Abstract
pH is tightly maintained at cellular, tissue, and systemic levels, and altered pH, particularly in the acidic range, is associated with infection, injury, solid tumors, and physiological and pathological inflammation. However, how pH is sensed, regulated, and influences immune responses remains poorly understood at the tissue level. Applying conceptual frameworks of homeostatic and inflammatory circuitries, we categorize cellular and tissue components engaged in pH regulation, drawing parallels from established cases in physiology. By expressing various intracellular and extracellular pH-sensing receptors, the immune system may integrate information of tissue and cellular states into the regulation of homeostatic and inflammatory programs. We introduce the novel concept of resistance and adaptation responses to rationalize pH-dependent immunomodulation intertwined with homeostatic equilibrium and inflammatory control. We discuss challenges and opportunities in understanding the immunological roles of pH sensing, which might reveal new strategies to combat inflammation and restore tissue homeostasis.
Keywords: pH homeostasis, acidic environment, pH sensing, inflammatory response, resistance, adaptation
pH in health and disease
Living organisms depend on an intricate network of biochemical reactions that are exquisitely sensitive to acid-base balance. Proper regulation of pH is thus indispensable for cells, tissues, and organisms (Box 1), while dysregulation of pH is associated with tissue pathology and certain human diseases (Figure 1). Acidosis, characterized by a decrease in homeostatic pH, has attracted considerable attention in immunology research due to its association with the immune response, inflammation, and the tumor microenvironment [1,2]. Although substantial evidence suggests that an acidic pH profoundly influences various immune activities, how pH-dependent immune reactions are functionally linked to the deviation in pH is seldom discussed. Leveraging the knowledge of human physiology and regulation of other environmental variables, we discuss the roles of pH-sensing in the novel framework of tissue homoeostasis and inflammation [3,4]. We provide evidence to argue that immune cells play a key role in detecting and regulating pH at the tissue level. In response to pH deviations, immune responses can act as either negative feedback to counter pH changes or an adaptation to the altered microenvironment. In most cases, these pH-dependent regulations curtail an inflammatory response as feedback control, while in others, maladaptation to acidic pH can worsen inflammatory disorders such as solid tumors and atherosclerosis. A functional understanding of pH regulation may offer valuable insights into the fundamental principles governing tissue homeostasis and inflammation.
Box 1. Homeostatic and pathological pH in mammalian tissues.
Cellular, tissue, and systemic functions rely on a carefully maintained pH environment. While cytosolic pH is kept at pH 7–7.2, compartmentalization of cellular organelles creates distinct pH niches tailored to various biochemical bioreactions [2]. Lysosomes operate optimally at a pH 4.5–4.7, supporting enzymatic activity and catabolism [90], while mitochondria maintain a transmembrane pH gradient at pH 7–8 to facilitate efficient ATP generation [91]. At the organismal level, blood pH is tightly controlled in the narrow range of 7.35 to 7.45 [22]. Deviation of 0.4–0.5 pH units is often considered incompatible with life [92]. In tissues, pH largely depends on the interstitial fluid bathing the cells, which interfaces with the blood circulation and is thought to be close to systemic pH. The fluid’s pH can vary substantially to support specialized tissue functions (Figure 1), influenced by local metabolism, and proton and bicarbonate excretion [7,8,93]. For example, the acid mantle on the human skin has pH 4–5 as an antimicrobial barrier [94]; the lumen in the epididymis is maintained at pH 6.5 to keep spermatozoa in a quiescent state [95]; and mouse bones have a mild acidic environment (pH 7.1) to support osteoclast remodeling [96]. Importantly, to maintain pH within various defined ranges, the body must employ intricate detection and control mechanisms to safeguard cellular, tissue, and organismal pH homeostasis.
Deviations from the normal tissue pH (7.3–7.4 represents the homeostatic pH for most tissue spaces) have been linked to various pathological conditions, such as cancer, ischemia/reperfusion, infections, wounding, autoimmune diseases such as multiple sclerosis, as well as local and systemic inflammatory disorders such as sepsis and inflammatory bowel disease [1,23,29]. For instance, in patients with intra-abdominal infections, the pH of the peritoneal fluid decreases from 7.5–8 to below 7.1 [97]. Patients with rheumatoid arthritis can experience joint fluid pH values as low as 6.0 [98], and solid tumors are characterized by an acidic microenvironment with a pH ranging from 6.5 to 7.0 [1]. Moreover, alterations in pH within specialized tissue compartments have been associated with diseases (Figure 1, e.g. severe sepsis [99]) and, in some cases, can act as contributing factors, such as in hypochlorhydria, vaginitis, increased risk of acne in high pH skin [100–102]. Consequently, the detection of pH perturbations holds particular significance in regulating disease pathology.
Figure 1: Examples of pH in mammalian health and disease.
Various tissues and organs in our body maintain a unique pH to support their physiological functions. Each tissue possesses distinct mechanisms for pH regulation, ensuring a balanced internal environment conducive to cellular activities. Dysregulation in pH homeostasis is associated with disease or pathology (red) specific for the illustrated tissue compartment. The acid mantle helps maintain the antimicrobial defense of the stratum corneum. Rosacea or acne is associated with an elevated pH in the skin. Blood pH is tightly regulated within a narrow range of 7.35–7.45. Acidosis in severe sepsis is around pH 7.1–7.35. The pancreatic fluid is basic with a of pH 7.6–8.8 to neutralize the gastric content released into the duodenum. Chronic pancreatitis can reduce pH to 7.2. In the brain, the cerebrospinal fluid bathing meningeal immune cells maintains a mild acidic pH ~ 7.3. During ischemic injury, the CSF becomes more acidified to pH 6.6. Stomach acids have a pH 1.5–3.5 to activate digestive enzymes, while hypochlorhydria (pH 3–5) leads to poor nutrient absorption. The lumen of the small intestine is slightly alkaline (pH 7–8.5) for nutrient absorption. The acidic pH in the vaginal tract helps prevents the growth of opportunistic pathogens. Higher vaginal pH increases the risk of vaginitis. Lymph node paracortical zones in mice maintain an acidic pH (6.3–7.1) to limit unwarranted T cell activation. Solid tumors exhibit acidic pH ranging between 5.6–7.0 due to increased metabolic activity and proton extrusion by cancer cells (see text and box 1 for references).
Homeostatic and inflammatory framework of pH control
Homeostasis embodies an active process that maintains a set of regulated variables within their defined range (Box 2), through negative feedback regulation involving homeostatic sensors, signals, and effectors (Figure 2) [3]. While the regulation of pH is best illustrated at the systemic level via respiratory and renal circuits (Figure 3, Box 3), the specific components involved in pH regulation at the tissue level remain largely speculative. Immune cells and neurons emerge as potential pH sensors, because they express several extracellular pH-sensing receptors, based on available expression data in mice and humans [5,6]. Candidate homeostatic signals and effectors may be inferred from physiological factors controlling tissue pH, such as proximity to capillaries, microvasculature density, blood flow, and local metabolic activity [7,8]. Thus, the effector cell types may include endothelial cells, smooth muscle cells, and metabolic driver cells within a specific tissue compartment. The homeostatic signals may include biological peptides and mediators that regulate vasoconstriction, vasodilation, and angiogenesis of blood vessels, as well as growth factors and differentiation signals that modulate proliferation and cellular metabolism. While many of these components are individually characterized, it is not understood which sensor cells produce homeostatic signals in response to pH deviation and how these signals act on effector cells to regulate pH in tissues. Frequent associations between abnormal pH and pathology highlight an emerging study area concerning the immunological roles of cells in pH-sensing and regulation at the tissue level.
Box 2. Regulation of tissue homeostasis and inflammation.
Homeostatic sensors detect the regulated variables in reference to a desired set-point (e.g., pancreatic α or β cells for blood glucose), and effectors either increase or decrease the regulated variables (e.g., muscle cells, hepatocytes) to correct the deviation through negative feedback [3]. For example, hypoglycemia triggers pancreatic α cells to release glucagon, which induces glycogenolysis and gluconeogenesis in hepatocytes to elevate blood glucose concentrations. Similarly, hypoxia (lack of O2) activates peritubular interstitial fibroblasts in the kidney through hypoxia-induced factors, leading to the production of erythropoietin, which promotes erythropoiesis in the bone marrow, thus increasing oxygen delivery [103,104]. These examples illustrate how an environmental variable can be maintained within a specific range by a homeostatic circuit. Similar to oxygen and glucose concentrations, the pH needs to be precisely regulated at cellular, tissue, and systemic levels. A significant disruption to homeostatic pH will initiate a regulatory cascade aiming to restore pH values to their proper range.
