Abstract
Background
Our study aimed to build a risk stratification system predicting the progression-free survival (PFS) to classify patients into diverse prognostic subgroups for advanced non-small-cell lung cancer patients treated with PD-(L)1 inhibitor.
Methods
404 patients from our center were enrolled in this study and 70% patients (n = 282) were randomly assigned into the training cohort and other 30% patients (n = 122) into the validation cohort. A testing cohort contained 81 patients from other centers were used to assess the generalizability of model. Cox regression analyses were used to identify the most significant clinical parameters. The model's performance was assessed by using concordance index (C-index), calibration curves, Decision Curve Analyses (DCAs), net reclassification improvement (NRI), integrated discrimination improvement (IDI) analyses, and survival curve.
Results
Five clinical parameters were identified as the most significant predictors by using cox regression. We then integrated them into a Nomogram to Evaluate the relative PFS of ICIs Treatment (NEPIT). The C-index of NEPIT in the training cohort, the validation cohort and testing cohort was 0.789 (95%CI: 0.750–0.828), 0.745 (95%CI: 0.706–0.784), and 0.766 (95%CI: 0.744–0.788), respectively. The calibration curves presented a good congruence between the predictions and actual observations. The Decision Curve Analyses (DCAs) reflected positive net benefits can be obtained for NEPIT. The results from NRI and IDI analyses showed that the NEPIT could improve predictive power of TPS. In addition, the further constructed risk stratification system could effectively categorize patients into different risk subgroups.
Conclusion
The tools developed in this study would have value in guiding the optimal patient selection for precision care.
Keywords: Non-small-cell lung cancer, Risk assessment, Progression-free survival, PD-(L)1 inhibitor, Immunotherapy
Novelty & impact statements
Although the progress of immune checkpoints inhibitors (ICIs) targeting the PD-1/PD-L1 axis has greatly revolutionized the treatment paradigm for advanced non-small-cell lung cancer (NSCLC), the difference in time-to-progression observed in clinical trials suggested high heterogeneity in patients. Hence, a risk stratification system that can reliably classify patients into diverse prognostic subgroups is important to determine high-risk progression patients. Herein, we conducted a thorough explorative analysis in advanced NSCLC patients treated with PD-(L)1 inhibitor by using a 6-year cohort from our institute. A total of 404 patients from our center were enrolled in this study and randomly assigned into the training and validation cohorts (7:3). In addition, a testing cohort contained 81 patients between January 2015 and December 2021 from other centers were used to assess the generalizability of model. We developed an online clinical model, NEPIT, to provide individualized prediction of PFS and a robust risk stratification system for progression evaluation. The NEPIT developed in this study was the primary reported online predictive model based on clinical routine parameters to predict PFS for NSCLC treated with PD-(L)1 inhibitor. The further risk stratification system presented outstanding discriminating power for different risk subgroups identification. The tools developed in this study would have value in guiding the optimal patient selection.
1. Background
The progress of immune checkpoints inhibitors (ICIs) targeting the PD-1/PD-L1 axis has greatly revolutionized the treatment paradigm for advanced non-small-cell lung cancer (NSCLC). Several randomized clinical trials revealed that PD-(L)1 inhibitor could achieve durable and long-term benefits in advanced patients in combination or as a single agent compared with conventional cytotoxic chemotherapy [[1], [2], [3], [4]]. Following these promising results, ICIs were gradually approved in the first-, second-, or later-line setting in the treatment of advanced NSCLC patients. Unfortunately, only 35% patients treated with ICIs could benefit from sustained response and majority of ICIs treated patients still relapse [5]. Most patients develop immune resistance after treatment discontinuation or during treatment and suffer disease progression ultimately [6,7]. Hence, a risk stratification system that can reliably classify patients into diverse prognostic subgroups is important to determine high-risk progression patients.
At present, the consideration of distinct diversity of clinical responses and benefits in lung cancer indicates that NSCLC have unique potential predictors to predict the long-term clinical benefits and the onset of drug resistance. Previous studies indicated that the PD-L1 expression level in tumors could be a better predictor for anti-PD-1/PD-L1 therapy [8,9]. However, many challenges are present in ongoing attempts to apply PD-L1 expression level for therapeutic benefit prediction. First, the role of PD-L1 expression level in treatments outcomes currently is still controversial. Two phase III trials showed that the PD-L1 expression was not correlated with progression-free survival (PFS), overall survival (OS) and response rate in NSCLC, but some studies presented different results [[10], [11], [12]]. Second, it remains unclear which cutoff point is the best value in predicting therapy response. The cutoff values in different clinical trials were diverse, and even there existed different cutoff values in the same study [13,14]. Moreover, the sensitivity and specificity of this method still remains unsatisfactory [15]. Therefore, how to find a way to improve the predictive power of PD-L1 expression level is still an open question.
Nomogram is a predictive statistical model that has been applied to integrate relevant risk factors and evaluate patient's outcomes [16]. As a convenient graphical representation of mathematical function, nomogram is popularly used to treatment stratification and outcome evaluation in NSCLC [17]. However, predictive models including routine clinical data for advanced NSCLC patients treated with PD-(L)1 inhibitor are scarce.
Herein, we conduct a thorough explorative analysis of routinely collected clinical baseline factors from advanced NSCLC patients treated with PD-(L)1 inhibitor at our institute, in an attempt to identify key predictor and build multivariable predictive models for individualized evaluation of PFS. Another cohort from Ziyang hospital was remained as testing cohort to assess the generalizability of model in different population. We also incorporate them into a risk stratification system that can categorize patients into different risk subgroups with distinct survival benefits for precision care.
