Abstract
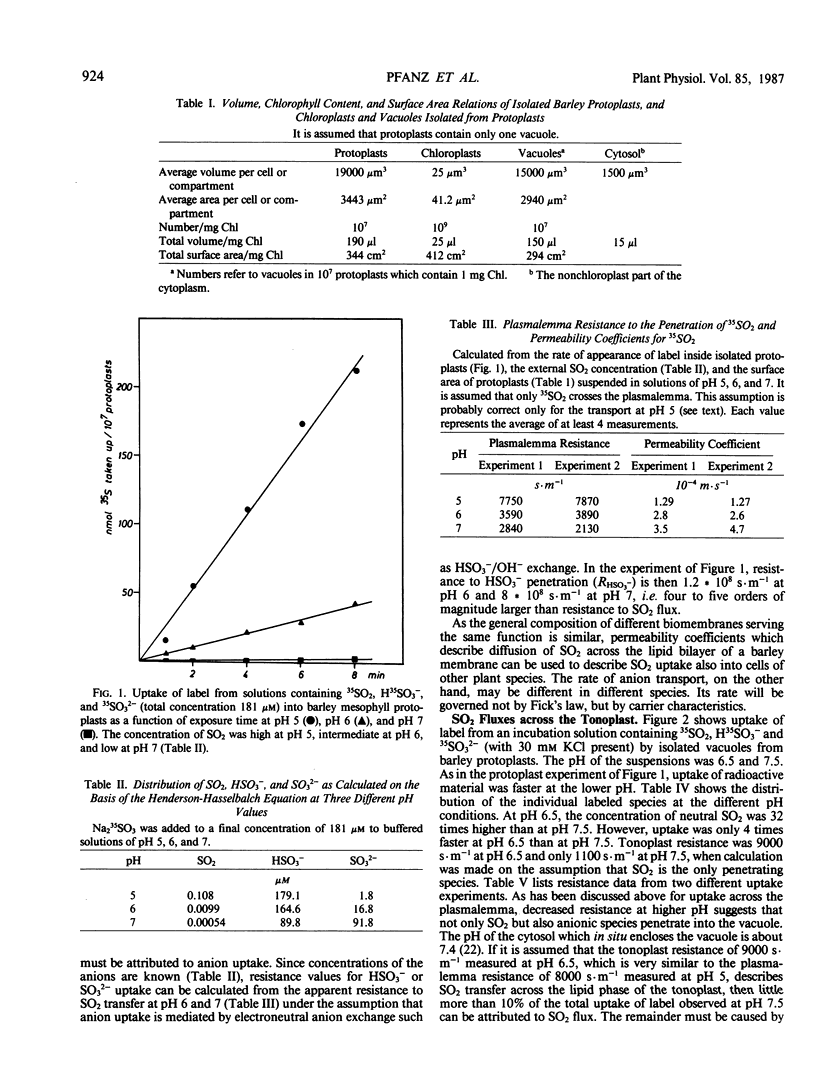
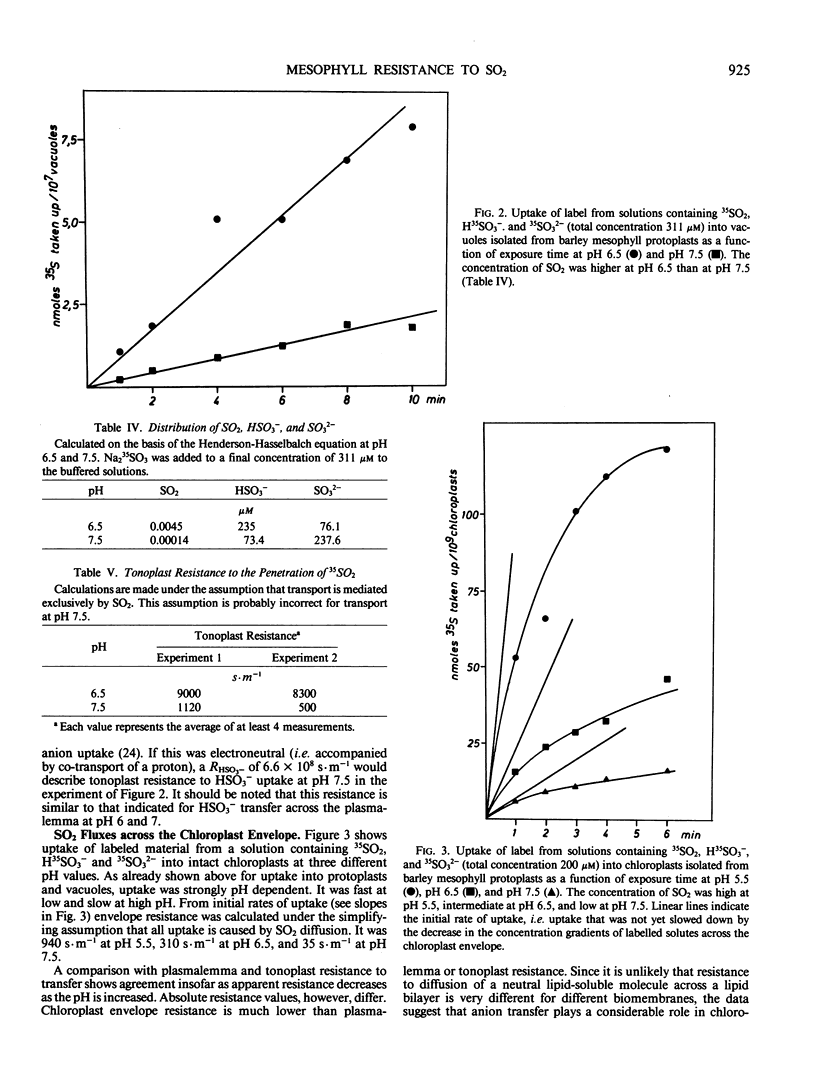
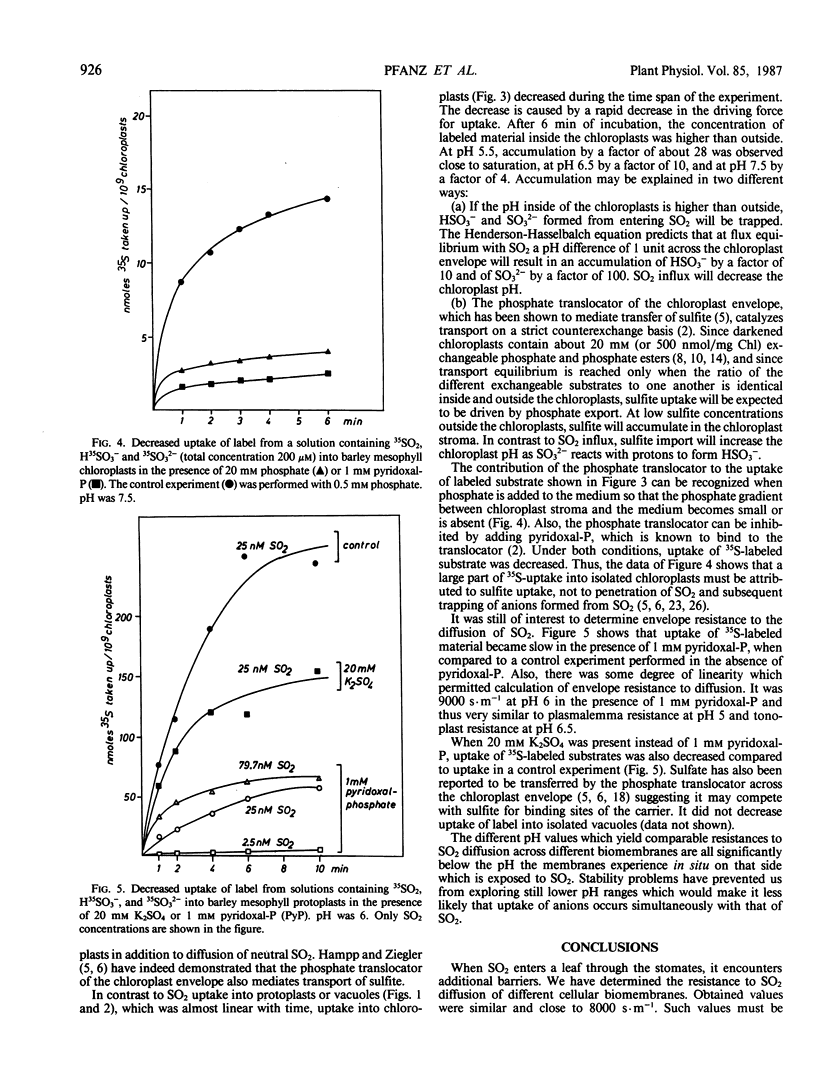
Uptake of label from solutions containing 35SO2, H35SO3− and 35SO32− into mesophyll protoplasts, vacuoles, and chloroplasts isolated from young barley leaves was measured at different pH values. Uptake was fast at low pH, when the concentration of SO2 was high, and low at high pH, when the concentration of SO2 was low. When the resistance (R) of plasmalemma, tonoplast, and chloroplast envelope to the penetration of SO2 was calculated from rates of uptake of label, comparable values were obtained for the different biomembranes at low pH values. R was close to 8000 seconds per meter and permeability coefficients were close to 1.25 × 10−4 meters per second. Under these conditions R may describe resistance to SO2 diffusion across a lipid bilayer. At higher pH values, R decreased. As R was calculated on the assumption that SO2 is the only penetrating molecular species, the data suggest that carrier-mediated anion transport contributes to the uptake of sulfur at physiological