Abstract
The potential of liposome-encapsulated antibiotics for prolonging drug application intervals was investigated by using a murine model of chronic lethal Mycobacterium avium infection. Liposomal encapsulation of amikacin, but not of ciprofloxacin, resulted in high and sustained drug levels in infected tissues, exceeding the minimal inhibitory concentration for M. avium for at least 28 days. As a consequence, once-weekly and even once-monthly treatments with liposomal amikacin significantly reduced bacterial replication in infected tissues and extended the survival time of infected mice.
Mycobacterium avium continues to cause life-threatening disseminated infections in AIDS patients (11, 12, 16, 21). Daily multiple-drug therapy must often be continued throughout life and is frequently accompanied by major side effects, resulting in poor patient compliance (2). Therefore, alternative strategies which reduce the toxicity of the drugs and allow for prolonged application intervals are needed (1, 15).
Previous studies demonstrated the general usefulness of liposome-encapsulated drugs in murine models of M. avium infection (6–9). To capitalize on these findings, the aims of our study were (i) to define suitable application intervals for intermittent therapy based on a pharmacokinetic evaluation of drug accumulation in infected organs and (ii) to establish whether intermittent therapy with prolonged application intervals would be effective in experimental M. avium infection.
Pharmacokinetic studies.
Female C57BL/6 mice (6 to 8 weeks old) were injected intravenously either with a single dose of 600 mg of free or liposome-encapsulated amikacin (Bristol) or with 1,000 mg of free or liposome-encapsulated ciprofloxacin (Bayer). Liposome encapsulation of the drugs was achieved by a modified ethanol injection method, as described previously (5).
Two and 24 h following injection, three mice per regimen were sacrificed and their livers, spleens, and lungs were homogenized in 0.1 N H3PO4. Amikacin levels in the samples were measured by a fluorescence polarization immunoassay (13), and ciprofloxacin was measured by high-performance liquid chromatography analysis (4). The thresholds of detection were 8 and 4 mg/kg of organ, respectively.
Drug concentrations measured 2 h after injection of liposome-encapsulated antibiotics were 35.6 ± 9.8 mg of amikacin/kg of liver, 162.0 ± 14.2 mg of amikacin/kg of spleen, 34.1 ± 2.4 mg of ciprofloxacin/kg of liver, and 69.2 ± 26.2 mg of ciprofloxacin/kg of spleen (means ± standard deviations). In contrast, 2 h after application of aqueous drug preparations, amikacin levels were below the level of detection, while ciprofloxacin was still detectable at 10.2 ± 2.1 mg/kg of liver and 10.7 ± 1.4 mg/kg of spleen. At 24 h postinjection, however, only liposome-encapsulated amikacin resulted in a sustained accumulation of drug in the livers and spleens (59.1 ± 7.5 and 194.0 ± 51.0 mg/kg, respectively), while ciprofloxacin concentrations had fallen below the threshold of detection. Thus, in contrast to previous studies demonstrating the therapeutic efficacy of liposomal ciprofloxacin in vitro (17, 18), our data obtained in vivo show a rapid redistribution of ciprofloxacin from the target tissues, limiting its therapeutic potential.
