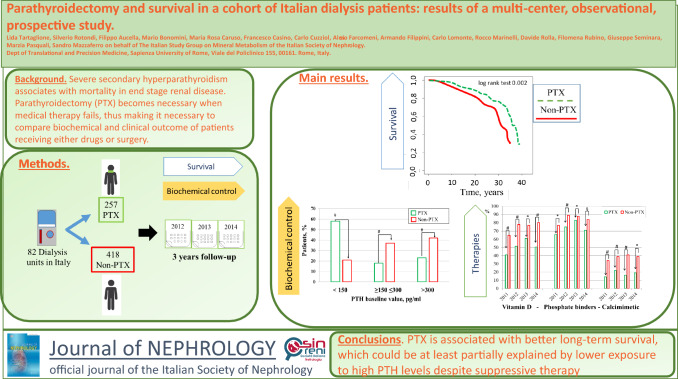
Abstract
Background
Severe secondary hyperparathyroidism (SHPT) is associated with mortality in end stage kidney disease (ESKD). Parathyroidectomy (PTX) becomes necessary when medical therapy fails, thus highlighting the interest to compare biochemical and clinical outcomes of patients receiving either medical treatment or surgery.
Methods
We aimed to compare overall survival and biochemical control of hemodialysis patients with severe hyperparathyroidism, treated by surgery or medical therapy followed-up for 36 months. Inclusion criteria were age older than 18 years, renal failure requiring dialysis treatment (hemodialysis or peritoneal dialysis) and ability to sign the consent form. A control group of 418 patients treated in the same centers, who did not undergo parathyroidectomy was selected after matching for age, sex, and dialysis vintage.
Results
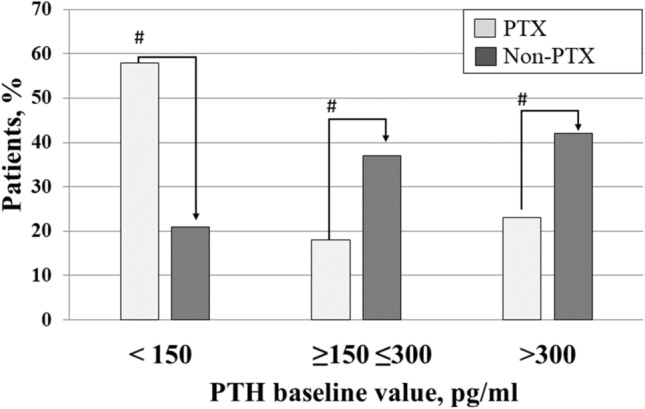
From 82 Dialysis units in Italy, we prospectively collected data of 257 prevalent patients who underwent parathyroidectomy (age 58.2 ± 12.8 years; M/F: 44%/56%, dialysis vintage: 15.5 ± 8.4 years) and of 418 control patients who did not undergo parathyroidectomy (age 60.3 ± 14.4 years; M/F 44%/56%; dialysis vintage 11.2 ± 7.6 y). The survival rate was higher in the group that underwent parathyroidectomy (Kaplan–Meier log rank test = 0.002). Univariable analysis (HR 0.556, CI: 0.387–0.800, p = 0.002) and multivariable analysis (HR 0.671, CI:0.465–0.970, p = 0.034), identified parathyroidectomy as a protective factor of overall survival. The prevalence of patients at KDOQI targets for PTH was lower in patients who underwent parathyroidectomy compared to controls (PTX vs non-PTX: PTH < 150 pg/ml: 59% vs 21%, p = 0.001; PTH at target: 18% vs 37% p = 0.001; PTH > 300 pg/ml 23% vs 42% p = 0.001). The control group received more intensive medical treatment with higher prevalence of vitamin D (65% vs 41%, p = 0.0001), calcimimetics (34% vs 14%, p = 0.0001) and phosphate binders (77% vs 66%, p = 0.002).
Conclusions
Our data suggest that parathyroidectomy is associated with survival rate at 36 months, independently of biochemical control. Lower exposure to high PTH levels could represent an advantage in the long term.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40620-023-01658-0.
Keywords: Parathyroidectomy, Hemodialysis, Mortality, CKD-MBD
Introduction
Secondary hyperparathyroidism (SHPT) in end-stage renal disease (ESRD) is associated with disturbances in mineral metabolism, metabolic bone disease and renal osteodystrophy, bone fractures, vascular calcifications [1–4] and the eventual increase of cardiovascular disease and mortality. Conventional treatment of SHPT with phosphate binders, vitamin D receptor activators (VDRAs) and calcimimetics [5–7] may not allow adequate biochemical control, and parathyroidectomy (PTX) is still recommended in severe cases failing to respond to medical therapy [8].
Parathyroidectomy rapidly lowers parathyroid hormone (PTH) serum levels with improvement of serum calcium and phosphate control, and has potentially favorable effects on cardiovascular survival. Indeed, a lower risk of mortality is reported when all three standard biochemical indicators of metabolic control (namely Ca, P and PTH) reach the target levels recommended by K-DOQI at least once [9]. However, targeting all three biomarkers is not easily accomplished after PTX [10–12]. In fact, in the long term after surgery, hypoparathyroidism is frequent and both low and high levels of PTH are associated with increased cardiovascular morbidity and mortality, in a typically U-shaped modality [13]. Notwithstanding, available observational studies in hemodialysis (HD) patients describe reduced all-cause and cardiovascular mortality rates after PTX in the long term[14–17], apparently regardless of sub-optimal biochemical control. The pathophysiological link between PTX and improved survival is not clear but may include the reported effects of PTH on left ventricular hypertrophy, blood pressure control, erythropoietin-resistant anemia, nutritional status and humoral and/or cellular immunity, independently of calcium and phosphate control and of prescribed specific therapies [18–21]. Regrettably, prospective, randomized-controlled trials comparing the mortality rates of HD patients receiving either medical or surgical therapy for severe SHPT are not available, and will never be carried out due to ethical issues [22]. Therefore, observational studies, despite suffering selection bias are still the main source of data that provide information on the relationship between PTX, biochemical control and mortality rates in HD patients. This paper reports the results of a multicenter, observational, prospective cohort study aimed at evaluating the impact of PTX on survival in an Italian cohort of HD patients.
