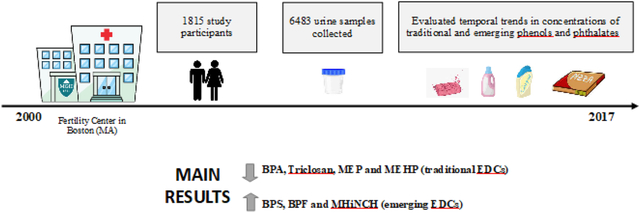
Abstract
Endocrine disrupting chemicals (EDCs) can adversely affect human health and are ubiquitously found in everyday products. We examined temporal trends in urinary concentrations of EDCs and their replacements. Urinary concentrations of 11 environmental phenols, 15 phthalate metabolites, phthalate replacements such as two di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH) metabolites, and triclocarban were quantified using isotope-dilution tandem mass spectrometry. This prospective ecological study included 996 male and 819 female patients who were predominantly White/Caucasian (83%) with an average age of 35 years and a BMI of 25.5 kg/m2 seeking fertility treatment in Boston, MA, USA. Patients provided a total of 6,483 urine samples (median=2, range=1–30 samples per patient) between 2000 and 2017. Over the study period, we observed significant decreases (% per year) in urinary concentrations of traditional phenols, parabens, and phthalates such as bisphenol A (β: −6.3, 95% CI: −7.2, −5.4), benzophenone-3 (β: −6.5, 95% CI: −1.1, −18.9), parabens ((β range:−5.4 to −14.2), triclosan (β: - 18.8, 95% CI: −24, −13.6), dichlorophenols (2.4-dichlorophenol β: −6.6, 95% CI: −8.8, −4.3); 2,5-dichlorophenol β: −13.6, 95% CI: −17, −10.3), di(2-ethylhexyl) phthalate metabolites (β range: − 11.9 to −22.0), and other phthalate metabolites including mono-ethyl, mono-n-butyl, and mono-methyl phthalate (β range: −0.3 to −11.5). In contrast, we found significant increases in urinary concentrations of environmental phenol replacements including bisphenol S (β: 3.9, 95% CI: 2.7, 7.6) and bisphenol F (β: 6, 95% CI: 1.8, 10.3), DINCH metabolites (cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester [MHiNCH] β: 20, 95% CI: 17.8, 22.2; monocarboxyisooctyl phthalate [MCOCH] β: 16.2, 95% CI: 14, 18.4), and newer phthalate replacements such as mono-3-carboxypropyl phthalate, monobenzyl phthalate, mono-2-ethyl-5-carboxypentyl phthalate and di-isobutyl phthalate metabolites (β range=5.3 to 45.1), over time. Urinary MHBP concentrations remained stable over the study period. While the majority of biomarkers measured declined over time, concentrations of several increased, particularly replacement chemicals that are studied.
Keywords: Endocrine disrupting chemicals, temporal trends, phthalates, phenols
Graphical Abstract
Introduction
Endocrine disrupting chemicals (EDC) — such as environmental phenols, phthalates, and triclosan — are commonly used and interfere with the endocrine system, with reported adverse developmental, reproductive, neurological, and immune effects in both humans and wildlife (Diamanti-Kandarakis, Bourguignon et al. 2009). Environmental phenols include a wide range of chemicals such as bisphenols, parabens, and triclosan. Bisphenol A (BPA) is one of the most widely utilized and studied phenols, present in synthetic polymers (Flint, Markle et al. 2012, Hoekstra and Simoneau 2013) building materials, thermal paper (Ehrlich, Calafat et al. 2014), toys, dental products (Flint, Markle et al. 2012, Huang, Wong et al. 2012) and food packaging (Yoshida, Horie et al. 2001, Niu, Zhang et al. 2012). Given the endocrine disrupting activities associated with BPA (Bonefeld-Jorgensen, Long et al. 2007, Matsushima, Kakuta et al. 2007, De Coster and van Larebeke 2012, Rochester 2013, Rezg, El-Fazaa et al. 2014), the replacement chemicals bisphenol S (BPS) and bisphenol F (BPF) were introduced in the market as potentially safer alternatives. Parabens — such as methylparaben, butylparaben, propylparaben, and ethylparaben — are used as food preservatives and shelf stabilizers (Soni, Taylor et al. 2002, Soni, Carabin et al. 2005) and within personal care products such as shampoos, creams (Guo, Wang et al. 2014) and pharmaceutical products (Moreta, Tena et al. 2015, Ma, Zhao et al. 2016). Triclosan and triclocarban are antimicrobial agents (Alfhili and Lee 2019) used in personal hygiene products such as soaps, mouthwashes, toothpastes, and hand sanitizers (Weatherly and Gosse 2017).
Phthalates are synthetic phthalic acids (Hauser and Calafat 2005) comprised of high molecular weight and low molecular weight phthalates. They have a wide range of uses as industrial agents – such as building materials, toys, food packaging, and medical devices (Hauser and Calafat 2005, Schettler 2006) – and daily use consumer products such perfumes, lotions and cosmetics, pharmaceuticals, lacquers, varnishes, coatings, adhesives, and pesticides (Hauser and Calafat 2005, Schettler 2006). Di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), a non-phthalate plasticizer, was introduced commercially in 2002 as a safer alternative because of a more favorable toxicological profile (EFSA 2006). Given their uses, phenols and phthalates are most commonly absorbed through ingestion, dermal contact (Moss, Howes et al. 2000, Chedgzoy, Winckle et al. 2002), inhalation (Rudel, Camann et al. 2003, Matsumoto, Adachi et al. 2005, Loganathan and Kannan 2011, Bledzka, Gromadzinska et al. 2014, Moos, Angerer et al. 2016) or mucosal absorption (Bagley and Lin 2000, Lin 2000, Sandborgh-Englund, Adolfsson-Erici et al. 2006) through the use of personal care and household products (Silva, Barr et al. 2004, Hauser and Calafat 2005, Schettler 2006, Zota, Calafat et al. 2014). Urine is the optimal matrix for quantifying phenols and phthalate metabolites because of their short half-lives (<24 hours), their metabolism and excretion, as well as being a non-invasive and convenient medium for biological monitoring (Calafat, Longnecker et al. 2015).
The United States has instituted bans and restrictions on use of certain phenols and phthalates due to health concerns from their exposures. Fetuses, infants, toddlers, and children have been found to be particularly vulnerable to BPA effects. In 2011, Europe (EFSA 2011) initially banned BPA in baby bottles, with Canada (Canada 2012), and the USA (USFDA 2012) passing similar bans in 2012 for baby bottles, formula packaging, and sippy cups. and In 2016, the US Food and Drug Administration (USFDA 2016) banned the use of triclosan in soap products such as liquids, gels, foams, and bars. Selected phthalates and parabens as well as BPA and benzophenones have been either banned or targeted for market-based campaigns to reduce their use in consumer products (Cosmetics 2005, Improvement 2008, FDA 2012, Commission 2014).
As legislation is implemented over time, with the introduction of new alternatives and reformulations, understanding temporal trends in exposures is essential to better identifying population-level exposures and the impact of legislative measures. Therefore, the present study aimed to describe temporal trends in concentrations of phenol, phthalate metabolites and their replacements in urine samples collected between 2000 and 2017 among male and female patients attending a fertility center in Boston, Massachusetts.
Methods
Study sample
This cohort study was designed to identify environmental chemicals and dietary factors that impact fertility. The study began in 2000 and voluntarily recruited only male patients of couples seeking fertility treatment at the Massachusetts General Hospital (MGH) Fertility Center (Minguez-Alarcon, Gaskins et al. 2016) in Boston, MA, USA. Starting in 2005, both female and male patients of subfertile couples were invited to voluntarily participate in the study (Hauser, Meeker et al. 2006). Study enrollment ended in 2019. Female patients aged 18–45 years, and male patients 18–56 years who had not had a vasectomy and who were not taking hormones at the time of enrollment, were eligible to join either independently or as a couple. Study participants were a convenience sample of the population seeking care at the clinic. After the study procedures were explained and all questions were answered, participants signed an informed consent form. The participant’s date of birth was collected at entry, and weight and height were measured by trained study staff at study entry. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared. At enrollment, research staff administered sociodemographic (e.g. race), lifestyle, and medical history questionnaires to participants. Study participants also completed a comprehensive questionnaire on family, medical, reproductive, and occupational history, consumer products use, smoking history, etc. The study was approved by the Human Subject Committees of the Harvard T.H. Chan School of Public Health, MGH, and the Centers for Disease Control and Prevention (CDC).