During inflammation, molecular cues from infections or injuries such as lipopolysaccharide, ATP, extracellular nucleic acids (inflammatory triggers) are sensed by innate immune cell types, such as macrophages and dendritic cells[105]. These cells respond by secreting inflammatory mediators such as IL-6, TNF, IFNs, and prostaglandins, which act on endothelial, stromal, and smooth muscle cells to coordinate the recruitment and activation of immune effectors, including neutrophils and monocytes, ultimately resolving the initial infection or wound (Fig. 2) [3]. [3][4]
Figure 2. Homeostatic and Inflammatory circuits and feedback control of environment sensing.
The homeostatic circuit comprises four major components: regulated variables, sensors, homeostatic signals, and effectors. Deviations in regulated variables (such as pH or oxygen) from a set-point can be monitored by homeostatic sensors and corrected by effectors through a feedback mechanism. In parallel, the inflammatory circuit is initiated by sensing an inflammatory trigger (such as molecular cues of an infection or injury) by the sensor cells (e.g., macrophages, dendritic cells, airway epithelial cells). Inflammatory signals produced from the sensor cells can act on various cell types within a target tissue, activating inflammatory cascades and modulating tissue physiology to eliminate the inflammatory triggers as negative feedback. As a consequence of inflammation, many environmental variables, including pH, oxygen, lactate are perturbed from homeostatic range. Sensing the deviation of critical tissue microenvironment variables can serve as tunable feedback to limit tissue damage.
Figure 3: The control circuits of homeostatic pH at systemic, cellular, and tissue levels.
In particular, the respiratory and renal homeostatic circuits control systemic pH regulation. In the respiratory circuit, pH alterations are sensed by receptors and ion channels, such as the G-protein coupled receptor (GPR4) and Two-Pore Domain Potassium Channels (TASK2) within central chemoreceptors and carotid glomus cells, which activate a neuronal reflex driving the modulation in the rate of ventilation and oxygen/carbon dioxide concentrations. In the renal circuit, luminal pH is sensed by GPCRs and Insulin-related like receptors (IRRs), and the proton/lactate flux is regulated by transporters and ion channels, ultimately driving bicarbonate resorption or proton excretion. Diseases, intoxication, and other disorders can threaten this balance. Severe metabolic or respiratory disorders, characterized by chronic acidosis or alkalosis, can lead to deviation in the blood pH from the normal range where pH < 7.35 is defined as acidosis and pH > 7.45 is defined as alkalosis. Acid base disturbances can be categorized as respiratory alkalosis/acidosis and metabolic alkalosis/acidosis. MCT: monocarboxylate transporter; NHE: Sodium proton exchanger. (see text and Boxes 2 and 3 for references).
Box 3. Homeostatic pH control circuits.
Homeostatic control is best illustrated using a control circuit[3,106]. The systemic pH is tightly maintained by two parallel and well-defined mechanisms operating in different functions and time scales: the respiratory circuit and the renal circuit (Figure 3) [22]. On the one hand, in the respiratory circuit, carotid body glomus cells are the central chemoreceptors (sensors) that detect changes in blood gas composition (pO2, pCO2 as proxy for pH) and signal through neuronal reflex to regulate cardiorespiratory activity and ventilation (effector) [107]. On the other hand, the renal circuit encompasses sensor cells that monitor pH and bicarbonate, and effector cells that reabsorb bicarbonate and excrete hydrogen[108]. The neuronal-based respiratory circuit occurs within minutes to restore an acute acid-base disturbance (e.g., running), while the renal circuit operate at hours to days to long-term disturbance. Despite having unique sets of sensors, signals, and effectors, both respiratory and renal circuits function as negative feedback control systems to ensure pH homeostasis.
At the cellular level, the mechanisms involved in maintaining pH remain relatively elusive, despite the well-known existence of numerous pH sensing receptors. In one case, a decrease in intracellular pH (pHi) triggered the activation of HIF-1a independently from the well-known hypoxia pathway in a human glioblastoma cell line [45]. In another case, lactic acids polarized bone marrow derived macrophages towards an M2-like phenotype of tumor associated macrophages, in a HIF-1a-dependent manner [109]. HIF-1a activation in turn increased the expression of H+/lactate co-transporters, NHE1 proton channels and Arg1, which are essential for polyamine synthesis [45,109,110], leading to a net proton extrusion, and acting as a negative feedback mechanism to restore proper pHi. However, how the majority of pH sensing receptors specifically contribute to pH homeostasis remains ambiguous.
At the opposite end of the response spectrum, the inflammatory response can be viewed as an extreme mechanism to restore tissue homeostasis in infections or injuries [3]. During inflammation, many physiological changes negatively impact tissue homeostasis, including substantial deviations in tissue pH (Figure 2). Without proper regulation of inflammatory responses, excessive or sustained deviation from homeostasis can lead to pathological changes as collateral damage, such as systemic hypotension and fibrosis (Figure 2, “Without feedback”) [3,4]. Sensing deviations in pH may offer a tunable feedback control to mitigate the deleterious effects of inflammation (“With feedback”). This feedback may act directly on sensor cells to control the amplitude and duration of the inflammatory response (anti-inflammatory) such as the concentrations of inflammatory cytokines. It can also act on target cells to reduce the responsiveness to inflammatory signals or reverse potential negative impact (counter-inflammatory), such as adaptation and tissue repair mechanisms in tissue tolerance [4]. Defining specific cellular roles of pH-sensing in anti-inflammatory and counter-inflammatory outcomes may present new opportunities in constraining dysregulated inflammatory responses, as well as reversing tissue damage and inflammation-associated pathology.
Immune cells as pH sensors at the tissue level.
Tissue-resident immune cells appear to be particularly well-suited as sensors for homeostatic variables at the tissue level. First, they are universally present in tissues and express pH sensing receptors. In mammals, macrophages, innate lymphoid cells, γδT cells, and mast cells, reside in tissues since birth [9–11]. Diverse mouse and human immune cell types possess a diverse array of receptors that monitor various environmental factors, such as oxygen concentrations (HIFs), pH (GPR65, GPR68), tissue stiffness (PIEZO1, PIEZO2, YAP/TAZ), and various metabolites (GPR81-lactate, GPR91-succinate, GPR99-α-ketoglutarate) (data from ImmGen Database) [6,9–11]. Second, resident immune populations are strategically distributed throughout tissues, often in close proximity to vascular and lymphatic vessels, making them prime candidates for pH sensing and subsequent communication with effector cell types. For example, the LYVE‐1high MHC-II low interstitial macrophages reside in tissue parenchyma near blood vessels, and inducible deletion of this population has exacerbated experimental lung and heart fibrosis in mice [12,13]. Third, as tissue-resident immune cells are well known to regulate responses to infections and injuries, accumulating evidence suggests that they can directly integrate changes in the chemical and physical microenvironment to shape inflammatory processes [14,15]. Fourth, many signals produced by immune cells also serve homeostatic functions. For instance, during episodes of hypoxia or osmotic stress, skin macrophages produce VEGF-C to remodel local vasculature, increasing blood flow or lymphatic drainage [16]. Regulatory T cells and innate lymphoid cells (ILCs) also secrete mediators with important regulatory roles in tissue physiology, such as amphiregulin and osteopontin [17–19]. Considering their pervasive presence and strategic localization in tissues, along with their capacity to detect environmental variables and regulate tissue physiology, we hypothesize that tissue-resident immune cells serve as key sensors in maintaining pH homeostasis and that these unique abilities make them central players in coordinating tissue responses and adaptations to environmental changes.
Sensing extracellular and intracellular pH in the immune system
Generally, cells tend to maintain a slightly acidic intracellular pH (pHi) compared to the extracellular pH (pHe) [2,20]. The intrinsic buffering capacity of cells allows for a stable pHi within a limited range of pHe, beyond which pHi largely mirror the fluctuations in pHe [21]. Although acidic pH modulates a wide spectrum of immune functions, in experimental conditions either in vitro or in pathological models in vivo, the acidic level of pH can impact both pHe and pHi, To distinguish between mechanisms sensing pHe or pHi, and the potential interconnectedness, we primarily focus on studies based on genetic models of specific pH-sensing receptors. While knowledge is significantly and specifically lacking in the immune system, we rely on work from other fields of research to discuss the potential implications for immune cells.
