Abstract
Background:
The co-use of nicotine and cannabis has been steadily rising in the United States. Rodent studies suggest that delta-9-tetrahydrocannabinol (THC) could increase addictive qualities of nicotine, but whether repeated THC exposure alters self-administration of nicotine has not been tested. We hypothesized that THC would increase the reinforcing effects of nicotine and alter nicotine intake.
Methods:
Adult male and female Sprague-Dawley rats were treated with THC (0, 3, 30 mg/kg) daily for 14 days prior to and during training for intravenous self-administration of nicotine. Rats were allowed to self-administer nicotine for several weeks, then tested for sensitivity to nicotine dose through multiple determinations of a nicotine dose-effect curve with or without THC pretreatment. A separate set of rats were trained on fixed ratio responding for sucrose and assessed for THC effects on behavior.
Results:
Post-session THC decreased nicotine self-administration in male and female rats throughout acquisition and maintenance and increased the latency to stable rates of nicotine intake during acquisition. Post-session THC shifted nicotine dose-effect curves downward, and pre-session THC suppressed responding at higher nicotine doses. Unlike nicotine, responding for sucrose was not affected by post-session THC. Pre-session THC decreased responding for sucrose, particularly for THC-naïve rats.
Conclusions:
Repeated post-session THC decreased nicotine-taking behaviors but did not alter sucrose responding. Thus, post-session THC may alter sensitivity to nicotine. Pre-session THC treatment decreased lever pressing in both sucrose and nicotine studies, indicating this effect was nonspecific. These studies show that THC modulates patterns of nicotine intake in rat models.
Keywords: Nicotine, Delta-9-tetrahydrocannabinol, Cannabis, Polysubstance use, Co-abuse, Self-administration
1.0. Introduction
Cannabis and tobacco polysubstance use (CT-PSU) is increasing in the United States (Ruglass et al., 2020; Schauer et al., 2015; Schauer et al., 2017; Weinberger et al., 2021; Weinberger et al., 2022). Compared to tobacco only use, CT-PSU can lead to greater dependence on tobacco (Patton et al., 2005; Rubinstein et al., 2014), poorer tobacco-cessation outcomes (Agrawal et al., 2012; Hindocha et al., 2015; Peters et al., 2012; Ream et al., 2008), increased tobacco relapse (Budney et al., 2008), and may be associated with additive health risks relative to use of either substance alone (Weinberger et al., 2020a; Weinberger et al., 2020b). While only a few published human laboratory studies have evaluated pharmacological and behavioral effects of CT-PSU, findings to date have suggested interactions between the effects of nicotine and the primary psychoactive constituent of cannabis, delta-9-tetrahydrocannabinol (THC). For example, nicotine, administered via transdermal patch 4 h before smoking a cannabis joint, did not alter plasma levels of THC; however, it enhanced cannabis-related increases in heart rate, subject ratings of feeling “stimulated,” and scores on the amphetamine scale of the Addiction Research Center Inventory (Penetar et al., 2005).
Similarly, preclinical research with rodents indicates that prior exposure to nicotine or THC enhances the rewarding or reinforcing effects of the other drug (Kohut, 2016; Panlilio et al., 2013; Stringfield et al., 2023). Co-administration of low doses of nicotine and THC produced conditioned place preference in rodents, while administration of the same doses of nicotine and THC individually did not produce place preference or place aversion (Valjent et al., 2002). Interestingly, nicotine self-administration was reduced on a fixed ratio (FR) schedule of reinforcement but enhanced on a progressive ratio (PR) schedule when the cannabinoid receptor 1 (CB1) agonist WIN 55,212–2 was administered pre-session (Gamaleddin et al., 2012). Furthermore, WIN 55,212–2 reinstated nicotine seeking and enhanced cue-induced nicotine seeking (Gamaleddin et al., 2012). To date, most rodent literature on CT-PSU indicates that co-administration of THC and nicotine may increase the rewarding or reinforcing effects of these drugs and/or decrease their aversive effects, but there are exceptions (Le Foll et al., 2006).
The present study examined the effects of co-administration of nicotine and THC in rodent models of human CT-PSU patterns. Due to inability to demonstrate reinforcing effects of THC in multiple past self-administration studies (Abraham et al., 2020; Deiana et al., 2007; Lefever et al., 2014), we focused on nicotine self-administration alone and in combination with experimenter-administered THC. It is important to note, however, that rats will self-administer THC at greater than vehicle levels under some conditions, such as when experimenter administered nicotine is delivered pre-session (Stringfield et al., 2023). We investigated the timing and sequence of intraperitoneal (i.p.) THC exposure on acquisition and maintenance of nicotine self-administration in rats. Our model encompassed several patterns of CT-PSU that have been identified in humans (Apollonio et al., 2019; Tucker et al., 2019): single substance (nicotine only) use; separate use (both tobacco and cannabis use during the last 30 days, but not within the same “session”); simultaneous use (use of cannabis and tobacco in sequence during the same “session” – i.e., “chasing”); and combinations of these patterns (e.g., separate and simultaneous use). Rats in this study were exposed to THC before nicotine, modeling initiation of cannabis use before tobacco use (Agrawal et al., 2011; Becker et al., 2015; Patton et al., 2005), which is becoming a more common pattern of initiation, particularly among youth (Fairman et al., 2019). Lastly, to confirm whether our observed effects were specific to nicotine self-administration, we assessed the effects of post- and pre-session i.p. THC exposure on sucrose self-administration.
2.0. Materials and Methods
2.1. Subjects
Adult male (N=48) and female (N=48) Sprague-Dawley rats (Envigo, Frederick, MD), aged postnatal day 60–70 at the start of the experiment, were singly housed in polycarbonate home cages. All males were fed 17 g of rodent chow daily and all females were fed 15 g, which allowed moderate weight gain throughout the study. This level of food restriction has been used in past nicotine self-administration studies (Caine et al., 2014; Marusich and Palmatier, 2023; Marusich et al., 2019; Palmatier et al., 2020). Rats were maintained in a temperature- (20–26°C) and humidity-controlled (30–70%) environment with a 12-hour light-dark cycle (lights on at 0700). Rats had free access to water in the home cage. Experiments complied with the ARRIVE guidelines, principles of laboratory animal care (National Research Council, 2011), and with the Institutional Animal Care and Use Committee for RTI.
2.2. Apparatus
Experimental sessions were conducted in operant conditioning chambers for rats (MED Associates, St. Albans, VT) housed inside sound-attenuating chambers (MED Associates). Each chamber contained two retractable levers (designated as active or inactive levers; counterbalanced for side across subjects), a stimulus light over each lever, a house light, a fan for ventilation, and speakers for white noise. Infusion pumps (MED Associates) were located outside the cubicle. MED-PC software (Med Associates) arranged experimental events and recorded data.
2.3. Drugs/Chemicals
THC (Δ9-tetrahydrocannabinol) was obtained from the National Institute on Drug Abuse (NIDA, Bethesda, MD) Drug Supply Program. (−)-Nicotine tartrate salt (Sigma-Aldrich, St. Louis, MO), was dissolved in physiological saline (Patterson Veterinary Supply, Blythewood, SC). Due to mild acidity, the pH of the nicotine solution was adjusted to approximately neutral (pH ≈ 7). Nicotine doses are expressed as μg/kg of the base. THC was suspended in 7.8% polysorbate 80 (Fisher Scientific, Pittsburgh, PA) and 92.2% saline (Patterson Veterinary Supply). Stock solutions of THC were typically made approximately every 3 months, and stock solutions of nicotine were typically made every 1–3 months. These stock solutions were frequently diluted to create the doses/concentrations needed for the study. All drug solutions were stored at approximately 4°C. Gentamicin and heparin were purchased from Patterson Veterinary Supply. Glycerol was obtained from Fisher Scientific. Experimenter-administered THC and vehicle were injected i.p. at a volume of 1 ml/kg.
2.4. Surgery for Nicotine Self-administration Rats (Experiment 1)
Rats were surgically implanted with chronic indwelling jugular catheters under isoflurane anesthesia. Surgeries were conducted at the rodent vendor (Envigo). Briefly, an incision was made on the chest to access the jugular vein. One end of the catheter (0.6 mm internal diameter) was inserted into the vein, while the other end was tunneled subcutaneously around the shoulder to exit between the scapula where it was attached to a quick connect harness (SAI Infusion Technologies, Lake Villa, IL). Rats were allowed to recover from surgery for a minimum of seven days before beginning the experiment. For three days following surgery, rats were administered 3 mg/kg ketoprofen daily. All catheters were checked for patency prior to the start of the experiment by attaching a syringe to the external end of the catheter and pulling back on the syringe to determine if blood could be extracted from the catheter. Catheters were not explicitly checked for patency later in the study because in our experience, non-patent catheters are evident through either an inability to infuse liquid into the catheter or pooling of liquid where the catheter exits the body between the scapula, both of which are noticeable to the experimenter. If animals displayed these signs of catheter malfunction, they were dropped from the study.
