Endoplasmic reticulum in oocytes
The storage and release of calcium ions (Ca2 +) in oocyte maturation and fertilization are particularly noteworthy features of the endoplasmic reticulum (ER). The ER is the largest organelle in the cell composed of rough ER, smooth ER, and nuclear envelope, and is the main site of protein synthesis, transport and folding, and lipid and steroid synthesis. An appropriate calcium signaling response can initiate oocyte development and embryogenesis, and the ER is the central link that initiates calcium signaling. The transition from immature oocytes to zygotes also requires many coordinated organelle reorganizations and changes. Therefore, the purpose of this review is to generalize information on the function, structure, interaction with other organelles, and spatiotemporal localization of the ER in mammalian oocytes. Mechanisms related to maintaining ER homeostasis have been extensively studied in recent years. Resolving ER stress through the unfolded protein response (UPR) is one of them. We combined the clinical problems caused by the ER in in vitro maturation (IVM), and the mechanisms of ER have been identified by single-cell RNA-seq. This article systematically reviews the functions of ER and provides a reference for assisted reproductive technology (ART) research.
Keywords: Oocyte maturation, Endoplasmic reticulum (ER), Calcium oscillations, Stress, Mitochondria
The structure of ER
The ER is the main site for the synthesis and transport of various biomolecules and is defined as an interconnected network with continuous membranes [1, 2]. The ER consists of a nuclear envelope, smooth tubules, and rough lamellae. The different structures that make up the ER perform very diverse and specialized functions within the cell. The nuclear envelope consists of two lipid bilayers, the inner nuclear membrane and the outer nuclear membrane, and shares a common lumen with the peripheral ER [3]. The nuclear envelope is attached to form part of the peripheral ER, and the lamellae are essentially composed of two lipid bilayers with an intermediate lumen, with curved regions located only at the membrane edge [4].
Specifically, the rough ER is defined by a high density of ribosomes on the cytoplasmic surface and is the primary site for synthesis, folding, and post-translational modification of secreted or membrane-bound proteins [5]. Far fewer ribosomes are present on the membrane surface of the smooth ER tubules, which are highly curved and smooth [5]. The tubular network is dynamic, constantly rearranging and growing, and consists of three-way junctions connecting individual tubules [6]. Tubules and sheets have very different structural features and thus play different roles in different cellular processes [7, 8].
ER tubules and lamellae are present in all eukaryotic cells, and the ratio of lamella to tubules varies in different cell types and reflects the different functions of these cells [9]. For example, the rough ER structure of specialized cells that synthesize a large number of secreted proteins, such as pancreatic secretory cells and B lymphocytes, is mainly composed of sheets. Cells involved in processes including lipid synthesis, calcium signaling, and other organelle contact sites have ERs that are mainly composed of tubules. Mural granulosa cells (MGCs), adrenal cells, hepatocytes, and muscle cells that synthesize lipid hormones are all examples of specialized cells dominated by a tubular network. Calcium signaling occurs at the site of contact between the plasma membrane (PM) and the adjacent cortical ER, and the morphology and intracellular location of ER subdomains contribute to the function of these structures and thus the specialized cells in which they reside.
The ER is also the major reservoir of intracellular Ca2+ [7, 10, 11]. The typical cytoplasmic concentration of Ca2+ is approximately 100 nM, while the Ca2+ concentration in the ER lumen is 100–800 μM, and the extracellular Ca2+ concentration is approximately 2 mM. The multifunctional nature of this organelle requires numerous proteins, unique physical structures, and coordination and response to changes in the intracellular environment [12]. The ER of oocytes also has similar structure and function, but oocytes need to undergo highly dynamic changes, and the structure and function of the ER will also have corresponding adaptation and coordination.