Extracellular sensing
Receptors responsible for sensing pHe have been extensively studied over past decades [22,23]. These receptors include G-protein coupled receptors (GPCRs), acid-sensing ion channels (ASICs), transient receptor potential cation channel subfamily V member (TRPVs), and alkali-sensing receptor tyrosine kinase (IRRs) [24,25]. We analyzed the expression of these receptors in ImmGen database based on groups of major immune cell types [6]. Notably, proton-sensing GPCRs, such as GPR65 (TDAG8), GPR68 (OGR1), and GPR132 (G2A), are abundantly expressed in various immune cell types, such as dendritic cells (DCs), γδT cells, NK cells, and ILCs (Figure 4A). GPR4, GPR65, and GPR68 share sequence homology [26] and the human homologs are activated at pH 7.6–8.4, 6.8–7.4, and 6.0–7.0, respectively (Figure 4B) [27], while the pH sensing capability of GPR132 remains debatable [28]. Among these receptors, the most compelling functions of pH-sensing in immune cells derive from studies of GPR65. GPR65 expression is mainly confined to hematopoietic lineages and a subset of vagal neurons [29,30]. Human Genome-wide association studies (GWAS) analyses have revealed significant associations between GPR65 and various inflammatory disorders, including ulcerative colitis, Crohn’s disease, asthma, eczema, and other inflammatory disorders, as well as autoimmune disorders such multiple sclerosis (MS) [31–37]. In animal models using whole body Gpr65−/− mice, GPR65 was demonstrated to play key roles in promoting survival of lung eosinophils in a mild acidic environment [38], enhancing experimental autoimmune encephalomyelitis by promoting Th17 cellular responses (EAE; mouse model for MS) [39], regulating autophagy and lipid metabolism in antimicrobial defense during gastric infections [15,34], as well as restraining inflammatory responses in lung, skin, and brain, in models of sepsis and chemical-induced injuries [36,40,41]. Overall, the largely restricted expression of GPR65 in the immune system and the dysregulated immune responses observed in animal models collectively suggest that importance of pH-sensing in the immune system. However, the level of pH that activates GPR65 in vivo, or potentially dysregulated tissue pH due to the lack of pH-sensing have not been tested in most studies. Apart from its aforementioned role in inflammation, GPR65 is fully activated in the range closest to the homeostatic pH. Its high expression in innate immune cell types such as macrophages, eosinophils, and mast cells hints that these cells might potentially contribute to the homeostatic control of pH [42].
Figure 4. Extracellular and intracellular pH sensors.
(A). For illustration purposes only, the bubble plot depicts the expression of extracellular and intracellular pH-sensing receptors in various immune populations in mice (RNA-sequencing (seq) data from GSE109125) [111]. Normalized counts of bulk RNA-seq were aggregated based on the major immune cell types in the Immgen database. The sub cell types that were combined into the indicated immune population include different stages of development, distinct populations, or different tissue of origins. The average expression, as well as the standardized variance of all sub-types (standard deviation / mean) were shown as the dot size, and the color spectrum in the bubble plot. High variation likely indicates a tissue-specific or sub-type specific expression pattern.
(B). The activation range of pH sensing receptors (from A) on the spectrum of homeostatic to pathological pH is shown.
Other than GPR65, an understanding of other extracellular pH-sensing receptors in the immune system remains limited. GPR68, while broadly expressed in various immune and non-immune cell types including DCs, NK cells, NKT cells, and γδ T cells, has recently been shown to respond to shear stress in endothelial cells, in addition to pH [43]. Its broad expression pattern may have contributed to an underappreciated focus compared to GPR65. In addition, ASICs are expressed in the central and peripheral nervous system and regulate various neuronal processes, including fluidic pH, synaptic plasticity, and pain perception [22]. IRRs are expressed in nephron tubular cells, which regulate physiological adaptation to metabolic alkalosis [44]. It remains to be determined whether ASICs or IRRs are expressed and function in selective subsets of immune populations. Therefore, models of cell-type specific deletion of these non-immune restricted receptors is needed to reveal their homeostatic and inflammatory functions in the immune system.
Intracellular sensing
Compared to extracellular sensing, there is less consensus for sensing receptors of pHi in the immune system. Several transcription factors, including ATOH1, SMAD5, HIF-1a, HIF-2a, and Sterol regulatory element-binding protein 2 (SREBP2) have been suggested to have pH-dependent activation and have mostly been studied in cancer cell lines [45–49]. In particular, acidosis prolonged HIF-1a activation in adenocarcinoma and leukemic cell lines [50]. Temperature, acidic pH, and osmolarity all impacted the nuclear-cytoplasmic shuttling of SMAD5 in a human embryonic kidney cell line [47]. Adaptation to an acidic environment activated HIF-2α in a SIRT1-deacetylase dependent manner to regulate the glutamine transporter and metabolic enzymes [51]. Yet, in almost all cases, the specific mechanisms of how pH regulates transcription factor activity and whether these transcription factors account for pH-dependent gene regulation remains largely unknown. In addition to transcription factors, histone acetylation and deacetylation have been suggested to act as putative rheostats of pHi [21] Specifically, acidic pH decreases histone acetylation and increases the intracellular acetate pool in HeLa cells, which is subsequently shuttled outside of cells by proton-dependent monocarboxylate transporters to alleviate acidosis [21]. Of note, activation of SREBP2 at acidic pH induces the expression of cholesterol metabolic genes, including Acss2, which encodes cytosolic Acyl-coenzyme A synthetase that catalyzes acetyl-CoA synthesis from lactate [47]. This suggests that an increased acetate pool due to decreased histone acetylation in acidic pH may feed into SREBP2-dependent Acyl-CoA synthesis in cholesterol metabolism. Moreover, GPR65 deficiency has led to downregulation of genes in cholesterol metabolism such as Cyp51, Msmo1, Npc2 and Hmgcr [15]. SREBP2 and acetylation- mediated intracellular sensing pathways may synchronize with GPR65-dependent extracellular sensing mechanisms to regulate a metabolic switch in cholesterol metabolism.
In the immune system, HIF-1a and SREBP2 are universally expressed while HIF-2a is expressed selectively in group 2 and group 3 ILCs (ILC2s and ILC3s, respectively) (Figure 4A). Among these transcription factors, HIF-1a and SREBP2 have been documented to modulate effector functions of various immune cell types [52–54], but it is not well understood how they modulate pH-dependent immune responses. Moreover, whether HIF-2a is functionally implicated in influencing immune responses, despite unique expression in ILCs, remains completely elusive. Therefore, defining the specific intracellular pH-sensing receptors and their roles in immune cells may present fruitful opportunities to understand pH-dependent immune regulation.
Recent work, predominantly in non-mammalian systems, has provided new insights into pH-sensitive intracellular processes. In budding yeasts, Sup35, a prion-like protein belonging to the translation termination factor eRF3 family, displayed pH-dependent liquid-liquid phase separation in response to heat-shock via a cluster of negatively charged residues adjacent to its N-terminal prion domain [55]. This work suggests that biological condensates might serve as intracellular pH-sensing receptors. Moreover, SNF5 in budding yeast, an essential component of the SWI/SNF chromatin remodeling complex, controls pH-dependent transcriptional responses to nutrient deprivation, through its glutamine-rich low-complexity sequences are embedded with pH-sensitive histidine residues. This study indicated that a general biological process, such as chromatin remodeling could be specifically tuned by pH [56]. Although both mechanisms are unique in fungi, they suggest a putative new mechanism whereby intrinsically disordered sequences and pH-sensitive amino acid residues constitute a pH-sensitive molecular machinery that can modulate cellular signaling and gene expression, although this remains conjectural. Of note, in mammalian skin, a sudden shift to acidic pHi during the development of keratinocytes leads to the disappearance of liquid-like keratohyalin granules in vivo [57]. This is relevant because such a phase transition is essential to squame formation [57], thus establishing a functional link between pH sensing via biological condensates and physiological tissue organization. Although none of these cases can be directly translated into the immune system, the emerging role of biological condensates in immune signaling and gene expression [58] demands investigation into a new frontier of pH- or environment-dependent condensates in immunity and inflammation.
Resistance and adaptation to the acidic environment
The homeostatic circuit provides a rationalization for cellular responses to pH perturbation as a means to restore a transient pH deviation. However, in many physiological and pathological conditions, cells also need to react to extreme and chronic pH perturbations. In fact, acidic pH has been shown to influence various aspects of immune responses that are not directly connected to pH homeostasis (Figure 5). For instance, acidosis selectively biases the myeloid lineage, promoting monocyte to DC differentiation by reducing mTORC1 activity [59]. Acidosis also enhances endocytic activity and T-cell priming in DCs, while attenuating pro-inflammatory cytokine production (e.g. TNF and IFN-γ ) [15,60,61]. In CD8+ T cells, acidic pH inhibits T cell proliferation, cytokine production, and cytotoxicity, while promoting a stemness-like memory state that is necessary for anti-tumor immunity [62]. Although the impact of pH has been portrayed in such studies, the lack of a general framework obscures biological understanding of how these responses are functionally related to pH perturbation.
