Abstract
Many studies have shown that gut microbiota is closely related to autoimmune diseases (ADs). Studies on gut microbiota and ADs have also increased significantly, but no bibliometric analysis has summarized the association between gut microbiota and ADs. This study aimed to conduct a bibliometric and visual analysis of published studies on gut microbiota and ADs. Based on the Web of Science Core Collection SCI-expanded database, we utilize Excel 2019 and visualization analysis tools VOSviewer and co-occurrence13.2 (COOC13.2) for analysis. A total of 2516 related kinds of literature were included, and the number of papers presented an overall increasing trend. The country/region with the most publications is the USA, the institution is the Harvard Medical School, and the author is Mikael Knip from the USA. Hot research areas include intestinal regulation (such as dysbiosis, short chain fatty acids, and probiotics), multisystem ADs (such as multiple sclerosis, rheumatoid arthritis, and inflammatory bowel disease), and immune-related cells (such as T cells, and dendritic cells). Psoriasis, dysbiosis, autoimmune liver disease, and fecal microbiota transplantation may be the future research direction. Our research results can help researchers grasp the current status of ADs and gut microbiota research and find new research directions in the future.
Keywords: Autoimmune diseases, Gut microbiota, Visual analysis, Hot spots, Frontiers, Keywords analysis
Introduction
In the past few decades, the prevalence of autoimmune diseases (ADs) has increased rapidly worldwide, affecting up to 10% of the population, and most human ADs are complex diseases caused by the interaction between genetic, epigenetic, and environmental factors [1–4]. ADs are also associated with psychological stress and gastrointestinal symptoms, namely microbiota dysregulation, intestinal hyperpermeability, and intestinal inflammation [2, 5, 6]. They are characterized by chronic, systemic, excessive immune activation and inflammation and involve almost all body tissues [7, 8]. Glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs), immunosuppressants, and biologics are currently used to treat ADs of different origins [3, 9]. Several dietary and natural products (including polyphenols, quercetin) have also been investigated as possible alternative therapeutic strategies for managing ADs [3, 10]. However, due to the complexity of ADs, the social burden caused by ADs is still severe.
The role of the gut microbiome in human disease has received much attention, and the understanding of the composition and function of the gut microbiome has increased exponentially [11, 12]. Primarily responsible for maintaining the balance between host defense and immune tolerance, the gut microbiota plays a crucial role in shaping the immune system [11, 13, 14]. Dysbiosis of the gut microbiota is associated with various alterations in the immune system [11, 13]. The possible causal relationship between the gut microbiota and the initiation or exacerbation of ADs, microbial dysbiosis, and intestinal leakage are common phenomena in human ADs and mouse models of autoimmunity [11, 15]. Gut commensal microbiota can contribute to the pathogenesis of ADs by altering the intestinal barrier [16, 17]. Among them, the effects on gut microbiota through probiotics and fecal transplantation may serve as novel targets for autoimmune therapy [18]. It can be seen that gut microbiota plays a vital role in ADs. Therefore, we want to understand the research hot spots and future trends of ADs and gut microbiota more intuitively and comprehensively through bibliometric methods.
The bibliometric analysis uses mathematical and statistical methods to study the distribution, structure, quantity, and content evolution of bibliographic information qualitatively or quantitatively. It is of great value to describe the status quo of various research disciplines, publishing trends, and scientific achievements of researchers, institutions, and countries, as well as future research hot spots, academic frontiers, and knowledge maps, which provide researchers and clinicians a comprehensive picture of the current state of development in a particular research area [19, 20]. Moreover, bibliometrics has been widely used in immunology [19–25]. And VOSviewer is also a commonly used software in various fields of bibliometrics [21–30]. At the same time, COOC is a software developed by Chinese scholars for bibliometrics and scientific mapping and is continuously iterating [26]. COOC software has also been increasingly used in SCI-E articles [26–29]. It can be seen that bibliometric analysis is an excellent choice for the study of ADs and gut microbiota. Still, there need to be relevant studies on analyzing the whole literature by bibliometrics of ADs and gut microbiota. This paper aims to make up for the shortcomings of this study and summarize the studies of ADs and gut microbiota to some extent.
Material and methods
Data retrieval strategy, data extraction, and cleaning
The research object of this paper is the correlation study of ADs and gut microbiota. The web of science core collection is one of the most comprehensive and authoritative databases, containing more than 12,000 high-quality journals [19, 20, 31]. Thus, we select the web of science core collection SCI-expanded (SCI-E) database as the search source and as the data source of the research object and select the advanced search, the search formula: TS = (gut OR intestine OR gastrointestine OR gastrointestine) AND TS = (microbiota OR microbiome OR flora OR microflora OR bacteria) AND TS = (autoimmunity OR autoimmune). The time limit was from 2004-01-01 to 2022-12-20, and 2659 papers were retrieved, excluding duplicate publications, conference abstracts, letters, etc., mainly leaving articles and reviews. And without consulting in advance, we read the title, abstract, and keywords of the searched literature simultaneously, exclude irrelevant literature, and only include what we think can be left. In the end, a total of 2516 articles were left.
Scientometric analysis methods
The 2516 pieces of literature were exported in plain text format. We utilize Excel 2019 and visualization analysis tools VOSviewer, co-occurrence13.2 (COOC13.2) for overall trend analysis, synonym merging, frequency of countries/regions, institutions, authors and funds, cluster analysis of co-occurrence matrix, dissimilarity matrix, two-mode matrix, burst keywords map to explore the research hot spot and frontier direction of ADs and gut microbiota.
Results
Annual analysis of publication
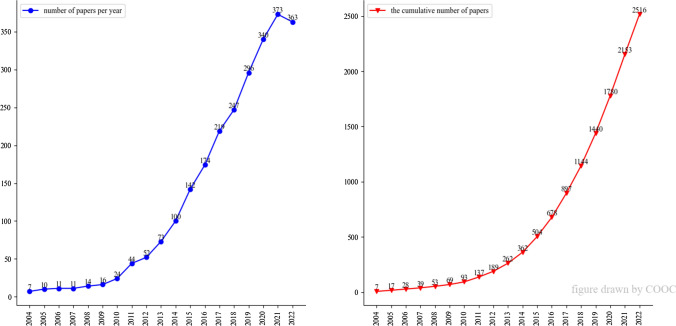
Since 2004, the research on ADs and gut microbiota has increased rapidly every year and only declined slightly in 2022 (Fig. 1). According to the increasing law of scientific literature, research in this direction is still rising.
Fig. 1.