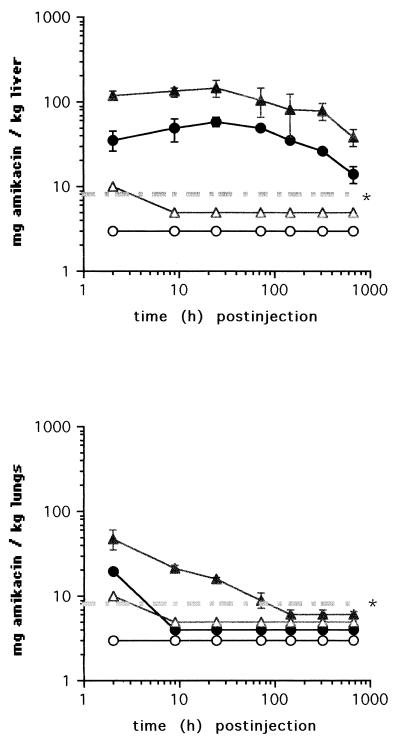
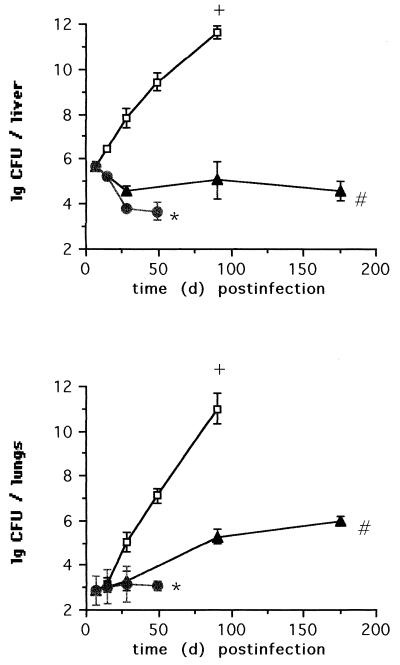
Extended pharmacokinetic studies were therefore performed with amikacin only. For this purpose, amikacin concentrations were determined in the livers, spleens, lungs, and kidneys of uninfected mice at intervals from 2 h to 28 days following injection of a single dose of 2,000 or 600 mg of liposome-entrapped or aqueous amikacin. Two hours after injection of the larger dose, the levels of liposome-encapsulated amikacin in the livers (Fig. 1) and spleens were up to 30-fold higher than those of aqueous amikacin. Throughout a period of 28 days after administration of liposomal amikacin, the drug concentrations in the livers and spleens after injection of either dose exceeded 8 mg/kg of organ. By contrast, 2 to 9 h after injection of aqueous amikacin, drug concentrations fell below the threshold of detection in both organs.
FIG. 1.
Concentrations of amikacin in the livers and lungs of C57BL/6 mice at indicated time points after a single intravenous injection of 600 (circles) or 2,000 (triangles) mg of either liposomal (closed symbols) or free (open symbols) amikacin. The threshold of detection is indicated (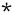 ).
).
With the lungs, administration of liposomal amikacin resulted in approximately a fivefold-higher drug level at 2 h postinjection than administration of aqueous amikacin did. Amikacin concentrations fell below the threshold of detection at 9 h after injection of either the lower dose of liposomal amikacin or the higher dose of the aqueous preparation or at 6 days after injection of the higher dose of liposomal amikacin (Fig. 1). These pharmacokinetic studies extend the original findings of Cynamon et al. (6). Using rats, Swenson et al. (19) previously showed that liposomal encapsulation of gentamicin resulted in the prolonged presence of the drug in the spleens (15 weeks) but not in the lungs (3 days).
Drug accumulation levels and retention times in the kidneys were similar after injection of liposomal and aqueous amikacin, showing a dose-dependent decline throughout the observation period (data not shown). The increased drug accumulation in reticuloendothelial system organs is therefore unlikely to be accompanied by nephrotoxicity. Similar conclusions were reached by Tomioka et al. (20), who studied the accumulation of free and liposome-entrapped kanamycin in the kidneys of mice.
Therapeutic efficacy of liposomal amikacin administered over prolonged treatment intervals.
To test the therapeutic efficacy of once-weekly and once-monthly administration of 2,000 mg of liposomal amikacin during infection, C57BL/6 mice were inoculated intravenously with 105 CFU of the virulent M. avium strain TMC 724 (a gift from F. Collins, Trudeau Institute, Saranac Lake, N.Y.). Liposomal amikacin was given intravenously for 6 consecutive weeks or months, starting on day 7 after infection, and bacterial loads were monitored in the livers, spleens, and lungs of treated and untreated mice, as described previously (9, 10). This model of infection was chosen because it leads to the death of even immunocompetent mice 120 to 150 days after infection (9).