Methods
Study population and data collection
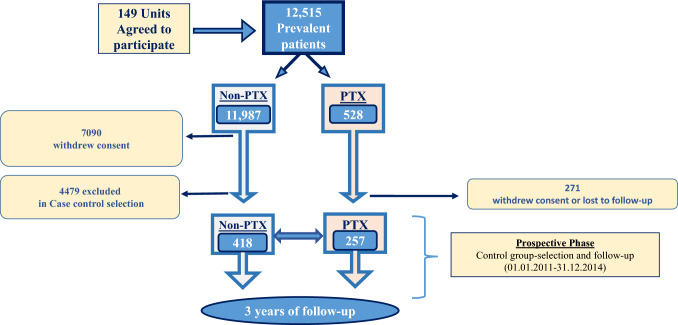
In this paper, we report the prospective, observational part of a multicenter cohort study on PTX that involved 149 Italian dialysis Units, whose protocol was approved by the Ethics Committee of the Policlinico Umberto I in Rome (prot. N° 888/09), and whose baseline data have already been published [12]. Briefly, in this study, inclusion criteria were age older than 18 years, renal failure requiring dialysis treatment (hemodialysis or peritoneal dialysis) and ability to sign the consent form. Data from each Unit of the 528 enrolled patients with PTX history were provided by a referent physician who recorded medical history, timing of PTX, type of surgery, laboratory data, and prescribed SHPT medications in a dedicated data sheet. In addition, information about sex, age and dialysis vintage of all 12,515 patients receiving treatment in the involved Units provided a population from which a control group could be selected.
Follow-up data
Further to the baseline descriptive phase, the protocol also included a prospective observational follow-up, lasting three years, which, however, did not include 67 units. Thus, as schematically reported in Fig. 1, for the follow up phase of the study (the results of which are reported in this paper) we had 257 PTX patients and 4897 controls, among whom we selected, in 2011, 418 non-PTX cases that were similar in terms of age, sex, and dialysis vintage to the study group. Clinical and therapeutic updates were then collected prospectively for the selected patients for three consecutive years (from 01.01.2012 to 31.12.2014). We recorded fatal events from any cause, prescribed medications for SHPT control (vitamin D and calcium-based therapies, calcimimetics, phosphate binders) and laboratory data pertinent to mineral metabolism (PTH, calcium and phosphate) during the three years of follow-up.
Fig. 1.
Flowchart for the study population. Abbreviations: PTX parathyroidectomy; PTH Parathyroid hormone; Ca calcium; P phosphate
The study complied with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Endpoints
The primary endpoint was the overall survival rate of PTX and non-PTX patients during the 36 months of follow-up.
The secondary endpoint was the prevalence of patients reaching biochemical targets for mineral metabolism, as defined by the K-DOQI ranges for Ca, P and PTH [9], in the two groups of PTX and non-PTX patients.
Statistical analysis
Data are expressed as mean ± SD for variables with a Gaussian distribution or median [25–75th percentiles] when the distribution was non Gaussian. We used the Kolmogorov–Smirnov test to evaluate normality of continuous measurements. Parametric tests, chi squared test for qualitative and t-test for quantitative variables, were used to compare measurements between the groups. When the normality assumption was not tenable, Mann–Whitney was used to test for significant differences. All tests were two-tailed and (adjusted) P-values < 0.05 were considered as statistically significant. When general r-by-c contingency tables yielded statistical significance, we proceeded to the evaluation of two-by-two sub-tables of interest. In that case, significance levels were Bonferroni-adjusted by multiplication by the number of two-by-two tables evaluated. The family-wise significance level was fixed at 5%, so that a Bonferroni adjusted p value below 0.05 was considered as statistically significant after taking into account multiplicity. The time-to-event outcomes association in PTX and non-PTX patients was evaluated starting from the date of hemodialysis inception, through stratified Kaplan–Meier curves and associated log-rank tests and/or univariable Cox regression models. As multivariable analyses, we used Cox regression models, where the final set of predictors was selected by means of forward selection based on Akaike Information Criterion. We further evaluated the effect of PTX through a propensity-score matched analysis. First we estimated the probability of obtaining the treatment based on gender, age, diabetes, albumin and hemoglobin levels. One-to-one matching was then performed based on the estimated propensity score, and the matched subset was used in a Cox regression model for estimation of the Average Treatment effect for the Treated (under selection-on-observables assumptions). Balance was evaluated through Standardized Mean Differences (SMD), where an SMD < 10% indicated a good balance.
Analyses were performed using the open source software package R version 4.2.1.