Number of papers per year and the cumulative number of papers. According to this figure, an uprising trend can be seen from 2012 to 2021, and the peak value appears in 2021
Country/region, institution, author, and journal frequency analysis
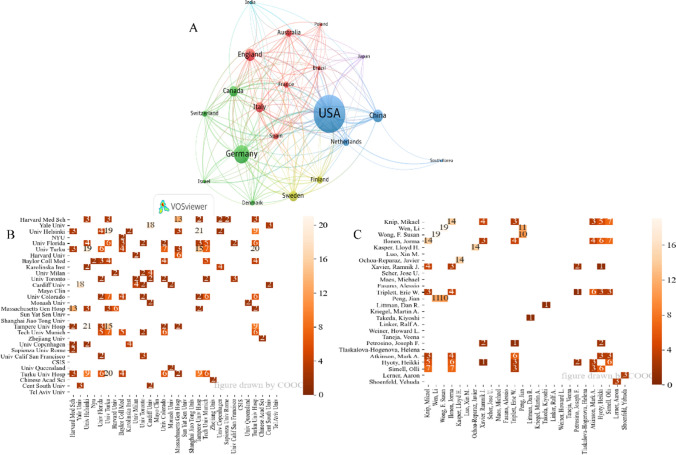
As seen from Table 1, the frequency analysis of countries/regions shows that the USA is the country with the most research on ADs and gut microbiota, and it is mainly concentrated in Europe and North America. In Asia and Oceania, only China, Japan, and Australia made the list. The most significant research institution is Harvard Medical School, with six from the USA and the other four from the University of Helsinki and the University of Turku in Finland, Karolinska Institute in Sweden, and the University of Milan in Italy. The most published authors are Mikael Knip from the University of Helsinki (23 articles). The second is Li Wen from Yale University. The third is F. Susan Wong from Cardiff University, and the fourth is Jorma Ilonen from the University of Turku. The fifth is Lloyd H. Kasper from the Geisel School of Medicine at Dartmouth College. Furthermore, the high-yield authors 6, 7, 8, and 10 were all from institutions in the USA. A total of seven high-yield authors were from US institutions. The top three journals were frontiers in immunology, international journal of molecular sciences, and frontiers in microbiology. The top 10 journals published a total of 574 articles. Meanwhile, three of the top 10 journals are immunology journals, three are microbiology journals, two are comprehensive journals, one is a biochemistry and molecular biology journal, and one is a nutrition journal. The types are not single, which proves to a certain extent that studies on ADs and gut microbiota involve multiple studies and have received attention from various fields.
Table 1.
Top 10 countries/regions, institutions, authors and journals
| Rank | Country/region | Count | Institution | Count | Author | Count | Journal | Count | 2022 impact factor /JCR partition |
|---|---|---|---|---|---|---|---|---|---|
| 1 | USA | 939 | Harvard Med Sch (USA) | 67 | Mikael Knip | 23 | Frontiers in immunology | 202 | 8.786/Q1 |
| 2 | China | 450 | Yale Univ (USA) | 48 | Li Wen | 22 | International journal of molecular sciences | 64 | 6.208/Q1 |
| 3 | Italy | 242 | Univ Helsinki (Finland) | 43 | F. Susan Wong | 21 | Frontiers in microbiology | 50 | 6.064/Q1 |
| 4 | Germany | 214 | NYU (USA) | 39 | Jorma Ilonen | 18 | Nutrients | 49 | 6.706/Q1 |
| 5 | England | 132 | Univ Florida (USA) | 39 | Lloyd H. Kasper | 18 | PloS One | 49 | 3.752/Q2 |
| 6 | Australia | 113 | Univ Turku (Finland) | 38 | Luo Xin M | 15 | Scientific reports | 42 | 4.996/Q2 |
| 7 | Japan | 112 | Harvard Univ (USA) | 35 | Javier Ochoa Reparaz | 15 | Gut microbes | 32 | 9.434/Q1 |
| 8 | Canada | 112 | Baylor Coll Med (USA) | 33 | Jose U. Scher | 13 | Journal of autoimmunity | 31 | 14.511/Q1 |
| 9 | Spain | 98 | Karolinska Inst (Sweden) | 31 | Michael Maes | 13 | Microorganisms | 28 | 4.926/Q2 |
| 10 | Netherlands | 92 | Univ Milan (Italy) | 30 | Ramnik J. Xavier | 13 | Journal of immunology | 27 | 5.426/Q2 |
Authors, institutions, countries/regions, and analysis of cooperation
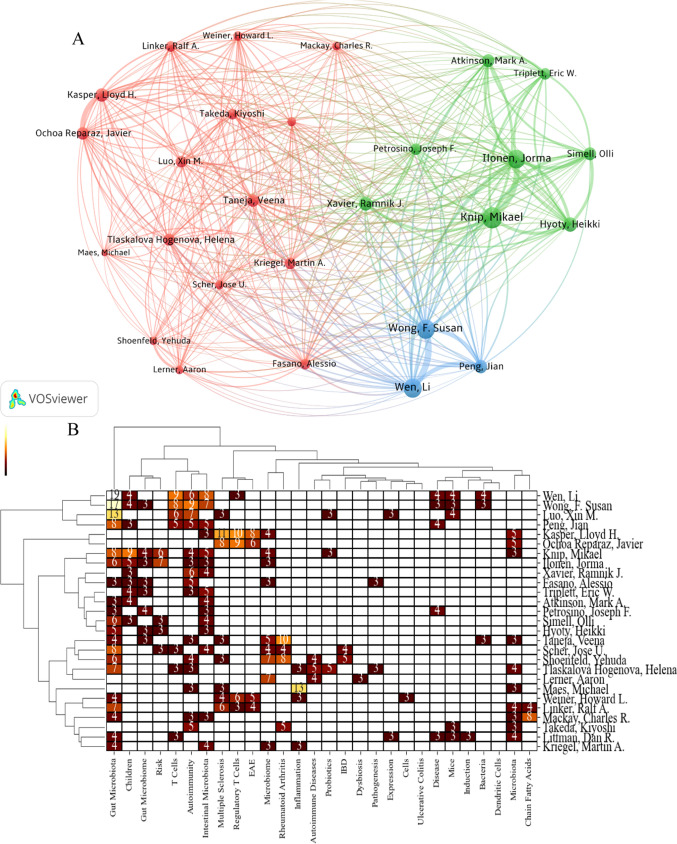
It can be seen from Fig. 2A that the largest number of cooperation is between the USA and China. Meanwhile, the USA also has a close collaboration with Germany, Italy, England, and Canada. In regards to institutions, Fig. 2B, the University of Helsinki cooperated with Tampere University Hospital most times. The University of Turku and Tampere University Hospital, Yale University, and Cardiff University also collaborate closely. The cooperation between them is transnational. The University of Helsinki and the University of Turku also have a tight cooperative relationship. Their collaboration with each other is concentrated in the corresponding country. Furthermore, The University of Turku closely cooperates with Turku University Hospital, and Harvard Medical School cooperates closely with Massachusetts General Hospital. They are all part of the Turku and Harvard health care system, so it is not surprising that there is much collaboration. There are more transnational cooperation and cooperation between the same system on this topic. According to Fig. 2C, professors Li Wen and F. Susan Wong have cooperated most frequently. Mikael Knip and Jorma Ilonen, as well as Lloyd H. Kasper and Javier Ochoa-Reparaz, have the second most times of cooperation. Li Wen and F. Susan Wong also closely cooperate with Jian Peng. In general, the cooperation among authors is relatively concentrated.
Fig. 2.
Countries/regions, institution, and author analysis. A Countries/regions cooperative network. B Institutions cooperative network. C Authors cluster map
Citation analysis
According to Table 2, the most cited article was a 2009 review in nature reviews immunology by June L. Round and Sarkis K. Mazmanian, about the strong connection between the immune system and the gut microbiome. It is proposed that microbial disorders are associated with immune dysregulation (associated with autoimmunity) and that the immune system may be engineered or controlled by microbes [32]. This was followed by an article by Ivaylo I. Ivanov et al. in Cell in 2009. They found that colonization of the small intestine of mice with commensal segmented filamentous bacteria (SFB) was beneficial for the expression of genes related to inflammation and antimicrobial defense and increased resistance to the intestinal pathogen citrobacter rodentgens. Manipulating this symbiotic regulation may provide new opportunities for enhancing mucosal immunity and treating ADs [33]. The third most cited, but most frequently cited per year on average, was a review published in Cell in 2014 by authors Yasmine Belkaid and Timothy W. Hand. The dramatic rise in autoimmune and inflammatory diseases in some parts of the world, possibly coupled with the overuse of antibiotics, changes in diet, and elimination of nematodes, results in a lack of the resilient and diverse microbiota needed to build a balanced immune response, which plays a vital role in the induction, training, and function of the host immune system [34].