Assessment of endocrine disrupting chemicals
Each participant provided one spot urine sample at enrollment and subsequent samples over the time they participated. Urinary concentrations of the biomarkers measured were used as an index of exposure to their respective parent compounds. Samples were collected in a sterile polypropylene specimen cup. Specific gravity (SG), which was used as a covariate in the models to account for urine dilution, was measured at room temperature at MGH using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) calibrated with deionized water before each measurement. The urine was divided into aliquots, frozen, and stored at −80°C. Samples were shipped overnight on dry ice to the CDC (Atlanta, GA) where they were stored at or below −40 °C until analysis. As previously described (Ye, Kuklenyik et al. 2005, Silva, Samandar et al. 2007, Hauser, Gaskins et al. 2016), online solid-phase extraction coupled with isotope dilution-high-performance liquid chromatography-tandem mass spectrometry was used to quantify the urinary concentrations of triclocarban, 11 phenols [bisphenol A (BPA), bisphenol S (BPS), bisphenol F (BPF), benzophenone-3, butylparaben, ethylparaben, methylparaben, propylparaben, triclosan, 2,4-dichlorophenol and 2,5-dichlorophenol], 15 phthalate metabolites [monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), mono-hydroxybutyl phthalate (MHBP), mono-isobutyl phthalate (MiBP), mono-3-carboxypropyl phthalate (MCPP), monobenzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), monocarboxyisooctyl phthalate (MCOP), monooxononyl phthalate (MONP), monocarboxyisononyl phthalate (MCNP), mono-2-ethyl-5-carboxypentyl (MECPTP), mono-2-ethyl-5-hydrohexyl terephthalate (MEHHTP), and two metabolites of DINCH (cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester (MHiNCH), and cyclohexane-1,2-dicarboxylic acid monocarboxyisooctyl ester (MCOCH)). The limit of detection (LOD) ranged from 0.1 to 2.3 μg/L, depending on the biomarker (Supplemental Table 1). Biomarker concentrations below the LOD were assigned a value equal to the LOD divided by the square root of 2 (Hornung and Reed 1990, Lubin, Colt et al. 2004). Analytical methods to quantify some of the EDCs included in this work at CDC were developed years after the study started, therefore, the measurement of certain biomarkers in urine did not begin at the study onset.
The CDC laboratory is certified by the Health Care Financing Administration to comply with the requirements set forth in the Clinical Laboratory Improvement Amendments of 1988 (CLIA’88). All analytical measurements follow strict quality control/quality assurance CLIA guidelines. For example, along with study samples, each analytical run includes a set of calibrators, reagent blanks, and high- and low-concentration quality control (QC) materials. Concentrations of the QCs are evaluated using standard statistical probability rules (Caudill, Schleicher et al. 2008). Accuracy (92%−105%, phthalates; 83%−109%, phenols) and precision (2.1%−14.0%, phthalates; 2.5%−8.4%, phenols), depending on the analyte, are included in the public documents describing the analytical approaches used (CDC 2018, CDC 2019); these approaches have been used since the early 2000s for the analyses of tens of thousands of biological specimens, including those collected as part of CDC’s ongoing national survey, the National Health and Nutrition Examination Survey (NHANES).
Statistical analysis
Demographic characteristics were summarized using median ± interquartile ranges (IQRs) or percentages. Kolmogorov-Smirnov tests were used to evaluate whether urinary biomarker concentrations were normally distributed. Urinary concentrations of all examined chemical biomarkers were natural log-transformed before analysis to better approximate a normal distribution. Multivariable mixed models were used to estimate the differences in biomarkers concentrations across the years, using a random intercept to account for correlation across multiple observations per study participant and adjusting for age, BMI, sex and SG. We adjusted for predictors of exposure biomarkers and also SG to account for urine dilution. The independent variable, years, was considered as a discrete categorical variable to obtain the marginal means per two-year period and as a continuous variable to estimate the decrease per year. To allow for better interpretation of results, population marginal means were obtained, back-transforming to the original scale (Searle, Speed et al. 1980) and were presented per two year period, at the mean level for other continuous covariates and the weighted frequency for categorical variables. We evaluated the robustness of the findings stratifying by sex (male vs. female), age (<35 vs ≥35 years), and BMI (<25 vs ≥25 kg/m2). We stratified by age and sex because younger people tend to have increased exposure to EDCs by using more personal care products (Park, Kim et al. 2019, Pagoni, Arvaniti et al. 2022) and consuming more processed foods (Buckley, Kim et al. 2019). Additionally, many EDCs are obesogenic (Darbre 2017, Xia, Zhu et al. 2018, Heindel and Blumberg 2019, Zhang, Dong et al. 2019) with preferential fat accumulation that may impact urinary biomarker concentrations. Statistical tests were two-tailed and all p-values<0.05 were conventionally regarded as statistically significant. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
We included 996 male and 819 female patients (N total=1,815 participants) who provided a total of 6,483 urine samples (median=2, range=1–30) between 2000 and 2017 in our evaluation. At study entry, participants were mostly white (83%), 29% had ever smoked and they had a median (interquartile range [IQR]) age of 35 (32, 39) years and BMI of 25.5 (22.8, 28.8) kg/m2 (Table 1).
Table 1.
Demographic characteristics [median (IQR) or counts (%)] of 1815 study participants between 2000 and 2017 at study entry.
| Characteristic | N (%) or Median (IQR) |
|---|---|
| Sex, n (%) | |
| Male | 996 (55) |
| Female | 819 (45) |
| Age, years | 35.0 (32.0, 39.0) |
| <35, n (%) | 812 (45) |
| ≥35, n (%) | 1003 (55) |
| Race, n (%) | |
| White/Caucasian | 1508 (83) |
| Black/Asian/Other | 307 (17) |
| Body Mass Index, kg/m2 | 25.5 (22.8, 28.8) |
| <25, n (%) | 794 (44) |
| ≥25, n (%) | 1021 (56) |
| Smoking, n (%) | |
| Never | 1280 (71) |
| Ever | 535 (29) |
Detection frequencies for some phenols such as BPA (90% in 2000–2001 vs. 74% in 2016–2017) and triclosan (76% in 2008–2009 vs. 59% in 2016–2017) decreased over the study period, whereas detection frequencies increased for BPS (69% in 2010–2011 vs. 76% in 2016–2017) (Supplemental Table 1). Detection frequency for the urinary phthalate metabolite concentrations remained stable over time. By contrast, detectable urinary concentrations of metabolites of the phthalate replacement DINCH increased over time: MHiNCH (6% in 2010–2011 vs. 60% in 2016–2017) and MCOCH (non-detectable in 2010–2011 vs. 53% in 2016–2017). Geometric mean (GM) urinary concentrations of benzophenone-3, some specific parabens, particularly methyl and propylparaben and di-2-ethylhexyl phthalate (DEHP) metabolites, were higher among females and participants with normal BMI, compared to males and participants having obesity (Supplemental Table 2). Most biomarkers had minimum urinary concentrations <LOD over the study period (Supplemental Table 3).
We observed decreasing trends for urinary concentrations of some environmental phenols, such as BPA, and increasing trends of urinary concentrations of BPA replacements, such as BPS and BPF, over the study period (Figure 1, Supplemental Table 4). Based on adjusted linear mixed effect models, the estimated mean (95% CI) urinary BPS increased from 0.23 (95% CI: 0.15, 0.34) μg/L in 2010–2011 to 0.35 (95% CI: 0.31, 0.39) μg/L in 2016–2017, while the estimated mean for urinary BPF increased from 0.28 (95% CI: 0.19, 0.41) μg/L to 0.31 (95% CI: 0.28, 0.34) μg/L over the same period. These trends correspond to yearly increases in urinary BPS and BPF of 3.9% (95% CI 2.7, 7.6) and 6.0% (95% CI: 1.8, 10.3), respectively (Table 2). Some decreasing trends in urinary phenol concentrations included, for example, BPA with an estimated 6.3% (95% CI −7.2, −5.4) decline per year between 2000 and 2017, and triclosan showing the largest decrease corresponding to an 18.8% per year (95% CI: −24.0, −13.6) (Table 2).
Figure 1.