2. Methods
2.1. Study cohort and patient selection
Between January 2015 and December 2021, patients with advanced NSCLC who underwent antiPD-1/PD-L1 inhibitor treatment in our institution and Ziyang hospital were retrospectively reviewed. The inclusion criteria were listed below: (1) NSCLC confirmed by pathology; (2) stage III and stage IV tumor; (3) treatment with ICIs (antiPD-1/PD-L1 inhibitor) as first or subsequent line-at least one cycle. The exclusion criteria consisted of (1) patients aged 0–18 years; (2) patients with a history of clinical trials or other cancers; (3) patients with missing data about follow-up information. Clinical information of eligible patients were obtained from hospital electronic medical records. Patient's baseline characteristics including age, gender, Eastern Cooperative Oncology Group performance status (ECOG-PS), smoking status, tumor location, pathologic subtype, stage, PD-L1 expression level and treatment information, were collected. We routinely follow patients 3 month 1 times or the patients have a progression symptoms. The endpoint of current study was progression-free survival (PFS) and the diagnosis of PFS for each individual was based on the results of radiographic progression.
2.2. Ethics statement
This study was approved by the ethical review board of West China Hospital of Sichuan University and The First People's Hospital of Ziyang (No. 2021-301). The requirement for written informed consent was waived due to the retrospective nature of the study.
2.3. Definitions
Pathological diagnosis was conducted immunologically and morphologically at our institution and Ziyang hospital. In this study, patients with squamous cell carcinoma or adenocarcinoma were recognized as common pathological subtype, and other pathological subtypes, such as large cell carcinoma, were recognized as uncommon pathological subtype. Monotherapy group refers to patients who only received ICIs monotherapy, and those who received combination therapy, such as platinum-based chemotherapy and ICIs or radiotherapy and ICIs, were recognized as combination group. The PD-L1 expression level in this study was presented by The PD-L1 tumor proportion Score (TPS). The acronym, NEPIT, in this study was the abbreviation for Nomogram to Evaluate the relative PFS of ICIs Treatment and also was the name of our model.
2.4. Descriptive analysis
A total of 404 eligible patients from our center and 81 patients from Ziyang hospital were enrolled in this study. Among 404 individuals from our center, 70% patients were randomly assigned into the training cohort and other 30% patients into the validation cohort by using computer-generated random numbers. Patients from Ziyang hospital (n = 81) were remained as testing cohort. The Pearson's Chi square test was used to compare the baseline characteristics between two groups.
2.5. Development of the clinical predictive model
Using univariable and multivariable Cox regression analyses, we identified the potential predictors to PFS in the training cohort. A forward stepwise manner was applied to perform the multivariable analyses. The hazards ratios (HR) of Cox regression with a corresponding 95% confidence intervals (95% CI) were reported. All predictors were compiled into a composite NEPIT (Nomogram to Evaluate the relative PFS of ICIs Treatment) to forecast the probability of PFS at 3-, 6-, 9-month.
2.6. Evaluation of NEPIT
To maximize the representativeness of NEPIT, bootstrap internal validation in the training cohort and external validation in the validation cohort and testing cohort were performed. We then evaluated the discrimination ability of NEPIT by using concordance index (C-index), and the accuracy of NEPIT by using calibration curves for 3-, 6-, and 9-month PFS with 1000 bootstrap resamples. Additionally, the clinical effects of NEPIT were explored by Decision Curve Analyses (DCAs) and clinical impact curves. Finally, we conducted the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) analyses for the comparison of model's performance between NEPIT and TPS. We also reported a corresponding 95% confidence intervals (95% CI) of NRI and IDI by using 1000 bootstrap resamples for point estimates of performance metrics. The NRI ≥0 and IDI ≥0 suggested NEPIT could improve net benefit as compared with TPS.
2.7. A risk stratification system
In order to construct a risk stratification system, we used NEPIT to compute the total score of risk factors for each participant in the training cohort. We then inputted the patients' total score of risk factors, patients' survival time and patients’ survival status into X-tile software to determine the best cutoff value of the total risk score. Subsequently, the best cut-off point for total risk score was automatically identified by X-tile analysis [17,18]. Using X-tile software, the participants from training cohort were assigned into low-risk (0–134), Intermediate-risk (134–215.4) and high-risk (>215.4) subgroups. Survival curves of PFS in different risk subgroups were evaluated by using Kaplan-Meier method, and the significance assessed by using log-rank test. The values generated by X-tile were then applied to the validation cohort and testing cohort to test the representativeness of model. All analyses were conducted via the R software, version 4.1.0 (R Foundation for Statistical Computing). P-value≤0.05 was recognized as statistically significant.
3. Results
3.1. Patient baseline data
A total of 404 patients from our center and 81 patients from Ziyang hospital who met the eligibility criteria were enrolled in this study. Of patients from our center, the median age was 61 years and the median (25%-75%IQR) PFS was 6.1 (2.9–11.8) months. Among patients from Ziyang hospital, the median age was 62 years and the median (25%-75%IQR) PFS was 6.8 (3.2–12.9) months. Patient demographics and characteristics of patients from two centers are summarized in Table 1.
Table 1.