Figure 5. The impact of acidic extracellular pH on immune responses.
The table summarizes pH-dependent modulation on various immune cell types. Cellular responses related to survival, differentiation, metabolism, cytokine signaling, migration, antigen presentation, cytotoxicity, and cell death are impacted by acidosis or lactic acidosis (pH<7) in a context- and cell-type dependent manner [36,38,59,60,62,64,66,70,109,112–130]. In many cases, the underlying molecular mechanisms remain to be defined.
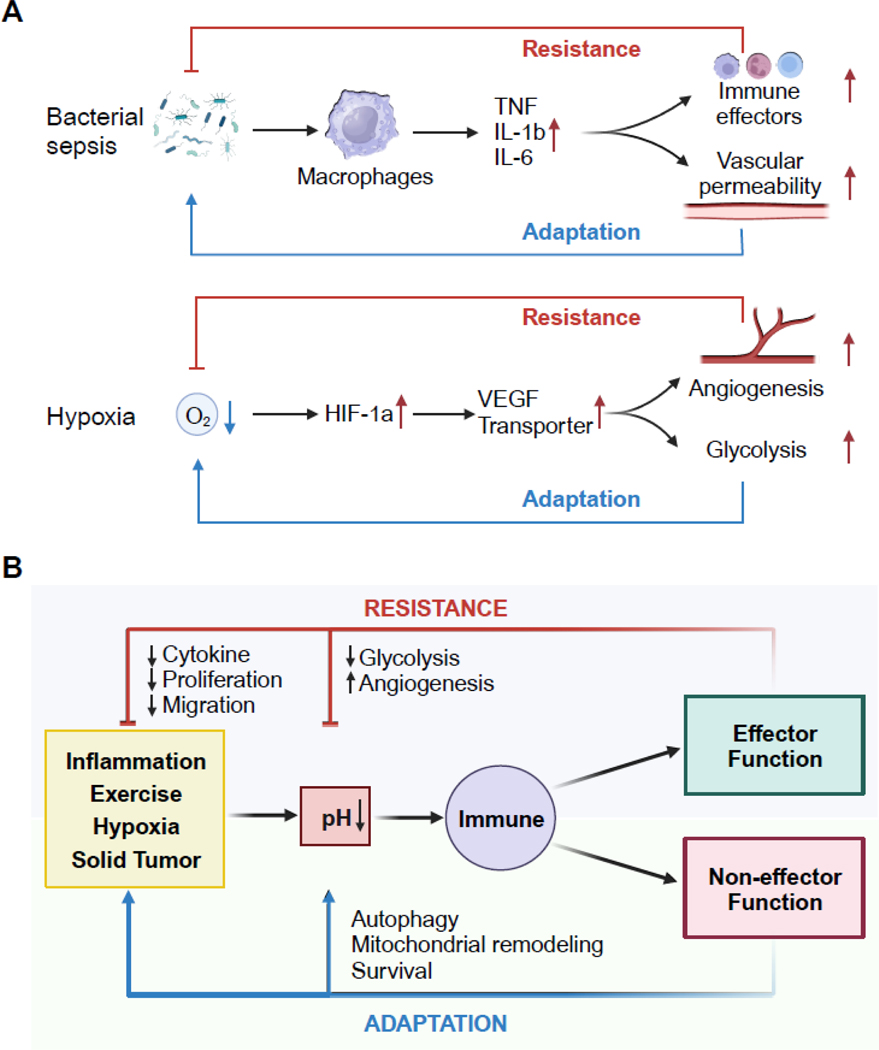
To understand the response to pH perturbations, we revisit two known cases of bacteria-induced sepsis and hypoxia response (Figure 6A). During the inflammatory response, innate immune cells sensing the environment can be classified into two kinds: the effectors that are directly involved in the elimination of inflammatory triggers, such as macrophages, neutrophils, monocytes, and the non-effector cells that enable and facilitate the inflammatory state [4]. The latter include, for example, capillary endothelial and smooth muscle cells that change capillary permeability during tissue inflammation, or adipocytes and hepatocytes that alter systemic metabolism in severe sepsis [3]. These cells do not directly remove the initial instigation of an immune response but facilitate the inflammatory response at tissue and organismal levels [3,4]. In other words, the effector response establishes negative feedback to the initial response triggers (similar to homeostatic control) as a resistance arm, and the supportive non-effector response constitutes an adaptation arm [4]. Failures in either the resistance arm or the adaptation arm may lead to vulnerability to disease. As a second example, activation of the HIF-1a pathway in macrophages in response to hypoxia induces VEGF to enhance local angiogenesis, thus improving oxygenation of tissues [3,63]. In parallel, HIF-dependent metabolic reprogramming ensures sufficient energy generation as an adaptation in hypoxic conditions [54]. In these two examples, bacteria and hypoxia trigger inflammatory and stress responses, respectively, because a homeostatic response is insufficient to eliminate either trigger [3]. The responses to such perturbations in tissues include effector and non-effector functions that could be categorized as either resistant or adaptive to the initial trigger of the response. We might also attempt to conceptualize a pH-dependent immune response as either resistance or adaptation to the environment (Figure 6B).
Key Figure, Figure 6. Model of resistance and adaptation responses to pH perturbations.
(A) In response to an infection, sensor cells (e.g., macrophages, dendritic cells) secrete inflammatory mediators (e.g., TNF, IL-1β and IL-6) leading to the recruitment of effector cells (e.g., neutrophils, monocytes) that directly eliminate the pathogen as the resistance arm. The pro-inflammatory cytokines can also modulate other tissue targets (e.g., endothelial cells) to enable the inflammatory state as the adaptation arm of the response. In a second example, low oxygen tension is sensed by transcription factor HIF-1a, which induces angiogenic factors and activates glycolytic metabolism. New blood vessels deliver oxygen to relieve hypoxia (resistance) and anerobic glycolysis supplies energy (adaptation).
(B) pH alterations from physiological and inflammatory perturbation can be sensed to regulate effector and non-effector functions in immune cells. The effector responses resist reduction in pH, by either directly regulating pH or the initial trigger that causes pH deviation. In contrast, pH responses can carry non-effector functions that are permissive for cells to adapt to a new pH environment. (see main text for references).
Resistance response
Consider that T cell activation acidifies the extracellular space due to the high glycolytic fluxes of lactic acids [57]. By in vivo fluorescence and MRI imaging in mouse lymph nodes, it has been demonstrated that the T-cell-rich paracortical zone establishes a naturally acidic niche to hinder glycolytic activity and suppress activated T cells. However, once outside the lymph nodes, acidosis-mediated inhibition of T cell becomes alleviated. This metabolic response serves as negative feedback to extracellular acidification, maintaining a specific pH level (“pH-stat”) [64]. Interestingly, tumor cells adapt specialized strategies to export protons and metabolic acids, creating an acidic extracellular environment while stabilizing an alkaline cytoplasmic pH to support increased metabolic activities [65]. This resistance mechanism of controlling T cell activity in lymph node is exploited by solid tumors to paralyze the anti-tumor response, impairing the cytotoxic activity of infiltrating CD8+ T cells [66]. In addition to directly reducing acidosis by inhibiting glycolysis, resistance can exist in another form: the regulation of triggers resulting from pH deviation. For instance, the influx and proliferation of immune cells can result in elevated concentrations of lactic acid; inflammatory cytokines can divert blood flow away from the site of infections leading to acid accumulation. The increases in immune cell populations and diverted blood flow are causes for acidosis in tissues, and acidic pH can repress the recruitment of immune cells or the production of inflammatory cytokines as a means to regulate the initial triggers of pH deviation [66]. We posit that the regulation of immune functions that perturb pH homeostasis (without directly contributing to pH) might represent a common form of immune resistance to pH deviations, although further investigation is warranted.
Adaptation response.