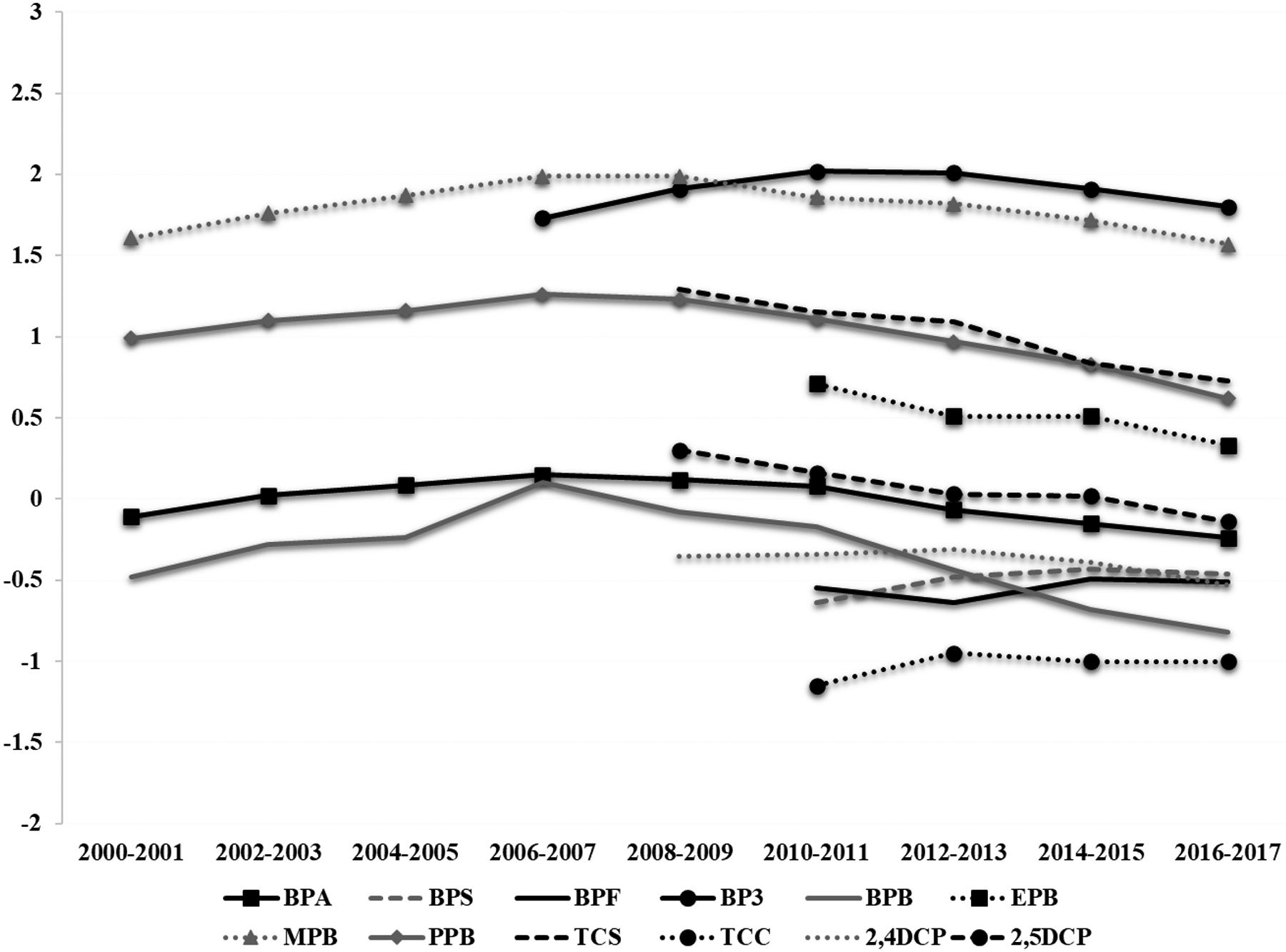
Estimated trendsa in urinary concentrations of phenol and other EDCs among study participants between 2000 and 2017, based on adjusted linear mixed effect models.
aLog-transformed mean urinary biomarker concentrations adjusted for age, BMI, sex and specific gravity.
Abbreviations: BPA, bisphenol A; BPS, bisphenol S; BPF, bisphenol F; BP3, benzophenone-3; BPB, butylparaben; EPB, ethylparaben; MPB, methylparaben; PPB, propylparaben; TCS, triclosan; TCC, triclocarban; 2,4-DCP, 2,4-dichlorophenol; 2,5-DCP, 2,5-dichlorophenol.
Table 2.
Adjusted change per year in% (95% CI) for urinary concentrations of phenols, phthalate metabolites and other EDCs among study participants between 2000 and 2017.
| Total cohorta | Sexb | Agec | BMId | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | <35 years | ≥35 years | <25 kg/m2 | ≥25 kg/m2 | ||
| triclocarban | −0.53 (−4.58, 3.53) | −1.04 (−5.08, 3.00) | 1.82 (−9.59, 1.32) | 1.61 (−2.62, 5.83) | −3.45 (−10.4, 3.48) | 1.73 (−2.37, 5.83) | −2.72 (−10.1, 4.61) |
| DINCH | |||||||
| MHiNCH | 20.0 (17.8, 22.2) | 19.0 (16.4, 21.6) | 22.8 (18.4, 27.1) | 20.4 (17.0, 23.8) | 19.1 (16.1, 22.1) | 20.4 (17.4, 23.5) | 20.3 (17.0, 23.6) |
| MCOCH | 16.2 (14.0, 18.4) | 16.3 (13.7, 18.8) | 15.5 (11.5, 19.6) | 17.1 (13.9, 20.4) | 15.0 (11.9, 18.2) | 15.4 (13.4, 19.3) | 16.9 (13.4, 20.3) |
| Phenols | |||||||
| BPA | −6.30 (−7.19, −5.40) | −9.46 (−10.6, −8.33) | −2.60 (−3.91, −1.29) | −6.12 (−7.44, −4.80) | −6.17 (−7.37, −4.97) | −7.94 (−9.23, −6.64) | −4.37 (−5.93, −3.53) |
| BPS | 3.92 (2.66, 7.57) | 4.66 (5.09, 8.81) | 1.64 (−5.99, 9.28) | 4.79 (−0.56, 10.2) | 3.56 (−1.60, 8.72) | 4.19 (−0.60, 8.98) | 4.17 (−1.38, 9.72) |
| BPF | 6.01 (1.78, 10.3) | 9.43 (4.47, 14.4) | −4.43 (−12.2, 3.37) | 5.86 (−0.16, 11.9) | 6.35 (0.35, 12.3) | 6.96 (1.45, 12.5) | 5.23 (−11.8, 11.7) |
| benzophenone-3 | −6.48 (−1.11, −18.9) | −8.87 (−14.7, −3.07) | 0.14 (−6.14, 6.42) | −6.71 (−13.4, −0.74) | −4.06 (−9.39, 12.8) | −11.9 (−18.8, −50.5) | −0.33 (−5.67, 5.61) |
| butylparaben | −14.2 (−16.2, −12.3) | −26.2 (−29.3, −23.0) | −4.96 (−6.93, −2.98) | −15.1 (−18.1, −12.1) | −13.5 (−16.1, −10.8) | −18.4 (−21.7, −15.1) | −10.9 (−13.2, −8.67) |
| ethylparaben | −11.8 (−18.3, −5.41) | −16.1 (−23.8, −8.25) | 1.03 (−8.39, 10.5) | −16.9 (−25.6, −8.34) | −9.78 (−18.7, −0.90) | −13.3 (−22.8, −3.73) | −9.91 (−18.4, −1.38) |
| methylparaben | −5.37 (−7.04, −3.69) | −13.2 (−15.5, −10.8) | 1.24 (−0.79, 3.28) | −4.84 (−7.53, −2.14) | −5.24 (−7.36, −3.11) | −8.04 (−10.7, −5.44) | −2.78 (−4.82, −0.75) |
| propylparaben | −10.2 (−12.5, −8.03) | −17.4 (−20.6, −14.2) | −3.91 (−6.78, −1.05) | −10.7 (−14.1, −7.30) | −9.22 (−12.0, −6.39) | −13.8 (−17.3, −10.3) | −7.12 (−9.85, −4.39) |
| 2,4-dichlorophenol | −6.57 (−8.80, −4.34) | −6.50 (−9.05, −3.96) | −6.22 (−10.8, −1.67) | −8.85 (−11.9, −5.03) | −4.21 (−7.03, −1.40) | −8.55 (−11.7, −5.45) | −4.47 (−7.63, −1.31) |
| 2,5-dichlorophenol | −13.6 (−17.0, −10.3) | −12.3 (−16.3, −8.38) | −17.0 (−22.9, −11.2) | −17.0 (−22.1, −11.9) | −10.7 (−15.1, −6.41) | −12.6 (−17.3, −7.79) | −14.8 (−19.1, −10.5) |
| triclosan | −18.8 (−24.0, −13.6) | −17.0 (−23.5, −10.6) | −24.1 (−32.1, −16.0) | −19.8 (−27.3 −12.3) | −16.5 (−23.2, −9.75) | −23.4 (−30.8, −16.0) | −15.6 (−22.7, −8.47) |
| Phthalates | |||||||
| MEP | −11.5 (−12.5, −9.67) | −11.2 (−13.4, −8.84) | −12.4 (−14.7, −10.2) | −9.47 (−11.5, −7.50) | −12.2 (−14.0, −10.3) | −8.69 (−10.8, −6.53) | −12.7 (−14.6, −10.8) |
| MBP | −3.33 (−4.44, −2.24) | −6.11 (−7.63, −4.60) | −3.74 (−6.09, −1.40) | −2.42 (−4.05, −0.80) | −3.83 (−5.20, −2.46) | −4.29 (−5.98, −2.60) | −3.22 (−4.83, −1.61) |
| MHBP | 0.47 (−2.42, 3.36) | −0.33 (−3.75, 3.09) | 3.11 (−2.05, 8.28) | 1.16 (−3.31, 5.62) | 0.06 (−3.37, 3.86) | 1.27 (−2.64, 5.19) | 0.09 (−4.32, 4.33) |
| MiBP | 5.53 (4.34, 6.73) | 5.54 (3.99, 7.10) | 5.35 (3.53, 7.18) | 5.70 (3.81, 7.59) | 5.78 (4.39, 7.23) | 6.16 (4.39, 7.92) | 4.66 (3.08, 6.23) |