2.5. Daily THC Administration (Experiments 1 and 2)
Rats used in all studies described below were randomly assigned to one of three THC dose groups (0, 3, or 30 mg/kg). Each group received its assigned vehicle or THC dose daily in the afternoon throughout the entire study. These THC doses were chosen to model chronic moderate and high THC use. In rats, 3 mg/kg THC (moderate dose) has psychoactive effects and is a common training dose for THC discrimination in rats (Wiley et al., 2021; Wiley et al., 2014), whereas 30 mg/kg THC (high dose) often suppresses responding in naïve rats when administered before an operant session (Wiley et al., 2017). Notably, these THC doses are higher than daily intake in past rat self-administration studies (Lefever et al., 2014; Neuhofer et al., 2020; Spencer et al., 2018; Stringfield et al., 2023; Stringfield and Torregrossa, 2021), but that may be because THC is not self-administered above vehicle levels in rats except under very specific circumstances (Stringfield et al., 2023). Two weeks prior to beginning operant sessions, rats began daily experimenter administration of their assigned THC dose to allow rats time to develop tolerance to potential rate-suppressant effects of THC (Table 1). During this time, rats with catheters had their catheters locked with 0.05 ml of a standard glycerol lock solution, which contained 50% glycerol and 50% heparin (500 IU heparin/ml). The catheter lock was flushed into the catheter with saline approximately once every four days, after which another 0.05 ml of catheter lock was infused.
Table 1.
Timetable for rats in the nicotine self-administration study.
| Pre-session Injection (For all THC Groups) | Experimental Session | Post-session Injection (Daily Throughout the Study) | Duration of this Phase |
|---|---|---|---|
| None | None | According to group assignment (0, 3, or 30 mg/kg THC) | 14 Days |
| None | Autoshaping (sucrose reinforcement) | According to group assignment | 3 Days |
| None | Acquisition of self-administration (FR1–5; 30 μg/kg/inf nicotine) | According to group assignment | 30 Days |
| None | FR5; 1.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| None | FR5; 7.5 μg/kg/inf nicotine | According to group assignment | 3 Days |
| None | FR5; 15.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| None | FR5; 30.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| None | FR5; Saline | According to group assignment | 5 Days |
| THC Vehicle on Day 6 | FR5; 30.0 μg/kg/inf nicotine | According to group assignment | 6 Days |
| 0.3 mg/kg THC on Day 3 | FR5; 1.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 0.3 mg/kg THC on Day 3 | FR5; 7.5 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 0.3 mg/kg THC on Day 3 | FR5; 15.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 0.3 mg/kg THC on Day 3 | FR5; 30.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 0.3 mg/kg THC on Day 5 | FR5; Saline | According to group assignment | 5 Days |
| THC Vehicle on Day 6 | FR5; 30.0 μg/kg/inf nicotine | According to group assignment | 6 Days |
| 3.0 mg/kg THC on Day 3 | FR5; 1.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 3.0 mg/kg THC on Day 3 | FR5; 7.5 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 3.0 mg/kg THC on Day 3 | FR5; 15.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 3.0 mg/kg THC on Day 3 | FR5; 30.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 3.0 mg/kg THC on Day 5 | FR5; Saline | According to group assignment | 5 Days |
| THC Vehicle on Day 6 | FR5; 30.0 μg/kg/inf nicotine | According to group assignment | 6 Days |
| 30.0 mg/kg THC on Day 3 | FR5; 1.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 30.0 mg/kg THC on Day 3 | FR5; 7.5 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 30.0 mg/kg THC on Day 3 | FR5; 15.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 30.0 mg/kg THC on Day 3 | FR5; 30.0 μg/kg/inf nicotine | According to group assignment | 3 Days |
| 30.0 mg/kg THC on Day 5 | FR5; Saline | According to group assignment | 5 Days |
2.6. Experiment 1: Nicotine Self-Administration
Throughout self-administration, catheter patency was maintained by flushing catheters daily with 0.2 ml of a solution (0.38% gentamicin, 2.88% heparin, 96.74% saline) after testing. Daily THC administration continued throughout the self-administration phase to provide daily exposure to both THC and nicotine. Daily experimenter administered injections of THC (or vehicle) were administered 3 h after the self-administration session ended to investigate effects of separate nicotine and THC exposure (Apollonio et al., 2019; Tucker et al., 2019) with minimal overlap in direct effects of THC and nicotine (Table 1). Experimental sessions were conducted seven days a week throughout the study.
2.6.1. Response lever training.
Rats were trained to respond on the active lever for sucrose through autoshaping (Brown and Jenkins, 1968; Marusich et al., 2019) for three sessions. During autoshaping, active lever extension was paired with delivery of a sucrose pellet (45 mg; Bioserv Inc., Frenchtown, NJ) on a random time 60 s schedule. Following 20 s of lever extension, or immediately after a lever press, a pellet was delivered. Pellet delivery was followed by a 20 s timeout signaled by illumination of both stimulus lights. Autoshaping sessions delivered approximately 60 pellets within the first 90 min, then rats remained in the chamber for 30 min with only the inactive lever present to provide additional exposure to the lack of contingency for responding on the inactive lever. Throughout all training and self-administration sessions, the inactive lever was extended, and presses on this lever were recorded, but had no programmed consequence.
2.6.2. Acquisition of nicotine self-administration.
Following autoshaping, nicotine (30 μg/kg/infusion) was available for i.v. self-administration on a fixed ratio (FR) 1 schedule of reinforcement for 5 days, on a FR2 for 3 days, and then on a FR5 for 22 days (Clemens et al., 2009; Donny et al., 1999; Donny et al., 1998; Marusich et al., 2019). The FR schedule of reinforcement meant that an infusion of nicotine was delivered when a specific number of responses were made on the active lever (e.g., on FR5, an infusion of nicotine was delivered for every fifth response). Rats were maintained on FR5 longer than most nicotine self-administration studies because of delays in attaining stable drug intake for some rats, particularly those in the 30 mg/kg THC group. This training dose was chosen because it reliably led to self-administration in previous research (Brennan et al., 2015; Donny et al., 1998; Marusich et al., 2019; Shram et al., 2008). Meeting the ratio requirement on the active lever produced an infusion of nicotine. Infusions were 0.1 ml, occurred over the course of 3.8 s, and were followed by a 20 s timeout signaled by illumination of both stimulus lights (Fowler and Kenny, 2011; Gomez et al., 2015; Karatayev et al., 2015; Morganstern et al., 2013; Sorge and Clarke, 2009). Self-administration sessions lasted 2 h (Arnold et al., 2014; Brennan et al., 2015; Brunzell et al., 2010). To avoid differential exclusion of rats across THC groups, there were no acquisition criteria for self-administration. All rats continued in the study, regardless of how much nicotine they self-administered.
2.6.3. Nicotine dose-effect curve determinations.
In the next phase of the experiment, the nicotine dose-effect curve was determined four times with different pre-session conditions for each curve (Table 1). For all nicotine dose-effect curves, rats had access to 1.0, 7.5, 15.0, and 30.0 μg/kg/infusion nicotine on an FR5 schedule of reinforcement. Doses were presented in ascending order, and each dose was tested for three consecutive sessions. Then, rats had access to saline for five sessions to provide a vehicle comparison. Subsequently, responding was reestablished on the training dose of nicotine for six sessions. On the last day of 30.0 μg/kg/infusion nicotine, THC vehicle was administered 30 min pre-session to ensure that it did not disrupt responding for the training dose of nicotine.
The first dose-effect curve determined the effects of nicotine alone, and no pre-session THC injections were given. This provided a comparison of the nicotine dose-effect curve in single use (0 mg/kg THC group) and separate nicotine and THC use conditions (3 and 30 mg/kg daily THC groups) during which the effects of nicotine and THC did not overlap (Table 2). During the subsequent three nicotine dose-effect curves, a dose of THC was administered prior to the self-administration session on some days, and modeled effects that might be associated with using tobacco as a “chaser” after cannabis use (vehicle group, e.g., simultaneous use) or simultaneous and separate use (daily THC 3 and 30 mg/kg groups) (Table 2). THC doses administered pre-session were 0.3, 3.0, and 30.0 mg/kg, and were evaluated in ascending order. These doses were chosen to provide one dose that substitutes for THC in THC discrimination (3.0 mg/kg), one dose that fails to substitute (0.3 mg/kg), and a third dose that decreases response rate in drug discrimination (30.0 mg/kg) (Wiley et al., 2021; Wiley et al., 2014; Wiley et al., 2017). During these simultaneous exposure dose-effect sessions, THC was administered on the last day of exposure to each dose of nicotine (or saline) 30 min before the session (Table 1), which minimized the development of tolerance to THC in the 0 mg/kg THC group.
Table 2.