By the end of therapy, weekly treatment with liposomal amikacin had reduced the bacterial counts in the livers by more than 2 orders of magnitude and replication of M. avium organisms in the spleens (data not shown) and lungs had stabilized (Fig. 2). Thus, a once-weekly treatment schedule proved to be as effective as previously reported regimens of twice-weekly or even daily injections (3, 6, 8, 9).
FIG. 2.
Time course of M. avium infection in the livers and lungs of C57BL/6 mice treated intravenously once weekly (closed circles) or once monthly (closed triangles) with 2,000 mg of liposome-encapsulated amikacin or left untreated (open squares). The time of death of control infected mice (+), the end of the observation period after treatment with weekly application intervals (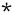 ), and the end of the observation period after treatment with monthly application intervals (#) are indicated.
), and the end of the observation period after treatment with monthly application intervals (#) are indicated.
One month after a single injection of liposomal amikacin, the bacterial loads in the livers were also found to be reduced. Continuation of the once-monthly therapy, however, did not further reduce the bacterial load (Fig. 2). In the spleens and lungs, bacterial counts increased during once-monthly treatment, albeit at a lower rate than that in untreated controls. As a reflection of the low drug levels achieved in the lungs after administration of liposomal amikacin, overall treatment efficacy in the lungs was poor (Fig. 2) (7, 8, 14).
Most importantly, monthly treatment led to a significantly prolonged survival time of the treated animals, which all survived the observation period of 7 months, whereas all control infected animals died approximately 4 months after time of infection. This benefit was also observed when treatment was initiated at an advanced stage of infection.
In conclusion, our pharmacokinetic data concerning drug accumulation in infected organs provided a sound basis for predicting treatment results. Since administration of a liposomal preparation of amikacin resulted in elevated drug concentrations in target tissues for up to 28 days, treatment was effective even when given only once a month. The potential of liposomal amikacin in the therapy of M. avium infection, i.e., in the substantial extension of application intervals, warrants further study with humans.
Acknowledgments
We thank Ellen Borner for expert technical assistance.
This work was supported by grant MU 708/9-1 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Bakker-Woudenberg I A J M, Storm G, Woodle M C. Liposomes in the treatment of infections. J Drug Target. 1994;2:363–371. doi: 10.3109/10611869408996811. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez L E, Inderlied C B, Young L S. Mycobacterium avium complex in AIDS. Curr Clin Top Infect Dis. 1992;12:257–281. [PubMed] [Google Scholar]
- 3.Bermudez L E, Yau-Young A O, Lin J-P, Cogger J, Young L S. Treatment of disseminated Mycobacterium avium complex infection of beige mice with liposome-encapsulated aminoglycosides. J Infect Dis. 1990;161:1262–1268. doi: 10.1093/infdis/161.6.1262. [DOI] [PubMed] [Google Scholar]
- 4.Borner K, Lode H, Höffken G, Prinzing C, Glatzel P, Wiley R. Liquid chromatographic determination of ciprofloxacin and some metabolites in human body fluids. J Clin Chem Clin Biochem. 1986;24:325–331. doi: 10.1515/cclm.1986.24.5.325. [DOI] [PubMed] [Google Scholar]
- 5.Bucke W E, Ehlers S, Leitzke S, Diederichs J E, Hahn H, Müller R H. Proceedings of the First World Meeting on Pharmaceutics, Biopharmaceutics and Biotechnology. 7th International Conference on Pharmacological Technology, Budapest, Hungary. 1995. Optimized liposomal amikacin for treatment of disseminated mycobacterial infections; pp. 545–546. [Google Scholar]