pH values thereby decreasing apparent RSO2. The contribution of anion transport appeared to be smaller for transfer across the plasmalemma than for transfer across the tonoplast. It was large for transfer across the chloroplast envelope. The phosphate translocator of the chloroplast envelope catalyzed uptake of SO32− into chloroplasts at neutral pH. Uptake was decreased in the presence of high levels of phosphate or sulfate and by pyridoxal phosphate. SO2 transfer into cells leads to the intracellular liberation of one or two protons, depending on pH and oxidizing conditions. When the divalent sulfite anion is exchanged across the chloroplast envelope, bisulfite formation results in proton uptake in the chloroplast stroma, whereas SO2 uptake into chloroplasts lowers the stroma pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliege R., Flügge U. I., Werdan K., Heldt H. W. Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim Biophys Acta. 1978 May 10;502(2):232–247. doi: 10.1016/0005-2728(78)90045-2. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Chon C. J., Mosbach A., Heldt H. W. The distribution of metabolites between spinach chloroplasts and medium during photosynthesis in vitro. Biochim Biophys Acta. 1977 May 11;460(2):259–272. doi: 10.1016/0005-2728(77)90212-2. [DOI] [PubMed] [Google Scholar]
- Mourioux G., Douce R. Transport du sulfate à travers la double membrane limitante, ou enveloppe, des chloroplastes d'épinard. Biochimie. 1979;61(11-12):1283–1292. [PubMed] [Google Scholar]
- Olszyk D. M., Tingey D. T. Interspecific Variation in SO(2) Flux : Leaf Surface versus Internal Flux, and Components of Leaf Conductance. Plant Physiol. 1985 Dec;79(4):949–956. doi: 10.1104/pp.79.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanz H., Martinoia E., Lange O. L., Heber U. Mesophyll Resistances to SO(2) Fluxes into Leaves. Plant Physiol. 1987 Dec;85(4):922–927. doi: 10.1104/pp.85.4.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. E., Tingey D. T. Sulfur Dioxide Flux into Leaves of Geranium carolinianum L. : Evidence for a Nonstomatal or Residual Resistance. Plant Physiol. 1983 May;72(1):237–244. doi: 10.1104/pp.72.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]