Results
Patient characteristics
Table 1 describes the main clinical and biochemical characteristics of the PTX and non-PTX groups which were similar with regard to age and sex distribution, but different concerning dialysis vintage (PTX = 15.5 ± 8.4 vs non-PTX = 11.2 ± 7.6 years, p < 0.0001) (Table 1). Patients in the PTX group, who underwent surgery on average 8 years after dialysis inception, showed a higher prevalence of glomerular diseases and tubulointerstitial nephropathies, as compared to the non-PTX group (Table 1). History of comorbidities did not differ between the two groups, in particular regarding the incidence of cardiovascular diseases (peripheral vascular disease, ischemic heart disease, and/or heart failure). In addition, no difference was observed in the prevalence of arterial hypertension (identified as current antihypertensive drug prescription), while diabetes was less frequently in PTX patients (6% vs 14%, p = 0.002) (Table 1).
Table 1.
Baseline characteristics in PTX and non-PTX patients
| PTX | Non-PTX | P | |
|---|---|---|---|
| Total Patients | 257 | 418 | |
| Age, years | 58.2 ± 12.8 | 60.3 ± 14.4 | 0.057 |
| Dialysis vintage, y | 15.5 ± 8.4 | 11.2 ± 7.6 | < .0001 |
| Female, % | 56 | 56 | 1 |
| Male, % | 44 | 44 | 1 |
| Causes of ESRD ( %) | |||
| Glomerular diseases | 43 | 30 | < .0001 |
| Tubulointerstitial nephropathies | 12 | 6.4 | < .0001 |
| Nephroangiosclerosis | 10 | 15 | 0.025 |
| ADPKD | 10 | 13.8 | 0.07 |
| Uncertain ESRD etiology | 25 | 34.8 | 0.001 |
| Comorbidities (%) | |||
| Arterial hypertension | 44 | 46 | 0.624 |
| Diabetes | 6 | 14 | 0.002 |
| Peripheral vascular disease, | 13 | 12 | 0.852 |
| Ischemic heart disease | 14 | 12 | 0.246 |
| Heart failure | 7 | 9 | 0.545 |
| Dyslipidemia | 32 | 30 | 0.644 |
| Laboratory tests | |||
| Ca, mg/dl | 8.79 ± 0.67 | 9.04 ± 0.68 | < .0001 |
| Phosphate, mg/dl | 4.98 ± 1.42 | 5.11 ± 1.34 | 0.294 |
| PTH, pg/ml | 102.0 (17.2–337.15) | 250.0 (163.0–400) | < .0001 |
| Albumin, gr/dl | 3.85 ± 0.52 | 3.69 ± 0.45 | < .0001 |
| Hemoglobin, gr/dl | 11.5 ± 1.0 | 11.6 ± 0.8 | 0.651 |
Data are shown as mean ± standard deviation, median (IQR) or percentage. Chi-squared test was used to test for any significant differences between qualitative variables. T-test or Mann–Whitney were used to test for any significant differences between quantitative variables
Abbreviations: D dialysis; PTX Parathyroidectomy; ESRD end stage renal disease; Ca calcium; P phosphate; PTH parathyroid hormone, ADPKD autosomal dominant polycystic kidney
Survival analysis
The survival curves in the two groups of patients, evaluated from the date of hemodialysis inception, clearly show the long-term lower mortality rate of the PTX group (Kaplan–Meier log rank test = 0.002 Fig. 2). This result was also confirmed when the survival curves were considered from the beginning of the three years of follow-up (Kaplan–Meier log rank test = 0.023, Fig. 1 supplemental material).
Fig. 2.
Overall survival, PTX vs. Non-PTX. Time 0 was the date of starting hemodialysis treatment. Kaplan–Meier log rank test = 0.002
Biochemical control and effect of therapies
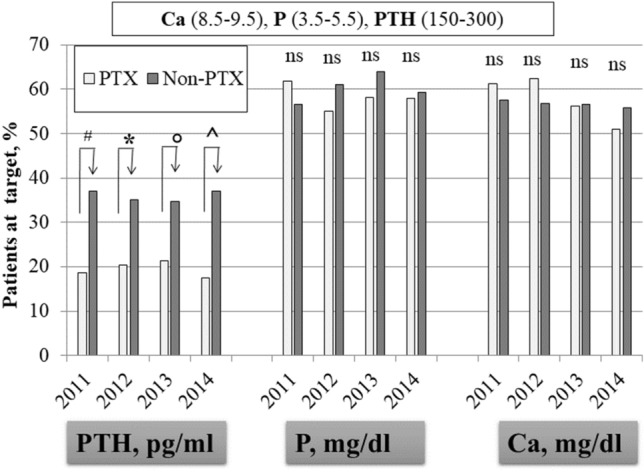
Serum calcium (8.79 ± 0.67 vs 9.04 ± 0.68 mg/dl, p < 0.0001) and intact PTH (102.0, IQR: 17.2–337.1 vs 250, IQR: 163–400 pg/ml, p < 0.0001) were significantly lower in the PTX compared to the non-PTX group of patients, while phosphate was not different (Table 1). We then compared biochemical values, therapies and any-cause mortality in the two groups during the three consecutive years of follow-up. During the observation period, fifty-four patients (21%) in the PTX group and 181 (43%) in the non-PTX group were lost to follow-up, leaving 203 and 237 cases, respectively, for comparison. As illustrated in Fig. 3, at baseline, the prevalence of cases below, at target or above the PTH KDOQI targets was different between the two groups. In particular the percentage of patients below 150 was higher in the PTX group (59 vs 21%, p = 0.001), while the percentage at target or above was significantly higher in the non-PTX group (18 vs 37%; p < 0.0001and 23 vs 42%, p = 0.0001, respectively). Notably, during follow-up, these differences were systematically confirmed (Fig. 4). No difference was evident for serum calcium and phosphate (Fig. 4). As for SHPT therapies, the non-PTX group received more aggressive treatment characterized by significantly higher prescriptions of vitamin D, phosphate binders and calcimimetics (Fig. 5). Moreover, PTX patients received more calcitriol and calcium-based phosphate binders than the control group, which, by comparison, received more vitamin D receptor activators and non-calcium-based phosphate binders (Table 2). Univariate analysis carried forward in the population of patients as a whole and adjusted for dialysis vintage, identified PTX as a protective factor for overall survival (Table 3; p = 0.002). Similarly, higher serum albumin (p = 0.0001), higher hemoglobin levels (p = 0.0001) and younger age (p = 0.0001) were associated with better survival. As reported in Table 3, multivariable analysis confirmed PTX as an independent factor of better survival while age and dialysis vintage were associated with worse outcome. On a subset of propensity-score matched patients, well balanced for sex, age, dialysis vintage, diabetes, albumin, hemoglobin, Calcium, Phospahte, PTH and therapies (Table 4), PTX was confirmed to be a protective factor for overall survival (HR: 0.404 [0.254–0.643]; p = 0.00132).