Overall experimental design of the nicotine self-administration experiment.
| Nicotine Dose-Effect Curves | |||
|---|---|---|---|
| 0 mg/kg | 3 mg/kg | 30 mg/kg | |
| 1st nicotine self-administration dose-effect curve (Figure 2) | Single (nicotine only) use | Separate use (low dose daily THC and nicotine at different times) | Separate use (high dose daily THC and nicotine at different times) |
| Subsequent three nicotine dose-effect curves (pre-injection with 0.3, 3, or 30 mg/kg THC before nicotine session) (Figure 3) | Simultaneous use (THC injection 30 min prior to nicotine self-administration session) | Simultaneous and separate use (THC injection 30 min prior to nicotine self-administration session AND low dose daily THC and nicotine at different times) | Simultaneous and separate use (THC injection 30 min prior to nicotine self-administration session AND high dose daily THC and nicotine at different times) |
2.7. Experiment 2: Sucrose Self-Administration
Separate groups of rats responded for sucrose pellets instead of nicotine. Rats in the sucrose self-administration procedure did not have surgery or jugular catheters, nor were they exposed to any chemicals used during surgery or catheter maintenance. Two weeks prior to beginning operant sessions, rats began experimenter administration of their assigned daily THC dose (0, 3, or 30 mg/kg). Post-session THC or vehicle administration continued throughout sucrose self-administration to provide daily exposure to both THC/vehicle and sucrose. Daily experimenter administered injections of THC (or vehicle) were administered 3 h after the self-administration session ended to maintain consistency with the procedures used in the nicotine study. Experimental sessions were conducted seven days a week throughout the study. The timeline for the sucrose self-administration experiment was the same as that shown in Table 1 except that rats had access to sucrose in all self-administration sessions instead of nicotine (or saline).
2.7.1. Response lever training.
Rats were trained to respond on the active lever for sucrose through autoshaping for three sessions. Session parameters were the same as those used during autoshaping for the nicotine self-administration group except that for sucrose rats, autoshaping sessions delivered approximately 20 pellets within the first 23 min. Rats then remained in the chamber for 7 min with only the inactive lever present.
2.7.2. Acquisition of sucrose self-administration.
Following autoshaping, sucrose pellets were available for self-administration on a FR1 schedule of reinforcement for five days, on a FR2 for three days, and then on a FR5 for 22 days. Meeting the ratio requirement on the active lever delivered a 45 mg sucrose pellet throughout the study, and pellet deliveries were followed by a 20 s timeout signaled by illumination of both stimulus lights. Sucrose self-administration sessions lasted 30 min to avoid satiation. There were no acquisition criteria for sucrose self-administration.
2.7.3. Sucrose self-administration with pre-session THC.
Due to the complexity of determining different magnitudes of sucrose (e.g., number of pellets) that mimicked the different levels of magnitude in nicotine doses used in the nicotine study, a single magnitude of sucrose was used throughout the study (e.g., meeting the ratio requirement on the active lever always delivered one 45 mg pellet). Therefore, in this phase of the experiment, rats continued to respond for sucrose pellets on a FR5 schedule of reinforcement for the same number of days that rats in the nicotine study were in the nicotine dose-effect determination phases. Thus, after acquisition, rats continued in the sucrose study for another 22 sessions with no pre-session injections (Table 1). This provided a comparison of responding for sucrose in the absence of THC (e.g., 0 mg/kg THC group) and the presence of daily post-session THC (3 and 30 mg/kg THC) during which the effects of THC did not overlap with the session. On the 23rd day, THC vehicle was administered 30 min pre-session to ensure that it did not disrupt responding for sucrose.
During the subsequent three sets of 23 sessions, a dose of THC was administered prior to the session on some days. This provided control conditions for the dose-effect curves in the nicotine study that modeled effects associated with using tobacco after cannabis use. Pre-session THC doses (0.3, 3.0, and 30.0 mg/kg) were evaluated in ascending order. During each set of 23 sucrose sessions, THC was administered 30 min before the session once every 3–5 days to mimic administering THC on the last day of exposure to each dose of nicotine (or saline) for a total of five pre-session administrations of each dose of THC to match the nicotine study. Prior to the 23rd session of each set, THC vehicle was administered 30 min pre-session. Throughout all phases of the sucrose study, rats continued to receive their assigned daily dose of THC or vehicle 3 h after the self-administration session.
2.8. Data Analysis
The number of infusions/pellets earned and responses on the active and inactive lever were recorded for each session. Analyses for lever presses are described in the supplement. Only the final session of exposure to each dose in the nicotine dose-effect curves was used in graphical and statistical analyses because it was the only session preceded by THC administration. For the sucrose study, each session with THC pretreatment was included in graphs and analyses. Sessions in the sucrose study from the same timepoints (e.g., same session number) as those in the nicotine dose-effect curve with no pretreatment were analyzed for comparison. Subjects dropped due to catheter problems and health issues are listed in the supplement.
2.8.1. Experiment 1: Nicotine self-administration.
A session x THC group x sex ANOVA comparing infusions over the course of the initial 22 sessions of FR5 responding during acquisition was conducted to determine if THC impacted the amount of nicotine earned during acquisition (Figure 1). Infusions earned during the initial dose-effect curve with no pretreatments were analyzed with a nicotine dose x THC group x sex ANOVA (Figure 2). Each set of nicotine dose-effect curves for a given THC post-session group were compared with separate three-way split-plot ANOVAs, with between factor as sex and within factors of THC pretreatment dose (including no pretreatment) and nicotine dose (Figure 3). A mean substitution procedure was used for cases of missing data when data were only missing for one rat (e.g., one male 0 mg/kg THC rat was dropped from the study after the first two dose-effect curves); however, the female 30 mg/kg THC group had a lot of attrition, thus, only data from the four females that completed the study were included in analyses for that group.
Figure 1.
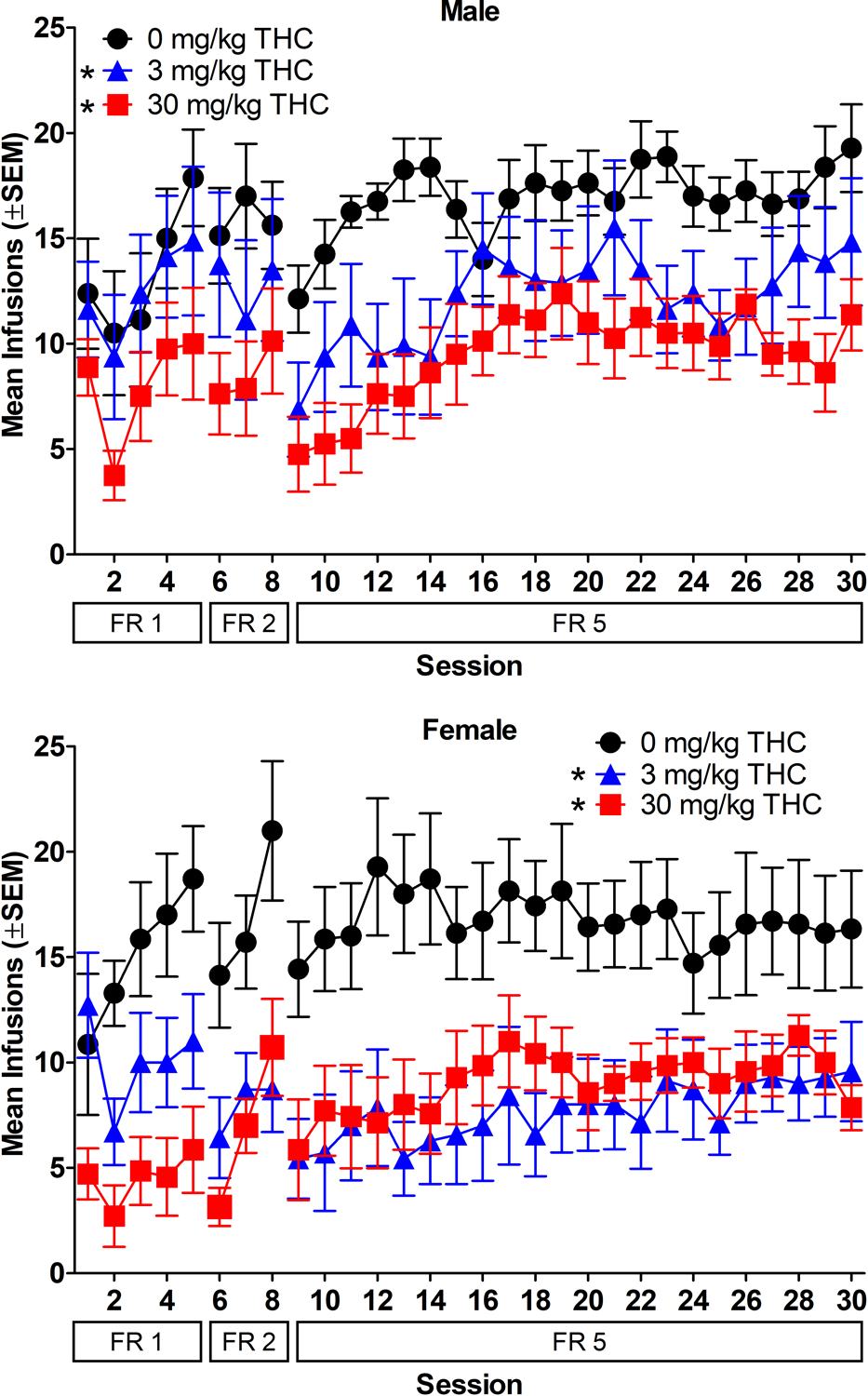
Acquisition of nicotine self-administration (30 μg/kg/infusion). Mean infusions earned as a function of session and FR value. Data for males are shown in the top panel and data for females are shown in the bottom panel. * indicates significant difference from 0 mg/kg THC group for FR5 (main effect of THC group) (p < 0.05). n=8/group for males and n=7/group for females.
Figure 2.