| MCPP | −3.19 (−4.64, −1.76) | −5.15 (−7.03, −3.26) | −0.34 (−2.52, 1.83) | −3.10 (−5.30, −0.91) | −2.83 (−4.67, −1.01) | −3.28 (−5.33, −1.22) | −3.02 (−5.04, −1.00) |
| MBzP | −2.99 (−4.18, −1.80) | −1.62 (−3.49, 0.26) | −5.82 (−8.11, −3.52) | −1.15 (−2.89, 0.59) | −4.43 (−5.97, −2.89) | −1.45 (−3.21, 0.33) | −4.79 (−6.43, −3.15) |
| MEHP | −11.9 (−13.1, −10.8) | −14.9 (−16.2, −13.5) | −11.0 (−13.2, −8.78) | −10.2 (−11.9, −8.46) | −13.3 (−14.8, −11.7) | −12.8 (−14.2, −11.3) | −11.7 (−13.4, −9.94) |
| MEHHP | −19.1 (−20.2, −18.1) | −20.4 (−21.8, −19.1) | −18.6 (−20.3, −16.8) | −19.0 (−20.6, −17.4) | −19.4 (−20.8, −18.1) | −19.0 (−20.1, 16.8) | −19.5 (−20.9, −18.0) |
| MEOHP | −19.6 (−20.6, −18.5) | −20.0 (−21.4, −18.7) | −19.9 (−21.8, −18.2) | −19.3 (−20.9, −17.7) | −20.0 (−21.4, −18.7) | −19.2 (−20.7, −17.7) | −20.3 (−21.8, −18.8) |
| MECPP | −22.0 (−23.1, −20.9) | −20.7 (−22.0, −19.4) | −25.2 (−27.2, −23.2) | −22.2 (−23.8, −20.6) | −21.8 (−23.2, −20.4) | −20.6 (−22.0, −19.1) | −23.8 (−25.3, −22.3) |
| MCOP | 2.13 (0.01, 4.26) | −2.14 (−0.47, 4.75) | 1.97 (−1.60, 5.54) | 2.11 (−1.07, 5.28) | 2.61 (−0.16, 5.38) | 3.43 (0.44, 6.41) | 0.49 (−2.47, 3.45) |
| MONP | −47.3 (−53.5, −41.1) | −42.8 (−50.0, −35.8) | −60.0 (−72.5, −47.6) | −44.8 (−54.3, −35.4) | −48.2 (−56.2, −40.2) | −47.4 (−55.3, −39.4) | −45.8 (−54.8, −36.8) |
| MCNP | −7.42 (−8.84, −5.99) | −7.16 (−8.88, −5.45) | −8.14 (−10.6, −5.64) | −7.40 (−9.54, −5.26) | −6.92 (−8.82, −5.02) | −7.13 (−9.08, −5.17) | −7.77 (−9.81, −5.72) |
| MECPTP | 45.1 (38.2, 52.0) | 46.0 (38.2, 53.9) | 42.0 (28.9, 55.1) | 40.8 (30.8, 50.7) | 50.3 (40.7, 59.9) | 47.4 (37.8, 57.0) | 44.8 (34.6, 55.0) |
| MEHHTP | 32.0 (26.4, 37.7) | 31.1 (24.7, 37.7) | 35.5 (24.7, 46.3) | 30.4 (22.0, 38.9) | 34.9 (27.2, 42.6) | 31.2 (23.6, 38.8) | 34.6 (26.1, 43.0) |
Adjusted for aage, BMI, sex and specific gravity; bage, BMI, and specific gravity; cBMI, sex and specific gravity; dage, sex and specific gravity.
Abbreviations: BPA, bisphenol A; BPS, bisphenol S; BPF, bisphenol F; MEP, monoethyl phthalate; MBP, mono-n-butyl phthalate; MHBP, mono-hydroxybutyl phthalate; MiBP, mono-isobutyl phthalate; MCPP, mono-3-carboxypropyl phthalate; MBzP, monobenzyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEHHP, mono-2-ethyl-5-hydroxyhexyl phthalate; MEOHP, mono-2-ethyl-5-oxohexyl phthalate; MECPP, mono-2-ethyl-5-carboxypentyl phthalate; MCOP, monocarboxyisooctyl phthalate; MONP, monooxononyl phthalate; MCNP, monocarboxyisononyl phthalate; MHiNCH, cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester; MCOCH, cyclohexane-1,2-dicarboxylic acid monocarboxyisooctyl ester; MECPTP, mono-2-ethyl-5-carboxypentyl; MEHHTP, mono-2-ethyl-5-hydrohexyl terephthalate.
Similarly, estimated mean urinary concentrations of the majority of phthalate metabolites decreased over the study period, while metabolites of alternative chemicals including DINCH have increased (Figure 2). For example, based on adjusted mixed effect models, the estimated mean (95% CI) urinary MHiNCH increased from 0.31 (95% CI: 0.29, 0.33) μg/L in 2010–2011 to 0.78 (95% CI: 0.69, 0.87) μg/L in 2016–2017 (Supplemental Table 3), corresponding to an overall increase of 20.0% (95% CI: 17.8, 22.2) per year (Table 2). While urinary MCOCH concentrations were not detected among our participants between 2010 and 2011, the mean (95% CI) urinary MCOCH was 0.69 (95% CI: 0.63, 0.75) μg/L between 2016 and 2017, increasing by 16.2% (95% CI: 14.0, 18.4) per year (Table 2). In contrast, estimated mean urinary concentrations of MEP and MEHP significantly decreased between 2000 and 2017 [−11.5% per year (95% CI: −12.−9.67) and −11.9 (95% CI: −13.1, −10.8) per year, respectively] (Table 2). Specifically, the estimated mean (95% CI) urinary MEP decreased from 109 (95% CI: 58.8, 138) μg/L in 2000-2001 to 22.0 (95% CI: 18.4, 26.3) μg/L in 2016–2017, and the estimated mean urinary MEHP decreased from 3.81 (95% CI: 2.90, 5.01) μg/L in 2000-2001 to 1.23 (95% CI: 1.13, 1.34) μg/L in 2016–2017 (Supplemental Table 4). Urinary MHBP concentrations remained stable over the study period (Table 2).
Figure 2.