Function of the oocyte ER
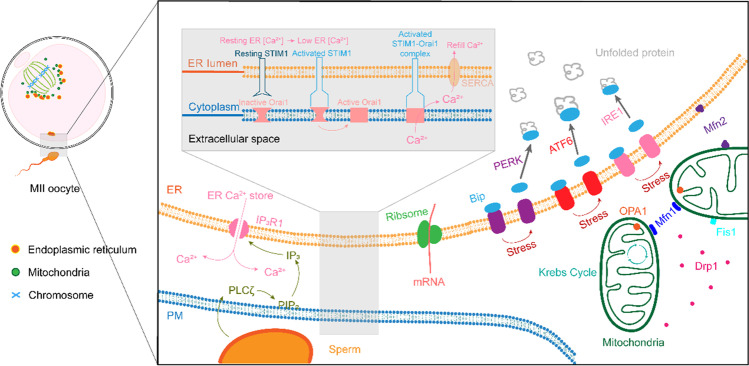
Functional protein synthesis occurs through translation of maternal messenger RNA (mRNA) and is critical for proper oocyte development and maturation. The ability of the ER to store and release free Ca2+ in the cytoplasm is also important. Therefore, maintenance of ER homeostasis may be a key mechanism for folliculogenesis and oocyte maturation (Fig. 1).
Fig. 1.
The function of ER in oocyte
A prevalent mode of calcium regulation upon oocyte activation is the release of PLC zeta by sperm into the oocyte cytoplasm. PLC zeta then cleaves PIP2 into IP3 and DAG. The binding of IP3 to IP3Rs located on the SER membrane causes a conformational change that allows the release of Ca2+, triggering Ca2+ oscillations. The combined action of STIM proteins in the ER membrane and Orai channels in the PM mediates the maintenance of cellular Ca2+ homeostasis. This coupling process is initiated in response to depletion of Ca2+ stores in the ER, which triggers STIM protein activation. Thereafter, the opening of the Orai1 Ca2+ channel located in the PM allows the influx of extracellular Ca2+. The accumulation of misfolded or unfolded proteins in the lumen disrupts ER homeostasis and activates ER stress. Activation of ER stress can trigger the UPR, a signal transduction pathway that senses the fidelity of protein folding in the ER lumen. The MAM is a physically coupled structure between mitochondria and the ER in cells. MFN includes two isoforms, MFN1 and MFN2. MFN2 on the ER membrane and MFN1 or MFN2 on the mitochondrial outer membrane can form a homotypic or heterotypic structural complex. MFN1/2 are located on the outer mitochondrial membrane and OPA1, located on the inner mitochondrial membrane, mediates mitochondrial fusion. MAM is involved in the regulation of mitochondrial fission. Fission 1 (FIS1), located on the mitochondrial outer membrane, recruits mitochondrial DRP1, which is mainly located in the cytoplasm, to the fission site of the mitochondrial outer membrane.This image was originally created by Kang Xin and Wang Jing.
ER stress in oocytes
The ER is the main intracellular organelle responsible for protein synthesis. During these processes, the ER plays a crucial role in meeting the increased protein demands of the oocyte. This task is accomplished through proper protein synthesis, folding, modification, and transport [13]. Developing gametes and embryos may experience various types of exogenous stress in in vitro culture systems, some of which adversely affect ER function and protein synthesis, leading to ER stress and UPR signaling being activated [14–16]. ER proteostasis surveillance is mediated by the UPR, a signal transduction pathway that senses the fidelity of protein folding in the ER lumen [17]. Misfolded proteins are retained in the ER for proper folding or targeted for degradation by ER-associated degradation (ERAD) mechanisms [18]. The accumulation of misfolded or unfolded proteins in the lumen disrupts ER homeostasis and activates ER stress. Activation of ER stress can trigger the UPR, which is designed to maintain cellular homeostasis and normal ER function [19].
Although the exact molecular mechanism of UPR in mammalian oocyte development is poorly described, it is generally believed that three ER transmembrane proteins, protein kinase R-like ER kinase (PERK), activating transcription factor 6 (ATF6), inositol-requiring enzyme 1 (IRE1), and the ER molecular chaperone-binding immunoglobulin protein BiP (also known as glucose-regulated protein 78 or GRP78), trigger the UPR response to ER stress [16]. As an adaptive response, the UPR mitigates misfolded protein accumulation and restores ER function. PERK signaling reduces the translocation of new proteins to the ER lumen and prevents protein overload, while the ATF6 and IRE1 pathways regulate the transcriptional activation of various genes including those responsible for increased ER translocation, protein folding, export, degradation, and other functions. However, if ER stress becomes prolonged or too severe for UPR-based relief, apoptosis is induced through activation of the C/EBP homologous protein (CHOP), Jun N-terminal kinase (JNK), and caspase 12 pathways [13].