Fig. 3.
Baseline percentage of patients distributed according to PTH K-DOQI target values.#PTX vs Non-PTX X2, p = 0.0001. Abbreviation: Parathyroid hormone
Fig. 4.
Patients at K-DOQI targets for calcium, phosphate and PTH during follow-up #PTX vs Non-PTX X2 p = 0.0001 *PTX vs Non-PTX X2 p = 0.0002; °PTX vs Non-PTX X2 p = 0.0025; ^PTX vs Non-PTX X2 p = 0.0003. Abbreviation: PTH Parathyroid hormone; Ca calcium; P phosphate
Fig. 5.
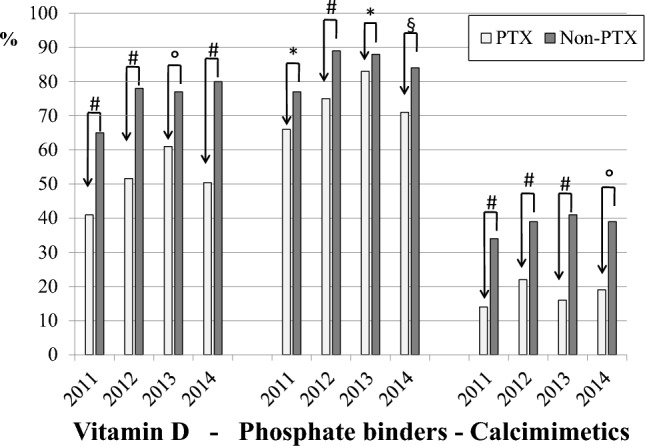
Therapies during follow-up. Vitamin D includes calcitriol, intravenous Vitamin D Receptor Activators and other forms. Phosphate binders include both calcium and non calcium based binders. #X2 p<0.0001; * X2 p= 0.002; X2 p = 0.0005; $ X2 p= 0.01
Table 2.
Prevalence of drug prescriptions during follow-up
| PTX | Non-PTX | P | |
|---|---|---|---|
| Baseline | |||
| Total patients | 257 | 418 | |
| Phosphate binders, % | 66 | 77 | 0.002 |
| Calcium binders, % | 66 | 36 | < .0001 |
| Non calcium based binders, % | 85 | 99 | < .0001 |
| Vitamin D, % | 41 | 65 | < .0001 |
| Calcitriol (p.o + e.v), % | 58 | 44 | 0.0039 * |
| Paricalcitol, % | 44 | 68 | 0.0384* |
| Other, % | 12 | 18 | 0.1313* |
| Cinacalcet, % | 14 | 34 | < .0001 |
| First-year follow-up | |||
| Total patients | 221 | 295 | |
| Phosphate binders, % | 75 | 89 | < .0001 |
| Calcium binders, % | 67 | 41 | 0.001 |
| Non calcium based binders, % | 80 | 87 | 0.001 |
| Vitamin D, % | 52 | 68 | < .0001 |
| Calcitriol (p.o + e.v), % | 57 | 30 | < .0001* |
| Paricalcitol, % | 43 | 62 | 0.036* |
| Other, % | 11 | 25 | 0.027* |
| Cinacalcet, % | 22 | 39 | < .0001 |
| Second-year follow-up | |||
| Total patients | 183 | 230 | |
| Phosphate binders, % | 83 | 88 | 0.001 |
| Calcium based binders, % | 68 | 35 | < 0.0001 |
| Non calcium based binders, % | 77 | 84 | < 0.0001 |
| Vitamin D, % | 61 | 77 | 0.0006 |
| Calcitriol (p.o + e.v), % | 57 | 25 | < .0001* |
| Paricalcitol, % | 48 | 67 | 0.018 * |
| Other, % | 16 | 29 | 0.060* |
| Cinacalcet, % | 16 | 41 | < .0001 |
| Third-year follow-up | |||
| Total patients | 132 | 144 | |
| Phosphate binders, % | 71 | 84 | 0.014 |
| Calcium based binders, % | 62 | 33 | 0.0143 |
| Non calcium based binders, % | 91 | 92 | 0.0143 |
| Vitamin D, % | 50 | 80 | < .0001 |
| Calcitriol (p.o + e.v), % | 61 | 21 | < .0001* |
| Paricalcitol, % | 28 | 75 | < .0001 * |
| Other, % | 36 | 27 | 0.9684* |
| Cinacalcet, % | 19 | 39 | 0.0004 |
Data are shown as percentage. Chi-squared test was used to test for significant differences between groups
*Bonferroni p-adjusted
Abbreviations: PTX parathyroidectomy
Table 3.