Table 2.
Ranking of the top 10 highest cited references
| Rank | Year | Title | Journal | First author | Citations |
|---|---|---|---|---|---|
| 1 | 2009 | The gut microbiota shapes intestinal immune responses during health and disease | Nature reviews immunology | June L. Round | 3045 |
| 2 | 2009 | Induction of intestinal Th17 cells by segmented filamentous bacteria | Cell | Ivaylo I. Ivanov | 2965 |
| 3 | 2014 | Role of the microbiota in immunity and inflammation | Cell | Yasmine Belkaid | 2307 |
| 4 | 2008 | Innate immunity and intestinal microbiota in the development of type 1 diabetes | Nature | Li Wen | 1393 |
| 5 | 2013 | Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity | Science | Janet G. M. Markle | 1145 |
| 6 | 2010 | Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells | Immunity | Hsin-Jung Wu | 1082 |
| 7 | 2014 | The microbiome in inflammatory bowel disease: current status and the future ahead | Gastroenterology | Aleksandar D. Kostic | 1024 |
| 8 | 2011 | Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis | Proceedings of the national academy of sciences of the United States of America | Yun Kyung Lee | 882 |
| 9 | 2017 | Interactions between the microbiota, immune and nervous systems in health and disease | Nature neuroscience | Thomas C. Fung | 849 |
| 10 | 2011 | Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination | Nature | Kerstin Berer | 808 |
The number of times used in 180 days reflects the number of times the article has met a user’s information needs, as demonstrated by clicking links to the full-length article on the publisher’s website or by saving the metadata for later use. High usage counts take time to translate into high citations. Still, they have the advantage of novelty, and researchers tend to use newer literature. Still, older literature with higher citations contributes to a secondary increase in its subsequent use [35]. The number of 180-day usages can reflect the current research hot spots and frontiers to a certain extent.
According to Table 3, the first and second most frequently used articles within 180 days were the third and first, respectively, highly cited articles. In third place is an article by Xuan Zhao et al., published in Microbiome in 2022, that disease-resistant phenotypes are related to immunomodulatory function and immune tolerance, with implications for animal husbandry and human health [36]. By analyzing a model of acute colitis developed by Min pigs and Yorkshire pigs, they found that host-microbiota crosstalk contributes to disease resistance phenotypes in three ways: By maintaining part of pattern recognition receptor (PRR) not activated, it can maintain Th2 immune dominance and immune tolerance mode and restore intestinal barrier function to prevent colon diseases [36]. The fourth is the article by Yangxin Li et al. in phytomedicine, where they found that Ershiwuwei Lvxue Pill (ELP), a prescription of Tibetan medicine, can improve joint damage in systemic autoimmune disease rheumatoid arthritis (RA) by inhibiting the production of matrix metalloproteinases (MMPs) and osteoclast activity and regulating intestinal microbiota and host metabolites [37]. The fifth is a review by Liying He et al. published in biomedicine and pharmacotherapy in 2022, in which they summarized the mechanism of interaction between diabetes and gut microbiota and also classified and summarized the natural compounds that treat diabetes through gut microbiota [37]. The sixth on the list, and also on the highly cited list, is a review by Thomas C. Fung, Christina A. Olson, and Elaine Y. Hsiao, published in nature neuroscience in 2017. Microbes influence the activation of peripheral immune cells, which regulate responses to neuroinflammation, brain injury, autoimmunity, and neurogenesis. They discuss the role of CNS resident and peripheral immune pathways in microbiota-gut-brain communication during health and neurological disease [38]. It can be seen that gut microbes impact ADs in multiple body systems.
Table 3.
Ranking of the top 10 highest 180 days usage
| Rank | Year | Title | Journal | First author | Usage count |
|---|---|---|---|---|---|
| 1 | 2014 | Role of the microbiota in immunity and inflammation | Cell | Yasmine Belkaid | 79 |
| 2 | 2009 | The gut microbiota shapes intestinal immune responses during health and disease | Nature reviews immunology | June L. Round | 66 |
| 3 | 2022 | Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs | Microbiome | Xuan Zhao | 57 |
| 4 | 2022 | Ershiwuwei Lvxue Pill alleviates rheumatoid arthritis by different pathways and produces changes in the gut microbiota | Phytomedicine | Yangxin Li | 47 |
| 5 | 2022 | Regulation of the intestinal flora: a potential mechanism of natural medicines in the treatment of type 2 diabetes Mellitus | Biomedicine and pharmacotherapy | Liying He | 46 |
| 6 | 2017 | Interactions between the microbiota, immune and nervous systems in health and disease | Nature neuroscience | Thomas C. Fung | 40 |
| 7 | 2021 | Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? | Nature reviews immunology | Cezmi A. Akdis | 38 |
| 8 | 2022 | Metabolite-based dietary supplementation in human type 1 diabetes is associated with microbiota and immune modulation | Microbiome | Kirstine J. Bell | 37 |
| 9 | 2021 | The gut-joint axis in rheumatoid arthritis | Nature reviews rheumatology | Mario M. Zaiss | 35 |
| 10 | 2021 | Polysaccharides confer benefits in immune regulation and multiple sclerosis by interacting with gut microbiota | Food research international | Ying Sun | 35 |
Keywords analysis
Keywords frequency analysis
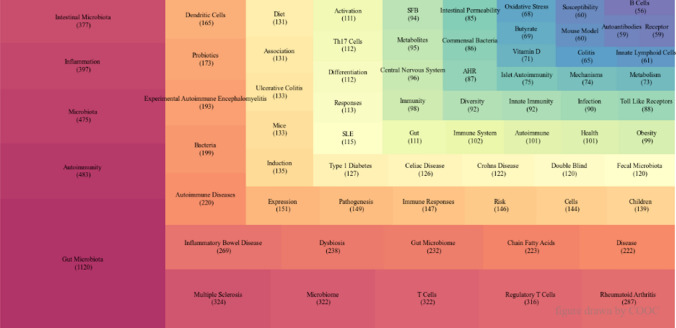
COOC13.2 was used to extract keywords, and the keywords were synonymously combined. Because keywords overlap in the image, the aryl hydrocarbon receptor, abbreviated as AHR, and the systemic lupus erythematosus abbreviated as SLE. Finally, the top 70 keyword frequencies are reflected in Fig. 3. Keyword frequency is an important index that directly demonstrates the research content, research hot spot, and frontier direction of a field.
Fig. 3.
Tree map of top 70 keywords
Keywords co-occurrence analysis
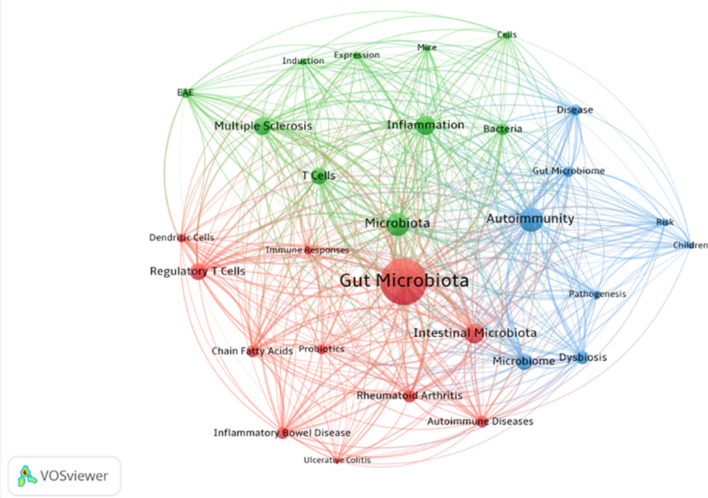
There must be some correlation among the keywords given in the paper, which can be expressed by the co-occurrence frequency. It is generally believed that the more lexical pairs appear in the same literature, the closer the relationship between these two topics will be. Experimental autoimmune encephalomyelitis was abbreviated as EAE because the complete keywords could not be displayed. As shown in Fig. 4, excluding the relationship between the five headings gut microbiota, autoimmunity, microbiota, intestinal microbiota, and microbiome, gut microbiota is associated with inflammation, multiple sclerosis (MS), T Cells, regulatory T Cells (Tregs), RA, inflammatory bowel disease (IBD), and short chain fatty acids (SCFAs). It can be seen that ADs and gut microbiota mainly focus on the immune response, immune cells, and related diseases.