Summary of patients from two centers. Cohort I: the patients from West China hospital, Cohort II: the patients from Ziyang hospital.
| Cohort I (n = 404) | Cohort II (n = 81) | |
|---|---|---|
| Age | ||
| ≥60 | 223 (55.20%) | 41 (50.62%) |
| <60 | 181 (44.80%) | 40 (49.38%) |
| Gender | ||
| Male | 311 (76.98%) | 68 (83.95%) |
| Female | 93 (23.02%) | 13 (16.05%) |
| ECOG-PS | ||
| 0 | 243 (60.15%) | 51 (62.96%) |
| 1 | 161 (39.85%) | 30 (37.04%) |
| Smoking status | ||
| Never | 58 (14.36%) | 12 (14.81%) |
| Former | 131 (32.42%) | 28 (34.57%) |
| Active | 215 (53.22%) | 41 (50.62%) |
| Location | ||
| Right | 236 (58.42%) | 44 (54.32%) |
| Left | 168 (41.58%) | 37 (45.68%) |
| Pathologic subtype | ||
| Uncommon pathological subtype | 47 (11.63%) | 10 (12.35%) |
| Common pathological subtype | 357 (88.37%) | 71 (87.65%) |
| Stage | ||
| Stage III | 51 (12.62%) | 7 (8.64%) |
| Stage IV | 353 (87.38%) | 74 (91.36%) |
| PDL1:TPS | ||
| <1% | 90 (22.28%) | 16 (19.75%) |
| 1–49% | 107 (26.49%) | 26 (32.10%) |
| ≥50% | 90 (22.28%) | 16 (19.75%) |
| Unknown | 117 (28.96%) | 23 (28.40%) |
| Line of treatments | ||
| 1st | 211 (52.23%) | 48 (59.26%) |
| 2nd | 129 (31.93%) | 23 (28.40%) |
| ≥3rd | 64 (15.84%) | 10 (12.35%) |
| Treatment | ||
| Monotherapy | 159 (39.36%) | 23 (28.40%) |
| Combination | 245 (60.64%) | 58 (71.60%) |
To develop and validate a predictive model, 70% patients (n = 282) from our center were randomly assigned into the training cohort and other 30% patients (n = 122) into the validation cohort. Table 2 compiled patient baseline characteristics in training and validation cohort. The results from Table 2 indicated that the training cohorts shared the similar clinical characteristics with the validation cohorts (P > 0.05). Contrary to patients from our center, all participants from Ziyang hospital were used as a testing set.
Table 2.
Demographics and characteristics of patient in the training and validation cohorts.
| Training cohort (n = 282) | Validation cohort (n = 122) | P-value | |
|---|---|---|---|
| Age | 0.8545 | ||
| ≥60 | 157 (55.67%) | 66 (54.10%) | |
| <60 | 125 (44.33%) | 56 (45.90%) | |
| Gender | 0.3792 | ||
| Male | 221 (78.37%) | 90 (73.77%) | |
| Female | 61 (21.63%) | 32 (26.23%) | |
| ECOG-PS | 0.979 | ||
| 0 | 169 (59.93%) | 74 (60.66%) | |
| 1 | 113 (40.07%) | 48 (39.34%) | |
| Smoking status | 0.5983 | ||
| Never | 39 (13.83%) | 19 (15.57%) | |
| Former | 90 (31.91%) | 41(33.61%) | |
| Active | 153 (54.26%) | 62 (50.82%) | |
| Location | 0.0703 | ||
| Right | 156 (55.32%) | 80 (65.57%) | |
| Left | 126 (44.68%) | 42 (34.43%) | |
| Pathologic subtype | 0.8148 | ||
| Uncommon pathological subtype | 34 (12.06%) | 13 (10.66%) | |
| Common pathological subtype | 248 (87.94%) | 109 (89.34%) | |
| Stage | 0.9742 | ||
| Stage III | 35 (12.41%) | 16 (13.11%) | |
| Stage IV | 247 (87.59%) | 106 (86.89%) | |
| PDL1:TPS | 0.6293 | ||
| <1% | 62 (21.99%) | 28 (22.95%) | |
| 1–49% | 73 (25.89%) | 34 (27.87%) | |
| ≥50% | 60 (21.28%) | 30 (24.59%) | |
| Unknown | 87 (30.85%) | 30 (24.59%) | |
| Line of treatments | 0.4339 | ||
| 1st | 144 (51.06%) | 67 (54.92%) | |
| 2nd | 89 (31.56%) | 40 (32.79%) | |
| ≥3rd | 49 (17.38%) | 15 (12.30%) | |
| Treatment | 0.9999 | ||
| Monotherapy | 111 (39.36%) | 48 (39.34%) | |
| Combination | 171 (60.64%) | 74 (60.66%) |
3.2. Integrated NEPIT model via routine clinical data
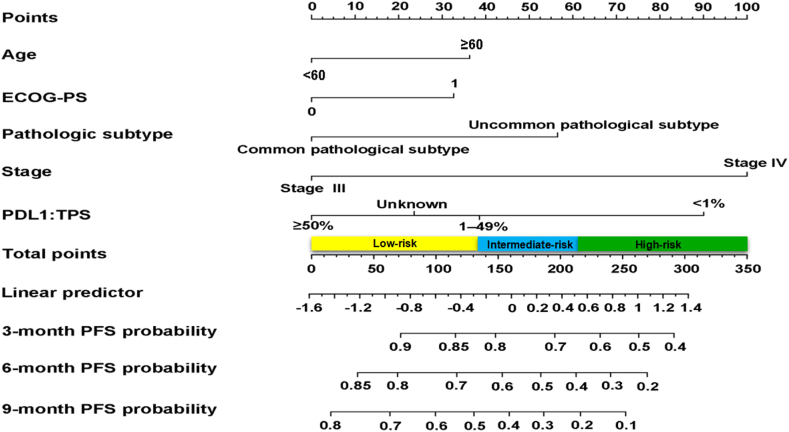
In the training cohort, variables including age, ECOG-PS, pathological subtype, stage and TPS were identified as predictive biomarkers by using the Cox proportional hazards model (Table 3). We further developed a comprehensive clinical predictive model, named as NEPIT, to integrate all predictors (Fig. 1). In NEPIT, each predictor was assigned a corresponding point according to its HR and we could obtain the probability of PFS at 3-, 6-, 9-month for patients by accumulating the total score for each item.
