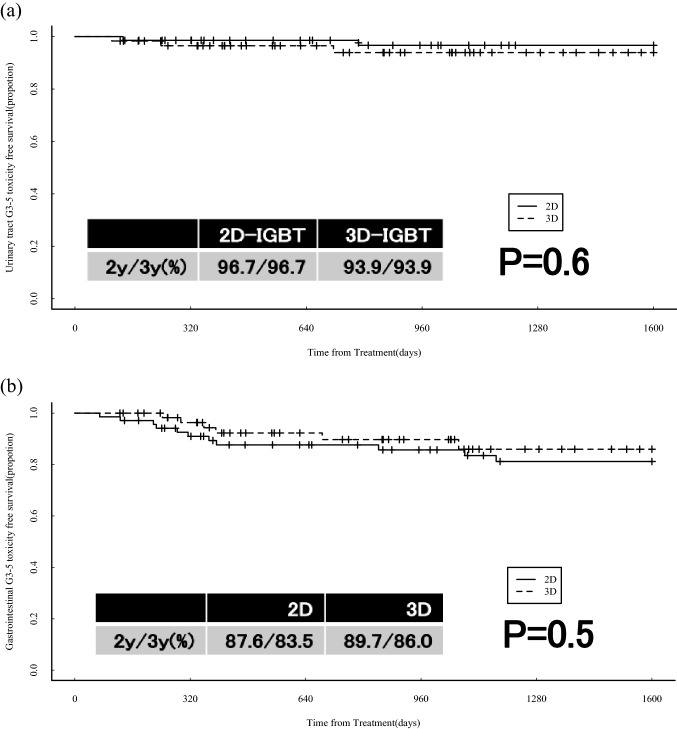
The rates of grade 3 (G3) or greater urinary tract and gastrointestinal (GI) adverse events (AE) were 3.3%/3.3% and 12.4%/16.5% in the 2D-IGBT group and 6.1%/6.1% and 10.3%/14.0% in the 3D-IGBT group after 2 and 3 years of follow-up, respectively. The results are shown in Fig. 2a, b; there was no significant difference between the two groups. The complication details are presented in Table 2a, b. There were two cases of bladder perforation in each of the two groups. All patients with fistula were not associated with bladder invasion. In the 2D-IGBT group, one patient underwent neoadjuvant CTx and the other underwent hysterectomy within 3 months after CCRT. In the 3D-IGBT group, one patient underwent treatment with bevacizumab and the other had related regional recurrence. There were four cases of intestinal perforation in each of the two groups. Three of the four patients in the 3D-IGBT group had previously been treated with bevacizumab. Eleven cases of recurrence occurred in the 3D-IGBT group, and intestinal perforation occurred in one out of six cases, which did not receive bevacizumab, and in three out of five cases of patients receiving bevacizumab (Table 3a, b).
Fig. 2.
Comparison of the rates of grade 3 or more adverse events between the 2D-IGBT and 3D-IGBT groups. a G3-5 urinary tract adverse events free survival, b G3-gastrointestinal adverse events free survival
Table 2.
Adverse events ≥ G3
| Adverse Events ≥ G3 | 2D-IGBT (n = 71) | 3D-IGBT (n = 61) |
|---|---|---|
| a. Urinary tract | ||
| Bleeding | 2 | 0 |
| Stenosis | 0 | 1 |
| Fistula | 2 | 2 |
| Total | 4 | 3 |
| b. Gastrointestinal tract | ||
| Bleeding | 4 | 2 |
| Ileus | 3 | 0 |
| Stenosis | 2 | 0 |
| Fistula | 4 | 4 |
| Total | 13 | 6 |
Table 3.
Perforation with or without bevacizumab in the 3D-IGBT group
| Recurrence in the 3D-IGBT group | Bladder perforation (No) | Bladder perforation (Yes) | Total |
|---|---|---|---|
| a. Urinary tract | |||
| With bevacizumab | 4 | 1 | 5 |
| Without bevacizumab | 5 | 1 | 6 |
| Total | 9 | 2 | 11 |
| Recurrence in the 3D-IGBT group | Intestinal perforation (No) | Intestinal perforation (Yes) | Total |
|---|---|---|---|
| b. Gastrointestinal tract | |||
| With bevacizumab | 2 | 3 | 5 |
| Without bevacizumab | 5 | 1 | 6 |
| Total | 7 | 4 | 11 |
IMRT
Eight patients were treated with IMRT or SIB-IMRT. Especially, seven of the eight patients were of Stage IIIb while the other patient was of Stage IIb. We confirmed the presence of uterine and cervical tumors within the planning target volume by daily cone beam CT. No adverse effects of acute gastroenteritis above G3, such as diarrhea, were observed. Although the follow-up period was short, as it started in 2020, no bowel perforation was observed.