Fig. 4.
Keywords co-occurrence network
Keywords, authors analysis
We use the keyword coupling strength of authors’ works to establish the relationship between authors, draw the corresponding two-mode matrix based on the number of two authors with the exact keywords, and directly display the correlation between authors and keywords in the visualization diagram, which should be able to explicitly and instantly discover the subject knowledge structure centered on the main author and can show the diversity of the author’s academic interests. Compared with the cooperative network of authors in Fig. 2C, Fig. 5A can better reflect the researcher’s research content and seek cooperation between authors in the same research field by coupling keywords with the author. Through Fig. 5A and B can intuitively display the author’s research content. For example, Professor Li Wen has researched gut microbiota, T cells, autoimmunity, intestinal microbiota, Tregs, children, disease, mice, and bacteria. Meanwhile, we can also reflect on the detailed research fields of the authors through Fig. 5A and B, for example, Mikael Knip, Jorma Ilonen, Ramnik J. Xavier, Eric W. Triplett, Mark A. Atkinson, Heikki Hyoty, Olli Simell, and Joseph F. Petrosino, these eight authors have similar research fields.
Fig. 5.
Keywords and authors analysis A keywords and authors coupling matrix. B Keywords and author two-modular matrix
Keywords cluster analysis
The high-frequency keywords were analyzed by cluster analysis, and the keywords were classified. According to the co-occurrence color of figure keywords and the understanding of self-related knowledge, the keywords gut microbiota, autoimmunity, microbiota, intestinal microbiota, microbiome, etc., were removed. And they were divided into three categories: A. intestinal regulation, B. immune diseases, C. immune-related cells.
Keywords, time analysis
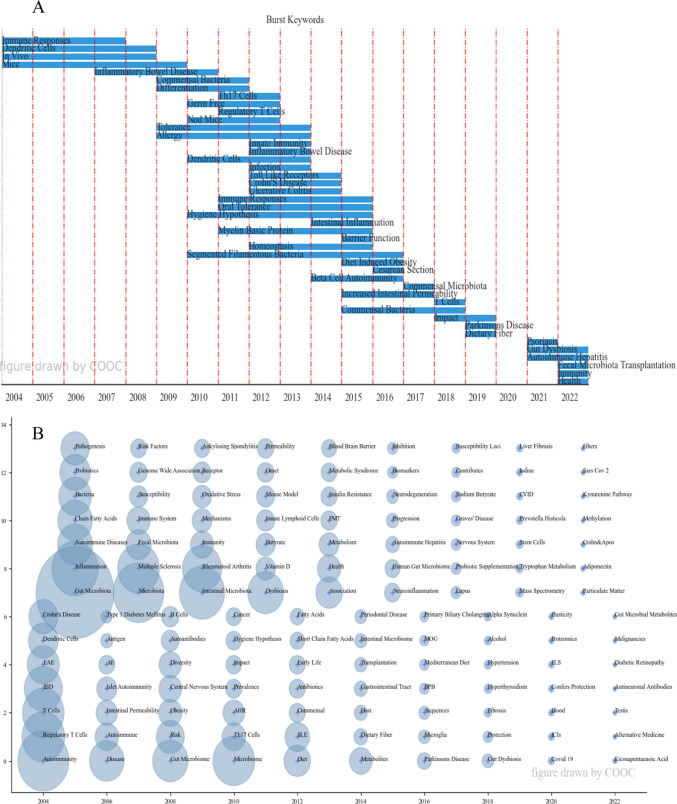
COOC13.2 software is used to draw Fig. 6, which can reflect the changing trend of research topics in the field over time. Figure 6A can focus on the annual keyword mutation, which can better grasp the annual hot issues, and provide a reference for the future research and development of the industry through the mutation words in recent years. By setting the interception frequency of 20 through COOC13.2, Fig. 6A is obtained. Figure 6B. Each circle represents a keyword, and the larger the circle, the higher the frequency of the critical word. The key word is in the year when it first appeared in the analyzed dataset. Once a keyword occurs, it will be fixed to the year it first appeared, although it will still appear in the paper afterward, and will no longer be shown in the figure, only in the year it first appeared. If the keyword appears again in the later years, it will increase the frequency to the position of the keyword for the first time, and the frequency will increase several times. Because keywords overlap in the image, ARS for acute respiratory syndrome, HDP for highly differentiated phenotype, FMT for fecal microbiota transplantation, ICIs for immune checkpoint inhibitors, MOG for myelin oligodendrocyte glycoprotein, BPB for butyrate-producing bacteria, FLS for fibroblast like synoviocytes, and CVID for common variable immunodeficiency. COOC software was used to draw Fig. 6B of topic evolution, reflecting the changing trend of research topics in the field over time.
Fig. 6.
Keywords and time analysis. A Burst keywords map. B Time zone diagram of theme evolution path
Discussion
General information
The overall number of studies on ADs and gut microbiota showed an upward trend, especially after 2012. The possible reason is that the National Institutes of Health of the United States issued the human microbiome project (HMP) in 2007, and the European Commission announced the launch of the “METAgenomics of the Human Intestinal Tract (MetaHIT)” in 2008. The publication of HMP results in 2012, which might lead to the rapid growth of gut microbiota related research. Afterward, many studies have shown that gut microbiota has a relationship with the immune system, which has also led researchers to focus on ADs with complex etiologies, resulting in the growth of related research on ADs and gut microbiota. From the countries/regions in Table 1, Fig. 2A, the USA (939 articles) has the highest productivity, surpassing second-place China (450 articles). There is close collaboration between countries, especially the USA, with 89 times and 66 times in China and Germany, respectively. In addition, according to Table 1 and Fig. 2B and C, six of the top 10 institutions are from the USA, and the other four were Finland (2), Sweden, and Italy. And seven of the top 10 authors are from the USA, which shows that the related research in the USA is more productive. Furthermore, through Fig. 5, we can know the authors with the same research direction, such as these eight professors, Mikael Knip, Jorma Ilonen, Ramnik J. Xavier, Eric W. Triplett, Mark A. Atkinson, Heikki Hyoty, Olli Simell, and Joseph F. Petrosino, which provides references for cooperation and communication between authors.
Analysis of research contents and hot spots
The research content can be divided into three categories: A. intestinal regulation, B. multisystem ADs, C. immune-associated cells.