Table 3.
Analyses of predictors of PFS in the training cohort. PFS progression-free survival, HR hazard ratio, CI confidence interval.
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Age | ||||||
| ≥60 | 1 | 1 | ||||
| <60 | 0.75 | 0.58–0.98 | 0.037 | 0.69 | 0.52–0.91 | 0.01 |
| Gender | ||||||
| Male | 1 | 1 | ||||
| Female | 1.29 | 0.94–1.77 | 0.114 | 1.39 | 0.94–2.06 | 0.095 |
| ECOG-PS | ||||||
| 0 | 1 | 1 | ||||
| 1 | 1.45 | 1.11–1.90 | 0.007 | 1.38 | 1.03–1.83 | 0.028 |
| Smoking status | ||||||
| Never | 1 | 1 | ||||
| Former | 0.76 | 0.54–1.08 | 0.083 | 0.86 | 0.61–1.11 | 0.326 |
| Active | 0.85 | 0.65–1.11 | 0.242 | 1.08 | 0.78–1.51 | 0.641 |
| Location | ||||||
| Right | 1 | 1 | ||||
| Left | 0.9 | 0.69–1.17 | 0.414 | 0.9 | 0.68–1.18 | 0.434 |
| Pathologic subtype | ||||||
| Uncommon pathological subtype | 1 | 1 | ||||
| Common pathological subtype | 0.67 | 0.46–0.98 | 0.038 | 0.59 | 0.40–0.87 | 0.009 |
| Stage | ||||||
| Stage III | 1 | 1 | ||||
| Stage IV | 2.68 | 1.63–4.42 | <0.001 | 2.72 | 1.63–4.55 | <0.001 |
| PDL1:TPS | ||||||
| <1% | 1 | 1 | ||||
| 1–49% | 0.57 | 0.39–0.83 | 0.003 | 0.64 | 0.44–0.94 | 0.023 |
| ≥50% | 0.38 | 0.25–0.57 | <0.001 | 0.45 | 0.30–0.68 | <0.001 |
| Unknown | 0.49 | 0.34–0.71 | <0.001 | 0.55 | 0.37–0.81 | 0.003 |
| Line of treatments | ||||||
| 1st | 1 | 1 | ||||
| 2nd | 1.4 | 1.04–1.89 | 0.025 | 1.1 | 0.80–1.50 | 0.57 |
| ≥3rd | 1.47 | 1.01–2.14 | 0.044 | 1.27 | 0.86–1.87 | 0.236 |
| Treatment | ||||||
| Monotherapy | 1 | 1 | ||||
| Combination | 0.77 | 0.59–1.01 | 0.064 | 0.8 | 0.60–1.06 | 0.124 |
Fig. 1.
The established NEPIT for predicting 3-, 6-, 9-month PFS in advanced NSCLC patients treated with PD-(L)1 inhibitor. In NEPIT, five clinical parameters, age, ECOG-PS, pathological subtype, stage and TPS were assigned a score. The sum of these points is located on the ‘Total Points’ axis. The risk group that the patient belongs to could be acquired based on the total score. PFS progression-free survival, NSCLC non-small-cell lung cancer, NEPIT Nomogram to Evaluate the relative PFS of ICIs Treatment, ICIs immune checkpoints inhibitors.
3.3. Evaluation of NEPIT
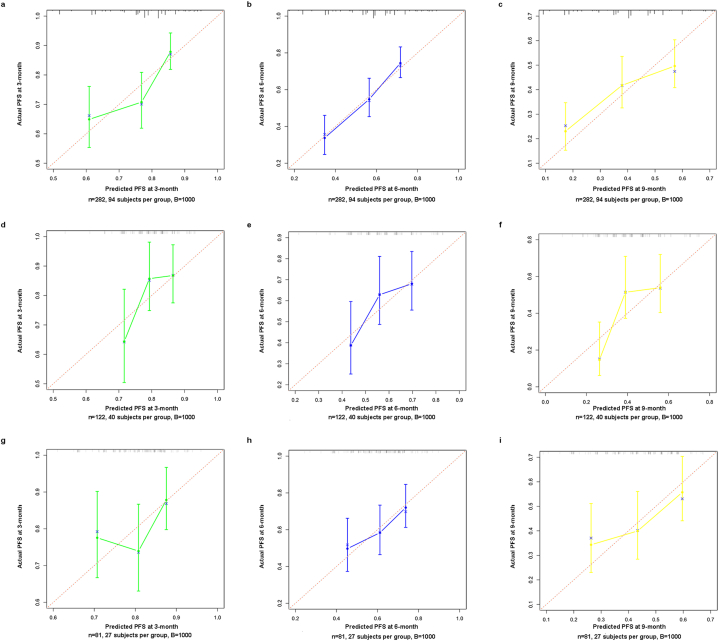
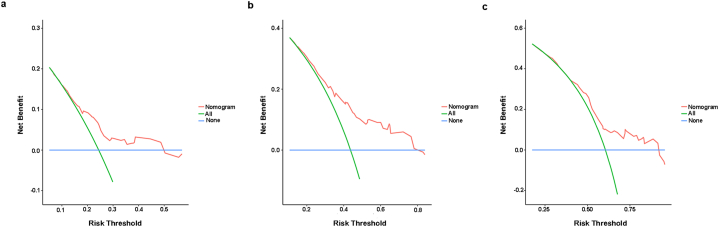
In the training cohort, the C-index of NEPIT in predicting PFS was 0.789 (95%CI: 0.750–0.828). In the validation cohort, the C-index of NEPIT in predicting PFS was 0.745 (95%CI: 0.706–0.784). In the testing cohort, the C-index of NEPIT in predicting PFS was 0.766 (95%CI: 0.744–0.788). The calibration curves showed good congruence in the probability of 3-, 6-, and 9-month PFS between the predicted rates and observation (Fig. 2a–i). The DCAs reflected positive net benefits can be made for NEPIT (Fig. 3a–c). In addition, we calculated the NRI and IDI by comparing the multivariate model, NEPIT, with the predictive model solely using TPS. In the training cohort, the NRI was 0.335 (95%CI: 0.017–0.467) at 3-month, 0.156 (95%CI: 0.031–0.316) at 6-month and 0.103 (95%CI: 0.007–0.248) at 9-month, with the IDI was 0.044 (95%CI:0.015–0.090) at 3-month, 0.072 (95%CI:0.036–0.127) at 6-month and 0.065 (95%CI:0.027–0.131) at 9-month (Table 4). In the validation cohort, the NRI was 0.120 (95%CI: 0.037–0.213) at 3-month, 0.093 (95%CI: 0.012–0.167) at 6-month, and 0.211 (95%CI: 0.184–0.269) at 9-month, with the IDI was 0.030 (95%CI: 0.009–0.111) at 3-month, 0.039 (95%CI: 0.007–0.123) at 6-month, and 0.041 (95%CI: 0.002–0.134) at 9-month (Table 4). In the testing cohort, the NRI was 0.240 (95%CI: 0.021–0.331) at 3-month, 0.132 (95%CI: 0.041–0.286) at 6-month, and 0.165 (95%CI: 0.053–0.276) at 9-month, with the IDI was 0.025 (95%CI: 0.004–0.132) at 3-month, 0.056 (95%CI: 0.013–0.168) at 6-month, and 0.086 (95%CI: 0.034–0.165) at 9-month (Table 4).