The oocyte ER affects Ca2+ oscillations during fertilization
Ca2+ oscillations are a hallmark of mammalian fertilization and regulate the transition of oocytes to early embryos [11, 20]. Decades of studies on different species have finally identified the origin of stimulation in sperm-carried phospholipase C zeta (PLC zeta), a pervasive pattern of calcium regulation upon oocyte activation involving sperm transfer of phospholipase. PLC zeta is released into the oocyte cytoplasm [21]. PLC zeta then cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) [22]. Downstream of this signaling pathway, the binding of IP3 to IP3 receptors (IP3Rs) located on the smooth endoplasmic reticulum (SER) membrane causes a conformational change that allows the release of Ca2+ from the organelle lumen, triggering the release of Ca2+ from the ER and leading to an increase in intracellular Ca2+ levels [23]. The initial rise in Ca2+ is followed by repeated Ca2+ transients, termed Ca2+ oscillations, that stimulate oocyte activation and embryonic development [24, 25]. The metaphase II (MII) oocytes respond to gamete fusion by Ca2+ oscillations. The amplitude, frequency, and duration of these oscillations are then decoded by downstream regulators into signals that trigger fertilization events, namely, further cumulus cell release, exit from MII, second polar body (PBII) extrusion, and pronucleus formation.
In oocytes, therefore, the ability of the ER to store and release free Ca2+ in the cytoplasm is important. The ER is central to the mechanism that generates intracellular Ca2+oscillations at the time of fertilization [26–29]. The mechanism of Ca2+ release in mature oocytes has been widely described, and in this context, this review will describe the changes that the ER undergoes during oocyte maturation as part of cytoplasmic reorganization and are associated with the acquisition of developmental competence.
Changes in the structure and distribution of the ER in oocytes
Mouse oocyte maturation is associated with a marked reorganization of the ER [30], during which the distribution of the ER undergoes major changes guided by microtubules and filaments, ranging from a network of cytoplasmic accumulation at the germinal vesicle (GV) stage to a distinctive cortical cluster network in MII [31, 32]. Multiple lines of evidence suggest that this redistribution of the ER is important in preparing the oocyte to trigger Ca2+ oscillations upon fertilization [28, 31–33]. What needs to be understood is to study the time course and mechanism of ER reorganization during oocyte maturation. The ER first reorganizes upon GV breakdown (GVBD) into a dense network of membranes that wrap and invade the developing meiotic spindle [28]. GVBD is critical for initiating ER reorganization, as ER structure does not change in GV-arrested oocytes of mice [31]. The microtubule inhibitor nocodazole and the inhibition of cytoplasmic dynein prevented ER reorganization. Thus, ER redistribution of GVBD is dynein-driven and cell cycle-dependent [32]. Some proteins play a critical role in microtubule-mediated organelle redistribution via cytoplasmic lattices (CPLs) [26, 34]. CPLs are ascribed with key roles in oocyte maturation, which is causatively related to microtubule and organelle dynamics by controlling the translational processes [35]. Peptidylarginine deiminase 6 (PADI6) and maternal antigen that embryos require (MATER), localized to CPL, are required for the formation of CPL [35, 36]. PADI6/CPL complex is involved in regulating microtubule-mediated organelle localization and redistribution during oocyte maturation. The PADI6/CPL complex may regulate organelle localization and redistribution by promoting the formation of stable acetylated microtubules [35]. MATER may work in concert with PADI6 and microtubule to orchestrate organelle redistribution during oocyte maturation [26]. Moreover, the mRNA vector Staufen and calreticulin are constantly expressed and selectively localize during oocyte maturation. At the GV stage both proteins display a dispersed distribution localization throughout the cytoplasm. Progressing to the MII stage, Staufen tends to aggregate to the cortical cluster of ER, while calreticulin assumes a pattern possibly coincidental with the position of the meiotic spindle that may mediate cytoskeletal remodeling during oocyte maturation [37]. After GVBD, a dense network of ER surrounds the spindle during its migration into the oocyte cortex. Later, the endoplasmic reticulum was reshaped into the characteristic cortical clusters of MII dependent not on microtubules but on microfilaments [32].