Category A includes intestinal regulation, such as dysbiosis, SCFAs, and probiotics. Dysbiosis refers to the disturbance of microbial composition and function associated with mucosal barrier dysfunction and inflammatory response [17, 39] and is associated with ADs, especially RA, type 1 diabetes (T1D), MS, and autoimmune liver disease (AILD), IBD [13, 39]. Dysbiosis can be induced by diet (such as chronic consumption of saturated or trans fats, meat proteins, reducing sugars and salt, and a diet low in fiber [13]), drugs (such as antibiotics [40]), and endogenous factors (such as antimicrobial peptides, S-IgA and mucin layers) [17], mutations in genes (i.e., NOD2 and XBP1) and environmental stress [41]. Therefore, in the relevant studies of dysbiosis in ADs and gut microbiota, the causes and effects of dysbiosis are hot topics. Fatty acids are divided into SCFAs, medium chain fatty acids (MCFAs) and long chain fatty acids (LCFAs). SCFAs are metabolites produced by intestinal microflora through the digestion of complex carbohydrates by fermentation [42]. SCFAs have a variety of mechanisms that inhibit intestinal inflammation and are an inhibitor of histone deacetylase (HDAC), and they can stimulate histone acetyltransferase activity and stabilize hypoxia-inducing factors (HIF) and may induce Tregs either by G-protein-coupled receptors or inhibition of HDAC [42, 43]. LCFAs enhanced the differentiation and proliferation of T helper cell 1 (Th1) and/or T helper cell 17(Th17) cells and damaged their intestinal isolation through the p38-MAPK pathway, which also promoted the autoimmune suppressive T(H)2 immune responses [44, 45]. Its research focuses on treating MS, an autoimmune disease in the nervous system. Probiotics are a common way to regulate gut flora. The main mechanisms of action include enhanced mucosal barrier function, increased abundance of beneficial bacteria, direct antagonism with pathogens, reduced abundance of potentially harmful bacteria in the gut, inhibition of bacterial adhesion and intestinal epithelial invasion, enhanced immune system, and regulation of the central nervous system [46, 47]. In patients with ADs, probiotics can be introduced as a support measure in addition to the standard treatment plan [11]. It has been suggested that the onset of reproductive dysfunction in microbiota can be treated by probiotics using typical species of the genus Lactobacillus [48]. Therefore, using probiotics to regulate intestinal flora to treat ADs is a hot spot.
Category B includes MS, RA, IBD, EAE, and ulcerative colitis (UC). MS is a spontaneous immune disease of the nervous system. The microbial-gut-brain axis provides new ideas for treating nervous system diseases such as MS by gut microbiota. The impact of the gut microbiome on immune function through regulation of serotonin production in the gut and complex interactions with immune system components such as T and B cells is important in the development and course of MS [49], and the association of specific gut microbiome with functional changes in MS risk, disease duration and progression, and treatment response [50]. Therefore, the exploration of different intestinal flora provides new ideas for the treatment of MS. Similarly, dietary-microbiome studies have demonstrated that for MS, diet therapy is also one of the directions. As mentioned earlier, SCFAs are one of the dietary modalities used to treat MS. Moreover, identifying gut microbiota-specific IgA cells as systemic mediators of MS has broad implications as a useful biomarker and IgA-producing cells as an immune subset for therapeutic intervention [51]. And, EAE is an animal model of MS [52]. For animal models, the main role is to simulate the effects of various experiments on MS and explore the mechanism of MS, including intestinal flora. For RA, which is a chronic autoimmune inflammatory disease that primarily affects joints, gut microbiota and/or gut barrier function may help prevent or treat RA [53]. DMARDs that regulate the gut-joint axis can change the composition of gut microbiota [53], and drugs that regulate gut microbiota or anti-inflammatory drugs that require intestinal activation also play a role in the development of drugs for the treatment of RA [54]. Additionally, diets such as acarbose, probiotics, and prebiotics play a role in the prevention of RA [55]. IBD, including Crohn’s disease (CD) and UC, is a multifactorial chronic disease of the gastrointestinal tract [56]. Specific classes of metabolites of gut microbes, especially bile acids, SCFAs, and tryptophan metabolites, have been implicated in the pathogenesis of IBD [57]. In terms of diet, there have been attempts to treat IBD by modulating the gut microbiome with probiotics, prebiotics, antibiotics, FMT, and genetic manipulation [58]. And several cross-sectional reports suggest that a gluten-free diet (GFD) may improve symptoms in patients with IBD [59]. For CD, the bacterial-metabolite interaction network of sulfur metabolism is a key mechanism associated with CD activity [60]. Nutritional strategies for children with CD are exclusive enteral nutrition (EEN), partial enteral nutrition (PEN), Crohn’s disease elimination diet (CDED), and Crohn’s disease with diet therapy (CD-Treat) [61]. For UC, the treatment of UC by specific strains is one of the directions. For example, engineered S. cerevisiae can be used as an effective and safe treatment strategy for UC by inhibiting macrophage pyroptosis and regulating intestinal microbiota [62]. And substances that affect gut microbiota have been isolated from medicinal herbs (e.g., SP2-1, from Scutellaria baicalensis georgi [63], rhein, from rhubarb [64], evodiamine, from evodia fructus [65]) to treat UC. Furthermore, FMT by colonoscopic infusion or enema or by oral administration may all be promising and feasible treatment options for UC [66], and multidonor FMT with an anti-inflammatory diet can effectively induce deep remission of mild-moderate UC for more than one year [67]. Moreover, T1D, celiac disease (CeD), CD, and SLE, although they did not appear in the keyword co-occurrence network (30 keywords), Fig. 3 shows that they ranked 32, 33, 34 and 37, respectively, in the list of high-frequency keywords, and there are many related studies. These are also key concerns in gut microbiota and ADs. Among them, T1D and CeD are also closely related to pediatric research [68–71], and CD belongs to IBD mentioned above.
Category C includes T cells, Tregs, and dendritic cells. T cells are closely related to intestinal microbes and ADs, affect the stability of intestinal microbes, and are also regulated by intestinal microbes [72]. Treg is a kind of T cells, which has clinical potential as a cell therapy for the treatment of autoimmunity [73]. The immune imbalance between anti-inflammatory Tregs and proinflammatory Th17 is associated with a variety of ADs [74, 75]. Attention has been paid to the influence of intestinal microbiota [74], and Tregs have also been associated with intestinal dysbiosis [76]. Tregs, as mentioned above, are regulated by SCFAs and LCFAs, and some altered commensal communities can enhance the mitochondrial fitness of intestinal Tregs [77]. Th17 cells are also a type of T cells, which also play an important role in ADs and gut microbiome research [72, 78], ranking 39 in the high-frequency keywords. Dendritic cells, one of the major professional antigen-presenting cells, are also affected by gut microbiota-derived metabolites [79], such as SCFAs [80], taurine deoxycholic acid (TCDCA) [81], and secondary bile acids (BAs)[82]. Among them, BAs regulate DC mainly through TGR5 [81, 82].
Frontier analysis
According to Fig. 6A, burst keywords can find annual hot issues. In 2012, when the data began to grow rapidly, we can see that the most prominent keywords are Th17 Cells (2011–2012), Germ Free (2010–2012), Tregs (2011–2012), Nod Mice (2010–2012), Tolerance (2009–2013), Allergy (2009–2013), dendritic cells (2010–2013), etc. As for 2021 and 2022, the most prominent keywords are psoriasis (2021), gut dysbiosis (2021–2022), AIH (2021–2022), and FMT (2022).
Psoriasis is an immune-mediated systemic disease that affects approximately 125 million people worldwide with profound skin and intestinal dysbiosis [83–85]. Tregs deficiency contributes to the pathogenesis of psoriasis and may be attributed to enhanced suppression and/or impaired stimulation of Tregs [86]. Oral probiotics, prebiotics, and fecal microbial transplantation are most evident in providing health benefits for patients with psoriasis [84, 87]. In addition, n-3 polyunsaturated fatty acids, vitamin D, vitamin B12, SCFAs, selenium, genistein, and dietary fibers are also beneficial for psoriasis, and deficiencies in vitamin D or selenium have also been associated with intestinal disorders [83]. As mentioned above, gut dysbiosis is related to a variety of ADs [13, 39]. Gut dysbiosis is not only a hot spot but also a frontier in ADs and gut microbiota.