Infusions earned during nicotine dose-effect curves with no pretreatment (Panels A-B) and 0.3 mg/kg THC pretreatment (Panels C-D). Data from males are shown in left panels and data from females are shown in right panels. S = saline, TV = self-administration of 30 μg/kg/infusion nicotine + THC vehicle pretreat. * = significant difference from 0 mg/kg THC group (main effect of THC group); # = significant difference from saline (main effect of nicotine dose) (p < 0.05). n=7/sex/group except that n=8/group for 3 and 30 THC males.
Figure 3.

Infusions earned during nicotine dose-effect curves with 3 mg/kg THC pretreatment (Panels A-B) and 30 mg/kg THC pretreatment (Panels C-D). Data from males are shown in left panels and data from females are shown in right panels. S = saline, TV = self-administration of 30 μg/kg/infusion nicotine + THC vehicle pretreat. For no pretreatment and 0.3 THC pretreatment, n=8 in Panels C and E, and n=7 in Panels A, B, D, and F; for 3 and 30 mg/kg THC pretreatment, n=6 in Panels A, B, and D, n=4 in Panel F; n=8 for 3 THC pretreatment in Panels C and E, and n=7 for 30 THC pretreatment in Panels C and E.
2.8.2. Experiment 2: Sucrose self-administration.
A session x THC group x sex ANOVA comparing pellets earned during the first 22 sessions of FR5 was conducted to determine if THC impacted the amount of sucrose rats earned during acquisition (Figure 4). Pellets earned during the no-pretreatment phase of the study that mimicked the initial nicotine dose-effect curve were analyzed with a session x THC group x sex ANOVA (Figure 5). Pellets earned during each THC pretreatment were compared with THC pretreatment dose (including no pretreatment) x sex x sucrose session ANOVAs with each THC post-session group analyzed separately (Figure 6). A mean substitution procedure was used for cases of missing data. All significant ANOVAs were further analyzed with Tukey post hoc tests (α = 0.05) to specify differences between means, and partial eta squared (η2p) was calculated as a measure of effect size. NCSS Statistical Software (NCSS Statistical Software, Kaysville, UT, USA) was used for all analyses.
Figure 4.
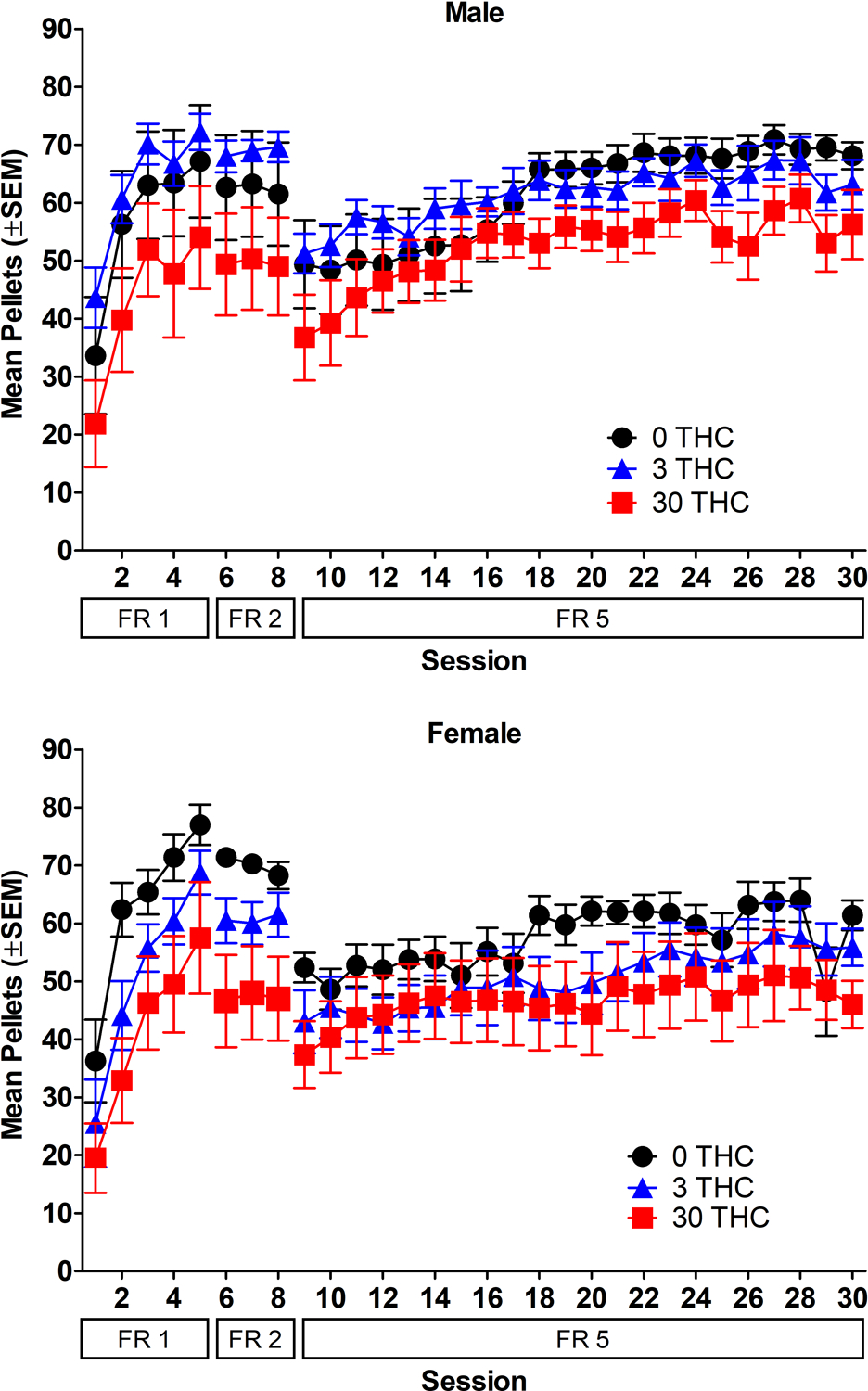
Acquisition of sucrose self-administration. Mean pellets earned as a function of session and FR value. Data for males are shown in the top panel and data for females are shown in the bottom panel. n=8/sex/group.
Figure 5.
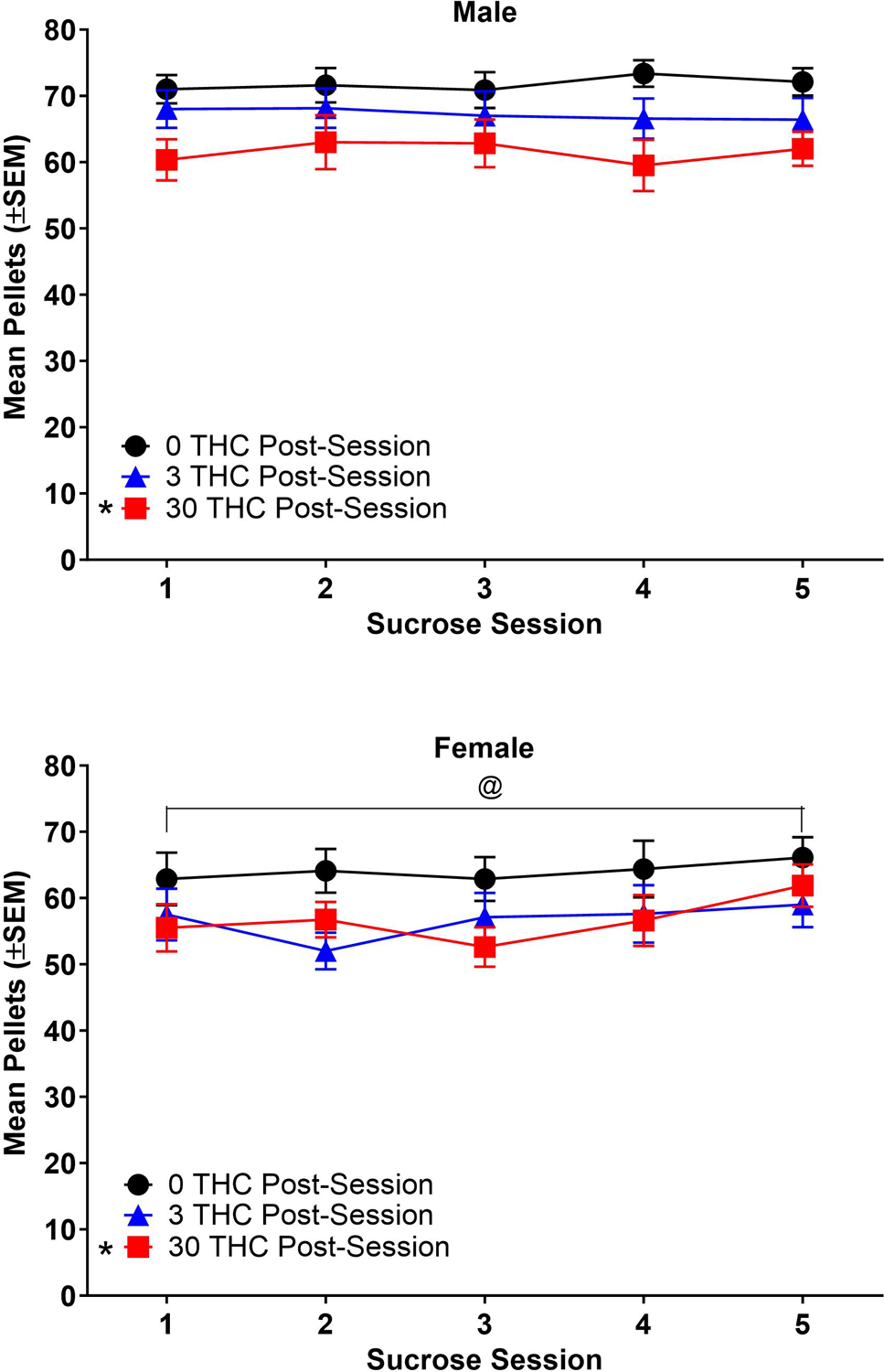
Pellets earned during sucrose self-administration sessions with no pretreatment (Panels A-B) and 0.3 mg/kg THC pretreatment (Panels C-D). Data from males are shown in left panels and data from females are shown in right panels. TV = sucrose self-administration + THC vehicle pretreat. * = significant difference from 0 mg/kg THC group (main effect of THC group); @ = significant difference from male (main effect of sex); % = significant difference from THC vehicle (sex x pretreat interaction) (p < 0.05). n=8/sex/group, except that n=7 for 3 THC males and n=6 for 30 THC males.
