Abstract
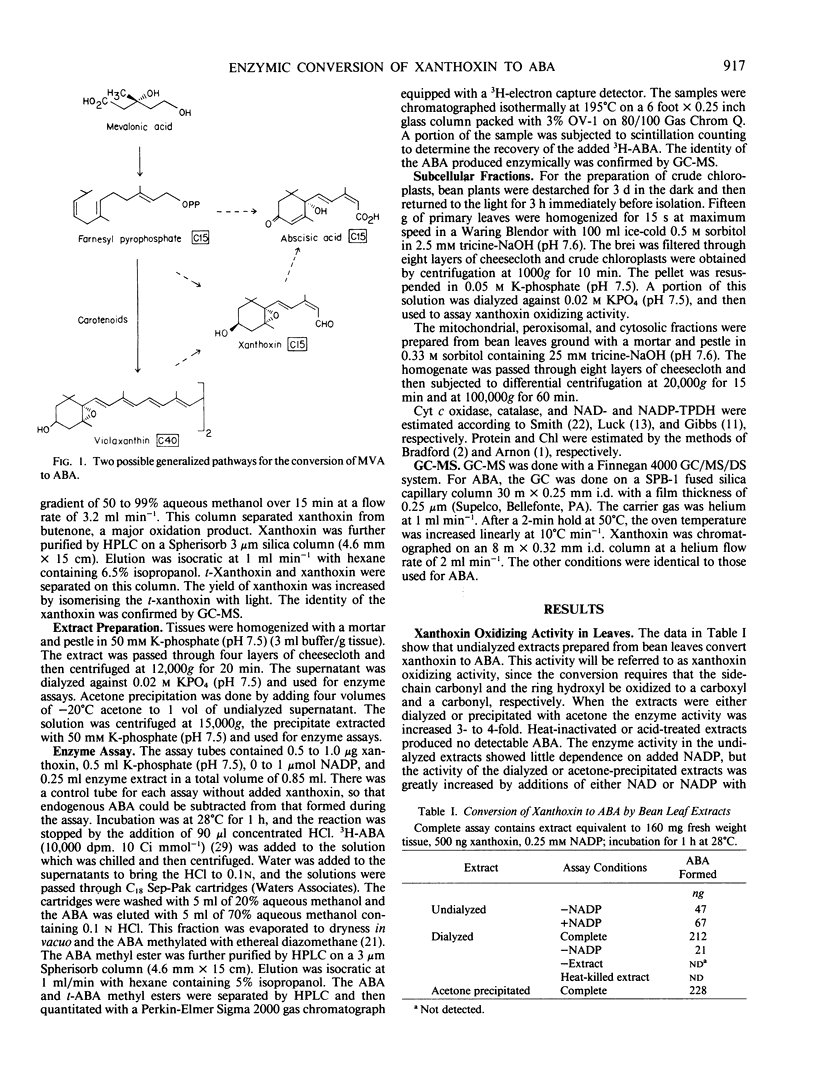
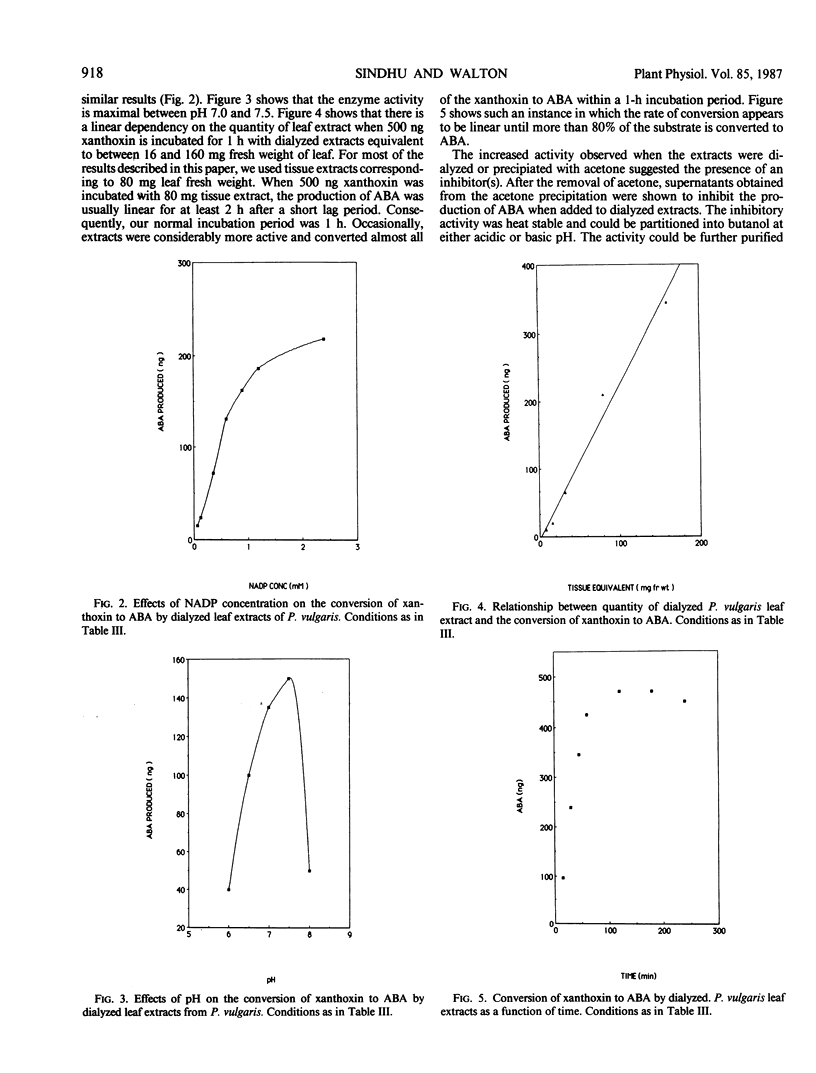
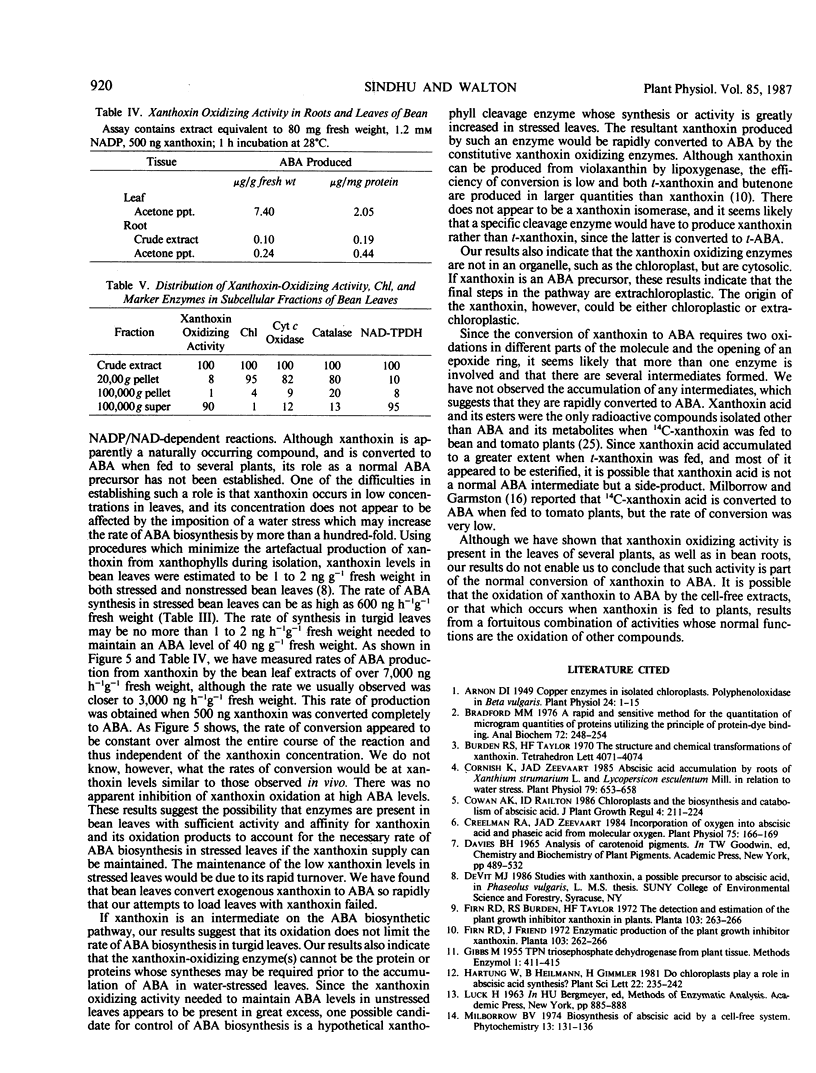
Cell-free extracts from the leaves of Phaseolus vulgaris L. convert xanthoxin to abscisic acid. The enzyme activity in dialyzed or acetone-precipitated extracts shows a strong dependence on either NAD or NADP. The enzyme activity appears to be cytosolic with no significant activity observed in chloroplasts. The activity was observed in extracts from roots of Phaseolus vulgaris, and also in extracts prepared from the leaves of Pisum sativum L., Zea mays L., Cucurbita maxima Duchesne, and Vigna radiata L. Neither water stress nor cycloheximide appear to significantly affect the level of enzyme activity in leaves. No intermediates between xanthoxin and abscisic acid were detected.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burden R. S., Taylor H. F. The structure and chemical transformations of xanthoxin. Tetrahedron Lett. 1970 Oct;(47):4071–4074. doi: 10.1016/s0040-4039(01)98668-x. [DOI] [PubMed] [Google Scholar]