Immune cells adapt to acidic conditions by reprogramming their internal environment. For instance, an acidic pH profoundly reprograms aerobic glycolysis toward fatty acid oxidation [67,68]. Such metabolic remodeling counters a reduced energy supply due to the prominent loss of mitochondrial membrane potential in acidic pH [69]. In the case of peripheral blood-derived human macrophages or monocytes, an acidic pH (6.5) triggers a pseudo starvation state despite the abundance of nutrients and oxygen. Starvation-induced ketone bodies protect mitochondria from acidosis-induced depolarization and mitophagy [70],because of a necessary adaptation to the acidic environment. Importantly, both insufficient and prolonged adaptation may lead to pathogenesis. For instance, while tumor-associated macrophages have the “flexibility” to adapt to the tumor microenvironment, the infiltrated lymphocytes may become dysfunctional due to disturbed mitochondrial dynamics and insufficient metabolic adaptation [71,72]. By contrast, prolonged adaptation of macrophages to endothelial acidic plaques can fuel inflammation in atherosclerosis. In this case, excessive ingestion of fatty deposits traps macrophages as cholesterol-laden foam cells, which initiate inflammatory cascades that lead to narrowing of the arteries and acidic atherosclerotic plaques [73]. Subsequently, the acidic pH in human atherosclerotic lesions (pH<6.5) further impairs cholesterol efflux from macrophages. However, for foam cells, such adaptation can perpetuate a positive feedback loop of cholesterol accumulation and inflammatory responses, leading to advanced atherosclerosis[74].
It is worth noting that pathways that regulate resistance and adaptation responses are intertwined and sometimes even in conflict. As discussed above, both hypoxia and acidic pH induce the HIF pathway [75]. While acidic pH induces HIF-dependent responses and HIF-1 activation (commonly studied in the context of hypoxia) promotes glycolysis, glycolytic activity is known to be directly inhibited by acidic pH. This apparent opposite impact on glycolysis, in this case between acidic pH and hypoxia, might potentially indicate a hierarchy in the response to the perturbation of different homeostatic variables. It remains to be determined how immune cells would prioritize their response to various signals that co-exist in tissues and how these responses are controlled by distinct or overlapping mechanisms.
Challenges and opportunities for better understanding tissue environments
With the advance of single-cell and spatial ‘omics technologies, we now possess unprecedented insights into cell types, sub-types, cell states, as well as their communication and interactions within tissues under various health and disease conditions. However, amidst this ever-increasing complexity and detailed information stemming from ‘big data’, essential information on a more comprehensive view of the characteristics of tissues seems to be missing—namely, the chemical and physical environments that profoundly influence immune cell differentiation, survival, activation, polarization, and effector functions. Measuring, perturbing, and understanding pH can begin to illuminate the unseen environment of tissues, ideally opening new avenues for developing selective and specific strategies in both preclinical and clinical settings for cancer and inflammatory disorders.
Measuring pH in tissues
It is plausible to assume that tissue pH is steadily maintained under healthy conditions, yet how pH changes temporally and within tissue regions during inflammation, infection, injury, or tumor development remains elusive. Such a knowledge gap rests on the challenge of accurately determining interstitial pH. Magnetic resonance spectroscopy (MRS) and Magnetic resonance imaging (MRI)-based approaches have revealed acidic niches in solid tumors and in the lymph node during immune activation [76,77], providing valuable information to predict the metastatic potential of primary tumors. However, the need for specialized instruments and nuclear magnetic resonance–active compounds often restrict the broad application in basic and translational research. A class of pH-Low Insertion Peptides (pHLIP) was recently developed to identify acidic tissue microenvironments. Specifically, these peptides undergo conformational changes at acidic pH and become reversibly inserted into the cellular plasma membrane [78–80]. While measuring pHLIP uptake potentially permits single-cell level identification of the pH environment in tissues, it offers limited quantitative assessment and dynamic information of pH fluctuations [80]. New fluorescent and chemiluminescent tools coupled with advanced imaging techniques might help uncover the heterogeneity and changes in tissue pH in a spatiotemporal manner, although this remains to be further explored. We argue that novel technological advances that integrate pH microenvironment readouts coupled to single-cell approaches might help inform our understanding of pH-dependent immune responses in a physiological context.
Manipulating pH-dependent responses
Systemic manipulation of pH is mostly hypothetical based on the sophisticated feedback pH control mechanisms that exist. However, modifying intracellular pH or targeting pH-sensors might provide effective strategies to modulate inflammation and tumor immunology and which remain to be robustly investigated. Nevertheless, a chemical agonist of GPR65 has improved ischemic stroke in murine models [81]. Targeting the lactate transporters MCT1 and MCT4 has been effective in limiting tumor growth in several leukemic cell lines and in primary leukemic blasts from patients with Acute Myeloid Leukemia [82]. Blocking Hv1, the voltage-gated proton extrusion channel that is highly expressed in various tumors and phagocytic cells, has also been shown to delay tumor development in a mouse model of granulocytic sarcoma, or neutrophil inflammatory responses in inflamed alveoli of LPS-treated mice [83,84]. In fact, mimicking the adaptation of certain cancer cells to an acidic environment by overexpressing Hv1 on CAR-T cells has enhanced antitumor functions [85]. Also, leveraging pH-sensitive processes can enable selective activation in a specific pH environment. For example, engineered antibodies with increased binding affinity in acidic pH can reduce on-target toxicities outside of solid tumors [86]. Recently, the signaling of numerous cytokines was reported to be profoundly impacted by acidic pH, including IL-2 that is critical for T cell activation [87]. By using direct protein evolution of cytokine engineering, researchers developed Switch-2, an IL-2 mutant that is fully functional in acidic pH and triggers potent CD8+ T cell effector functions due to a stronger binding affinity to IL-2Ra and STAT5 activation than wildtype IL-2 [87]. Thus, boosting the bioactivity and stability of cytokine signaling by engineering pH-dependent protein-protein interactions may hold a promising therapeutic potential for antitumor and anti-inflammatory immune responses.
Understanding pH regulation
As described above, with the exception of HIF-2a, pH sensing receptors exhibit broad expression within most immune cell types, yet the cell-type-specific functions of pH sensing remain largely unknown. Moreover, the expression patterns of pH-sensing receptors can display surprising selectivity among tissues. For instance, murine splenic macrophages exhibit the highest GPR65 expression, while alveolar macrophages and microglia that reside in mildly acidic tissues, lose GPR65 expression entirely. In contrast, different subsets of murine T cells and B cells display consistent expression (Figure 4A) [6]. Thus, we propose that macrophages might act as pH sensors via GPR65 in the spleen, while other immune cell types such as mast cells and ILCs might detect pH in the lung or brain. Evidently, a combinatorial understanding of cell types, sensing receptors and tissue circuits will be needed to understand the dependencies and disease susceptibilities of pH sensing.
The fact that different pH-sensing receptors are activated across a broad range of pH (Figure 3B) suggests that there may be a hierarchy in the response to pH deviation. For instance, a mild perturbation in physiological pH activates GPR65 on immune cells at pH 7.2 and GPR68 at pH 7.0 [27]. At pH 6.7, ASIC3, expressed on cardiac sympathetic afferent neurons, is induced to half-maximum and is thought to contribute to pain sensation during cardiac ischemia, followed by ASIC1a at pH 6.4 and ASIC1b at pH 5.9 [88,89]. Acidity below pH 5.9 activates TRPV1 channels that are broadly expressed on sensory neurons, including nociceptors that detect noxious stimuli. These receptors can induce cell-type specific responses within a tissue at different ranges of pH deviation [88,89]. We thus argue that a deeper understanding of the coordination between different pH sensing pathways, either within the same cell or between different cells in the form of response circuitry, might provide key insights into tissue-level responses and adaptations to acidic environments.
Limitations
Manipulating pH systemically presents various challenges related to the complexity of the acid-base disturbance and physiological compensatory mechanisms. In addition, the homeostatic pH in various tissue compartments (e.g., lymph nodes) may lie within the range of a pathological pH in other tissues (e.g. solid tumor) (Figure 1). A tissue- or cell-type specific pH targeting strategy may be needed to overcome these limitations. Moreover, studies relying on approaches to perturb pH need to consider the implications of both extracellular and intracellular sensing mechanisms. Developing agonists and antagonists that are independent of pH, in combination with genetic models to demonstrate necessity and sufficiency, can help better illustrate the mechanistic control of pH-sensing in the immune system and beyond.
Concluding Remarks
pH is a critical environmental variable for the control of inflammatory responses. Yet the regulation and response to pH at the tissue level is obscure due to the lack of clear definition of key pH sensing and effector cell types. Furthermore, changes in pH may trigger both extracellular and intracellular sensing pathways that may have distinct and synergistic immunomodulatory functions. A deeper understanding of the molecular and cellular circuits that accurately detect and regulate interstitial pH in a cell type and tissue-specific manner can uncover fundamental mechanisms of pH-dependent regulation in tissue homeostasis and inflammation (see Outstanding Questions Box).