Univariate and multivariate survival analyses
| Univariate analysis | ||||
|---|---|---|---|---|
| Variable | HR | CI, Low | CI, Up | p |
| PTX | 0.556 | 0.387 | 0.800 | 0.002 |
| Age, years | 1.047 | 1.032 | 1.062 | 0.0001 |
| Gender (Male) | 0.799 | 0.572 | 1.116 | 0.189 |
| Ca, mg/dl | 1.269 | 0.974 | 1.654 | 0.078 |
| Phosphate, mg/dl | 0.950 | 0.891 | 1.100 | 0.491 |
| PTH, pg/ml | 1.000 | 0.999 | 1.001 | 0.966 |
| Albumin, g/dl | 0.372 | 0.249 | 0.555 | 0.0001 |
| Hb, g/dl | 0.672 | 0.558 | 0.810 | 0.0001 |
| Multivariate analysis | ||||
| PTX | 0.6719 | 0.4652 | 0.9706 | 0.034 |
| Dialysis vintage, years | 1.0345 | 1.0143 | 1.0551 | 0.0001 |
| Age, years | 1.0438 | 1.0286 | 1.0592 | 0.0001 |
Abbreviations: PTX Parathyroidectomy; Ca calcium; P phosphate; PTH parathyroid hormone; Hb hemoglobin; CI confidence interval
Table 4.
Balance measures pre- and post- matching
| Variable | Pre–matching SMD | Post–matching SMD |
|---|---|---|
| Age, years | – 0.1734 | 0.0432 |
| Gender (Male) | – 0.0012 | – 0.0748 |
| Dialysis vintage, years | – 0.4237 | – 0.0394 |
| Diabetes | – 0.2985 | – 0.0610 |
| Albumin, g/dl | 0.3369 | 0.0074 |
| Hb, g/dl | 0.0619 | – 0.0705 |
| Ca, mg/dl | – 0.3366 | 0.0531 |
| Phosphate, mg/dl | – 0.1489 | – 0.0419 |
| PTH, pg/ml | – 0.5105 | 0.0659 |
| Cinacalcet | – 0.5822 | 0.0254 |
| Phosphate binders | ||
| Calcium based binders | 0.5411 | 0.0841 |
| Non calcium based binders | – 0.2777 | 0.0489 |
| Vitamin D | ||
| Calcitriol | 0.5179 | – 0.0111 |
| Paricalcitol | – 0.8194 | – 0.0066 |
| Other | – 0.4859 | 0.0262 |
Threshold for well-balanced variable: > .1
Abbreviations SMD standardized mean difference; Hb hemoglobin; Ca calcium; P phosphate; PTH parathyroid hormone
Discussion
The main result of our study is that PTX was associated with a better survival rate in our HD population of 257 PTX patients compared with 418 matched non-PTX patients, prospectively followed-up for three years. This result was confirmed in both univariable and multivariable-adjusted survival analyses (Table 3). Interestingly, the percentage of patients with adequate calcium and phosphate control did not differ between the PTX and non-PTX groups, while PTH levels were less frequently at target during the three years of follow-up in the PTX group (Fig. 4). In particular, during the three years of follow-up, the PTX group was mostly and invariably exposed to very low PTH levels. In our study, given the time frame, we used the KDOQI target ranges. As a comparison, we also used the more recent KDIGO PTH targets, which confirmed the significant differences between PTX and non-PTX patients (below target: 65 vs 24%, p < 0.0001; at target 30 vs 66%, p < 0.0001; above target 5 vs 10%, p < 0.01. Supplemental material, Fig. 2). Therefore, the association between PTX and a better overall survival rate appears to be independent of the attained biochemical profile. Notably, the similar biochemical control of calcium, phosphate and PTH resulted from a lower prescription of active vitamin D, phosphate binders and calcimimetics in the PTX group (Fig. 5), thus pointing to the role of still poorly known but commonly recognized limitations of the widely employed therapeutic strategies for SHPT. The Kaplan–Meier survival analysis showed better survival rates of the PTX group, in particular in the long term (Fig. 2). Indeed, the PTX group had undergone surgery on average 8 years after hemodialysis inception and the survival curves progressively diverged over time, remaining significant even after 30 years of follow-up. It is also interesting to notice that although multivariable analysis identified dialysis vintage as a risk factor of mortality, the PTX group had better survival, despite a longer dialysis vintage. Overall, our data suggest that PTX has a long-term protective effect on survival in HD patients. Our results are in agreement with the available evidence in the literature showing reduced all-cause and cardiovascular mortality in the long term after PTX [13–15, 21], again independently of the biochemical control of mineral metabolism. We can therefore look back at the concept of PTH as a uremic toxin [23]. In fact, we are aware that PTH may have several extra-mineral negative effects in dialysis patients, spanning from increased left ventricular hypertrophy and higher blood pressure to erythropoietin-resistant anemia and poor nutrition and quality of life [24–26]. In our opinion, it is possible that PTX, by shortening exposure to high PTH levels, reduces the effects of extra-mineral damage.