AIH is a chronic immune-mediated liver disease that is distributed in all ethnic groups worldwide with increasing prevalence [88]. The gut microbiota can be used as a non-invasive biomarker to assess the potential of autoimmune hepatitis [89]. Probiotics and FMT, which may be involved in the regulation of the immune imbalance of follicular regulatory T(TFR) and helper T(TFH) cells and the recovery of IM composition, as well as targeting signaling pathways associated with the gut microbiome, which has provided new insights into the treatment of patients with AIH [88, 90]. The emergence of AIH also reflects the in-depth and related research on the gut-liver axis/liver-microbiome axis and AIH to a certain extent. In addition to AIH, AILD also contains primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) [91]. For PBC and PSC, they may also become the frontier of ADs and gut microbiota like AIH.
FMT was mentioned above as a potentially promising and feasible treatment option for the treatment of UC [66]. For psoriasis, FMT is one of the most effective modalities [84], as well as one of the treatment modalities for AIH [88, 90]. It is also effective against systemic sclerosis and T1D [92]. FMT can be optimized as a tailored dietary intervention pair, facilitating a pathway for precise engineering of the gut microbiome using the diet in ADs [93]. It can be seen that FMT is effective and relatively safe in the treatment of ADs and is expected to be used as a method to induce remission of active ADs [92]. Thus, from Fig. 6A, we think that psoriasis, gut dysbiosis, AILD, and FMT may be the future research direction.
Strength and limitation
This study provides the first intuitive, objective, accurate, and comprehensive systematic analysis of ADs and gut microbiota publications and their trends, which can provide comprehensive guidance for clinicians and scholars in the field. Literature metrology and visual analysis can help researchers intuitively understand the research hot spot, evolution, and development trend of ADs and gut microbiota. Inevitably, there are some limitations to the study. First, the literature included in our study may not be exhaustive. For one thing, our study only examined data from the Web of Science SCI-E database. Therefore, the articles identified may not adequately reflect all ADs and gut microbiota studies, and more detailed studies are expected in the future.
Conclusion
In conclusion, from the annual publication volume of related literature, gut microbiota has attracted more and more attention to ADs. Europe and the USA have made the greatest contribution to this field, and the cooperation between them is closer, and the publications are more concentrated. The research focus is mainly on dysbiosis, SCFAs, and probiotics for the regulation of gut microbiota, the impact of gut microbiota on MS, RA, IBD, EAE, UC, as well as T1D, CeD, CD, SLE, and related immune cells involved in ADs and gut microbiota, such as T cells, Tregs, Dendritic cells, as well as Th17. As for the ADs and gut microbiota research frontier, it is possible to focus on gut dysbiosis, which is also involved in hot spots. In addition, psoriasis, AILD (including AIH, PBC, PSC), and FMT may also be future research directions. These findings can help clinicians and researchers understand ADs and gut microbiota research hot spots and provide references for future research directions.
Author contributions
All authors contributed to the study conception and design. Conceptualization, methodology, investigation, writing, reviewing and editing were performed by YZ. Reviewing and revising were performed by YP. Conceptualization, methodology, investigation, reviewing and editing were performed by XX. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by (Special Fund Project for Science and Technology Innovation Strategy of Guangdong Province [Grant Number pdjh2023b0107) and National College Students Innovation and Entrepreneurship Training Program [Grant Number 202212121008]).
Availability of data and material
The datasets generated during the current study are available in the Web of Science (http://www.webofknowledge.com).
Declarations
Conflict of interest
The authors state that this study was conducted without any commercial or financial relationship that could be interpreted as a potential conflict of interest.
Ethical approval and consent to participate
The data are all from the public database web of science, which does not involve ethical issues.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Youao Zhang, Email: 1372330529@qq.com.
Yongzheng Peng, Email: yzpeng1981@126.com.
Xu Xia, Email: 853877559@qq.com.
References
- 1.Marson A, Housley WJ, Hafler DA. Genetic basis of autoimmunity. J Clin Investig. 2015;125(6):2234–2241. doi: 10.1172/JCI78086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilchmann-Diounou H, Menard S. Psychological stress, intestinal barrier dysfunctions, and autoimmune disorders: an overview. Front Immunol. 1823;2020:11. doi: 10.3389/fimmu.2020.01823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan H, Sureda A, Belwal T, et al. Polyphenols in the treatment of autoimmune diseases. Autoimmun Rev. 2019;18(7):647–657. doi: 10.1016/j.autrev.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyauchi E, Shimokawa C, Steimle A, Desai MS, Ohno H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat Rev Immunol. 2023;23(1):9–23. doi: 10.1038/s41577-022-00727-y. [DOI] [PubMed] [Google Scholar]
- 5.Stojanovich L, Marisavljevich D. Stress as a trigger of autoimmune disease. Autoimmun Rev. 2008;7(3):209–213. doi: 10.1016/j.autrev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Rossi CM, Lenti MV, Merli S, Santacroce G, Di Sabatino A. Allergic manifestations in autoimmune gastrointestinal disorders. Autoimmun Rev. 2022;21(1):102958. doi: 10.1016/j.autrev.2021.102958. [DOI] [PubMed] [Google Scholar]
- 7.Xiao ZX, Miller JS, Zheng SG. An updated advance of autoantibodies in autoimmune diseases. Autoimmun Rev. 2021;20(2):102743. doi: 10.1016/j.autrev.2020.102743. [DOI] [PubMed] [Google Scholar]
- 8.Wu F, Gao J, Kang J, et al. Knowledge mapping of exosomes in autoimmune diseases: a bibliometric analysis (2002–2021) Front Immunol. 2022;13:939433. doi: 10.3389/fimmu.2022.939433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konforte D, Diamandis EP, van Venrooij WJ, Lories R, Ward MM. Autoimmune diseases: early diagnosis and new treatment strategies. Clin Chem. 2012;58(11):1510–1514. doi: 10.1373/clinchem.2012.189480. [DOI] [PubMed] [Google Scholar]
- 10.Shen P, Lin W, Deng X, et al. Potential Implications of Quercetin in Autoimmune Diseases. FRONT IMMUNOL. 2021;12:689044. doi: 10.3389/fimmu.2021.689044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccioni A, Cicchinelli S, Valletta F, et al. Gut microbiota and autoimmune diseases: a charming real world together with probiotics. Curr Med Chem. 2022;29(18):3147–3159. doi: 10.2174/0929867328666210922161913. [DOI] [PubMed] [Google Scholar]
- 12.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao Y, Wu L, Huntington ND, Zhang X. Crosstalk between gut microbiota and innate immunity and its implication in autoimmune diseases. Front Immunol. 2020;11:282. doi: 10.3389/fimmu.2020.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geva-Zatorsky N, Sefik E, Kua L, et al. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168(5):928–943. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christovich A, Luo XM. Gut microbiota, leaky gut, and autoimmune diseases. Front Immunol. 2022;13:946248. doi: 10.3389/fimmu.2022.946248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martel J, Chang SH, Ko YF, Hwang TL, Young JD, Ojcius DM. Gut barrier disruption and chronic disease. Trends Endocrinol Metab. 2022;33(4):247–265. doi: 10.1016/j.tem.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Kinashi Y, Hase K. Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front Immunol. 2021;12:673708. doi: 10.3389/fimmu.2021.673708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol. 2019;195(1):74–85. doi: 10.1111/cei.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J, Lyu W, Chen N. A bibliometric analysis of diffuse large B-cell lymphoma research from 2001 to 2020. Comput Biol Med. 2022;146:105565. doi: 10.1016/j.compbiomed.2022.105565. [DOI] [PubMed] [Google Scholar]