Figure 6.
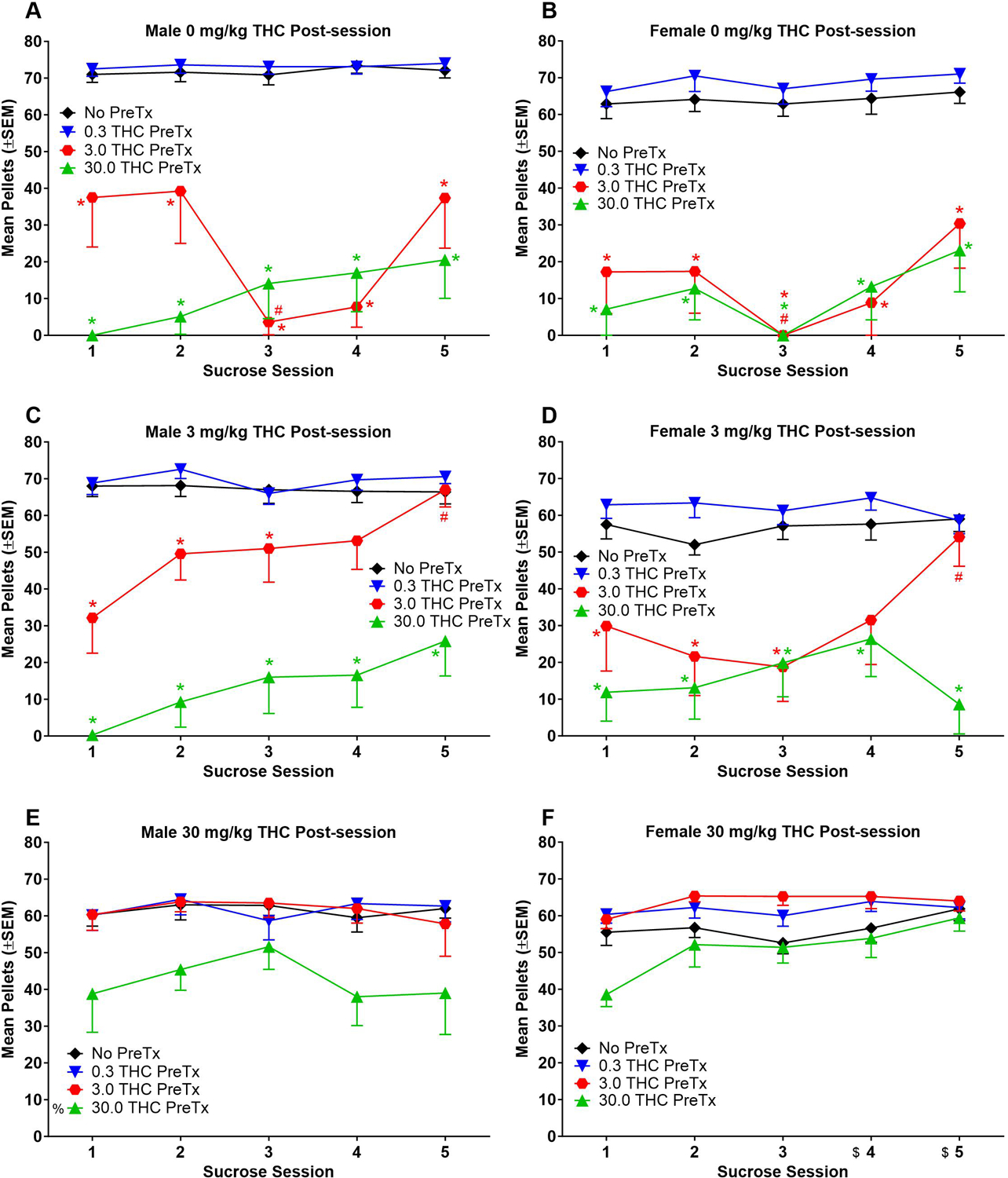
Pellets earned during sucrose self-administration sessions with 3 mg/kg THC (Panels A-B) and 30 mg/kg THC pretreatment (Panels C-D). Data from males are shown in left panels and data from females are shown in right panels. TV = sucrose self-administration + THC vehicle pretreat. * = significant difference from 0 mg/kg THC group (main effect of THC group); % = significant difference from THC vehicle (sex x pretreat interaction) (p < 0.05). n=8 in Panels A, B, D; n=7 in Panel C; n=6 in Panel E except for 30 THC pretreatment, which was n=5; n=8 in Panel F except for 30 THC pretreatment, which was n=7.
3.0. Results
3.1. Experiment 1: Nicotine self-administration
3.1.1. Acquisition.
Acquisition of nicotine self-administration is shown in Figure 1. Results for lever pressing are presented in the supplement. Post-session THC lowered nicotine intake, with both the 3 and 30 mg/kg groups self-administering significantly fewer nicotine infusions than the 0 mg/kg THC group [THC group: F(2, 39)=13.02, p<0.05, η2p=0.40]. Rats increased their nicotine intake while self-administering on FR5, with significantly greater infusions earned in sessions 4–22 compared to session 1 of FR5 [session: F(21, 814)=4.94, p<0.05, η2p=0.11]. There were no significant sex differences, nor were the interactions between sex and THC group and between THC group and session significant (p>0.05).
All rats continued in the study, regardless of how much nicotine they self-administered or how much they responded on each lever; however, their data were compared to standard acquisition criteria to determine how many rats in each group met acquisition criteria. The standard acquisition criteria used to examine the data were: 1) ≥ 10 active responses, and 2) ≥ 2:1 ratio of active to inactive responses, both of which needed to be met for five consecutive sessions on FR5 (Donny et al., 1999; Donny et al., 1998; Marusich et al., 2019). Table 3 shows the percentage of rats in each sex x THC group that met these criteria. Most rats in the study met criteria. The 3 mg/kg THC groups for both sexes and the 30 mg/kg THC male group each had one rat that did not meet criteria.
Table 3.
Percentage of rats in each group that met standard acquisition criteria for nicotine self-administration. All rats were retained in the study, regardless of whether they met acquisition criteria.
| Sex | |||
|---|---|---|---|
| 0 mg/kg | 3 mg/kg | 30 mg/kg | |
| Male | 100% | 87.5% | 87.5% |
| Female | 100% | 85.7% | 100% |
3.1.2. Nicotine dose-effect curve with no THC pretreatment.
Figure 2 shows infusions during the nicotine dose-effect curve with no pretreatment. Rats in 0 mg/kg THC groups earned more infusions than rats in 3 or 30 mg/kg THC groups [THC group: F(2, 38)=8.90, p<0.05, η2p=0.32], and rats earned significantly more infusions of 7.5, 15, and 30 μg/kg/infusion nicotine than saline [nicotine dose: F(4, 152)=22.10, p<0.05, η2p=0.37]. There was no significant effect of sex, or significant interaction between any factors (THC group, nicotine dose, sex) (p>0.05).
3.1.3. Comparison of nicotine dose-effect curves with THC pretreatments.
Figure 3 shows infusions during all nicotine dose-effect curves. Rats in 0 mg/kg THC groups earned more 7.5, 15, and 30 μg/kg/infusion nicotine than saline when no THC pretreatment was given (Figure 3A,B) [nicotine dose: F(4, 48)=11.22, p<0.05, η2p=0.48; THC pretreatment: F(3, 36)=20.34, p<0.05, η2p=0.63; nicotine dose x THC pretreatment interaction: F(12, 142)=2.89, p<0.05, η2p=0.19]. Pre-session 3 mg/kg THC lowered infusions of 15 and 30 μg/kg/infusion nicotine, and pre-session 30 mg/kg THC lowered infusions of 7.5, 15, and 30 μg/kg/infusion nicotine compared to no pretreatment (Figure 3A,B). Male 0 mg/kg THC rats earned more 7.5 μg/kg/infusion nicotine than saline when data were collapsed across THC pretreatments (Figure 3A) [nicotine dose x sex interaction: F(4, 48)=2.66, p<0.05, η2p=0.18]. There was no significant effect of sex or sex x THC pretreatment interaction for 0 mg/kg THC groups (p>0.05).