Outstanding Questions Box.
What are the dynamics and heterogeneity of tissue pH? Defining the spatial and temporal variation of pH can open a new dimension for understanding the relationship between cell states and tissue microenvironment in disease.
Are there novel pH sensors controlling immunological functions? If so, are there unique protein motifs or domains, or particular biological processes that are more sensitive to pH perturbations? Addressing this can formulate knowledge-based models to predict immune responses under pathological conditions associated with various pH.
What is the homeostatic role of pH sensing in the immune system? Only a few known pH-sensing receptors are activated close to homeostatic pH. How do these receptors regulate homeostasis of immune populations and the tissues they reside in? Small deviation in homeostasis may be amplified towards disease susceptibility in conditions of stress, infection, or injury.
What are the active non-immune signals produced from immune cells and do they regulate pH, at tissue homeostasis, during inflammation, tissue repair, or tumor development?
What is the cell-type specific response to pH deviation and how does pH-sensing in different immune cell types contribute to disease pathology? Employing conditional genetic knockout animal models and cell-type-specific targeting strategies can shed light on diverse roles in pH immune sensing.
What are the molecular mechanisms underlying the integration of pH sensing and inflammatory responses within immune cell types?
Does sensing intracellular pH change a signal at a different level of emergency from sensing extracellular pH changes? Will the responses elicited by extracellular and intracellular sensing receptors differ in functions, time scale, and categories in adaptation or resistance responses ?
Is there a distinction between immune sensing of pH and stromal or parenchymal sensing of pH during inflammation? Can we leverage pH-dependent sensing mechanisms to program immune responses and tissue tolerance?
Revisiting the homeostatic and inflammatory circuits, the versatility of immune cells empowers them to serve in multifaceted capacities to respond efficiently to homeostatic and inflammatory challenges. While they have been mostly studied in the context of resistance and tolerance to inflammatory triggers, immune cells can respond to environmental perturbations by means of resistance and adaptation. Variance in the regulation of immune resistance and adaptation may depend on the actual demands of the environment. Further studies might provide insights into both regulatory and adaptive mechanisms that determine the strength, endurance, and functions of the immune response under acidic conditions. A fine line between resistance and adaptation may reflect the need to delicately balance controlling immune responses to resolve inflammatory triggers while ensuring their optimal functioning in stressful acidic environments.
Highlights.
pH is a homeostatic variable that is tightly regulated at the cellular, tissue, and systemic levels. The regulation and responses to tissue pH remain poorly understood.
Mammalian immune cells may be ideal sensors for tissue pH at the interface between homeostatic and inflammatory responses.
pH can be sensed by extracellular and intracellular receptors. Recent findings illustrate the crucial roles of immune sensing, but the functions of many pH sensors remain to be uncovered.
Responses to pH can be classified into two categories: effector functions that counter the pH deviation as negative feedback, and non-effector functions that facilitate adaptation to altered pH environments. Dysregulated pH detection, effector, and non-effector functions can create vulnerability to pathological outcomes.
Understanding the immune mechanisms to pH perturbations might spur the development of novel candidate therapeutic approaches in cancer and inflammatory disorders.
Significance.
Novel conceptual frameworks of both homeostatic and inflammatory circuits can provide a coherent rationale for pH regulation in mammalian immune responses. These can offer a comprehensive exploration of the complex relationships between pH sensing and immune responses, as well as their broader implications in health and disease. This type of exploration can stimulate further research into the area of pH regulation and contribute to advancing our understanding of immune-related and inflammatory disorders.
Acknowledgments
We thank Ruslan Medzhitov for numerous insightful discussions on this topic. We thank M Rao, J Thiagaraph, and current Zhou lab members for comments on the manuscript, and C Sacristan for editorial feedback. This work was supported by NIGMS 1R35GM151000, the Kenneth Rainin foundation Innovator award, Charles H. Hood Foundation Health award, the G. Harold & Leila Y. Mathers Foundation to X.Z.
Glossary
- Autophagy
cellular process where cells degrade and recycle certain unnecessary or dysregulated components
- Carotid body glomus cells
cluster of sensory neurons positioned on the posterior site of bifurcation of the common carotid artery, which detects blood oxygen, carbon dioxide, an
- CAR-T cells: chimeric antigen receptor T cells
used as a type of immunotherapy, where T cells are harvested from patient blood, genetically engineered to express a CAR that is antigen-specific (e.g. tumor) and delivered back to patients
- Keratohyalin granules
keratinocyte cytoplasmic granules in the stratum granulosum of the epidermis; filled with histidine- and cysteine-rich proteins that contribute to the formation of the outermost skin barrier
- Ketone bodies
referred to acetone, acetoacetic acid, and beta-hydroxybutyric acid produced from fatty acids in ketogenesis
- Liquid-Liquid phase separation
segregation of biomolecules such as proteins and nucleic acids from a homogeneous environment into two distinct phases, usually a condensate phase and an aqueous phase
- pH-Low Insertion Peptide
a short peptide containing a mixture of hydrophobic and protonable amino acid residues; the protonated peptide forms a transmembrane helix to reversibly insert into the plasma membrane at acidic pH
- Squame
cells nearing the surface of the skin that lose their organelles and die
- Stemness-like memory state
State at which multipotent progenitors can both self-renew and differentiate into memory T cells
Footnotes
Declaration of interests
The authors declare no competing interests.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used ChatGPT in order to improve readability. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corbet C and Feron O. (2017) Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer 17, 577–593 [DOI] [PubMed] [Google Scholar]
- 2.Casey JR et al. (2010) Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11, 50–61 [DOI] [PubMed] [Google Scholar]
- 3.Meizlish ML et al. (2021) Tissue Homeostasis and Inflammation. Annu Rev Immunol 39, 557–581 [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R. (2021) The spectrum of inflammatory responses. Science 374, 1070–1075 [DOI] [PubMed] [Google Scholar]
- 5.Uhlén M et al. (2015) Proteomics. Tissue-based map of the human proteome. Science 347, 1260419. [DOI] [PubMed] [Google Scholar]
- 6.Heng TSP et al. (2008) The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 9, 1091–4 [DOI] [PubMed] [Google Scholar]
- 7.Street D et al. (2001) Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J Physiol 537, 993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh S-CA et al. (2022) Quantification of bone marrow interstitial pH and calcium concentration by intravital ratiometric imaging. Nat Commun 13, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasteiger G et al. (2015) Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribot JC et al. (2021) γδ T cells in tissue physiology and surveillance. Nat Rev Immunol 21, 221–232 [DOI] [PubMed] [Google Scholar]
- 11.Jenkins SJ and Allen JE. (2021) The expanding world of tissue-resident macrophages. Eur J Immunol 51, 1882–1896 [DOI] [PubMed] [Google Scholar]
- 12.Gibbings SL et al. (2017) Three Unique Interstitial Macrophages in the Murine Lung at Steady State. Am J Respir Cell Mol Biol 57, 66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakarov S et al. (2019) Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363 [DOI] [PubMed] [Google Scholar]
- 14.Solis AG et al. (2019) Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 573, 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X et al. (2022) pH sensing controls tissue inflammation by modulating cellular metabolism and endo-lysosomal function of immune cells. Nat Immunol 23, 1063–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacHnik A et al. (2009) Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15, 545–52 [DOI] [PubMed] [Google Scholar]
- 17.Monticelli LA et al. (2011) Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12, 1045–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burzyn D et al. (2013) A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi L et al. (2021) Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity 54, 1527–1542.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parks SK et al. (2011) pH control mechanisms of tumor survival and growth. J Cell Physiol 226, 299–308 [DOI] [PubMed] [Google Scholar]