Fig. 2.
Calibration curves showing the probability of 3-, 6-, and 9-month PFS between the predicted rates and the actual observation. a-c. Calibration curves in training cohort, d-f. Calibration curves in validation cohort, g-i Calibration curves in testing cohort.
Fig. 3.
Decision curves analysis. a. 3-month PFS DCAs of NEPIT, b. 6-month PFS DCAs of NEPIT, c. 9-month PFS DCAs of NEPIT. The horizontal solid blue line exhibits the hypothesis that no patients experienced the presence of progression, and the solid green line exhibits the hypothesis that all patients met the endpoint. The red line assumes the net benefit of using the NEPIT. NEPIT Nomogram to Evaluate the relative PFS of ICIs Treatment, ICIs immune checkpoints inhibitors, PFS progression-free survival, DCAs Decision Curve Analyses.
Table 4.
Comparison of performance metrics between TPS and NEPIT. NRI, net reclassification improvement. IDI, integrated discrimination improvement. The values between parentheses is 95% confidence interval.
| Training cohort | Validation cohort | Testing cohort | ||||
|---|---|---|---|---|---|---|
| PDL1:TPS | NEPIT | PDL1:TPS | NEPIT | PDL1:TPS | NEPIT | |
| NRI | ||||||
| 3-month | Reference | 0.335 (0.017–0.467) | Reference | 0.120 (0.037–0.213) | Reference | 0.240 (0.021–0.331) |
| 6-month | Reference | 0.156 (0.031–0.316) | Reference | 0.093 (0.012–0.167) | Reference | 0.132 (0.041–0.286) |
| 9-month | Reference | 0.103 (0.007–0.248) | Reference | 0.211 (0.184–0.269) | Reference | 0.165 (0.053–0.276) |
| IDI | ||||||
| 3-month | Reference | 0.044 (0.015–0.090) | Reference | 0.030 (0.009–0.111) | Reference | 0.025 (0.004–0.132) |
| 6-month | Reference | 0.072 (0.036–0.127) | Reference | 0.039 (0.007–0.123) | Reference | 0.056 (0.013–0.168) |
| 9-month | Reference | 0.065 (0.027–0.131) | Reference | 0.041 (0.002–0.134) | Reference | 0.086 (0.034–0.165) |
3.4. The web server for easy access to NEPIT
An online version of NEPIT was deployed on the web server (https://weloveoncology.shinyapps.io/NEPIT/). It can easily present the computed survival probabilities (unit: days) and generate relevant figures and tables if we input the actual values of predictors for patients on the web server.
3.5. Development and validation of a risk stratification system
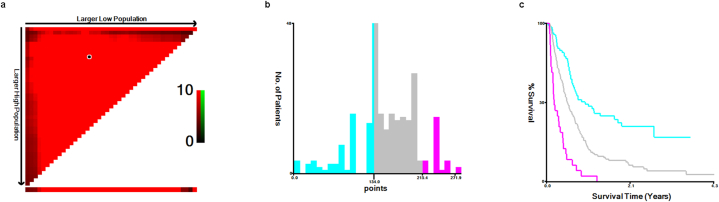
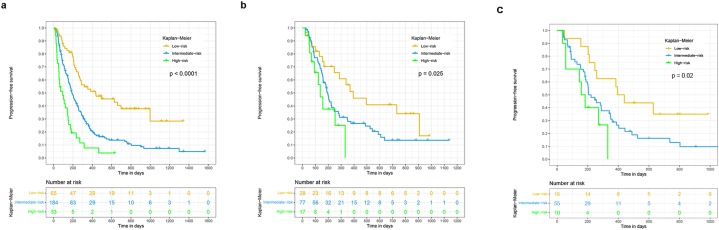
Based on the X-Tile program, patients can be assigned into three prognostic subgroups: low-risk (0–134), Intermediate-risk (134–215.4) and high-risk (>215.4) subgroups when applying 134 and 215.4 as the best cutoff points (Fig. 4a–c). Subsequently, we used patients from training cohort, validation cohort and testing cohort to validate this novel stratification system. The Kaplan-Meier curves showed that PFS in the different groups were accurately differentiated by this novel risk stratification system (P < 0.05) (Fig. 5a–c).