- 20.Meng T, Wang P, Ding J, et al. Global research trends on ventricular remodeling: a bibliometric analysis from 2012 to 2022. Curr Probl Cardiol. 2022;47(11):101332. doi: 10.1016/j.cpcardiol.2022.101332. [DOI] [PubMed] [Google Scholar]
- 21.Wan Y, Shen J, Ouyang J, et al. Bibliometric and visual analysis of neutrophil extracellular traps from 2004 to 2022. Front Immunol. 2022;13:1025861. doi: 10.3389/fimmu.2022.1025861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang ST, Liu YG, Zhang L, Sang XT, Xu YY, Lu X. Immune-related adverse events: a bibliometric analysis. Front Immunol. 2022;13:1096806. doi: 10.3389/fimmu.2022.1096806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei N, Xu Y, Li Y, et al. A bibliometric analysis of T cell and atherosclerosis. Front Immunol. 2022;13:948314. doi: 10.3389/fimmu.2022.948314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Yin Z, Du H, Huang K, Zhang F, Chen H. The latest research trends in primary biliary cholangitis: a bibliometric analysis. Clin Exp Med. 2022 doi: 10.1007/s10238-022-00825-0. [DOI] [PubMed] [Google Scholar]
- 25.Wan C, Kong X, Liao Y, et al. Bibliometric analysis of the 100 most-cited papers about the role of gut microbiota in irritable bowel syndrome from 2000 to 2021. Clin Exp Med. 2022 doi: 10.1007/s10238-022-00971-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhou T, Qu J, Sun H, Xue M, Shen Y, Liu Y. Research trends and hotspots on montessori intervention in patients with dementia from 2000 to 2021: a bibliometric analysis. Front Psychiatry. 2021;12:737270. doi: 10.3389/fpsyt.2021.737270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu Q, Ge J, Xu Y, et al. The evolution of research on depression during COVID-19: a visual analysis using co-occurrence and VOSviewer. Front Public Health. 2022;10:1061486. doi: 10.3389/fpubh.2022.1061486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan H, Li J, He M, et al. Global evolution of research on green energy and environmental technologies: a bibliometric study. J Environ manag. 2021;297:113382. doi: 10.1016/j.jenvman.2021.113382. [DOI] [PubMed] [Google Scholar]
- 29.Qin F, Li J, Zhang C, et al. Biochar in the 21st century: a data-driven visualization of collaboration, frontier identification, and future trend. Sci Total Environ. 2022;818:151774. doi: 10.1016/j.scitotenv.2021.151774. [DOI] [PubMed] [Google Scholar]
- 30.van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Zhao S, Tan L, et al. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens bioelectron. 2022;201:113932. doi: 10.1016/j.bios.2021.113932. [DOI] [PubMed] [Google Scholar]
- 32.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang XW, Fang ZC, Sun XL. Usage patterns of scholarly articles on web of science: a study on Web of Science usage count. Scientometrics. 2016;109(2):917–926. [Google Scholar]
- 36.Zhao X, Jiang L, Fang X, et al. Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs. Microbiome. 2022;10(1):115. doi: 10.1186/s40168-022-01303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Liu C, Luo J, et al. Ershiwuwei Lvxue pill alleviates rheumatoid arthritis by different pathways and produces changes in the gut microbiota. Phytomedicine. 2022;107:154462. doi: 10.1016/j.phymed.2022.154462. [DOI] [PubMed] [Google Scholar]
- 38.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li B, Selmi C, Tang R, Gershwin ME, Ma X. The microbiome and autoimmunity: a paradigm from the gut-liver axis. Cell Mol Immunol. 2018;15(6):595–609. doi: 10.1038/cmi.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Y, Huang Y, Wang Y, Wang P, Song H, Wang F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE. 2019;14(6):e218384. doi: 10.1371/journal.pone.0218384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salzman NH, Bevins CL. Dysbiosis—a consequence of Paneth cell dysfunction. Semin Immunol. 2013;25(5):334–341. doi: 10.1016/j.smim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Yoo JY, Groer M, Dutra S, Sarkar A, McSkimming DI. Gut microbiota and immune system interactions. Microorganisms. 2020;8(10):1587. doi: 10.3390/microorganisms8101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haase S, Haghikia A, Wilck N, Müller DN, Linker RA. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology. 2018;154(2):230–238. doi: 10.1111/imm.12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haghikia A, Jörg S, Duscha A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43(4):817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Berer K, Martínez I, Walker A, et al. Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. Sci Rep. 2018;8(1):10431. doi: 10.1038/s41598-018-28839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stavropoulou E, Bezirtzoglou E. Probiotics in medicine: a long debate. Front Immunol. 2020;11:2192. doi: 10.3389/fimmu.2020.02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Q, Tian H, Kang Y, et al. Probiotics alleviate autoimmune hepatitis in mice through modulation of gut microbiota and intestinal permeability. J Nutr Biochem. 2021;98:108863. doi: 10.1016/j.jnutbio.2021.108863. [DOI] [PubMed] [Google Scholar]
- 48.López-Moreno A, Aguilera M. Probiotics dietary supplementation for modulating endocrine and fertility microbiota dysbiosis. Nutrients. 2020;12(3):757. doi: 10.3390/nu12030757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol. 2022;18(9):544–558. doi: 10.1038/s41582-022-00697-8. [DOI] [PubMed] [Google Scholar]
- 50.Zhou X, Baumann R, Gao X, et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. 2022;185(19):3467–3486. doi: 10.1016/j.cell.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pröbstel AK, Zhou X, Baumann R, et al. Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. 2020 doi: 10.1126/sciimmunol.abc7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin X, Liu Y, Ma L, et al. Constipation induced gut microbiota dysbiosis exacerbates experimental autoimmune encephalomyelitis in C57BL/6 mice. J Transl Med. 2021;19(1):317. doi: 10.1186/s12967-021-02995-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaiss MM, Joyce WH, Mauro D, Schett G, Ciccia F. The gut-joint axis in rheumatoid arthritis. Nat Rev Rheumatol. 2021;17(4):224–237. doi: 10.1038/s41584-021-00585-3. [DOI] [PubMed] [Google Scholar]
- 54.Xu H, Zhao H, Fan D, et al. Interactions between gut microbiota and immunomodulatory cells in rheumatoid arthritis. Mediat Inflamm. 2020;2020:1430605. doi: 10.1155/2020/1430605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horta-Baas G, Sandoval-Cabrera A, Romero-Figueroa M. Modification of gut microbiota in inflammatory arthritis: highlights and future challenges. Curr Rheumatol Rep. 2021;23(8):67. doi: 10.1007/s11926-021-01031-9. [DOI] [PubMed] [Google Scholar]
- 56.Franzosa EA, Sirota-Madi A, Avila-Pacheco J, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4(2):293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 58.Glassner KL, Abraham BP, Quigley E. The microbiome and inflammatory bowel disease. J Allergy Clin Immun. 2020;145(1):16–27. doi: 10.1016/j.jaci.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Weaver KN, Herfarth H. Gluten-free diet in IBD: Time for a recommendation? Mol Nutr Food Res. 2021;65(5):e1901274. doi: 10.1002/mnfr.201901274. [DOI] [PubMed] [Google Scholar]