Discussion
In this study, we confirmed that the radiation dose can be escalated and that it shows good LC with 3D-IGBT. The 2/3-year LC rate was 94.2%/94.2% in the 3D-IGBT group. The previous study in RetroEMBRACE, a multicenter cohort study, reported that the overall 3/5 year LC rate was 91%/89% [8]. Our results were consistent, although it was still a short follow-up period. The global standard CTV-HR prescription dose is > 85 Gy [12], but the prescription dose in this study was low. Previous studies by Toita et al. [13, 14] have demonstrated that CCRT with a low cumulative RT dose schedule achieved comparable outcomes to global dose schedules. Our study also included cases treated with doses similar to those reported by Toita et al. If necessary, we referred patients to receive a post-treatment diffusion-weighted magnetic resonance imaging (DW-MRI) and gynecological examination for additional BT.
Evaluation of local recurrence immediately after RT can sometimes be difficult. Kalash et al. reported that patients at risk for persistent disease may be accurately diagnosed by DW-MRI and positron emission tomography/computed tomography [15]. Several studies have demonstrated the usefulness of the apparent diffusion coefficient in DW-MRI [16, 17].
A Cochrane meta-analysis of several trials performed showed that CCRT was associated with a 6% improvement in 5-year survival compared with RT alone [18]. However, these groups also reported that more advanced stages had less of a CTx benefit. In contrast, according to a randomized clinical trial by Shrivastava et al., CCRT using weekly cisplatin compared with definitive RT alone still elicited a beneficial response among patients with LACC with Stage IIIb, with an absolute benefit of 8.5% in DFS and 8% in OS [19]. A previous study by Schmid et al. also reported that decreasing the number of cycles of cisplatin may increase DM in patients with Stage IIIb–IVa tumors or positive lymph nodes [20] and that the frequency of CTx during RT was considered to be the most important factor for DM. In our study, the cases in the 3D-IGBT group received a greater number of CTx treatments. Although the difference was not significant, we observed improved DMFS in the 3D-IGBT group over the 2D-IGBT group. PFS was significantly better in the 3D-IGBT group due to improved LC and DMFS.
There was a relatively higher incidence of GI tract AEs > G3. Reduction in this number is expected by 3D-IGBT in the future, as reported by Charra–Brunaud et al. [21]. However, in our study, the 2/3 year rates of GI tract AEs > G3 were 12.4%/16.5% in the 2D-IGBT group and 10.3%/14.0% in the 3D-IGBT group, respectively. GI toxicity was not reduced, even in the 3D-IGBT group. Four patients in the 3D-IGBT group had intestinal perforation, three of whom had been previously treated with bevacizumab. In recent years, the usefulness of bevacizumab for advanced cervical cancer and its combination with an immune checkpoint inhibitor has also been reported [22, 23], and a further increase in treatment opportunities is expected. In the future, caution should be exercised with this combination therapy.
In some cases, much better outcomes may be obtained by treatment with IMRT, as reported by Gandhi et al. [24] and Yeung et al. [25]. We would like to use modern techniques, such as IMRT, to further reduce side effects. Currently, we have started curative treatment of cervical cancer using IMRT and 3D-IGBT as a prospective study. By lowering the dose in the OARs, we aim to reduce the development of severe late adverse effects.
Conclusion
The 2/3-year LC of the 3D-IGBT group was excellent and PFS also tended to improve. Care should be taken with the concomitant use of bevacizumab after radiotherapy.
Acknowledgements
This work was supported by JSPS KAKENHI [Grant number JP17K10481].