Fig. 4.
Risk stratification of patients with the total risk score cutting by X-tile. a. The black circle is the best cut-off values of the total risk score computed by the computer program, b. The best cut-off values of the total risk score are shown in histograms of the training cohort, c. Kaplan-Meier plots of the training cohort by using the best cut-off values. PFS progression-free survival.
Fig. 5.
Survival curves of patients in the training, validation, and testing cohort stratified by risk stratification system. a. Survival curves of patients in the training cohort, b. Survival curves of patients in the validation cohort, c. Survival curves of patients in the testing cohort. PFS progression-free survival.
4. DISSCUSSION
So far, anti-PD-1/PD-L1 therapy has been widely used in metastatic or advanced NSCLC patients and provides hope that durable, long-term benefit may be feasible. Such therapies could result in up to 15% with non-squamous and 16% of patients with squamous advanced NSCLC surviving for up to 5 years or more [19]. However, the median OS for metastatic patients with NSCLC still remains less than 3 years [2]. The difference in time-to-progression observed in clinical trials suggested high heterogeneity in patients, necessitating additional predictive factors to redefine personalized ICIs therapy.
In our study, we found five clinical parameters including age, ECOG-PS, pathological subtype, stage and TPS, could independently predict the relative benefit in anti-PD-1/PD-L1 therapy. Although the underlying mechanisms between age and the PFS of PD-(L)1 blockade are unknown, for elderly patients, they may have a reduced cytokine secretion, a slower lymphocyte proliferation after antigen stimulation and a reduced variability of T cell populations [20]. A multicenter international cohort study reported immune-related adverse events (irAEs) were more frequent with increasing age, and older patients were more likely to suffer ICIs discontinuation [21]. ECOG-PS as a predictor for the efficacy of ICIs in NSCLC was confirmed by many studies [22,23]. Patients with a good ECOG-PS can have better general condition with adequate treatments and a poor general condition may present the deterioration of the general immune state and weakness of effector T cells [24]. As a target of PD-1/PD-L1 antibodies, PD-L1 expression level was a commonly examined predictive factor in clinical practice [25,26]. However, challenges to clinical application of this approach consist of a limited understanding of the relationship between PFS and PD-L1 expression level, how to find an optimal cut-off points and how to find a way to improve predictive power of PD-L1 expression level.
To address these problems, we built a multivariable predictive model. The composite NEPIT integrated these clinical parameters including age, ECOG-PS, pathological subtype, stage and TPS to evaluate the relative PFS of ICIs treatment. We also enrolled patients from other centers for independently external validation to evaluate the model's generalizability and robustness to an unrelated population. The C-index of NEPIT in the training cohort, the validation cohort and the testing cohort was 0.789 (95%CI: 0.750–0.828), 0.745 (95%CI: 0.706–0.784), and 0.766 (95%CI: 0.744–0.788), respectively, which indicating the model's good discrimination. The calibration curves showed that NEPIT was able to precisely predict 3-, 6-, and 9-month PFS for patients, whether in the training cohort, the validation cohort or testing cohort. Thus, the NEPIT model shows excellent performance to predict PFS in advanced NSCLC patients treated with ICIs. The DCAs can assess whether NEPIT can improve clinical decision making [27]. The result from DCAs showed that NEPIT can achieve a net benefit, which indicating its good clinical applicability. The NRI and IDI analyses were two commonly used methods for quantifying the added value from the new predictors [28,29]. In this study, we also used NRI and IDI to evaluate improvement in risk prediction and measure the usefulness of NEPIT. The results from NRI and IDI analyses presented that NEPIT could improve net benefit as compared with TPS, which indicating that a multivariable predictive model including TPS enhanced the predictive ability of TPS. To put it another way, the composite NEPIT could improve predictive power of PD-L1 expression level.
Other models and predictors to predict ICIs efficacy were also developed in many studies [[30], [31], [32], [33]]. Tumor mutation burden (TMB) has become another potential marker for predicting prognosis because it is a substitute for neoepitopes that can be presented to T cells [34,35]. The RNA signatures were also developed and validated in other studies [36,37]. However, these predictors were not routinely detected in clinical practice. The model presented in these studies may be not applicable. For our model, NEPIT, five predictors in it were clinical routine parameters, which can be easily acquired in clinical practice. Furthermore, we deployed an online version of NEPIT on the web server (https://weloveoncology.shinyapps.io/NEPIT/) to easily access to NEPIT.