- 60.Metwaly A, Dunkel A, Waldschmitt N, et al. Integrated microbiota and metabolite profiles link Crohn’s disease to sulfur metabolism. Nat Commun. 2020;11(1):4322. doi: 10.1038/s41467-020-17956-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verburgt CM, Ghiboub M, Benninga MA, de Jonge WJ, Van Limbergen JE. Nutritional therapy strategies in pediatric Crohn’s disease. Nutrients. 2021;13(1):212. doi: 10.3390/nu13010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun S, Xu X, Liang L, et al. Lactic acid-producing probiotic Saccharomyces cerevisiae attenuates ulcerative colitis via suppressing macrophage pyroptosis and modulating gut microbiota. Front Immunol. 2021;12:777665. doi: 10.3389/fimmu.2021.777665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui L, Guan X, Ding W, et al. Scutellaria baicalensis georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int J Biol Macromol. 2021;166:1035–1045. doi: 10.1016/j.ijbiomac.2020.10.259. [DOI] [PubMed] [Google Scholar]
- 64.Wu J, Wei Z, Cheng P, et al. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics. 2020;10(23):10665–10679. doi: 10.7150/thno.43528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang MX, Lin L, Chen YD, et al. Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol Res. 2020;159:104978. doi: 10.1016/j.phrs.2020.104978. [DOI] [PubMed] [Google Scholar]
- 66.Haifer C, Paramsothy S, Kaakoush NO, et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. 2022;7(2):141–151. doi: 10.1016/S2468-1253(21)00400-3. [DOI] [PubMed] [Google Scholar]
- 67.Kedia S, Virmani S, Vuyyur SK, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut. 2022;71(12):2401–2413. doi: 10.1136/gutjnl-2022-327811. [DOI] [PubMed] [Google Scholar]
- 68.Levi MS, Marzano V, Vernocchi P, et al. Functional and taxonomic traits of the gut microbiota in type 1 diabetes children at the onset: a metaproteomic study. Int J Mol Sci. 2022;23(24):15982. doi: 10.3390/ijms232415982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mokhtari P, Metos J, Anandh BP. Impact of type 1 diabetes on the composition and functional potential of gut microbiome in children and adolescents: possible mechanisms, current knowledge, and challenges. Gut Microbes. 2021;13(1):1–18. doi: 10.1080/19490976.2021.1926841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torun A, Hupalowska A, Trzonkowski P, Kierkus J, Pyrzynska B. Intestinal microbiota in common chronic inflammatory disorders affecting children. Front Immunol. 2021;12:642166. doi: 10.3389/fimmu.2021.642166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan X, Wang R, Han B, et al. Functional and metabolic alterations of gut microbiota in children with new-onset type 1 diabetes. Nat Commun. 2022;13(1):6356. doi: 10.1038/s41467-022-33656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 73.Junius S, Mavrogiannis AV, Lemaitre P, et al. Unstable regulatory T cells, enriched for naïve and Nrp1(neg) cells, are purged after fate challenge. Sci Immunol. 2021 doi: 10.1126/sciimmunol.abe4723. [DOI] [PubMed] [Google Scholar]
- 74.Chen P, Tang X. Gut microbiota as regulators of Th17/Treg balance in patients with myasthenia gravis. Front Immunol. 2021;12:803101. doi: 10.3389/fimmu.2021.803101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan JB, Luo MM, Chen ZY, He BH. The function and role of the Th17/Treg cell balance in inflammatory bowel disease. J Immunol Res. 2020;2020:8813558. doi: 10.1155/2020/8813558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Tran DQ, Lindsey JW, Rhoads JM. The association of gut microbiota and Treg dysfunction in autoimmune diseases. Adv Exp Med Biol. 2021;1278:191–203. doi: 10.1007/978-981-15-6407-9_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun S, Luo L, Liang W, et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci USA. 2020;117(44):27509–27515. doi: 10.1073/pnas.1921223117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schnell A, Huang L, Singer M, et al. Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell. 2021;184(26):6281–6298. doi: 10.1016/j.cell.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang W, Cong Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol Immunol. 2021;18(4):866–877. doi: 10.1038/s41423-021-00661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan J, McKenzie C, Vuillermin PJ, et al. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep. 2016;15(12):2809–2824. doi: 10.1016/j.celrep.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 81.Ichikawa R, Takayama T, Yoneno K, et al. Bile acids induce monocyte differentiation toward interleukin-12 hypo-producing dendritic cells via a TGR5-dependent pathway. Immunology. 2012;136(2):153–162. doi: 10.1111/j.1365-2567.2012.03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu J, Wang C, Huang X, et al. Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling. Cell Rep. 2021;36(12):109726. doi: 10.1016/j.celrep.2021.109726. [DOI] [PubMed] [Google Scholar]
- 83.Olejniczak-Staruch I, Ciążyńska M, Sobolewska-Sztychny D, Narbutt J, Skibińska M, Lesiak A. Alterations of the skin and gut microbiome in psoriasis and psoriatic arthritis. Int J Mol Sci. 2021;22(8):3998. doi: 10.3390/ijms22083998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Polak K, Bergler-Czop B, Szczepanek M, Wojciechowska K, Frątczak A, Kiss N. Psoriasis and gut microbiome-current state of art. Int J Mol Sci. 2021;22(9):4529. doi: 10.3390/ijms22094529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hao Y, Zhu YJ, Zou S, et al. Metabolic syndrome and psoriasis: mechanisms and future directions. Front Immunol. 2021;12:711060. doi: 10.3389/fimmu.2021.711060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanda N, Hoashi T, Saeki H. The defect in regulatory T Cells in psoriasis and therapeutic approaches. J Clin Med. 2021;10(17):3880. doi: 10.3390/jcm10173880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buhaș MC, Gavrilaș LI, Candrea R, et al. Gut microbiota in psoriasis. Nutrients. 2022;14(14):2987. doi: 10.3390/nu14142970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng Z, Yang L, Chu H. The gut microbiota: a novel player in autoimmune hepatitis. Front Cell Infect Microbiol. 2022;12:947382. doi: 10.3389/fcimb.2022.947382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei Y, Li Y, Yan L, et al. Alterations of gut microbiome in autoimmune hepatitis. Gut. 2020;69(3):569–577. doi: 10.1136/gutjnl-2018-317836. [DOI] [PubMed] [Google Scholar]
- 90.Liang M, Liwen Z, Jianguo S, et al. Fecal microbiota transplantation controls progression of experimental autoimmune hepatitis in mice by modulating the TFR/TFH immune imbalance and intestinal microbiota composition. Front Immunol. 2021;12:728723. doi: 10.3389/fimmu.2021.728723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trivedi PJ, Hirschfield GM. Recent advances in clinical practice: epidemiology of autoimmune liver diseases. Gut. 2021;70(10):1989–2003. doi: 10.1136/gutjnl-2020-322362. [DOI] [PubMed] [Google Scholar]
- 92.Zeng L, Deng Y, Yang K, Chen J, He Q, Chen H. Safety and efficacy of fecal microbiota transplantation for autoimmune diseases and autoinflammatory diseases: a systematic review and meta-analysis. Front Immunol. 2022;13:944387. doi: 10.3389/fimmu.2022.944387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolter M, Grant ET, Boudaud M, et al. Leveraging diet to engineer the gut microbiome. Nat Rev Gastroentero Hepatol. 2021;18(12):885–902. doi: 10.1038/s41575-021-00512-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available in the Web of Science (http://www.webofknowledge.com).