- Cornish K., Zeevaart J. A. Abscisic Acid Accumulation by Roots of Xanthium strumarium L. and Lycopersicon esculentum Mill. in Relation to Water Stress. Plant Physiol. 1985 Nov;79(3):653–658. doi: 10.1104/pp.79.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Zeevaart J. A. Incorporation of oxygen into abscisic Acid and phaseic Acid from molecular oxygen. Plant Physiol. 1984 May;75(1):166–169. doi: 10.1104/pp.75.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Smith J. D. Graviresponsiveness and abscisic-acid content of roots of carotenoid-deficient mutants of Zea mays L. Planta. 1985;164:126–128. [PubMed] [Google Scholar]
- Moore R., Smith J. D. Growth, graviresponsiveness and abscisic-acid content of Zea mays seedlings treated with fluridone. Planta. 1984;162:342–344. [PubMed] [Google Scholar]
- Robinson D. R., Ryback G. Incorporation of tritium from [(4R)-4-3H]mevalonate into abscisic acid. Biochem J. 1969 Aug;113(5):895–897. doi: 10.1042/bj1130895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. F., Smith T. A. Production of plant growth inhibitors from xanthophylls: a possible source of dormin. Nature. 1967 Sep 30;215(5109):1513–1514. doi: 10.1038/2151513a0. [DOI] [PubMed] [Google Scholar]