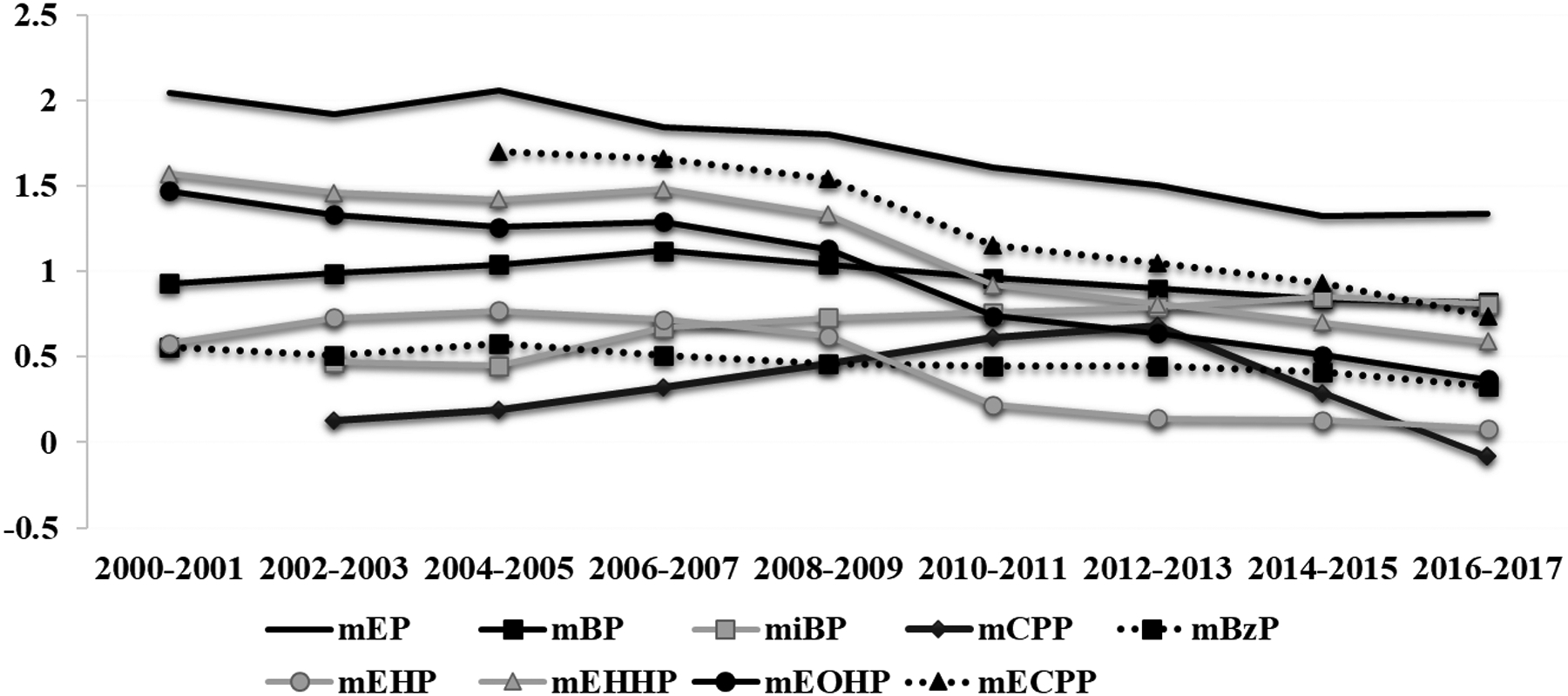
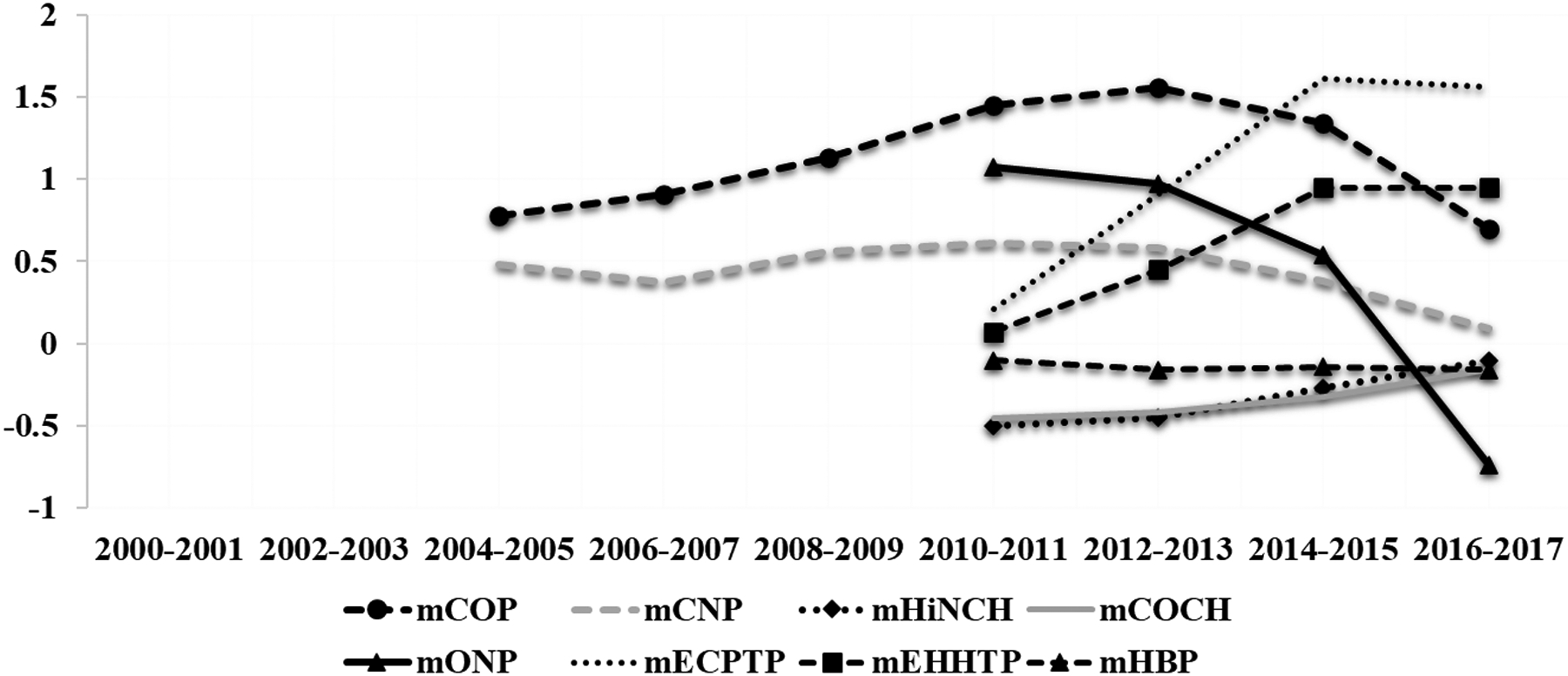
Estimated trendsa in urinary concentrations in phthalate metabolites among study participants between 2000 and 2017.
aLogtransformed mean urinary biomarker concentrations adjusted for age, BMI, sex and specific gravity.
Abbreviations: monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP), mono-hydroxybutyl phthalate (MHBP), mono-isobutyl phthalate (MiBP), mono-3-carboxypropyl phthalate (MCPP), monobenzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), monocarboxyisooctyl phthalate (MCOP), monooxononyl phthalate (MONP), monocarboxyisononyl phthalate (MCNP), mono-2-ethyl-5-carboxypentyl (MECPTP), mono-2-ethyl-5-hydrohexyl terephthalate (MEHHTP), and two metabolites of DINCH (cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester (MHiNCH), and cyclohexane-1,2-dicarboxylic acid monocarboxyisooctyl ester (MCOCH)
We evaluated the robustness of our findings by performing stratified analyses by sex (male vs. female), age (<35 vs. ≥35 years) and BMI (<25 vs. ≥25 kg/m2) (Table 2). Larger overall declines in urinary BPA (interaction test p-value=0.01) and increases in urinary BPS (interaction test p-value=0.32) and BPF (interaction test p-value<0.001) per year were observed in male participants compared to females. We also found that compared to heavier (participants having obesity), those who were leaner (normal BMIs) also had larger adjusted decreases in urinary BPA (interaction test p-value=0.07) and increases in BPF (interaction test p-value=0.95) per year. Estimated decreases in urinary concentrations of triclosan were larger for females (interaction test p-value≤0.001), younger (p of interaction=0.02) and leaner (normal BMI) participants (interaction test p-value=0.90), compared to males, and those who were older and having obesity, respectively. We also observed similar estimated yearly increases in urinary concentrations of MHiNCH and MCOCH for all the stratified groups (Table 2).
Discussion
In this ecological study among male and female patients seeking fertility care in Boston,MA, USA, we describe temporal trends of urinary concentrations of select exposure biomarkers, including environmental phenols, triclosan, phthalates and DINCH metabolites, and biomarkers of some replacement chemicals that are found in every-day consumer products. Overall, we observed declines in urinary concentrations of most of the examined EDCs (e.g. BPA, parabens, triclosan, most phthalate metabolites), but observed increases in estimated mean urinary concentrations of the replacement chemicals (e.g. BPS, BPF, DINCH metabolites). The decreased mean urinary biomarker concentrations in some of these chemicals likely reflect substitution by industry as well as legislative measures to regulate their use in consumer products. Given the increasing trends in urinary concentrations of the relatively new EDCs along with emerging studies linking their exposure with health problems, further studies should focus on exposures to these chemicals.
Biomonitoring data on chemical biomarkers from NHANES can be found for about 350 of the more than 40,000 chemicals used in the U.S (EPA 2023). The increasing concentrations of exposure biomarkers for replacement chemicals like BPS, BPF, and DINCH raise concerns for the need to better understand health impacts of new chemical replacements (Minguez-Alarcon, Souter et al. 2016). Some of the new replacements were introduced into the market without extensive testing as to whether they may cause adverse health effects. Some of the replacements, for instance BPS and BPF, have been associated with obesogenic and diabetogenic effects (Alharbi, Algonaiman et al. 2022). Temporal trends can help to better understand both individual and combined potential impacts of EDCs on human health.
These declining trends in mean concentrations of EDCs with increased concentrations of replacement compounds has been demonstrated in many populational cohorts. Most notably, the Environmental influences on Child Health Outcomes (ECHO) program recently published findings among their cohort of pregnant women demonstrating such temporal trends spanning 2008 to 2020. The ECHO cohort noted these inverse relationships among BPA with BPF/BPS and phthalates (Buckley, Kuiper et al. 2022). In agreement with our results, similar declining trends in mean concentrations of urinary BPA, and increased urinary BPS and BPF have been demonstrated among California pregnant women between 2007–2014 (Kim, Shin et al. 2021), the general population from the U.S. National Health and Nutrition Examination Survey (NHANES) between 2003–2012 (LaKind and Naiman 2015) and from the Canadian Health Measures Survey (CHMS) between 2007–2017 (Pollock, Karthikeyan et al. 2021), a Danish men’s cohort between 2009–2017 (Frederiksen et al), and an Australian population cohort between 2012–2018 (Tang, He et al. 2020). These downward trends in paraben concentrations have also been reported in a Massachusetts pregnancy cohort between 2006–2008 (Ferguson, Meeker et al. 2018), the CHMS cohort between 2007–2017 (Pollock, Karthikeyan et al. 2021), and in a cohort of Puerto Rican pregnant women between 2010–2016 (Ashrap, Watkins et al. 2018).