The main strength of our paper is the multicenter, observational, prospective study design which allows the evaluation of real-life therapeutic strategies. On the other hand, we acknowledge that the enrollment of prevalent PTX hemodialysis patients that underwent surgery during their dialytic history represents a limitation. In fact, enrolling prevalent instead of incident PTX patients carries a number of potential selection biases, as clearly reported in the literature [22]. However, randomized controlled trials comparing patients receiving surgical or medical therapy at the time of surgical indication for severe SHPT do not exist and, most likely, will never be carried out [22]. In conclusion, PTX can be regarded as an effective and safe therapy for refractory SHPT in dialysis patients even though the metabolic control reached after surgery may not be optimal.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was sponsored with an unrestricted grant by AMGEN under the auspices of the Italian Society of Nephrology and the contribution of its Study Group of Trace Elements and Mineral Metabolism. We acknowledge the contribution of the following physicians who provided clinical data: Piemonte. Dr. Bagnis C. MD, Ordine Mauriziano Hospital; Lombardia. Dr. Bisegna S. Melegnano Hospital; Dr. Conte F. Presidio Cernusco sul Naviglio, ASST Melegnano and Martesana; Dr. Camerini C. MD, Ospedali Riuniti di Brescia; Dr. Caruso M.R. MD, Papa Giovanni XXIII Hospital; Prof. Messa P., MD, Maggiore Policlinico Hospital; Dr. Corghi E. MD, Bassini Hospital; Dr. Farina M. MD, Lodi Hospital; Dr. Galassi A. MD, Desio Hospital; Dr. Malberti F. MD, Istituti Ospitalieri di Cremona; Dr. Poiatti P. MD, Dialysis Unit Nuovo Ronco; Dr. Ruggiero P., MD, Bolognini Hospital; Dr. Vezzoli G. MD, San Raffaele Hospital; Prof. Cozzolino M. Nephrology and Dialysis Unit, ASST Santi Paolo e Carlo, Presidio San Paolo; Veneto. Dr. Nordio M. MD, Nephrology and Dialysis Unit, Provincial Hospital, Camposampiero; Dr. Meneghel G., MD, Poli Ospedalieri di Dolo-Noale; Liguria. Dr Chiappini N. MD, Sant’Andrea Hospital; Dr. Icardi A. MD, la Colletta Hospital; Dr. Nordio M Dr. Rolla D., MD, Hospital San Martino; Toscana. Dr. Grimaldi C. NephrologyUnit, Firenze 1 ed Empoli, Florence; Emilia Romagna. Dr. Cianciolo G. MD, Policlinico Sant’Orsola Malpighi; Sardegna. Dr. Casu D. MD, Presidio Ospedaliero di Alghero, Presidio Ospedaliero di San Gavino; Dr. Pilloni D. MD, hospital Sirai; Dr. Scrivano M. MD, Dialysis Unit Nuova Casa di Cura di Decimomannu; Dr. Sini G., MD, dialysis unit Casa di Cura Lay; Dr. Sechi M.A. MD, hospital San Martino; Dr. Contu B. MD, dialysis unit Nostra Signora della Mercede di Lanusei; Dr. Grussu S. MD, Distretto Ospedaliero di Tempio Pausania; Dr. Gazzanelli L. MD, hospital La Maddalena; Dr. Pani A. Division of Nephrology and Dialysis, hospital G. Brotzu; Abruzzo. Dr. Bonomini M. MD, hospital SS Annunziata; Dr. Polidoro M. MD, ASL di Teramo, Atri, Bernabeo, Popoli, Giulianova; Dr. Gaetano Bernabeo hospital Di Vito; Lazio. Dr Pasquali M. M.D. Nephrology Unit, Azienda Policlinico Umberto I; Dr. Onorato L. MD, Casa di Cura Città di Roma; Dr. Leonardi M. MD, Nephrology and Dialysis Unit, Ospedale Santa Maria Goretti, Latina; Dr. Amoroso F., MD, Polo Ospedaliero Dono Svizzero Formia; Dr. Baldinelli M., MD, Dialysis Unit Monte San Biagio; Dr. Morosetti M. Nephrology Unit, G.B. Grassi Hospital; Dr. Boccia E., MD, Dialysis Unit Diaverum Latina; Dr. Chicca S., MD, Hospital Pertini; Dr. Martina P., MD, Dialysis Unit Italian Hospital group; Dr. Di Silva A., MD, Hospital Fiorini; Dr. Ordonez D.A., MD, Dialysis Unit Nephrocare Cer. Lab; Dr. Filippini A. MD, Policlinico Casilino; Dr. Marinelli A. MD, dialysis Unit Clinica Madonna della Fiducia; Dr. Massimetti C. MD, Hospital Belcolle; Prof. Menè P. MD, Azienda Ospedaliera sant’Andrea; Dr. Napoletano I. MD, Hospital Civita Castellana; Dr. De Cicco, Guarnieri Hospital; Dr. Sfregola P. MD, dialysis Unit Casa di cura Madonna delle Grazie; Dr. Retico E. MD, dialysis Unit Diagest; Dr. Bondatti F. MD, Hospital San Benedetto; Dr. Cannula F. MD, Casa di Cura Nomentana Hospital; Dr. Rubino F. MD, Hospital Santissima Trinità; Dr. Cuzziol C. MD, Dialysis Unit Casa di cura ARS Medica; Dr. Di Cicco C. MD, Dialysis Unit Casa di Cura Guarnieri; Dr. Flammini A., MD, Dialysis Unit Diaverum Ladispoli; Dr. Mantella D., MD, Hospital San Camillo Forlanini; Dr. Nacca R. MD, Hospital Civile Santa Scolastica; Dr. Violi