During maturation, human oocytes show altered ER distribution patterns and changes in IP3R abundance, similar to those observed in mice [28]. Human GV-stage oocytes are characterized by an ER organized into a fine mesh that extends throughout the cortex and cell interior, whereas MII oocytes have large and distinct clusters of 2–3 mm in diameter throughout the cortex and cell interior. Moreover, in MII oocytes, ER clusters are not absent in the cortex near the MII spindle, consistent with the presence of microvilli on the spindle surface of oocytes in humans, unlike in mice [28]. In addition, during the GV-MII transition, Western blot analysis shows that oocytes have a 50% increase in IP3R, while their capacity to release Ca2+ in response to IP3 nearly doubles [31]. Thus, in a manner similar to mouse models, during maturation, human oocytes undergo changes in the distribution and composition of ER elements, which explains the increased capacity to release Ca2+. However, human MII oocytes matured in vitro from GV stage oocytes are unable to support this increase in IP3R abundance and Ca2+ releasing capacity despite the normal distribution of ER clusters of 2–3 mm. This suggests that the response to IP3 may be reduced [28]. The similar distribution of the ER clusters and IP3R further suggests that the ER clusters are specialized sites for the initiation and propagation of Ca2+ oscillations in oocytes [38].
Some research results suggest that the diabetic condition adversely affects the ER distribution pattern during mouse oocyte maturation and early embryo development [33]. In vitro matured oocytes have been reported to have lower developmental capacity. However, these results should be interpreted with caution as they are obtained using residual GV stage oocytes from stimulated in vitro fertilization (IVF) cycles, i.e., oocytes that are not representative of normal humans. Furthermore, in such experiments, GV-stage oocytes are cultured in the absence of cumulus cells, a condition known to profoundly affect many cellular and biochemical aspects of the maturation process [30].
These experiments demonstrate that ER reorganization during oocyte maturation is a complex multistep process involving distinct microtubule-dependent and filament-dependent stages and suggest a role for dynein in cytoplasmic changes [33]. These changes are an indispensable step in oocyte fertilization.
ER and mitochondria cooperate to complete oocyte maturation
Organelle rearrangement is another major theme of oocyte maturation [39]. Organelle positioning and movement in oocytes are largely mediated by microtubules and their associated motor proteins [30, 32]. The coordination of cytoskeleton and ER has been well summarized in previous reviews and will not be discussed here [30, 40]. Elements of the ER and other organelles whose functions are regulated during maturation are also redistributed according to precise spatiotemporal control [35]. For example, during spindle migration into the cortex, a clump of mitochondrial and ER elements surrounds the spindle, providing energetic and spatial information for the localization of the actin nucleation factor FMN2, which facilitates spindle displacement [41, 42].
Mitochondria and ER are involved in a variety of cellular processes including regulation of lipid biosynthesis and metabolic energy, Ca2+ homeostasis, and apoptosis [18, 27, 43, 44]. Interactions and functional interactions between these two organelles have also been reported in mouse oocytes. The ER forms a fine reticular network with large ER-rich clusters in the cortex of MII oocytes, as mitochondria appear to facilitate Ca2+ oscillations upon fertilization through a Ca-ATPase-driven Ca2+ pumping mechanism [45].
The mitochondrion-associated ER membrane (MAM) is a physically coupled structure between mitochondria and the ER in cells. and ER stress and other processes play a key role. These physiological functions of MAM are very important for the maturation of oocytes [45–47]. In recent years, studies have found that a variety of proteins on MAMs are involved in the maturation of oocytes (Fig. 1).
MAM is involved in regulating the fusion process of mitochondria. In MAM, the GTPase mitofusin (MFN) is mainly located on the outer mitochondrial membrane, and MFN includes two isoforms, MFN1 and MFN2. MFN1 or MFN2 on the mitochondrial outer membrane can form a homotypic or heterotypic structural complex, thereby building a bridge between the ER and mitochondria. MFN1/2 are located on the outer mitochondrial membrane, and another mitochondrial fusion GTPase, optic atrophy 1 (OPA1), located on the inner mitochondrial membrane mediates mitochondrial fusion [47]. Using live-cell fluorescence imaging analysis, it is found that the ER is attracted to sites where Mfn is overexpressed, suggesting that MFN contributes to the bridge connecting mitochondria and ER [47]. In particular, MFN2-induced mitochondrial aggregation resulted in discrete ER networks and reduced ER Ca2+ storage. The lack or increase inMFN2 can lead to ER morphology and mitochondrion-ER physical and functional problems [48]. Aberrant ER morphology may impair ER Ca2+ homeostasis through ER-mitochondrion communication.