- 21.McBrian MA et al. (2013) Histone Acetylation Regulates Intracellular pH. Mol Cell 49, 310–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin LR and Buck J. (2015) Physiological Roles of Acid-Base Sensors. Annu Rev Physiol 77, 347–362 [DOI] [PubMed] [Google Scholar]
- 23.Damaghi M et al. (2013) pH sensing and regulation in cancer. Front Physiol 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holzer P. (2008) The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol 155, 1145–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludwig M-G et al. (2003) Proton-sensing G-protein-coupled receptors. Nature 425, 93–8 [DOI] [PubMed] [Google Scholar]
- 26.Rowe JB et al. (2021) The evolution and mechanism of GPCR proton sensing. J Biol Chem 296, 100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang XP et al. (2015) Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature 527, 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radu CG et al. (2005) Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proceedings of the National Academy of Sciences 102, 1632–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Justus CR et al. (2013) Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol 4, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang RB et al. (2015) Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell 161, 622–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tcymbarevich IV et al. (2019) The impact of the rs8005161 polymorphism on G protein-coupled receptor GPR65 (TDAG8) pH-associated activation in intestinal inflammation. BMC Gastroenterol 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franke A et al. (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet DOI: 10.1038/ng.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jostins L et al. (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lassen KG et al. (2016) Genetic Coding Variant in GPR65 Alters Lysosomal pH and Links Lysosomal Dysfunction with Colitis Risk. Immunity 44, 1392–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan Y et al. (2019) Genetic polymorphisms of G protein-coupled receptor 65 gene are associated with ankylosing spondylitis in a Chinese Han population: A case-control study. Hum Immunol DOI: 10.1016/j.humimm.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 36.Xie L et al. (2021) pH and Proton Sensor GPR65 Determine Susceptibility to Atopic Dermatitis. J Immunol 207, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diogo D et al. Phenome-wide association studies across large population cohorts support drug target validation. DOI: 10.1038/s41467-018-06540-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kottyan LC et al. (2009) Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood 114, 2774–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaublomme JT et al. (2015) Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell 163, 1400–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato K et al. (2020) The protective role of proton-sensing TDAG8 in the brain injury in a mouse ischemia reperfusion model. Sci Rep 10, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsurumaki H et al. (2015) Protective role of proton-sensing TDAG8 in lipopolysaccharide-induced acute lung injury. Int J Mol Sci 16, 28931–28942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heng TSP et al. (2008) The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 9, 1091–4 [DOI] [PubMed] [Google Scholar]
- 43.Xu J et al. (2018) GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 173, 762–775.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deyev IE et al. (2011) Deficient Response to Experimentally Induced Alkalosis in Mice with the Inactivated insrr Gene. Acta Naturae 3, 114–7 [PMC free article] [PubMed] [Google Scholar]
- 45.Filatova A et al. (2016) Acidosis Acts through HSP90 in a PHD/VHL-Independent Manner to Promote HIF Function and Stem Cell Maintenance in Glioma. Cancer Res 76, 5845–5856 [DOI] [PubMed] [Google Scholar]
- 46.Corbet C et al. (2014) The SIRT1/HIF2α Axis Drives Reductive Glutamine Metabolism under Chronic Acidosis and Alters Tumor Response to Therapy. Cancer Res 74, 5507–5519 [DOI] [PubMed] [Google Scholar]
- 47.Fang Y et al. (2017) Smad5 acts as an intracellular pH messenger and maintains bioenergetic homeostasis. Cell Res 27, 1083–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y et al. (2023) Intracellular pH dynamics regulates intestinal stem cell lineage specification. Nat Commun 14, 3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kondo A et al. (2017) Extracellular Acidic pH Activates the Sterol Regulatory Element-Binding Protein 2 to Promote Tumor Progression. Cell Rep 18, 2228–2242 [DOI] [PubMed] [Google Scholar]
- 50.Mekhail K et al. (2004) HIF activation by pH -dependent nucleolar sequestration of VHL. Nat Cell Biol 6, 642–647 [DOI] [PubMed] [Google Scholar]
- 51.Corbet C et al. (2014) The SIRT1/HIF2α Axis Drives Reductive Glutamine Metabolism under Chronic Acidosis and Alters Tumor Response to Therapy. Cancer Res 74, 5507–5519 [DOI] [PubMed] [Google Scholar]
- 52.Guo C et al. (2018) Cholesterol Homeostatic Regulator SCAP-SREBP2 Integrates NLRP3 Inflammasome Activation and Cholesterol Biosynthetic Signaling in Macrophages. Immunity 49, 842–856.e7 [DOI] [PubMed] [Google Scholar]
- 53.Kusnadi A et al. (2019) The Cytokine TNF Promotes Transcription Factor SREBP Activity and Binding to Inflammatory Genes to Activate Macrophages and Limit Tissue Repair. Immunity 51, 241–257.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGettrick AF and O’Neill LAJ. (2020) The Role of HIF in Immunity and Inflammation. Cell Metab 32, 524–536 [DOI] [PubMed] [Google Scholar]
- 55.Franzmann TM et al. (2018) Phase separation of a yeast prion protein promotes cellular fitness. Science 359 [DOI] [PubMed] [Google Scholar]
- 56.Gutierrez JI et al. (2022) SWI/SNF senses carbon starvation with a pH-sensitive low-complexity sequence. Elife 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quiroz FG et al. (2020) Liquid-liquid phase separation drives skin barrier formation. Science 367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao Q et al. (2022) Phase separation in immune signalling. Nat Rev Immunol 22, 188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erra Díaz F et al. (2020) Extracellular Acidosis and mTOR Inhibition Drive the Differentiation of Human Monocyte-Derived Dendritic Cells. Cell Rep 31, 107613. [DOI] [PubMed] [Google Scholar]
- 60.Martínez D et al. (2007) Extracellular Acidosis Triggers the Maturation of Human Dendritic Cells and the Production of IL-12. The Journal of Immunology 179, 1950–1959 [DOI] [PubMed] [Google Scholar]
- 61.Tong J et al. (2011) Acid-sensing ion channels contribute to the effect of acidosis on the function of dendritic cells. J Immunol 186, 3686–92 [DOI] [PubMed] [Google Scholar]
- 62.Cheng H et al. (2023) Extracellular acidosis restricts one-carbon metabolism and preserves T cell stemness. Nat Metab 5, 314–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shweiki D et al. (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843–845 [DOI] [PubMed] [Google Scholar]
- 64.Wu H et al. (2020) T-cells produce acidic niches in lymph nodes to suppress their own effector functions. Nat Commun 11, 4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neri D and Supuran CT. (2011) Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 10, 767–777 [DOI] [PubMed] [Google Scholar]
- 66.Calcinotto A et al. (2012) Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res 72, 2746–56 [DOI] [PubMed] [Google Scholar]
- 67.Corbet C et al. (2016) Acidosis Drives the Reprogramming of Fatty Acid Metabolism in Cancer Cells through Changes in Mitochondrial and Histone Acetylation. Cell Metab 24, 311–23 [DOI] [PubMed] [Google Scholar]
- 68.Khacho M et al. (2016) Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell Stem Cell 19, 232–247 [DOI] [PubMed] [Google Scholar]
- 69.Solaini G et al. (2010) Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta 1797, 1171–7 [DOI] [PubMed] [Google Scholar]
- 70.Adam C et al. (2021) Acetoacetate protects macrophages from lactic acidosis-induced mitochondrial dysfunction by metabolic reprograming. Nat Commun 12, 7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu Y-R et al. (2020) Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat Immunol 21, 1540–1551 [DOI] [PubMed] [Google Scholar]
- 72.Scharping NE et al. (2021) Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol 22, 205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu C-L et al. (2019) Na+-H+ exchanger 1 determines atherosclerotic lesion acidification and promotes atherogenesis. Nat Commun 10, 3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee-Rueckert M et al. (2020) Acidic extracellular pH promotes accumulation of free cholesterol in human monocyte-derived macrophages via inhibition of ACAT1 activity. Atherosclerosis 312, 1–7 [DOI] [PubMed] [Google Scholar]
- 75.Filatova A et al. (2016) Acidosis Acts through HSP90 in a PHD/VHL-Independent Manner to Promote HIF Function and Stem Cell Maintenance in Glioma. Cancer Res 76, 5845–5856 [DOI] [PubMed] [Google Scholar]
- 76.Longo DL et al. (2016) In Vivo Imaging of Tumor Metabolism and Acidosis by Combining PET and MRI-CEST pH Imaging. Cancer Res 76, 6463–6470 [DOI] [PubMed] [Google Scholar]