F., MD, Hospital Santa Scolastica; Dr. Pulcinelli G., MD, ASL Rieti; Dr. Balducci A., MD, Hospital San Giovanni Addolorata; Dr. Ciccciarelli Dialysis Unit Santissima Trinità Hospital; Marche. Dr. Baldini S., MD, Dialysis Unit IRCCS Istituto Nazionale Riposo e Cura per Anziani; Dr. Martello M., Di Luca M. Nephrology and Dialysis Unit, Azienda Ospedaliera Ospedali Riuniti Marche Nord, Presidio S. Salvatore, Pesaro. Molise. Dr. Brigante M., MD, Hospital Agostino Cardarelli; Umbria. Dr. Nunzi E., MD, Hospital S. Maria Della Misericordia; Puglia. Dr. Aucella F., MD, Dialysis Unit Casa Sollievo della Sofferenza; Dr. Lo Monte C., MD, Hospital Francesco Miulli, UAL Castellaneta; Dr. Magarelli P., MD, ASL Bari, Andria,Trani; Basilicata. Dr. Casino F., MD, Azienda sanitaria locale di Matera; Campania. Dr. D’Apice L., MD, Hospital Sant’Anna e San Sebastiano; Dr. Morrone L., MD, Hospital Gaetano Rummo; Sicilia. Dr. Battaglia G.G., MD, Hospital S. Marta e S.Venera Acireale; Dr. Savica V., MD, Hospital Papardo; Calabria. Dr. Roberti R., MD, Dialysis Unit ASP Cosenza;
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. This study was funded with an unrestricted grant by Amgen.
Declarations
Conflict of interest
SM received honoraria for Congress presentations by Viforpharma and Amgen.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lida Tartaglione and Silverio Rotondi have contributed equally to this work.
Contributor Information
Sandro Mazzaferro, Email: sandro.mazzaferro@uniroma1.it.
on behalf of The Italian Study Group on Mineral Metabolism of the Italian Society of Nephrology:
C. Bagnis, S. Bisegna, F. Conte, C. Camerini, M. R. Caruso, P. Messa, E. Corghi, M. Farina, A. Galassi, F. Malberti, P. Poiatti, P. Ruggiero, G. Vezzoli, M. Cozzolino, M. Nordio, G. Meneghel, N. Chiappini, A. Icardi, M. Nordio, D. Rolla, C. Grimaldi, G. Cianciolo, D. Casu, D. Pilloni, M. Scrivano, G. Sini, M. A. Sechi, B. Contu, S. Grussu, L. Gazzanelli, A. Pani, G. Brotzu, M. Bonomini, M. Polidoro, L. Onorato, M. Leonardi, F. Amoroso, M. Baldinelli, M. Morosetti, E. Boccia, S. Chicca, P. Martina, A. Di Silva, D. A. Ordonez, A. Filippini, A. Marinelli, C. Massimetti, P. Menè, I. Napoletano, P. Sfregola, E. Retico, F. Bondatti, F. Cannula, F. Rubino, C. Cuzziol, C. Di Cicco, A. Flammini, D. Mantella, R. Nacca, F. Violi, G. Pulcinelli, A. Balducci, S. Baldini, M. Martello, M. Di Luca, M. Brigante, E. Nunzi, F. Aucella, C. Lo Monte, P. Magarelli, F. Casino, L. D’Apice, L. Morrone, G. G. Battaglia, V. Savica, and R. Roberti
References
- 1.Torres PA, De Broe M. Calcium-sensing receptor, calcimimetics, and cardiovascular calcifications in chronic kidney disease. KidneyInt. 2012;82:19–25. doi: 10.1038/ki.2012.69. [DOI] [PubMed] [Google Scholar]
- 2.Bover J, Evenepoel P, Urena-Torres P, et al. Pro: Cardiovascular calcifications are clinically relevant. Nephrol Dial Transpl. 2015;30(3):345–351. doi: 10.1093/ndt/gfv020. [DOI] [PubMed] [Google Scholar]
- 3.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am SocNephrol. 2004;15(7):1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 4.Mace ML, Egstrand S, Morevati M, Olgaard K, Lewin E. New insights to the crosstalk between vascular and bone tissue in chronic kidney disease-mineral and bone disorder. Metabolites. 2021;11(12):849. doi: 10.3390/metabo11120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzaferro S, Brancaccio D, Messa P, et al. Management of secondary hyperparathyroidism in Italy: results of the Italian FARO survey. J Nephrol. 2011;24(02):225–235. doi: 10.5301/JN.2010.6193. [DOI] [PubMed] [Google Scholar]
- 6.Komaba H, Ketteler M, Cunningham J, Fukagawa M. Old and new drugs for the management of bone disorders in CKD. Calcif Tissue Int. 2021;108(4):486–495. doi: 10.1007/s00223-020-00788-y. [DOI] [PubMed] [Google Scholar]
- 7.Komaba H, Hamano T, Fujii N, Moriwaki K, Wada A, Masakane I, Nitta K, Fukagawa M. Parathyroidectomy vs cinacalcet among patients undergoing hemodialysis. J Clin Endocrinol Metab. 2022;107(7):2016–2025. doi: 10.1210/clinem/dgac142. [DOI] [PubMed] [Google Scholar]