MAM is involved in the regulation of mitochondrial fission. Fission 1 (FIS1), located on the mitochondrial outer membrane, recruits mitochondrial dynamin-related protein 1 (DRP1), which is mainly located in the cytoplasm, to the fission site of the mitochondrial outer membrane, and DRP1 forms a ring structure through oligomerization, enabling the mitochondrial membrane to undergo fracture. BAP31 is a protein located on the ER membrane and can regulate the degradation and apoptosis pathways of misfolded proteins. When FIS1 binds to BAP31 in MAM, apoptotic signals are transmitted to the ER, thereby initiating the apoptotic pathway.
Clinical problems caused by the distribution of oocyte ER
In addition to the aforementioned genetic changes, ER has attracted clinical attention in terms of the different distribution and morphology of ER during in vitro maturation and fertilization of human oocytes [49]. Aggregates of SER (SERa) in oocytes are one of the cytoplasmic malformations of the oocyte. Several researchers reported that oocytes with SERa (SERa +) resulted in significantly lower fertilization rate, blastocyst rate, and pregnancy rate than controls [50]. Pregnancies in women with affected gametes are accompanied by a higher incidence of obstetric problems [50]. Indeed, partially mature oocytes recovered from treatment cycles of ovulation induction with gonadotropins displayed large aggregates of smooth ER, manifesting as single or multiple translucent vacuole-like structures 10–80 μm in diameter. The cause of this deformity is unknown. Their incidence is related to the duration and dose of gonadotropins used for stimulation. No SERa is observed in oocytes from unstimulated patients [51]. This, further, may support that exposure to in vivo (IVO) gonadotropins during the final stages of oocyte maturation and may induce the emergence of SERa, but it does not affect pregnancy outcomes or increase the newborn malformation rate [52].
SERa is thought to contribute to chromosome segregation errors and abnormal cell division [53]. SERa is associated with cytoskeletal changes, including increased spindle length and cortical actin disturbance [54]. Overall, SERa may negatively affect fertilization and post-fertilization events, with effects on embryo quality, implantation, and fetal development [55, 56]. The presence of SERa is associated with lower chances of successful pregnancy [57]. A reduction in the live birth rate with an increasing proportion of SERa oocytes is observed [58]. For these reasons, the effect of SERa on embryonic and obstetric outcomes has been assessed. In several studies, the presence of SERa + oocytes or their use in IVF has been described to be associated with malformations or genetic abnormalities in neonates [55, 56]. ESHRE guidelines recommend against using SERa + oocytes in IVF [49]. However, a recent study showed that healthy babies can be born from oocytes that display ER abnormalities [59]. Knowledge about this issue is still limited, and more systematic research is needed [60]. At present, transcriptomic research on various stages of oocyte development is increasing [61]. This review integrates the research results in recent years to provide a reference.
Gene abnormalities associated with the ER in oocytes
Transcriptomics
The ER may play an important role in the deterioration of oocyte quality during oocyte aging [62]. An analysis of transcriptomic data from single cell RNA-seq has shown that 32-week mice are involved in protection in GV oocytes compared to 5-week mice with downregulation of a group of genes under ER stress, including adiponectin receptor 2 (AdipoR2), interleukin 1 receptor-associated kinase 1 (Irak1), regulator of calcineurin 1 (Rcan1), and methionine sulfoxide reduction regulator of enzyme B1 (Msrb1) [62]. The expression of AdipoR2 has been shown in cow, pig, goat, and rat oocytes. Multiple studies have shown that adiponectin supplementation positively affects meiotic progression and initial embryonic development during in vitro maturation (IVM) of human, mouse, goat, and porcine oocytes [63].
Calcium normally acts as an activator of enzymes through the ER or mitochondria. By analyzing transcriptomic data, it was found that calcium signaling in IVM oocytes may be dysfunctional. The Kyoto Encyclopedia of Genes and Genomes (KEGG) results show that IVM and IVO oocytes can be clearly separated according to the expression of 52 genes involved in the calcium signaling pathway. In addition, the mean intensity of calcium signals is also reduced in human IVM oocytes compared to human IVO oocytes. Genes encoding PM proteins related to calcium transport are all downregulated; however, genes encoding ER and mitochondrial membrane proteins are upregulated [64].