Temporal trends in triclosan exposure are more variable among reports within the literature. In our study, urinary concentrations of triclosan declined from 19.6 μg/L to 5.4 μg/L from 2008–2017, which differs from previously reported temporal trends. For example, data from the 2003–2012 NHANES (Han, Lim et al. 2016) showed no changes in triclosan concentrations among 10,232 U.S. participants except transiently between 2005–2006. Unlike BPA, among pregnant Puerto Rican women between 2010–2016, triclosan and triclocarban mean urinary concentrations increased during that time period, with women most likely exposed through liquid soap and bar soap products, respectively (Ashrap, Watkins et al. 2018). Some of the observed trends in urinary triclosan in this and the aforementioned studies may be due to its ban by the FDA in September 2016: triclosan and another 18 ingredients used in over-the-counter consumer antiseptic soaps are misbranded and are new drugs for which approved new drug applications are required for marketing (2016). Similar policies have been implemented in Canada and in the European Union (EU-Commission 2014, Canadian-Environmental-Protection 2016, EU-Commission 2016).
Phthalate and chemical replacements showed similar trends as BPA and its replacements, BPS and BPF. Some phthalates showed increasing concentrations, peaking in 2010–2011, with substantially decreasing mean concentrations thereafter through 2017. Additionally, urinary concentrations of MECPTP and MEHHTP, metabolites of di(2-ethylhexyl) terephthalate, a phthalate used to replace others such as DEHP, show dramatic increases from 2010–2017 noted to be 1.63 μg/L to 39.6 μg/L and 1.18 μg/L to 9.11 μg/L, respectively. Similar trends of decreasing mean concentrations of phthalate biomarkers with increasing replacement compounds were noted in NHANES between 2001–2010 (Zota, Calafat et al. 2014), among U.S. women aged 18–22 years in NHANES between 2005–2016 (Beckingham, Wischusen et al. 2022), the ECHO pregnancy women cohort (Buckley, Kuiper et al. 2022) and pregnant women in California between 2007–2013. Internationally, trends towards decreasing phthalate metabolites in Canada and Europe (Dominguez-Romero, Komprdova et al. 2023, Vogel, Frederiksen et al. 2023) reflect the positive impact of industrial phaseout on population exposure risk. For example, data from the German Environmental Specimen Bank suggest that exposures to regulated phthalates, such as DEHP, BBzP, DnBP, and DiBP, have decreased over time while exposure to phthalate substitutes, such as DINCH and DEHTP, appear, to have increased (Vogel, Frederiksen et al. 2023). Conversely, other studies have shown contradictory trends in Chinese populations (Dominguez-Romero, Komprdova et al. 2023), particularly for DEHP, suggesting the continued industrial prevalence of industrial phthalates, a particular concern given the market share that China occupies in global plasticizer use. Overall, these time-trends can be useful in evaluating global differences in exposures to industrial phthalates, allowing for geographic and temporal comparisons. However, special attention needs to be taken when directly comparing urinary biomarker concentrations which are adjusted using different methods (e.g. creatinine vs. SG) as results are only comparable if the urinary biomarkers are in the same scale. For example, it has been demonstrated that SG-adjusted concentrations could be higher than those adjusted for creatinine (Carrieri, Trevisan et al. 2001).
In stratified analyses, we found larger declines in estimated mean concentrations of BPA and increases in BPS and BPF in male participants, potentially due to differential environmental exposures by sex. Additionally, in vitro, the tissue:blood partition coefficient of BPA for fat was 3.31, higher than for kidney, muscle, and other organs (Csanady, Oberste-Frielinghaus et al. 2002), suggesting preferential bioaccumulation within fat cells. Also, it may contribute to the observed sex differences, given the sexual dimorphism of fat distribution in females versus males. Conversely, estimated triclosan declines were larger in participants <35 years of age, with an underweight BMI, and/or female sex. Female participants may have had larger overall exposure as women typically use more personal hygiene products compared to men (Wu, Bennett et al. 2010, Park, Nam et al. 2018), leading to steeper declines following legislative measures and bans.
Limitations of this study include the narrow geographic range of participants within the Boston and New England area, which may reflect local exposures and not necessarily general population exposures throughout the United States over the time period. Also, geographic restriction to the New England region may introduce confounders such as socioeconomic factors, racial or ethnic variations, and educational background that may affect the use of EDC-containing products. Additionally, all samples were collected from patients in a fertility clinic, which may limit generalizability to the general population. Even among states that provide mandated insurance coverage for fertility treatments, patients seeking fertility care tend to skew towards predominantly non-Hispanic white patients with graduate level education and high household incomes (Galic, Negris et al. 2021). Related to this point, we did not evaluate trends in EDCs by race (or other important socioeconomic factors) as sample sizes were small for minority groups. However, median urinary biomarker concentrations were overall similar to those found among participants in NHANES, which is a nationally representative sample of US population (CDC 2022). Strengths of this study include its large sample size, collection spanning 17 years, and prospective study design with samples analyzed by the same CDC laboratory. By collecting multiple samples per participant over the span of several years, we expect that exposure misclassification would be reduced, which is a major limitation in epidemiologic studies of EDCs with short half-lives. Quantification of the EDC biomarkers was conducted by a single CDC laboratory, which allows for consistency and uniformity in analytical chemistry techniques, equipment, and personnel skills. Additionally, we evaluated multiple classes of EDCs, including common, omnipresent chemicals and their replacements. Through this study, we evaluated well-studied commercially available chemicals, including environmental phenols and phthalates, with known health risks (Hauser and Calafat 2005, Matsumoto, Adachi et al. 2005, Keri, Ho et al. 2007, Kamrin 2009, Lyche, Gutleb et al. 2009, Meeker, Ehrlich et al. 2010) to quantify subtle changes in urinary concentrations over time. This is one of the first ecological studies to evaluate multiple EDC classes over 17 years, with a large cohort of male and female patients.
Conclusion
While estimated mean urinary concentrations of most EDCs declined over time among male and female patients attending a fertility center in Boston, concentrations of some of them, including chemical replacements, have increased in this ecological study. Additional studies can help to further explore the potential health effects of these replacement chemicals whose uses appear to be on the rise. Also, additional studies evaluating within-subject temporal trends in EDCs are advised.
Supplementary Material
Highlights.
We evaluated temporal trends in urinary phenols, phthalate metabolites and phthalate replacements between 2000 and 2017.
The majority of urinary exposure biomarkers declined over time.
Urinary concentrations of several replacement chemicals have increased.
Acknowledgments and grant information:
The project was funded by grants R01ES022955, R01ES009718, R01ES033651 and P30ES000002 from the National Institute of Environmental Health Sciences (NIEHS). The authors gratefully acknowledge all members of the EARTH study team, specifically the Harvard T. H. Chan School of Public Health research staff Ramace Dadd and Myra Keller, physicians and staff at Massachusetts General Hospital Fertility Center. We also gratefully acknowledge Manori Silva, Ella Samandar, James Preau, Xiaoliu Zhou, Tao Jia, and the late Xiaoyun Ye (CDC, Atlanta, GA) for technical assistance in measuring the urinary concentrations of the chemical biomarkers. A special thank you to all of the study participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: None of the authors has any conflicts of interest to declare.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC), the US Government, the Department of Health and Human Services (DHHS) or the National Institutes of Health (NIH). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or DHHS.
Declaration of Interest Statement
The authors have no conflicts of interest to disclose.