In addition, a risk stratification system based on the total risk score of each patient calculated by NEPIT in the training cohort was developed for different risk subgroups identification, which can help with optimal patient selection. We applied X-tile to evaluate the best cut-off values of the total risk score [18]. Based on the X-Tile program, we selected “134”and “215.4” as best values. All patients from the training cohort then were assigned into three subgroups: low-risk (0–134), Intermediate-risk (134–215.4) and high-risk (>215.4) subgroups. To test whether this risk stratification can have a better discriminating power, the Kaplan-Meier curves in different risk subgroups were executed and the significance evaluated by using log-rank test. The results from Kaplan-Meier analyses showed that our risk stratification system could be effectively differentiated patients into different risk subgroups, and the high-risk group presented a significantly poor survival than the Intermediate-risk and low-risk group in training cohort. Notably, the validation cohort and the testing cohort also presented similar findings. Therefore, this novel risk stratification system have outstanding discriminating power for different risk subgroups identification. The three risk subgroups separated using this risk stratification system counteracted the controversial impermanence of PFS benefit with exciting classification of the risks of progression.
This study is subject to multiple weaknesses. First, although there is a testing cohort from other centers to assess the model's generalizability and robustness to an unrelated population, the development and validation of NEPIT was only based on Chinese population. So, studies involving population from other countries are needed to further validate NEPIT in the future. Second, as other popular predictors, such as TMB and RNA sequence, were not routinely detected in our center, we were unable to conduct any further comparison between NEPIT and other predictive model. Finally, this retrospective study is susceptible to selection bias.
Even so, the NEPIT developed in this study was the primary reported online predictive model based on clinical routine parameters to predict PFS for NSCLC treated with PD-(L)1 inhibitor. The further risk stratification system presented outstanding discriminating power for different risk subgroups identification. The tools developed in this study would have value in guiding the optimal patient selection for precision care.
5. Conclusion
We constructed an online clinical model, NEPIT, to provide individualized prediction of PFS for advanced non-small-cell lung cancer patients treated with PD-(L)1 inhibitor using five clinical routine parameters. The validation of NEPIT presented its excellent performance and good clinical applicability. The further risk stratification system presented excellent discriminating power for different risk subgroups identification. The tools in this study would have value in guiding the optimal patient selection for precision care.
Consent for publication
Not applicable.
Availability of data and materials
The source data supporting the findings of this study are available from the corresponding author for reasonable request.
Authors’ contributions
Zegui Tu: Conceived and designed the experiments; Analyzed and interpreted the data; Performed the experiments; Wrote the paper.
Yang Yu: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
abrTian Tian: Contributed reagents, materials, analysis tools or data.
Caili Li: Conceived and designed the experiments; Analyzed and interpreted the data.
Jieyan Luo: Conceived and designed the experiments; Performed the experiments.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- ICIs
immune checkpoints inhibitors
- NSCLC
non-small-cell lung cancer
- OS
overall survival
- PFS
progression-free survival
- ECOG-PS
Eastern Cooperative Oncology Group performance status
- TPS
The PD-L1 tumor proportion Score
- HR
hazards ratios
- CI
confidence interval
- NEPIT
Nomogram to Evaluate the relative PFS of ICIs Treatment
- C-index
concordance index
- DCAs
Decision Curve Analyses
- NRI
net reclassification improvement
- IDI
integrated discrimination improvement
- IQR
Inter-Quartile Range
- irAEs
immune-related adverse events
- TMB
Tumor mutational burden
References
- 1.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 2.Garon E.B., Hellmann M.D., Rizvi N.A., Carcereny E., Leighl N.B., Ahn M.J., Eder J.P., Balmanoukian A.S., Aggarwal C., Horn L., et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J. Clin. Oncol. : Offl. J. Am. Soc. Clin. Oncol. 2019;37:2518–2527. doi: 10.1200/jco.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbst R.S., Giaccone G., de Marinis F., Reinmuth N., Vergnenegre A., Barrios C.H., Morise M., Felip E., Andric Z., Geater S., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N. Engl. J. Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 4.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Sosman J.A., Atkins M.B., Leming P.D., et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5:1411–1420. doi: 10.1001/jamaoncol.2019.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadgeel S., Rodríguez-Abreu D., Speranza G., Esteban E., Felip E., Dómine M., Hui R., Hochmair M.J., Clingan P., Powell S.F., et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J. Clin. Oncol. : Offl. J. Am. Soc. Clin. Oncol. 2020;38:1505–1517. doi: 10.1200/jco.19.03136. [DOI] [PubMed] [Google Scholar]
- 6.Passaro A., Brahmer J., Antonia S., Mok T., Peters S. Managing resistance to immune checkpoint inhibitors in lung cancer: treatment and novel strategies. J. Clin. Oncol. : Offl. J. Am. Soc. Clin. Oncol. 2022 doi: 10.1200/jco.21.01845. Jco2101845. [DOI] [PubMed] [Google Scholar]