Transcription of VDAC family members is significantly reduced in human IVM oocytes relative to IVO oocytes [64]. Voltage-dependent anion channel (VDAC) is a mitochondrion-associated Ca2+ transporter that regulates oocyte activation and is also known as mitochondrial porin [65]. Located on the outer mitochondrial membrane, VDACs act as gatekeepers for the entry and exit of mitochondrial metabolites, thereby controlling cross-talk between mitochondria and the rest of the cell. VDACs are also key players in mitochondria-mediated apoptosis [66]. VDAC2 is an autophagy inhibitor that exerts its function by inhibiting ovarian autophagy [67].
The mRNA expression levels of genes (ITPR1, ITPR2, ITPR3, STIM1, and SERCA) encoding ER membrane proteins are elevated in IVM oocytes [64]. ITPR encodes IP3R, as previously described [68]. The combined action of STIM proteins in the ER membrane and Orai channels in the PM mediates the maintenance of cellular Ca2+ homeostasis [69]. The two proteins undergo a dynamic coupling process within the ER-PM junction region. This coupling process is initiated in response to depletion of Ca2+ stores in the ER, which triggers STIM protein activation. Thereafter, the opening of the Orai1 Ca2+channel located in the PM allows the influx of extracellular Ca2+ [70–72]. However, it has actually been shown not to be necessary for store-operated Ca2+ entry in fertilized mouse eggs by research, in which they found that Ca2+ influx was not perturbed in fertilized eggs from double knockout STIM1/STIM2 mice or in Orai knockouts [73]. Rather, TRPM7 and Cav3.2 channels appear to mediate Ca2+ influx.
The mRNA expression levels of genes (ORAI1, ORAI2, ORAI3, PKA) encoding PM proteins are elevated in IVO oocytes, and the temporal and spatial regulation of PKA activity is critical for oocyte meiosis recovery [64, 74].
Furthermore, genes encoding calmodulin (CAM) and calcium/calmodulin-dependent protein kinase II (CAMKII) exhibited different expression profiles between IVM and IVO oocytes. The mRNA expression levels of CAM1, CAM2, and CAM3 are elevated in IVO oocytes, and the mRNA expression levels of CAMK2A are elevated in IVM oocytes [64]. These differences persist at the protein level according to immunostaining and dot blot analysis. CAM is associated with oocyte maturation [75]. The inhibition of cell death mediated by the pentose phosphate pathway is due to the inhibitory phosphorylation of caspase-2 by CaMKII [76].
To demonstrate the effect of calcium concentration on human oocyte development, human IVO oocytes are fertilized, and the embryos are cultured in calcium-free medium. Fertilized embryos develop to the blastocyst stage at a lower rate than normal controls. Studies have shown that nearly all genes encoding membrane proteins in calcium metabolism pathways are downregulated, suggesting a potential barrier to uptake. Excessive calcium release leads to ER stress, which induces apoptosis and autophagy. In this study, genes involved in the ER stress pathway are not stably expressed, suggesting that ER is involved in releasing calcium and maintaining calcium concentrations in the cytoplasm, inducing apoptosis, and blocking development due to stress. Therefore, inhibiting the ER stress response will improve the maturation and development of human IVM oocytes.
Characteristic transcriptomic changes in oocytes with SER
The transcriptomes of human MII SERa + oocytes and normal MII oocytes without SERa (SERa-) have been analyzed by a microarray method, and gene expression profiles have been also analyzed. In SERa + oocytes, the most significantly enriched Gene Ontology (GO) term for upregulated genes is the GoLoco (otherwise known as GPR or “G-protein regulatory”) motif, including the RAP1GAP, GPSM3, and GPSM1 genes. Proteins containing the GoLoco motif control mitotic spindle organization, microtubule interactions, and chromosome segregation during cell division. Among them, GPSM1 (G protein signaling regulator 1) localizes to the ER membrane of the Golgi apparatus and controls spindle orientation, while RAP1GAP (RAP1 GTPase-activating protein) is involved in cell proliferation, differentiation, and embryogenesis [61].