Rats in 3 mg/kg THC groups earned more 30 μg/kg/infusion nicotine than saline when no THC pretreatment was given (Figure 3C,D) [nicotine dose: F(4, 52)=11.43, p<0.05, η2p=0.47; THC pretreatment: F(3, 39)=15.45, p<0.05, η2p=0.54; nicotine dose x THC pretreatment interaction: F(12, 156)=3.73, p<0.05, η2p=0.22]. Pre-session 30 mg/kg THC lowered infusions of 30 μg/kg/infusion nicotine compared to no pretreatment (Figure 3C,D). There was no significant effect of sex, sex x THC pretreatment interaction, or sex x nicotine dose interaction for 3 mg/kg THC groups (p>0.05).
Rats in 30 mg/kg THC groups earned more 15 and 30 μg/kg/infusion nicotine than saline when no THC pretreatment was given (Figure 3E,F) [nicotine dose: F(4, 40)=5.29, p<0.05, η2p=0.35; nicotine dose x THC pretreatment interaction: F(12, 120)=3.93, p<0.05, η2p=0.28]. Interestingly, 30 mg/kg THC pre-session increased saline infusions compared to no pretreatment for rats administered 30 mg/kg THC post-session (Figure 3E,F). There was no significant effect of sex, THC pretreatment, sex x THC pretreatment interaction, or sex x nicotine dose interaction for 30 mg/kg THC groups (p>0.05).
3.2. Experiment 2: Sucrose self-administration
3.2.1. Acquisition.
Acquisition of sucrose self-administration is shown in Figure 4. Results for lever pressing are presented in the supplement. Post-session THC did not significantly alter the number of pellets earned on FR5 (p>0.05). Rats increased their sucrose intake while self-administering on FR5, with significantly greater pellets earned in sessions 6–22 compared to session 1 of FR5 [session: F(21, 881)=19.30, p<0.05, η2p=0.32]. There were no significant sex differences, nor was the interaction between sex and THC group and between THC group and session significant (p>0.05).
3.2.2. Sucrose self-administration with no pretreatment.
Figure 5 shows sucrose pellets earned with no pretreatment during the 22 sessions after acquisition. The graph shows data from one session every 3–5 days to mimic timepoints in a nicotine dose-effect curve with pre-session THC. Males earned significantly more pellets than females [sex: F(1, 39)=11.71, p<0.05, η2p=0.23], and 0 mg/kg THC rats earned significantly more pellets than 30 mg/kg THC groups [THC group: F(2, 39)=5.41, p<0.05, η2p=0.22]. There was no significant effect of session, or significant interaction between any of the three factors (THC group, sex, session) (p>0.05).
3.2.3. Comparison of sucrose self-administration with THC pretreatments.
Figure 6 shows pellets earned during each batch of 22 sessions. Graphs show data from one session every 3–5 days. For rats in 0 mg/kg THC groups, pre-session 3 and 30 mg/kg THC decreased pellets earned compared to no pretreatment (Figure 6A,B) [session: F(4, 56)=4.87, p<0.05, η2p=0.26; THC pretreatment: F(3, 42)=149.86, p<0.05, η2p=0.91; session x THC pretreatment interaction: F(12, 168)=2.62, p<0.05, η2p=0.16]. Pre-session 3 mg/kg THC lowered pellets earned during session 3 compared to session 1 (Figure 6A,B). There was no significant effect of sex, sex x session interaction, or sex x THC pretreatment interaction for 0 mg/kg THC groups (p>0.05).
For rats in 3 mg/kg THC groups, pre-session 3 mg/kg THC lowered pellets earned during sessions 1–3, and pre-session 30 mg/kg THC lowered pellets earned during all sessions compared to no pretreatment (Figure 6C,D) [session: F(4, 52)=3.35, p<0.05, η2p=0.20; THC pretreatment: F(3, 39)=77.26, p<0.05, η2p=0.86; session x THC pretreatment interaction: F(12, 156)=2.60, p<0.05, η2p=0.17]. Pre-session 3 mg/kg THC increased pellets earned during session 5 compared to session 1 (Figure 6C,D). There was no significant effect of sex, sex x session interaction, or sex x THC pretreatment interaction for 3 mg/kg THC groups (p>0.05).
For rats in 30 mg/kg THC groups, females earned more pellets in sessions 4–5 than session 1 (Figure 6E,F) [session: F(4, 48)=3.53, p<0.05, η2p=0.23; session x sex interaction: F(4, 48)=3.18, p<0.05, η2p=0.21]. Pre-session 30 mg/kg THC significantly decreased pellets earned compared to no pretreatment for males but not females (Figure 6E,F) [THC pretreatment: F(3, 36)=25.66, p<0.05, η2p=0.68; THC pretreatment x sex interaction: F(3, 36)=3.72, p<0.05, η2p=0.24]. There was no significant effect of sex, or sex x THC pretreatment interaction for 30 mg/kg THC groups (p>0.05).
4.0. Discussion
Overall, results showed that repeated post-session THC decreased nicotine intake; however, post-session THC did not affect sucrose responding. Thus, results suggest that these effects may be due to a specific interaction between drugs of abuse, rather than THC broadly affecting all reward-seeking or motivated behaviors. There were also complex interactions between pre-session THC administration and behavioral outcomes, suggesting that temporal relationships between drug treatments are important variables.
In previous research, a modest three-day regimen of THC pre-exposure increased the subsequent likelihood of male rats acquiring nicotine self-administration and increased the value of nicotine (Panlilio et al., 2013). In contrast, the present results showed that repeated THC administration prior to the start of operant training and continuation of post-session THC throughout nicotine self-administration decreased nicotine intake during acquisition and maintenance of nicotine self-administration in male and female rats. Interestingly, post-session THC also caused an apparent delay in acquisition of nicotine self-administration (Figure 1). Further, the dose of post-session THC (3 or 30 mg/kg) did not substantially alter the magnitude of these decreases in nicotine intake. While methodological differences between the two studies (present study; Panlilio et al., 2013) (e.g., dose and timing of THC, continuation of post-session THC during nicotine self-administration) preclude direct comparison, these findings together suggest that effects of THC prior to initiation of nicotine self-administration may differ from effects of THC and nicotine exposure during the same time span. Interestingly, the effects of THC on drug self-administration appear selective for nicotine-cannabinoid interactions: pre-exposure to THC did not affect acquisition of heroin (Solinas et al., 2004) or cocaine self-administration (Panlilio et al., 2007) and post-session THC minimally affected sucrose self-administration in the present study. These results also suggest that the changes in nicotine self-administration in both studies (present study; Panlilio et al., 2013) were not due to non-specific increases/decreases in operant responding.
In humans, intake of tobacco and cannabis can occur in varying orders and may be intermingled with periods of individual drug use (Agrawal et al., 2012). For example, in addition to separate use of nicotine and THC at different times of day or on different days during the same time span (as discussed in the previous paragraph), nicotine and THC may be used simultaneously during the same session (e.g., chasing) (Apollonio et al., 2019; Tucker et al., 2019). In the present study, pre-session administration of THC followed by access to nicotine self-administration dose-dependently flattened the nicotine dose-effect curve in both male and female rats that were receiving post-session vehicle. The decreased nicotine intake observed in the simultaneous-only nicotine-THC group (0 mg/kg THC post-session) agree with human studies showing that active cannabis delayed initiation of tobacco smoking and increased the duration of inter-tobacco bout intervals, thereby decreasing daily tobacco smoking bouts (Kelly et al., 1990). In humans, the decreased intake was also accompanied by decreased inter-puff intervals, which could suggest altered drug-seeking patterns (i.e., increased compulsive-like behaviors) or altered drug valuation. In rats, pre-session THC-induced decreases in responding were more likely related to THC-induced suppression of operant responding rather than an effect of THC on nicotine’s reinforcing efficacy, as similar dose-dependent decreases for sucrose reinforcement were noted in rats that did not receive post-session THC. These results were consistent with those of previous studies in which THC dose-dependently decreased operant responding for food in rats and suggest locomotor suppression and/or amotivational effects (Carriero et al., 1998), which have also been reported in humans (Karoly et al., 2022; Lawn et al., 2016).