References
- (2016). “Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Overthe-Counter Human Use. Final rule.” Fed Regist 81(172): 61106–61130. [PubMed] [Google Scholar]
- Alfhili MA and Lee MH (2019). “Triclosan: An Update on Biochemical and Molecular Mechanisms.” Oxid Med Cell Longev 2019: 1607304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi HF, Algonaiman R, Alduwayghiri R, Aljutaily T, Algheshairy RM, Almutairi AS, Alharbi RM, Alfurayh LA, Alshahwan AA, Alsadun AF and Barakat H (2022). “Exposure to Bisphenol A Substitutes, Bisphenol S and Bisphenol F, and Its Association with Developing Obesity and Diabetes Mellitus: A Narrative Review.” Int J Environ Res Public Health 19(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrap P, Watkins DJ, Calafat AM, Ye X, Rosario Z, Brown P, Vélez-Vega CM, Alshawabkeh A, Cordero JF and Meeker JD (2018). “Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in Northern Puerto Rico: Predictors and trends.” Environ Int 121(Pt 1): 990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley DM and Lin YJ (2000). “Clinical evidence for the lack of triclosan accumulation from daily use in dentifrices.” Am J Dent 13(3): 148–152. [PubMed] [Google Scholar]
- Beckingham BA, Wischusen K and Walker JP (2022). “Phthalate exposure among U.S. college-aged women: Biomonitoring in an undergraduate student cohort (2016–2017) and trends from the National Health and Examination Survey (NHANES, 2005–2016).” PLoS One 17(2): e0263578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledzka D, Gromadzinska J and Wasowicz W (2014). “Parabens. From environmental studies to human health.” Environ Int 67: 27–42. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Long M, Hofmeister MV and Vinggaard AM (2007). “Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review.” Environ Health Perspect 115 Suppl 1: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Kim H, Wong E and Rebholz CM (2019). “Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014.” Environ Int 131: 105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Kuiper JR, Bennett DH, Barrett ES, Bastain T, Breton CV, Chinthakindi S, Dunlop AL, Farzan SF, Herbstman JB, Karagas MR, Marsit CJ, Meeker JD, Morello-Frosch R, O’Connor TG, Romano ME, Schantz S, Schmidt RJ, Watkins DJ, Zhu H, Pellizzari ED, Kannan K and Woodruff TJ (2022). “Exposure to Contemporary and Emerging Chemicals in Commerce among Pregnant Women in the United States: The Environmental influences on Child Health Outcome (ECHO) Program.” Environ Sci Technol 56(10): 6560–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Rudel RA, Engel SM, Teitelbaum SL, Whyatt RM and Wolff MS (2015). “Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology.” Environ Health Perspect 123(7): A166168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada, P. o. (2012). Canada Consumer Product Safety Act. M. o. Justice S.C. 2010, c.21.
- Canadian-Environmental-Protection (2016). “Department of the Environment and Department of Health. Order adding a toxic substance to Schedule 1 to the Canadian Environmental Protection Act, 1999. Canada Gazette, Part I 2016;150:88–102 Available at: http://www.gazette.gc.ca/rp-pr/p1/2016/2016-12-10/pdf/g1-15050.pdf.”.
- Carrieri M, Trevisan A and Bartolucci GB (2001). “Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine.” Int Arch Occup Environ Health 74(1): 63–67. [DOI] [PubMed] [Google Scholar]
- Caudill SP, Schleicher RL and Pirkle JL (2008). “Multi-rule quality control for the age-related eye disease study.” Stat Med 27(20): 4094–4106. [DOI] [PubMed] [Google Scholar]
- CDC (2018). “Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Questionnaires, Datasets, and Related Documentation. NHANES 2015–2016. 2015–2016 Lab Methods. Metabolites of Phthalates and Phthalate Alternatives Laboratory Procedure Manual Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/PHTHTE_I_MET.pdf.”
- CDC (2019). “Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Questionnaires, Datasets, and Related Documentation. NHANES 2015–2016. 2015–2016 Lab Methods. Personal Care and Consumer Product Chemicals and Metabolites Laboratory Procedure Manual Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/EPHPP_I_MET.pdf.”
- CDC (2022). “Centers for disease control and prevention. National report on human exposure to environmental chemicals, updated tables, (December 2022) Atlanta, ga: U.S. Department of health and human services, centers for disease control and prevention. Available at https://www.Cdc.Gov/exposurereport/ [accessed January 2023].”. [Google Scholar]
- Chedgzoy P, Winckle G and Heard CM (2002). “Triclosan: release from transdermal adhesive formulations and in vitro permeation across human epidermal membranes.” Int J Pharm 235(1–2): 229–236. [DOI] [PubMed] [Google Scholar]
- Commission, C. P. S. (2014). “Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives Final Report.”
- Cosmetics, C. S. (2005). “Act of 2005.”
- Csanady GA, Oberste-Frielinghaus HR, Semder B, Baur C, Schneider KT and Filser JG (2002). “Distribution and unspecific protein binding of the xenoestrogens bisphenol A and daidzein.” Arch Toxicol 76(5–6): 299–305. [DOI] [PubMed] [Google Scholar]
- Darbre PD (2017). “Endocrine Disruptors and Obesity.” Curr Obes Rep 6(1): 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster S and van Larebeke N (2012). “Endocrine-disrupting chemicals: associated disorders and mechanisms of action.” J Environ Public Health 2012: 713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT and Gore AC (2009). “Endocrine-disrupting chemicals: an Endocrine Society scientific statement.” Endocr Rev 30(4): 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Romero E, Komprdova K, Kalina J, Bessems J, Karakitsios S, Sarigiannis DA and Scheringer M (2023). “Time-trends in human urinary concentrations of phthalates and substitutes DEHT and DINCH in Asian and North American countries (2009–2019).” J Expo Sci Environ Epidemiol 33(2): 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2006). “European Food Safety Authority. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to the 12th list of substances for food contact materials Available at: https://www.efsa.europa.eu/en/efsajournal/pub/395 [accessed Sept 2021].”
- EFSA (2011). “European Union Directive 2011/8/EU.”
- Ehrlich S, Calafat AM, Humblet O, Smith T and Hauser R (2014). “Handling of thermal receipts as a source of exposure to bisphenol A.” JAMA 311(8): 859–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (2023). Environmental Protection Agency. TSCA Chemical Substance Inventory. https://www.epa.gov/tsca-inventory [accessed May 2023]. [Google Scholar]
- EU-Commission (2014). “COMMISSION REGULATION (EU) No 358/2014 of 9 April 2014 amending Annexes II and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products.” Official Journal of the European Union. [Google Scholar]
- EU-Commission (2016). “Commission Implementing Decision (EU) 2016/110 of 27 January 2016 not approving triclosan as an existing active substance for use in biocidal products for product-type 1. Official Journal of the European Union, February 28, 2016 Available at: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016D0110&from=EN.”.
- FDA (2012). “Food and Drug Administration, Indirect Food Additives: Polymers. Vol. 77 41,899”.
- Ferguson KK, Meeker JD, Cantonwine DE, Mukherjee B, Pace GG, Weller D and McElrath TF (2018). “Environmental phenol associations with ultrasound and delivery measures of fetal growth.” Environ Int 112: 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S, Markle T, Thompson S and Wallace E (2012). Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manage. 104: 19–34. [DOI] [PubMed] [Google Scholar]
- Galic I, Negris O, Warren C, Brown D, Bozen A and Jain T (2021). “Disparities in access to fertility care: who’s in and who’s out.” F S Rep 2(1): 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wang L and Kannan K (2014). “Phthalates and parabens in personal care products from China: concentrations and human exposure.” Arch Environ Contam Toxicol 66(1): 113–119. [DOI] [PubMed] [Google Scholar]
- Han C, Lim YH and Hong YC (2016). “Ten-year trends in urinary concentrations of triclosan and benzophenone-3 in the general U.S. population from 2003 to 2012.” Environ Pollut 208(Pt B): 803–810. [DOI] [PubMed] [Google Scholar]
- Hauser R and Calafat AM (2005). “Phthalates and human health.” Occup Environ Med 62(11): 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, Meeker JD, Calafat AM and Williams PL (2016). “Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing in Vitro Fertilization: Results from the EARTH Study.” Environ Health Perspect 124(6): 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ and Calafat AM (2006). “Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites.” Epidemiology 17(6): 682–691. [DOI] [PubMed] [Google Scholar]
- Heindel JJ and Blumberg B (2019). “Environmental Obesogens: Mechanisms and Controversies.” Annu Rev Pharmacol Toxicol 59: 89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra EJ and Simoneau C (2013). “Release of bisphenol A from polycarbonate: a review.” Crit Rev Food Sci Nutr 53(4): 386–402. [DOI] [PubMed] [Google Scholar]
- Hornung RW and Reed LD (1990). “Estimation of Average Concentration in the Presence of Nondetectable Values.” Applied Occupational and Environmental Hygiene 5(1): 46–51. [Google Scholar]
- Huang YQ, Wong CK, Zheng JS, Bouwman H, Barra R, Wahlstrom B, Neretin L and Wong MH (2012). “Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts.” Environ Int 42: 91–99. [DOI] [PubMed] [Google Scholar]
- Improvement, C. P. S. (2008). “Act of 2008. In 2008; pp 122 STAT. 3016–122 STAT. 3077”.