Since SER is an important component of calcium signaling, differentially expressed genes that may have an effect on calcium signaling are valuable. Study found that in SERa + oocytes, 4 downregulated (FAT1, ITGA10, LMAN2, and TGM4) and 7 upregulated (DLK1, DSPP, EYS, MMP28, PCDHB13, PCDHB8, and PCDHGA12) genes encode proteins that bind at least a calcium atom or proteins whose function is calcium-dependent [61].
In addition, SERa + oocytes and SERa- oocytes have the following differences: a group of genes downregulated in SERa + oocytes are genes involved in cell division and mitotic/meiotic regulation, spindle assembly, and chromosome division. Specifically, low expression levels of the NEK2 and CROCC genes may alter the centrosome cycle in which centrosome duplication and segregation occur. Decreased expression of HAUS8, MAU2, and BIRC5 may alter microtubule formation, sister chromatid cohesion, chromosome alignment and segregation, and cell division within the mitotic spindle [61].
SERa + oocytes, compared with normal oocytes, involved mitochondrial structure and respiratory activity, suggesting that SERa may also reflect perturbations in the functional connection between the ER and mitochondrial network. Defects in calcium regulation of mitochondrial and energetic ATP homeostasis may have negative downstream developmental effects in SERa + oocytes.
Conclusion and perspectives
The ER plays an important role in both oocyte maturation and embryonic development and is a major site for protein synthesis, trafficking, and folding. For development to proceed properly, the oocyte also undergoes an appropriate calcium signaling response to initiate development and embryogenesis.
In terms of omics data, the correlation between mRNA and protein expression is weaker in oocytes than in other somatic cells. This insignificant correlation has been observed in many cell types but is especially pronounced in oocytes probably because of the storage of the mRNA pool [77]. In addition, intrinsic differences between individual oocytes still exist, and extrapolation of conclusions requires confirmation from subsequent experiments, as shown by mouse and human transcriptome studies. Additionally, it is difficult to reconcile the results described about transcriptomic analyses in oocytes matured in vitro vs in vivo, because fully-grown oocytes are transcriptionally silent up until the 2-cell stage or beyond depending on the species and regardless of the method of stimulating maturation (in vitro or in vivo). A comprehensive meta-analysis involving multiple datasets is still lacking. Great care is also needed when performing this analysis and only comparable elements should be included. Continued refinement of computer analysis tools will ultimately help improve our understanding of biological complexity. The proposed studies need to be considered and further validated to confirm that the results obtained are still applicable after implementation in humans, facilitating translation to the clinic.
Acknowledgements
We thank Yanxiang Zhao for her excellent beautification of figure.
Author contribution
All authors contributed to the study conception and design. Xin Kang and Jing Wang performed the literature search and wrote the manuscript and prepared the figure. LiyingYan revised the manuscript.
Funding
This work was funded by the Beijing Municipal Science and Technology Commission (Z191100006619075, Z191100006619073) , Project funded by China Postdoctoral Science Foundation (2021M690257) and National Natural Science Foundation of China (82201828, 82125013, 31871447 and 31871482).
Data Availability
The data and material in this article are available.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Kang and Jing Wang contributed equally to this work.
References
- 1.FagoneS. P. Jackowski, Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res. 2009;50:S311–6. doi: 10.1194/jlr.R800049-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jan CH, Williams CC, Weissman JS. Principles of ER cotranslational translocation revealed by proximity-specific ribosome profiling. Science. 2014;346(6210):1257521. doi: 10.1126/science.1257521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter KR, Claude A, Fullam EF. A study of tissue culture cells by electron microscopy: methods and preliminary observations. J Exp Med. 1945;81(3):233–246. doi: 10.1084/jem.81.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.English AR, Voeltz GK. Endoplasmic reticulum structure and interconnections with other organelles. Cold Spring Harb Perspect Biol. 2013;5(4):a013227. doi: 10.1101/cshperspect.a013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata Y, Voeltz GK, Rapoport TA. Rough sheets and smooth tubules. Cell. 2006;126(3):435–439. doi: 10.1016/j.cell.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Zucker B, and Kozlov MM, Mechanism of shaping membrane nanostructures of endoplasmic reticulum. Proc Natl Acad Sci U S A, 2022; 119(1). [DOI] [PMC free article] [PubMed]