Whereas THC dose-dependently reduced nicotine and sucrose intake in rats that were not exposed to THC post-session, a different pattern was observed in rats exposed to THC both simultaneously (pre-session) and separately (post-session) with nicotine. Interestingly, in male rats receiving 3 mg/kg THC post-session, simultaneous administration of 0.3 mg/kg THC pre-session increased nicotine intake (Figure 3C), with decreased intake only observed with pre-session 30 mg/kg THC. This trend was also observed in females but was less robust. In contrast, rats of both sexes in 3 mg/kg post-session THC groups exhibited dose-dependent suppression of responding for sucrose with increasing doses of simultaneous (pre-session) THC, albeit tolerance to this effect was seen at 3 mg/kg THC pre-session during later sessions. In rats regularly receiving a higher (30 mg/kg) daily THC dose, overall suppression of responding for nicotine was seen, regardless of the pre-session THC dose, suggesting that the consequences of heavy use of THC may differ from more moderate use. Responding for sucrose occurred at greater rates, with substantial decreases only at the highest (30 mg/kg) pre-session THC dose. Thus, simultaneous administration of a lower dose of THC (0.3 mg/kg), but not higher doses (3 and 30 mg/kg), may have selectively enhanced the reinforcing effects of nicotine (vs. sucrose) in rats that received a moderate daily dose (3 mg/kg) of post-session THC, with a more pronounced effect in males. Increasing the daily dose of THC obviated this effect through overall suppression of responding for nicotine (but not sucrose) across all pre-session THC doses. In studies with a single drug, upward shifts in dose-response curves (such as seen in the daily 3 mg/kg THC group that received simultaneous 0.3 mg/kg THC pre-session) are associated with increased abuse liability (Piazza et al., 2000). However, our studies show that CT-PSU produces complex interactions that can cause THC to either suppress or enhance nicotine self-administration under certain conditions and highlights the need for further investigation into the specific motivational features of drug seeking that are altered by interactions between THC and nicotine.
A caveat to the interpretations of the data in this study is that rats in 3 and 30 mg/kg THC groups had lower nicotine intake during acquisition than the 0 mg/kg THC groups. Thus, the outcomes of later phases of the study (e.g., during dose-response curves) could potentially be a result of lower nicotine intake earlier in the study rather than a result of THC exposure, or both factors could have contributed to the results. Prior studies showed that the frequency of drug reinforcement contributes to the maintenance of drug seeking (Quick and Shahan, 2009). Future studies could provide additional information on this topic by allowing rats to acquire nicotine self-administration prior to beginning daily post-session THC administration to determine if a history of greater nicotine intake and/or greater behavioral momentum affects the impact of post-session THC on nicotine self-administration.
One limitation of the present study is that self-administration was only assessed using FR schedules of reinforcement. THC-induced alterations in self-administration under FR schedules of reinforcement can be difficult to interpret because an increase or decrease in responding could be due to an increase or decrease in the reinforcing efficacy of the drug (Arnold and Roberts, 1997). In contrast, progressive ratio (PR) schedules of reinforcement, in which the response requirement increases for each subsequent infusion, evaluate the reinforcing efficacy of a drug by establishing the breakpoint (Richardson and Roberts, 1996; Roberts et al., 1989) and measure the motivation to self-administer drug (Arnold and Roberts, 1997). Breakpoints are also sensitive to pharmacological manipulations including pre- (or post-) treatment with another drug (Richardson and Roberts, 1996; Stafford et al., 1998). Given that nicotine self-administration was reduced on a FR schedule of reinforcement but enhanced on a PR schedule when the CB1 agonist WIN 55,212–2 was administered in a past study (Gamaleddin et al., 2012), THC may also have differential effects on nicotine self-administered on a FR vs PR schedule. Use of a PR schedule may also provide a more accurate comparison of effects of THC on nicotine vs sucrose self-administration as prior studies have used PR schedules to compare reinforcing efficacy of different stimuli (Stafford et al., 1998). Therefore, it will be important for future studies to examine the impact of THC on nicotine self-administration when behavior is maintained on a PR schedule of reinforcement.
Another limitation of the present study is that nicotine and sucrose yielded different response rates despite the sucrose sessions only being 30 min, whereas nicotine sessions were 2 h. Hence, we cannot rule out the possibility that the effects of repeated post-session THC administration on responding for a reinforcer may be rate-dependent (vs. reinforcer dependent). This difference in response rates across reinforcers is difficult to address without employing less commonly used food types such as food pellets with lower palatability (e.g., grain-based pellets), and since these foods are not commonly used, the response rate they may elicit is unknown. Another possibility that may equate response rates across reinforcers is to alter the schedule of reinforcement for sucrose to another schedule that elicits lower rates of responding such as a fixed interval (FI) schedule. However, comparing nicotine self-administration maintained on a FR schedule to sucrose self-administration maintained on a FI schedule presents additional challenges in that the nature of behavior maintained on FR vs FI schedules of reinforcement may produce fundamental differences in the brain (Barrett and Hoffmann, 1991). Thus, THC may have different effects on behavior maintained on one schedule vs the other (Barrett, 2002; Thompson et al., 1970).
5.0. Conclusion
Polysubstance use is a significant problem in humans, but rodent studies have primarily focused on modeling how individual drugs affect behavior. Our studies suggest that the effects of THC on nicotine self-administration depend upon the pattern of co-use in rats. Daily separate use of nicotine and THC (3 h post-session) decreased acquisition and maintenance of nicotine self-administration in both sexes, and delayed acquisition of nicotine self-administration when THC and nicotine exposure occurred at different times. In contrast, daily separate THC and nicotine use increased nicotine intake when low doses of THC were administered simultaneously with access to nicotine self-administration. CT-PSU is associated with worse health outcomes in humans, suggesting that the risks associated with drug use may not be solely dependent on amount of drug intake. Instead, synergistic and antagonistic interactions between cannabis and tobacco, and the timing of their co-use, could produce long-lasting changes in motivated behavior that increase the addictive potential of co-used drugs.
Supplementary Material
Acknowledgements
Role of Funding Source: This research was supported by U.S. National Institute of Health [grant number R33DA044377]. The funding source had no other role other than financial support. Contributors: Antony Abraham, Julie Marusich, Jenny Wiley. Declarations of interest: none. Acknowledgements: The authors thank Mariah Chapman, Kimberly Custer, Amanda Pons, Nikita Pulley, and Shanequa Taylor for technical assistance, and Ryan Vandrey for his assistance in conceptualization of this project.
References
- Abraham AD, Leung EJY, Wong BA, Rivera ZMG, Kruse LC, Clark JJ, Land BB, 2020. Orally consumed cannabinoids provide long-lasting relief of allodynia in a mouse model of chronic neuropathic pain. Neuropsychopharmacology 45(7), 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Budney AJ, Lynskey MT, 2012. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction 107(7), 1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Scherrer JF, Lynskey MT, Sartor CE, Grant JD, Haber JR, Madden PAF, Jacob T, Bucholz KK, Xian H, 2011. Patterns of use, sequence of onsets and correlates of tobacco and cannabis. Addict. Behav 36(12), 1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apollonio DE, Spetz J, Schmidt L, Jacobs L, Kaur M, Ramo D, 2019. Prevalence and Correlates of Simultaneous and Separate 30-Day Use of Tobacco and Cannabis: Results from the California Adult Tobacco Survey. Subst. Use Misuse 54(10), 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC, 1997. A Critique of Fixed and Progressive Ratio Schedules Used to Examine the Neural Substrates of Drug Reinforcement. Pharmacology Biochemistry and Behavior, 57(3), 441–447. [DOI] [PubMed] [Google Scholar]
- Arnold MM, Loughlin SE, Belluzzi JD, Leslie FM, 2014. Reinforcing and neural activating effects of norharmane, a non-nicotine tobacco constituent, alone and in combination with nicotine. Neuropharmacology 85, 293–304. [DOI] [PubMed] [Google Scholar]
- Barrett JE, 2002. The emergence of behavioral pharmacology. Mol. Interv 2(8), 470–475. [DOI] [PubMed] [Google Scholar]
- Barrett JE, Hoffmann SM, 1991. Neurochemical changes correlated with behavior maintained under fixed-interval and fixed-ratio schedules of reinforcement. J. Exp. Anal. Behav 56(2), 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Schaub MP, Gmel G, Haug S, 2015. Cannabis use and other predictors of the onset of daily cigarette use in young men: what matters most? Results from a longitudinal study. BMC Public Health 15, 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan KA, Crowther A, Putt F, Roper V, Waterhouse U, Truman P, 2015. Tobacco particulate matter self-administration in rats: differential effects of tobacco type. Addict. Biol 20(2), 227–235. [DOI] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM, 1968. Auto-shaping of the pigeon’s key-peck. J. Exp. Anal. Behav 11(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM, 2010. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology 35(3), 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z, 2008. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J. Subst. Abuse Treat 35(4), 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK, 2014. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp. Clin. Psychopharmacol 22(1), 9–22. [DOI] [PubMed] [Google Scholar]
- Carriero D, Aberman J, Lin SY, Hill A, Makriyannis A, Salamone JD, 1998. A detailed characterization of the effects of four cannabinoid agonists on operant lever pressing. Psychopharmacology (Berl.) 137(2), 147–156. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M, 2009. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int. J. Neuropsychopharmacol 12(10), 1355–1366. [DOI] [PubMed] [Google Scholar]