- Kamrin MA (2009). “Phthalate risks, phthalate regulation, and public health: a review.” J Toxicol Environ Health B Crit Rev 12(2): 157–174. [DOI] [PubMed] [Google Scholar]
- Keri RA, Ho SM, Hunt PA, Knudsen KE, Soto AM and Prins GS (2007). “An evaluation of evidence for the carcinogenic activity of bisphenol A.” Reprod Toxicol 24(2): 240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Shin HM, Busgang SA, Barr DB, Panuwet P, Schmidt RJ, Hertz-Picciotto I and Bennett DH (2021). “Temporal Trends of Phenol, Paraben, and Triclocarban Exposure in California Pregnant Women during 2007–2014.” Environ Sci Technol 55(16): 11155–11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaKind JS and Naiman DQ (2015). “Temporal trends in bisphenol A exposure in the United States from 2003–2012 and factors associated with BPA exposure: Spot samples and urine dilution complicate data interpretation.” Environ Res 142: 84–95. [DOI] [PubMed] [Google Scholar]
- Lin YJ (2000). “Buccal absorption of triclosan following topical mouthrinse application.” Am J Dent 13(4): 215–217. [PubMed] [Google Scholar]
- Loganathan SN and Kannan K (2011). “Occurrence of bisphenol A in indoor dust from two locations in the eastern United States and implications for human exposures.” Arch Environ Contam Toxicol 61(1): 68–73. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L and Hartge P (2004). “Epidemiologic evaluation of measurement data in the presence of detection limits.” Environ Health Perspect 112(17): 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M and Skaare JU (2009). “Reproductive and developmental toxicity of phthalates.” J Toxicol Environ Health B Crit Rev 12(4): 225–249. [DOI] [PubMed] [Google Scholar]
- Ma WL, Zhao X, Lin ZY, Mohammed MO, Zhang ZF, Liu LY, Song WW and Li YF (2016). “A survey of parabens in commercial pharmaceuticals from China and its implications for human exposure.” Environ Int 95: 30–35. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Adachi S and Suzuki Y (2005). “Bisphenol A in ambient air particulates responsible for the proliferation of MCF-7 human breast cancer cells and Its concentration changes over 6 months.” Arch Environ Contam Toxicol 48(4): 459–466. [DOI] [PubMed] [Google Scholar]
- Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M and Shimohigashi Y (2007). “Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma.” J Biochem 142(4): 517–524. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, Ye X and Hauser R (2010). “Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic.” Reprod Toxicol 30(4): 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Gaskins AJ, Chiu YH, Souter I, Williams PL, Calafat AM, Hauser R and Chavarro JE (2016). “Dietary folate intake and modification of the association of urinary bisphenol A concentrations with in vitro fertilization outcomes among women from a fertility clinic.” Reprod Toxicol 65: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Souter I, Chiu YH, Williams PL, Ford JB, Ye X, Calafat AM and Hauser R (2016). “Urinary concentrations of cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester, a metabolite of the non-phthalate plasticizer di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), and markers of ovarian response among women attending a fertility center.” Environ Res 151: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RK, Angerer J, Dierkes G, Bruning T and Koch HM (2016). “Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage.” Arch Toxicol 90(11): 2699–2709. [DOI] [PubMed] [Google Scholar]
- Moreta C, Tena MT and Kannan K (2015). “Analytical method for the determination and a survey of parabens and their derivatives in pharmaceuticals.” Environ Res 142: 452–460. [DOI] [PubMed] [Google Scholar]
- Moss T, Howes D and Williams FM (2000). “Percutaneous penetration and dermal metabolism of triclosan (2,4, 4’-trichloro-2’-hydroxydiphenyl ether).” Food Chem Toxicol 38(4): 361–370. [DOI] [PubMed] [Google Scholar]
- Niu Y, Zhang J, Wu Y and Shao B (2012). “Analysis of bisphenol A and alkylphenols in cereals by automated on-line solid-phase extraction and liquid chromatography tandem mass spectrometry.” J Agric Food Chem 60(24): 6116–6122. [DOI] [PubMed] [Google Scholar]
- Pagoni A, Arvaniti OS and Kalantzi OI (2022). “Exposure to phthalates from personal care products: Urinary levels and predictors of exposure.” Environ Res 212(Pt A): 113194. [DOI] [PubMed] [Google Scholar]
- Park GH, Nam C, Hong S, Park B, Kim H, Lee T, Kim K, Lee JH and Kim MH (2018). “Socioeconomic factors influencing cosmetic usage patterns.” J Expo Sci Environ Epidemiol 28(3): 242–250. [DOI] [PubMed] [Google Scholar]
- Park M, Kim S, Kim Y, Nam DJ, Ryoo JH and Lim S (2019). “Relationship between personal care products usage and triclosan exposure: the second Korean National Environmental Health Survey (KoNEHS 2012–2014).” Ann Occup Environ Med 31: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock T, Karthikeyan S, Walker M, Werry K and St-Amand A (2021). “Trends in environmental chemical concentrations in the Canadian population: Biomonitoring data from the Canadian Health Measures Survey 2007–2017.” Environ Int 155: 106678. [DOI] [PubMed] [Google Scholar]
- Rezg R, El-Fazaa S, Gharbi N and Mornagui B (2014). “Bisphenol A and human chronic diseases: current evidences, possible mechanisms, and future perspectives.” Environ Int 64: 83–90. [DOI] [PubMed] [Google Scholar]
- Rochester JR (2013). “Bisphenol A and human health: a review of the literature.” Reprod Toxicol 42: 132–155. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR and Brody JG (2003). “Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust.” Environ Sci Technol 37(20): 4543–4553. [DOI] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G and Ekstrand J (2006). “Pharmacokinetics of triclosan following oral ingestion in humans.” J Toxicol Environ Health A 69(20): 1861–1873. [DOI] [PubMed] [Google Scholar]
- Schettler T (2006). “Human exposure to phthalates via consumer products.” Int J Androl 29(1): 134–139; discussion 181–135. [DOI] [PubMed] [Google Scholar]
- Searle SR, Speed FM and Milliken GA (1980). “Population marginal means in the linear model: an alternative to least square means.” Am Stat 34(4): 216–221. [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL and Calafat AM (2004). “Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000.” Environ Health Perspect 112(3): 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr., Reidy JA, Needham LL and Calafat AM (2007). “Quantification of 22 phthalate metabolites in human urine.” J Chromatogr B Analyt Technol Biomed Life Sci 860(1): 106–112. [DOI] [PubMed] [Google Scholar]
- Soni MG, Carabin IG and Burdock GA (2005). “Safety assessment of esters of p-hydroxybenzoic acid (parabens).” Food Chem Toxicol 43(7): 985–1015. [DOI] [PubMed] [Google Scholar]
- Soni MG, Taylor SL, Greenberg NA and Burdock GA (2002). “Evaluation of the health aspects of methyl paraben: a review of the published literature.” Food Chem Toxicol 40(10): 1335–1373. [DOI] [PubMed] [Google Scholar]
- Tang S, He C, Thai PK, Heffernan A, Vijayasarathy S, Toms L, Thompson K, Hobson P, Tscharke BJ, O’Brien JW, Thomas KV and Mueller JF (2020). “Urinary Concentrations of Bisphenols in the Australian Population and Their Association with the Per Capita Mass Loads in Wastewater.” Environ Sci Technol 54(16): 10141–10148. [DOI] [PubMed] [Google Scholar]
- USFDA (2012). “Food Additive Petition (FAP) 1B4783.”
- USFDA (2016). Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. U. F. a. D. Administration. 81 FR 61106: 61106–61130. [PubMed] [Google Scholar]
- Vogel N, Frederiksen H, Lange R, Jorgensen N, Koch HM, Weber T, Andersson AM and Kolossa-Gehring M (2023). “Urinary excretion of phthalates and the substitutes DINCH and DEHTP in Danish young men and German young adults between 2000 and 2017 - A time trend analysis.” Int J Hyg Environ Health 248: 114080. [DOI] [PubMed] [Google Scholar]
- Weatherly LM and Gosse JA (2017). “Triclosan exposure, transformation, and human health effects.” J Toxicol Environ Health B Crit Rev 20(8): 447–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XM, Bennett DH, Ritz B, Cassady DL, Lee K and Hertz-Picciotto I (2010). “Usage pattern of personal care products in California households.” Food Chem Toxicol 48(11): 3109–3119. [DOI] [PubMed] [Google Scholar]
- Xia B, Zhu Q, Zhao Y, Ge W, Zhao Y, Song Q, Zhou Y, Shi H and Zhang Y (2018). “Phthalate exposure and childhood overweight and obesity: Urinary metabolomic evidence.” Environ Int 121(Pt 1): 159–168. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL and Calafat AM (2005). “Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine.” Anal Chem 77(16): 5407–5413. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Horie M, Hoshino Y and Nakazawa H (2001). “Determination of bisphenol A in canned vegetables and fruit by high performance liquid chromatography.” Food Addit Contam 18(1): 69–75. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dong T, Hu W, Wang X, Xu B, Lin Z, Hofer T, Stefanoff P, Chen Y, Wang X and Xia Y (2019). “Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models.” Environ Int 123: 325–336. [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM and Woodruff TJ (2014). “Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010.” Environ Health Perspect 122(3): 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