- 6.Cynamon M H, Swenson C E, Palmer G S, Ginsberg R S. Liposome-encapsulated-amikacin therapy of Mycobacterium avium complex in beige mice. Antimicrob Agents Chemother. 1989;33:1179–1183. doi: 10.1128/aac.33.8.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Düzgünes N, Ashtekar D R, Flasher D L, Ghori N, Debs R J, Friend D S, Gangadharam P R J. Treatment of Mycobacterium avium-intracellulare complex infection in beige mice with free and liposome-encapsulated streptomycin: role of liposome type and duration of treatment. J Infect Dis. 1991;164:143–151. doi: 10.1093/infdis/164.1.143. [DOI] [PubMed] [Google Scholar]
- 8.Düzgünes N, Perumal V K, Kesavalu L, Goldstein J A, Debs R J, Gangadharam P R J. Enhanced effect of liposome-encapsulated amikacin on Mycobacterium avium-M. intracellulare complex infection in beige mice. Antimicrob Agents Chemother. 1988;32:1404–1411. doi: 10.1128/aac.32.9.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehlers S, Bucke W, Leitzke S, Smith D, Fortmann L, Hänsch H, Hahn H, Bancroft G, Müller R. Liposomal amikacin for treatment of experimental Mycobacterium avium infections mimicking clinically relevant disease. Int J Med Microbiol Virol Parasitol Infect Dis. 1996;284:218–231. doi: 10.1016/s0934-8840(96)80097-1. [DOI] [PubMed] [Google Scholar]
- 10.Hänsch H C R, Smith D A, Mielke M E A, Hahn H, Bancroft G J, Ehlers S. Mechanisms of granuloma formation in murine Mycobacterium avium infection: the contribution of CD4+ T cells. Int Immunol. 1996;8:1299–1310. doi: 10.1093/intimm/8.8.1299. [DOI] [PubMed] [Google Scholar]
- 11.Horsburgh C R, Jr, Selik R M. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS) Am Rev Respir Dis. 1989;139:4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- 12.Inderlied C B, Kemper C A, Bermudez L E M. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iolley M E, Stroupe S D, Wang C-H J, Panas H N, Keegan C L, Schmidt R L, Schwenzer K S. Fluorescence polarisation immunoassay. Monitoring aminoglycoside antibiotics in serum and plasma. Clin Chem. 1981;27:1190–1197. [PubMed] [Google Scholar]
- 14.Klemens S P, Cynamon M H, Swenson C E, Ginsberg R S. Liposome-encapsulated-gentamicin therapy of Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1990;34:967–970. doi: 10.1128/aac.34.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreuter, J. 1991. Liposomes and nanoparticles as vehicles for antibiotics. Infection 19(Suppl. 4):224–228. [DOI] [PubMed]
- 16.Low N, Pfluger D, Egger M. Disseminated Mycobacterium avium complex disease in the Swiss HIV Cohort Study: increasing incidence, unchanged prognosis. AIDS. 1997;11:1165–1171. doi: 10.1097/00002030-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Majumdar S, Flasher D, Friend D S, Nassos P, Yajko D, Hadley W K, Düzgünes N. Efficacies of liposome-encapsulated streptomycin and ciprofloxacin against Mycobacterium avium-M. intracellulare complex infections in human peripheral blood monocyte/macrophages. Antimicrob Agents Chemother. 1992;36:2808–2815. doi: 10.1128/aac.36.12.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh Y-K, Nix D E, Straubinger R M. Formulation and efficacy of liposome-encapsulated antibiotics for therapy of intracellular Mycobacterium avium infection. Antimicrob Agents Chemother. 1995;39:2104–2111. doi: 10.1128/aac.39.9.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swenson C E, Stewart K A, Hammett J L, Fitzsimmons W E, Ginsberg R S. Pharmacokinetics and in vivo activity of liposome-encapsulated gentamicin. Antimicrob Agents Chemother. 1990;34:235–240. doi: 10.1128/aac.34.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomioka H, Saito H, Sato K, Yoneyama T. Therapeutic efficacy of liposome-encapsulated kanamycin against Mycobacterium intracellulare infection induced in mice. Am Rev Respir Dis. 1991;144:575–579. doi: 10.1164/ajrccm/144.3_Pt_1.575. [DOI] [PubMed] [Google Scholar]
- 21.Young L S. Mycobacterium avium complex infection. J Infect Dis. 1988;157:863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]