- Deiana S, Fattore L, Spano MS, Cossu G, Porcu E, Fadda P, Fratta W, 2007. Strain and schedule-dependent differences in the acquisition, maintenance and extinction of intravenous cannabinoid self-administration in rats. Neuropharmacology 52(2), 646–654. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE, 1999. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl.) 147, 135–142. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF, 1998. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl.) 136(1), 83–90. [DOI] [PubMed] [Google Scholar]
- Fairman BJ, Furr-Holden CD, Johnson RM, 2019. When Marijuana Is Used before Cigarettes or Alcohol: Demographic Predictors and Associations with Heavy Use, Cannabis Use Disorder, and Other Drug-related Outcomes. Prevention science : the official journal of the Society for Prevention Research 20(2), 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ, 2011. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology 61(4), 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, Goldberg SR, Le Foll B, 2012. Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict. Biol 17(1), 47–61. [DOI] [PubMed] [Google Scholar]
- Gomez AM, Sun WL, Midde NM, Harrod SB, Zhu J, 2015. Effects of environmental enrichment on ERK1/2 phosphorylation in the rat prefrontal cortex following nicotine-induced sensitization or nicotine self-administration. Eur. J. Neurosci 41(1), 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Shaban ND, Freeman TP, Das RK, Gale G, Schafer G, Falconer CJ, Morgan CJ, Curran HV, 2015. Associations between cigarette smoking and cannabis dependence: a longitudinal study of young cannabis users in the United Kingdom. Drug Alcohol Depend. 148, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatayev O, Lukatskaya O, Moon SH, Guo WR, Chen D, Algava D, Abedi S, Leibowitz SF, 2015. Nicotine and ethanol co-use in Long-Evans rats: Stimulatory effects of perinatal exposure to a fat-rich diet. Alcohol 49(5), 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Milburn MA, Brooks-Russell A, Brown M, Streufert J, Bryan AD, Lovrich NP, DeJong W, Cinnamon Bidwell L, 2022. Effects of High-Potency Cannabis on Psychomotor Performance in Frequent Cannabis Users. Cannabis and cannabinoid research 7(1), 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Rose AJ, Fischman MW, Brady JV, 1990. Smoked marijuana effects on tobacco cigarette smoking behavior. J. Pharmacol. Exp. Ther 252(3), 934–944. [PubMed] [Google Scholar]
- Kohut SJ, 2016. Interactions between nicotine and drugs of abuse: A review of preclinical findings. Am. J. Drug Alcohol Abuse, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn W, Freeman TP, Pope RA, Joye A, Harvey L, Hindocha C, Mokrysz C, Moss A, Wall MB, Bloomfield MA, Das RK, Morgan CJ, Nutt DJ, Curran HV, 2016. Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: an evaluation of the cannabis ‘amotivational’ hypotheses. Psychopharmacology (Berl.) 233(19–20), 3537–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Wiggins M, Goldberg SR, 2006. Nicotine pre-exposure does not potentiate the locomotor or rewarding effects of Delta-9-tetrahydrocannabinol in rats. Behav. Pharmacol 17(2), 195–199. [DOI] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Antonazzo KR, Wiley JL, 2014. Evaluation of WIN 55,212–2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacol. Biochem. Behav 118, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Palmatier MI, 2023. Development of a nicotine aerosol self-administration model in rats and the effects of e-liquid flavors. Behav. Pharmacol 34(2–3), 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Wiley JL, Silinski MAR, Thomas BF, Meredith SE, Gahl RF, Jackson KJ, 2019. Comparison of Cigarette, Little Cigar, and Waterpipe Tobacco Smoke Condensate and E-Cigarette Aerosol Condensate in a Self-administration Model. Behav. Brain Res 372, 112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Lukatskaya O, Moon SH, Guo WR, Shaji J, Karatayev O, Leibowitz SF, 2013. Stimulation of nicotine reward and central cholinergic activity in Sprague-Dawley rats exposed perinatally to a fat-rich diet. Psychopharmacology (Berl.) 230(4), 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the Care and Use of Laboratory Animals, 8th ed. National Academies Press (US), Washington, D.C. [Google Scholar]
- Neuhofer D, Spencer SM, Chioma VC, Beloate LN, Schwartz D, Kalivas PW, 2020. The loss of NMDAR-dependent LTD following cannabinoid self-administration is restored by positive allosteric modulation of CB1 receptors. Addict. Biol 25(6), e12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Smith AL, Odineal EM, Williams EA, Sheppard AB, Bradley CA, 2020. Nicotine Self-Administration With Tobacco Flavor Additives in Male Rats. Nicotine & Tobacco Research 22(2), 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Solinas M, Matthews SA, Goldberg SR, 2007. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology 32(3), 646–657. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Zanettini C, Barnes C, Solinas M, Goldberg SR, 2013. Prior exposure to THC increases the addictive effects of nicotine in rats. Neuropsychopharmacology 38(7), 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M, 2005. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction 100(10), 1518–1525. [DOI] [PubMed] [Google Scholar]
- Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, Lukas SE, 2005. Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend. 79, 211–223. [DOI] [PubMed] [Google Scholar]
- Peters EN, Budney AJ, Carroll KM, 2012. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction 107(8), 1404–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M, 2000. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J. Neurosci 20(11), 4226–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick SL, Shahan TA, 2009. Behavioral momentum of cocaine self-administration: effects of frequency of reinforcement on resistance to extinction. Behav. Pharmacol 20(4), 337–345. [DOI] [PubMed] [Google Scholar]
- Ream GL, Benoit E, Johnson BD, Dunlap E, 2008. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 95(3), 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC, 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods 66(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G, 1989. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl.) 97(4), 535–538. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Rait MA, Prochaska JJ, 2014. Frequent marijuana use is associated with greater nicotine addiction in adolescent smokers. Drug Alcohol Depend. 141, 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruglass LM, Espinosa A, Fitzpatrick S, Meyer MK, Cadet K, Sokolovsky A, Jackson KM, White HR, 2020. Prevalence and Correlates of Concurrent and Simultaneous Cannabis and Cigarette Use among Past-Year Cannabis-Using US College Students. Subst. Use Misuse 55(2), 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M, 2015. Assessing the overlap between tobacco and marijuana: Trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. Addict. Behav 49, 26–32. [DOI] [PubMed] [Google Scholar]
- Schauer GL, Rosenberry ZR, Peters EN, 2017. Marijuana and tobacco co-administration in blunts, spliffs, and mulled cigarettes: A systematic literature review. Addict. Behav 64, 200–211. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD, 2008. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology 33(4), 739–748. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Goldberg SR, 2004. Exposure to delta-9-tetrahydrocannabinol (THC) increases subsequent heroin taking but not heroin’s reinforcing efficacy: a self-administration study in rats. Neuropsychopharmacology 29(7), 1301–1311. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Clarke PB, 2009. Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J. Pharmacol. Exp. Ther 330(2), 633–640. [DOI] [PubMed] [Google Scholar]
- Spencer S, Neuhofer D, Chioma VC, Garcia-Keller C, Schwartz DJ, Allen N, Scofield MD, Ortiz-Ithier T, Kalivas PW, 2018. A Model of Δ(9)-Tetrahydrocannabinol Self-administration and Reinstatement That Alters Synaptic Plasticity in Nucleus Accumbens. Biol. Psychiatry 84(8), 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR, 1998. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl.) 139(3), 169–184. [DOI] [PubMed] [Google Scholar]
- Stringfield SJ, Sanders BE, Suppo JA, Sved AF, Torregrossa MM, 2023. Nicotine Enhances Intravenous Self-administration of Cannabinoids in Adult Rats. Nicotine Tob Res 25(5), 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfield SJ, Torregrossa MM, 2021. Intravenous self-administration of delta-9-THC in adolescent rats produces long-lasting alterations in behavior and receptor protein expression. Psychopharmacology (Berl.) 238(1), 305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, Trombley J, Luke D, Lott D, 1970. Effects of morphine on behavior maintained by four simple food-reinforcement schedules. Psychopharmacologia 17(2), 182–192. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Pedersen ER, Seelam R, Dunbar MS, Shih RA, D’Amico EJ, 2019. Types of cannabis and tobacco/nicotine co-use and associated outcomes in young adulthood. Psychol. Addict. Behav 33(4), 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R, 2002. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br. J. Pharmacol 135(2), 564–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Delnevo CD, Wyka K, Gbedemah M, Lee J, Copeland J, Goodwin RD, 2020a. Cannabis Use Is Associated With Increased Risk of Cigarette Smoking Initiation, Persistence, and Relapse Among Adults in the United States. Nicotine Tob Res 22(8), 1404–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Dierker L, Zhu J, Levin J, Goodwin RD, 2021. Cigarette dependence is more prevalent and increasing among US adolescents and adults who use cannabis, 2002–2019. Tob. Control [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Pacek LR, Wall MM, Gbedemah M, Lee J, Goodwin RD, 2020b. Cigarette smoking quit ratios among adults in the USA with cannabis use and cannabis use disorders, 2002–2016. Tob. Control 29(1), 74–80. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Wyka K, Goodwin RD, 2022. Impact of cannabis legalization in the United States on trends in cannabis use and daily cannabis use among individuals who smoke cigarettes. Drug Alcohol Depend. 238, 109563. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrus DG, Farquhar CE, Lefever TW, Gamage TF, 2021. Sex, species and age: Effects of rodent demographics on the pharmacology of Δ(9)-tetrahydrocanabinol. Prog. Neuropsychopharmacol. Biol. Psychiatry 106, 110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Cortes RA, Marusich JA, 2014. Cross-substitution of Delta9-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol. Biochem. Behav 124, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Marusich JA, Craft RM, 2017. Comparison of the discriminative stimulus and response rate effects of Delta9-tetrahydrocannabinol and synthetic cannabinoids in female and male rats. Drug Alcohol Depend. 172, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