- 8.Cianciolo G, Tondolo F, Barbuto S, Angelini A, Ferrara F, Iacovella F, Raimondi C, La Manna G, Serra C, De Molo C, Cavicchi O, Piccin O, D’Alessio P, De Pasquale L, Felisati G, Ciceri P, Galassi A, Cozzolino M. A roadmap to parathyroidectomy for kidney transplant candidates. Clin Kidney J. 2022;15(8):1459–1474. doi: 10.1093/ckj/sfac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S202. doi: 10.1016/S0272-6386(03)00905-3. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro S, Pasquali M, Farcomeni A, Vestri AR, Filippini A, Romani AM, Barresi G, Pugliese F. Parathyroidectomy as a therapeutic tool for targeting the recommended NKF-K/DOQI ranges for serum calcium, phosphate and parathyroid hormone in dialysis patients. Nephrol Dial Transpl. 2008;23(7):2319–2323. doi: 10.1093/ndt/gfm931. [DOI] [PubMed] [Google Scholar]
- 11.Cozzolino M, Messa P, Brancaccio D, et al. Achievement of NKF/K-DOQI recommended target values for bone and mineral metabolism in incident hemodialysis patients: results of the FARO-2 cohort. Blood Purif. 2014;38(1):37–45. doi: 10.1159/000365386. [DOI] [PubMed] [Google Scholar]
- 12.Mazzaferro S, Tartaglione L, Cascone C, et al. Multicenter study on parathyroidectomy (PTX) in Italy: preliminary results. J Nephrol. 2018;31(5):767–773. doi: 10.1007/s40620-018-0527-x. [DOI] [PubMed] [Google Scholar]
- 13.Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int. 2004;66(5):2010–2016. doi: 10.1111/j.1523-1755.2004.00972.x. [DOI] [PubMed] [Google Scholar]
- 14.Ivarsson KM, Akaberi S, Isaksson E, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant. 2015;30(12):2027–2033. doi: 10.1093/ndt/gfv334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato S, Ohta M, Kawaguchi Y, et al. Effects of parathyroidectomy on left ventricular mass in patients with hyperparathyroidism. Miner Electrolyte Metab. 1995;21:67–71. [PubMed] [Google Scholar]
- 16.Huang QX, Pang J, Shi CK, Huang XW, Chen XF, Luo YF, An HW, Jian JL, Liu L, Li YL. Impact of parathyroidectomy among nondiabetic hemodialysis patients with severe hyperparathyroidism. Ren Fail. 2022;44(1):1160–1168. doi: 10.1080/0886022X.2022.2098768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danese MD, Fox KM, Duryea JL, Desai P, Rubin RJ. The rate, cost and outcomes of parathyroidectomy in the united states dialysis population from 2016–2018. BMC Nephrol. 2022;23(1):220. doi: 10.1186/s12882-022-02848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rault R. Magnone M (1996) The effect of parathyroidectomy on hematocrit and erythropoietin dose in patients on hemodialysis. ASAIO J (Am Soc Artif Intern Organs) 1992;42(5):M901–M903. doi: 10.1097/00002480-199609000-00123. [DOI] [PubMed] [Google Scholar]
- 19.Yasunaga C, Nakamoto M, Matsuo K, Nishihara G, Yoshida T, Goya T. Effects of a parathyroidectomy on the immune system and nutritional condition in chronic dialysis patients with secondary hyperparathyroidism. Am J Surg. 1999;178(4):332–336. doi: 10.1016/S0002-9610(99)00194-4. [DOI] [PubMed] [Google Scholar]
- 20.Yasunaga C, Matsuo K, Yanagida T, Matsuo S, Nakamoto M, Goya T. Early effects of parathyroidectomy on erythropoietin production in secondary hyperparathyroidism. Am J Surg. 2002;183(2):199–204. doi: 10.1016/S0002-9610(01)00865-0. [DOI] [PubMed] [Google Scholar]
- 21.Lin HC, Chen CL, Lin HS, Chou KJ, Fang HC, Liu SI, Hsu CY, Huang WC, Huang CW, Huang CK, Chang TY, Chang YT, Lee PT. Parathyroidectomy improves cardiovascular outcome in nondiabetic dialysis patients with secondary hyperparathyroidism. Clin Endocrinol (Oxf) 2014;80(4):508–511. doi: 10.1111/cen.12333. [DOI] [PubMed] [Google Scholar]
- 22.Scialla JJ, Wolf M. When there will never be a randomized controlled trial. Kidney Int. 2015;88(2):220–222. doi: 10.1038/ki.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duque EJ, Elias RM, Moysés RMA. Parathyroid hormone: a uremic toxin. Toxins (Basel) 2020;12(3):189. doi: 10.3390/toxins12030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kono K, Fujii H, Watanabe K, Goto S, Nishi S. Relationship between parathyroid hormone and renin-angiotensin-aldosterone system in hemodialysis patients with secondary hyperparathyroidism. J Bone Miner Metab. 2021;39(2):230–236. doi: 10.1007/s00774-020-01139-5. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Komaba H, Fukagawa M. Emerging association between parathyroid hormone and anemia in hemodialysis patients. Ther Apher Dial. 2018;22(3):242–245. doi: 10.1111/1744-9987.12685. [DOI] [PubMed] [Google Scholar]
- 26.Yamada S, Tsuruya K, Kitazono T, Nakano T. Emerging cross-talks between chronic kidney disease-mineral and bone disorder (CKD-MBD) and malnutrition-inflammation complex syndrome (MICS) in patients receiving dialysis. Clin Exp Nephrol. 2022;26(7):613–629. doi: 10.1007/s10157-022-02216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.