- 7.Xing S., Hu K., Wang Y. Tumor immune microenvironment and immunotherapy in non-small cell lung cancer: update and new challenges. Aging and disease. 2022;13:1615–1632. doi: 10.14336/ad.2022.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibney G.T., Weiner L.M., Atkins M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. doi: 10.1016/s1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian S.L., Taube J.M., Anders R.A., Pardoll D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone D.P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., Felip E., van den Heuvel M.M., Ciuleanu T.E., Badin F., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gettinger S., Rizvi N.A., Chow L.Q., Borghaei H., Brahmer J., Ready N., Gerber D.E., Shepherd F.A., Antonia S., Goldman J.W., et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J. Clin. Oncol. : Offl. J. Am. Soc. Clin. Oncol. 2016;34:2980–2987. doi: 10.1200/jco.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powles T., O'Donnell P.H., Massard C., Arkenau H.T., Friedlander T.W., Hoimes C.J., Lee J.L., Ong M., Sridhar S.S., Vogelzang N.J., et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3 doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prelaj A., Tay R., Ferrara R., Chaput N., Besse B., Califano R. vol. 106. European journal of cancer; Oxford, England : 1990: 2019. pp. 144–159. (Predictive Biomarkers of Response for Immune Checkpoint Inhibitors in Non-small-cell Lung Cancer). [DOI] [PubMed] [Google Scholar]
- 16.Iasonos A., Schrag D., Raj G.V., Panageas K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. : Offl. J. Am. Soc. Clin. Oncol. 2008;26:1364–1370. doi: 10.1200/jco.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 17.Tu Z., Li C., Tian T., Chen Q. vol. 161. Lung cancer; Amsterdam, Netherlands: 2021. A Risk Classification System Predicting the Cancer-specific Survival for Postoperative Stage IB Non-small-cell Lung Cancer Patients without Lymphovascular and Visceral Pleural Invasion; pp. 114–121. [DOI] [PubMed] [Google Scholar]
- 18.Camp R.L., Dolled-Filhart M., Rimm D.L. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. : An Offl. J. Am. Assoc. Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.Ccr-04-0713. [DOI] [PubMed] [Google Scholar]
- 19.Gettinger S., Horn L., Jackman D., Spigel D., Antonia S., Hellmann M., Powderly J., Heist R., Sequist L.V., Smith D.C., et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the ca209-003 study. J. Clin. Oncol. : Offl. J. Am. Soc. Clin. Oncol. 2018;36:1675–1684. doi: 10.1200/jco.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 20.Galli G., De Toma A., Pagani F., Randon G., Trevisan B., Prelaj A., Ferrara R., Proto C., Signorelli D., Ganzinelli M., et al. vol. 137. Lung cancer; Amsterdam, Netherlands: 2019. Efficacy and Safety of Immunotherapy in Elderly Patients with Non-small Cell Lung Cancer; pp. 38–42. [DOI] [PubMed] [Google Scholar]
- 21.Nebhan C.A., Cortellini A., Ma W., Ganta T., Song H., Ye F., Irlmeier R., Debnath N., Saeed A., Radford M., et al. Clinical outcomes and toxic effects of single-agent immune checkpoint inhibitors among patients aged 80 Years or older with cancer: a multicenter international cohort study. JAMA Oncol. 2021;7:1856–1861. doi: 10.1001/jamaoncol.2021.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita R., Okishio K., Shimizu J., Saito H., Sakai H., Kim Y.H., Hataji O., Yomota M., Nishio M., Aoe K., et al. vol. 140. Lung cancer; Amsterdam, Netherlands: 2020. Real-world Effectiveness and Safety of Nivolumab in Patients with Non-small Cell Lung Cancer: A Multicenter Retrospective Observational Study in Japan; pp. 8–18. [DOI] [PubMed] [Google Scholar]
- 23.Crinò L., Bidoli P., Delmonte A., Grossi F., De Marinis F., Ardizzoni A., Vitiello F., Lo Russo G., Parra H.S., Cortesi E., et al. Italian cohort of nivolumab expanded access program in squamous non-small cell lung cancer: results from a real-world population. Oncol. 2019;24:e1165–e1171. doi: 10.1634/theoncologist.2018-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shankar B., Zhang J., Naqash A.R., Forde P.M., Feliciano J.L., Marrone K.A., Ettinger D.S., Hann C.L., Brahmer J.R., Ricciuti B., et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952–1956. doi: 10.1001/jamaoncol.2020.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V., et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanmamed M.F., Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Calster B., Wynants L., Verbeek J.F.M., Verbakel J.Y., Christodoulou E., Vickers A.J., Roobol M.J., Steyerberg E.W. Reporting and interpreting decision curve analysis: a guide for investigators. Eur. Urol. 2018;74:796–804. doi: 10.1016/j.eururo.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uno H., Tian L., Cai T., Kohane I.S., Wei L.J. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat. Med. 2013;32:2430–2442. doi: 10.1002/sim.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pencina M.J., D'Agostino R.B., Sr, Steyerberg E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat. Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S., et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, N.Y.) 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (New York, N.Y.) 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng Z., Gao Y., Li J., Zhang J., Li Y., He F., Huang Z., Han L., Gong Y., Xie C. SETD2 mediates immunotherapy and radiotherapy efficacy via regulating DNA damage responses and genomic stability in lung adenocarcinoma. Gene.Diseas. 2023;10:336–339. doi: 10.1016/j.gendis.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S., Jiang M., Yang Z., Huang X., Li N. The role of distinct co-mutation patterns with TP53 mutation in immunotherapy for NSCLC. Gene. Diseas. 2022;9:245–251. doi: 10.1016/j.gendis.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.L., et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Sci. (New York, N.Y.) 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 36.Kong S.K., Kim B.S., Lim H., Kim H.J., Kim Y.S. Dissection of PD-L1 promoter reveals differential transcriptional regulation of PD-L1 in VHL mutant clear cell renal cell carcinoma. Labor. Invest.; A J. Techn. Meth. Pathol. 2021 doi: 10.1038/s41374-021-00703-5. [DOI] [PubMed] [Google Scholar]
- 37.Davis-Marcisak E.F., Fitzgerald A.A., Kessler M.D., Danilova L., Jaffee E.M., Zaidi N., Weiner L.M., Fertig E.J. Transfer learning between preclinical models and human tumors identifies a conserved NK cell activation signature in anti-CTLA-4 responsive tumors. Genome Med. 2021;13:129. doi: 10.1186/s13073-021-00944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The source data supporting the findings of this study are available from the corresponding author for reasonable request.