- 77.Anemone A et al. (2021) Tumour acidosis evaluated in vivo by MRI-CEST pH imaging reveals breast cancer metastatic potential. Br J Cancer 124, 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reshetnyak YK et al. (2008) Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane. Proc Natl Acad Sci U S A 105, 15340–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Narayanan T et al. (2016) pHLIP Peptide Interaction with a Membrane Monitored by SAXS. J Phys Chem B 120, 11484–11491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wyatt LC et al. (2017) Applications of pHLIP Technology for Cancer Imaging and Therapy. Trends Biotechnol 35, 653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma XD et al. (2017) TDAG8 activation attenuates cerebral ischaemia-reperfusion injury via Akt signalling in rats. Exp Neurol 293, 115–123 [DOI] [PubMed] [Google Scholar]
- 82.Saulle E et al. (2020) Targeting Lactate Metabolism by Inhibiting MCT1 or MCT4 Impairs Leukemic Cell Proliferation, Induces Two Different Related Death-Pathways and Increases Chemotherapeutic Sensitivity of Acute Myeloid Leukemia Cells. Front Oncol 10, 621458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao R et al. (2023) Protection from acute lung injury by a peptide designed to inhibit the voltage-gated proton channel. iScience 26, 105901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El Chemaly A et al. (2023) Discovery and validation of new Hv1 proton channel inhibitors with onco-therapeutic potential. Biochim Biophys Acta Mol Cell Res 1870, 119415 [DOI] [PubMed] [Google Scholar]
- 85.Navarro F et al. (2022) Overcoming T cell dysfunction in acidic pH to enhance adoptive T cell transfer immunotherapy. Oncoimmunology 11, 2070337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sulea T et al. (2020) Structure-based engineering of pH-dependent antibody binding for selective targeting of solid-tumor microenvironment. MAbs 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaggero S et al. (2022) IL-2 is inactivated by the acidic pH environment of tumors enabling engineering of a pH-selective mutein. Sci Immunol 7, eade5686 [DOI] [PubMed] [Google Scholar]
- 88.Chen CC et al. (1998) A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci U S A 95, 10240–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sutherland SP et al. (2001) Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A 98, 711–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu Y-B et al. (2015) The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl Neurodegener 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abad MFC et al. (2004) Mitochondrial pH Monitored by a New Engineered Green Fluorescent Protein Mutant. Journal of Biological Chemistry 279, 11521–11529 [DOI] [PubMed] [Google Scholar]
- 92.Smith T et al. (2009) Lote CJ. Renal physiology. In: Fundamentals of Anaesthesia, ((3rd edn) ), Cambridge University Press [Google Scholar]
- 93.Gerweck LE and Seetharaman K. (1996) Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res 56, 1194–8 [PubMed] [Google Scholar]
- 94.Zlotogorski A. (1987) Distribution of skin surface pH on the forehead and cheek of adults. Arch Dermatol Res 279, 398–401 [DOI] [PubMed] [Google Scholar]
- 95.RODRIGUEZ-MARTINEZ H et al. (1990) Acidification of epididymal fluid in the boar. Int J Androl 13, 238–243 [DOI] [PubMed] [Google Scholar]
- 96.Bushinsky DA. (1995) Stimulated osteoclastic and suppressed osteoblastic activity in metabolic but not respiratory acidosis. Am J Physiol 268, C80–8 [DOI] [PubMed] [Google Scholar]
- 97.Simmen HP et al. (1994) Analysis of pH, pO2 and pCO2 in drainage fluid allows for rapid detection of infectious complications during the follow-up period after abdominal surgery. Infection 22, 386–9 [DOI] [PubMed] [Google Scholar]
- 98.Lombardi AF et al. (2022) AcidoCEST-UTE MRI Reveals an Acidic Microenvironment in Knee Osteoarthritis. Int J Mol Sci 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wernly B et al. (2020) Acidosis predicts mortality independently from hyperlactatemia in patients with sepsis. Eur J Intern Med 76, 76–81 [DOI] [PubMed] [Google Scholar]
- 100.McColl KE et al. (1997) Alterations in gastric physiology in Helicobacter pylori infection: causes of different diseases or all epiphenomena? Ital J Gastroenterol Hepatol 29, 459–64 [PubMed] [Google Scholar]
- 101.Mania-Pramanik J et al. (2008) Use of vaginal pH in diagnosis of infections and its association with reproductive manifestations. J Clin Lab Anal 22, 375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prakash C et al. (2017) Skin Surface pH in Acne Vulgaris: Insights from an Observational Study and Review of the Literature. J Clin Aesthet Dermatol 10, 33–39 [PMC free article] [PubMed] [Google Scholar]
- 103.Asada N et al. (2011) Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest 121, 3981–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rankin EB et al. (2007) Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 117, 1068–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fitzgerald KA and Kagan JC. (2020) Toll-like Receptors and the Control of Immunity. Cell 180, 1044–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kotas ME and Medzhitov R. (2015) Homeostasis, Inflammation, and Disease Susceptibility. Cell 160, 816–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ortega-Sáenz P and López-Barneo J. (2020) Physiology of the Carotid Body: From Molecules to Disease. Annu Rev Physiol 82, 127–149 [DOI] [PubMed] [Google Scholar]
- 108.Brown D and Wagner CA. (2012) Molecular mechanisms of acid-base sensing by the kidney. J Am Soc Nephrol 23, 774–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Colegio OR et al. (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimoda LA et al. (2006) HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. American Journal of Physiology-Lung Cellular and Molecular Physiology 291, L941–L949 [DOI] [PubMed] [Google Scholar]
- 111.Yoshida H et al. (2019) The cis-Regulatory Atlas of the Mouse Immune System. Cell 176, 897–912.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abebayehu D et al. (2016) Lactic Acid Suppresses IL-33–Mediated Mast Cell Inflammatory Responses via Hypoxia-Inducible Factor-1α–Dependent miR-155 Suppression. The Journal of Immunology 197, 2909–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Caslin HL et al. (2019) Lactic Acid Inhibits Lipopolysaccharide-Induced Mast Cell Function by Limiting Glycolysis and ATP Availability. The Journal of Immunology 203, 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trevani AS et al. (1999) Extracellular acidification induces human neutrophil activation. J Immunol 162, 4849–57 [PubMed] [Google Scholar]
- 115.Rotstein OD et al. (1987) The Bacteroides by-product succinic acid inhibits neutrophil respiratory burst by reducing intracellular pH. Infect Immun 55, 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cao S et al. (2015) Extracellular Acidification Acts as a Key Modulator of Neutrophil Apoptosis and Functions. PLoS One 10, e0137221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bilyy R et al. (2016) Neutrophil Extracellular Traps Form a Barrier between Necrotic and Viable Areas in Acute Abdominal Inflammation. Front Immunol 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maueröder C et al. (2016) Ménage-à-Trois: The Ratio of Bicarbonate to CO2 and the pH Regulate the Capacity of Neutrophils to Form NETs. Front Immunol 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Torres IM et al. (2018) Acidosis exacerbates in vivo IL-1-dependent inflammatory responses and neutrophil recruitment during pulmonary Pseudomonas aeruginosa infection. American Journal of Physiology-Lung Cellular and Molecular Physiology 314, L225–L235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paolini L et al. (2020) Lactic Acidosis Together with GM-CSF and M-CSF Induces Human Macrophages toward an Inflammatory Protumor Phenotype. Cancer Immunol Res 8, 383–395 [DOI] [PubMed] [Google Scholar]
- 121.Dietl K et al. (2010) Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol 184, 1200–9 [DOI] [PubMed] [Google Scholar]
- 122.Rajamäki K et al. (2013) Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem 288, 13410–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jancic CC et al. (2012) Low extracellular pH stimulates the production of IL-1β by human monocytes. Cytokine 57, 258–68 [DOI] [PubMed] [Google Scholar]
- 124.Mogi C et al. (2009) Involvement of Proton-Sensing TDAG8 in Extracellular Acidification-Induced Inhibition of Proinflammatory Cytokine Production in Peritoneal Macrophages. The Journal of Immunology 182, 3243–3251 [DOI] [PubMed] [Google Scholar]
- 125.Puig-Kröger A et al. (2003) Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. J Leukoc Biol 73, 482–92 [DOI] [PubMed] [Google Scholar]
- 126.Vermeulen M et al. (2004) Acidosis Improves Uptake of Antigens and MHC Class I-Restricted Presentation by Dendritic Cells. The Journal of Immunology 172, 3196–3204 [DOI] [PubMed] [Google Scholar]
- 127.Walton ZE et al. (2018) Acid Suspends the Circadian Clock in Hypoxia through Inhibition of mTOR. Cell 174, 72–87.e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fischer K et al. (2007) Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–9 [DOI] [PubMed] [Google Scholar]
- 129.Fischer B et al. (2000) Acidic pH Inhibits Non-MHC-Restricted Killer Cell Functions. Clinical Immunology 96, 252–263 [DOI] [PubMed] [Google Scholar]
- 130.Brand A et al. (2016) LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab 24, 657–671 [DOI] [PubMed] [Google Scholar]