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [TK], [ST], [MA], and [TN]. The first draft of the manuscript was written by [TK] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For retrospective studies using data from non-patient identifiable databases, the review committee/ethics committee will waive the need to obtain consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society, Cancer Facts & Figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/special-section-cancer-in-adolescents-and-young-adults-2020.pdf (Accessed 10 2020) [DOI] [PMC free article] [PubMed]
- 3.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 4.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 5.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 6.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a gynecologic oncology group and southwest oncology group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 7.Peters WA, III, Liu PY, Barrett RJ, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation cisplatin-based radiotherapy and chemotherapy therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 8.Sturdza A, Pötter R, Fokdal LU, Haie-Meder C, Tan LT, Mazeron R, et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120:428–433. doi: 10.1016/j.radonc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Pötter R, Haie-Meder C, Van Limbergen E, Barillot E, Brabandere MD, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Ohno T, Wakatsuki M, Toita T, Kaneyasu Y, Yoshida K, Kato S, et al. Recommendations for high-risk clinical target volume definition with computed tomography for three-dimensional image-guided brachytherapy in cervical cancer patients. J Radiat Res. 2017;58:341–350. doi: 10.1093/jrr/rrw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Common Terminology Criteria for Adverse Events (CTCAE). Protocol Development. CTEP. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.html. (Accessed Date 10 2020)
- 12.Tanderup K, Fokdal LU, Sturdza A, Haie-Meder C, Mazeron R, Van Limbergen E, et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol. 2016;120:441–446. doi: 10.1016/j.radonc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Toita T, Kato S, Niibe Y, Ohno T, Kazumoto T, Kodaira T, et al. Prospective multi-institutional study of definitive radiotherapy with high-dose-rate intracavitary brachytherapy in patients with nonbulky (<4-cm) stage I and II uterine cervical cancer (jarog0401/jrosg04-2) Int J Radiation Oncol Biol Phys. 2012;82:e49–56. doi: 10.1016/j.ijrobp.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Toita T, Kitagawa R, Hamano T, Umayahara K, Hirashima Y, Aoki Y, et al. Phase II study of concurrent chemoradiotherapy with high-dose-rate intracavitary brachytherapy in patients with locally advanced uterine cervical cancer: efficacy and toxicity of a low cumulative radiation dose schedule. Gynecol Oncol. 2012;126:211–216. doi: 10.1016/j.ygyno.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Kalash R, Glaser SM, Rangaswamy B, Horne ZD, Kim H, Houser C, et al. Use of functional magnetic resonance imaging in cervical cancer patients with incomplete response on positron emission tomography/computed tomography after image-based high-dose-rate brachytherapy. Int J Radiation Oncol Biol Phys. 2018;102:1008–1013. doi: 10.1016/j.ijrobp.2018.01.092. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, Kajitani S, Joja I, Haruma T, Fukushima C, Kusumoto T, et al. The posttreatment mean apparent diffusion coefficient of primary tumor is superior to pretreatment ADC mean of primary tumor as a predictor of prognosis with cervical cancer. Cancer Med. 2013;2:519–525. doi: 10.1002/cam4.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho JC, Fang P, Cardenas CE, Mohamed AS, Fuller CD, Allen PK, et al. Volumetric assessment of apparent diffusion coefficient predicts outcome following chemoradiation for cervical cancer. Radiother Oncol. 2019;135:58–64. doi: 10.1016/j.radonc.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: Individual patient data meta-analysis. Cochrane Database Syst Rev. 2010;1:CD008285. doi: 10.1002/14651858.CD008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrivastava S, Mahantshetty U, Engineer R, Chopra S, Hawaldar R, Hande V, et al. Cisplatin chemoradiotherapy vs radiotherapy in FIGO stage IIIB squamous cell carcinoma of the uterine cervix a randomized clinical trial. JAMA Oncol. 2018;4:506–513. doi: 10.1001/jamaoncol.2017.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid MP, Franckena M, Kirchheiner K, Sturdza A, Georg P, Dörr W, et al. Distant metastasis in patients with cervical cancer after primary radiotherapy with or without chemotherapy and image guided adaptive brachytherapy. Gynecol Oncol. 2014;133:256–262. doi: 10.1016/j.ygyno.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Charra-Brunaud C, Harter V, Delannes M, Haie-Meder C, Quetin P, Kerr C, et al. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: results of the French STIC prospective study. Radiother Oncol. 2012;103:305–313. doi: 10.1016/j.radonc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (gynecologic oncology group 240) Lancet. 2017;390:1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 24.Gandhi AK, Sharma DN, Rath GK, Julka PK, Subramani V, Sharma S, et al. Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: a prospective randomized study. Int J Radiation Oncol Biol Phys. 2013;87:542–548. doi: 10.1016/j.ijrobp.2013.06.2059. [DOI] [PubMed] [Google Scholar]
- 25.Yeung AR, Pugh SL, Klopp AH, Gil KM, Wenzel L, Westin SN, et al. Improvement in patient-reported outcomes with intensity-modulated radiotherapy (RT) compared with standard RT: a report from the NRG Oncology RTOG 1203 study. J Clin Oncol. 2020;38:1685–1692. doi: 10.1200/JCO.19.02381. [DOI] [PMC free article] [PubMed] [Google Scholar]