- 7.Schwarz DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73(1):79–94. doi: 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.English AR, Zurek N, Voeltz GK. Peripheral ER structure and function. Curr Opin Cell Biol. 2009;21(4):596–602. doi: 10.1016/j.ceb.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kepp O, and Galluzzi L. Preface: endoplasmic reticulum in health and disease. Int Rev Cell Mol Biol, 2020; 350: xiii-xvii. [DOI] [PubMed]
- 10.Kline D. Attributes and dynamics of the endoplasmic reticulum in mammalian eggs. Curr Top Dev Biol. 2000;50:125–154. doi: 10.1016/S0070-2153(00)50007-6. [DOI] [PubMed] [Google Scholar]
- 11.Homa ST, Carroll J, Swann K. The role of calcium in mammalian oocyte maturation and egg activation. Hum Reprod. 1993;8(8):1274–1281. doi: 10.1093/oxfordjournals.humrep.a138240. [DOI] [PubMed] [Google Scholar]
- 12.Wakai T, Mehregan A, and Fissore RA. Ca(2+) Signaling and homeostasis in mammalian oocytes and eggs. Cold Spring Harb Perspect Biol, 2019; 11(12). [DOI] [PMC free article] [PubMed]
- 13.Guzel E, et al. Endoplasmic reticulum stress and homeostasis in reproductive physiology and pathology. Int J Mol Sci, 2017; 18(4). [DOI] [PMC free article] [PubMed]
- 14.Takehara I, et al. Impact of endoplasmic reticulum stress on oocyte aging mechanisms. Mol Hum Reprod. 2020;26(8):567–575. doi: 10.1093/molehr/gaaa040. [DOI] [PubMed] [Google Scholar]
- 15.Pan MH, et al. Bisphenol A exposure disrupts organelle distribution and functions during mouse oocyte maturation. Front Cell Dev Biol. 2021;9:661155. doi: 10.3389/fcell.2021.661155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin T, et al. Endoplasmic reticulum (ER) stress and unfolded protein response (UPR) in mammalian oocyte maturation and preimplantation embryo development. Int J Mol Sci, 2019. 20(2). [DOI] [PMC free article] [PubMed]
- 17.Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21(8):421–438. doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz JG, Nabi IR. Interaction of the smooth endoplasmic reticulum and mitochondria. Biochem Soc Trans. 2006;34(Pt 3):370–373. doi: 10.1042/BST0340370. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, et al. A primary effect of palmitic acid on mouse oocytes is the disruption of the structure of the endoplasmic reticulum. Reproduction. 2021;163(1):45–56. doi: 10.1530/REP-21-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee B, Palermo G, Machaca K. Downregulation of store-operated Ca2+ entry during mammalian meiosis is required for the egg-to-embryo transition. J Cell Sci. 2013;126(Pt 7):1672–1681. doi: 10.1242/jcs.121335. [DOI] [PubMed] [Google Scholar]
- 21.Machaty Z. Signal transduction in mammalian oocytes during fertilization. Cell Tissue Res. 2016;363(1):169–183. doi: 10.1007/s00441-015-2291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu YR, Yang WX. Calcium influx and sperm-evoked calcium responses during oocyte maturation and egg activation. Oncotarget. 2017;8(51):89375–89390. doi: 10.18632/oncotarget.19679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machaty Z, et al. Fertility: store-operated Ca(2+) entry in germ cells: role in egg activation. Adv Exp Med Biol. 2017;993:577–593. doi: 10.1007/978-3-319-57732-6_29. [DOI] [PubMed] [Google Scholar]
- 24.Szpila M, et al. Postovulatory ageing modifies sperm-induced Ca(2+) oscillations in mouse oocytes through a conditions-dependent, multi-pathway mechanism. Sci Rep. 2019;9(1):11859. doi: 10.1038/s41598-019-48281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakai T, et al. Regulation of endoplasmic reticulum Ca(2+) oscillations in mammalian eggs. J Cell Sci. 2013;126(Pt 24):5714–5724. doi: 10.1242/jcs.136549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim B, et al. The role of MATER in endoplasmic reticulum distribution and calcium homeostasis in mouse oocytes. Dev Biol. 2014;386(2):331–339. doi: 10.1016/j.ydbio.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Rovere RM, et al. Intracellular Ca(2+) signaling and Ca(2+) microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium. 2016;60(2):74–87. doi: 10.1016/j.ceca.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Mann JS, Lowther KM, Mehlmann LM. Reorganization of the endoplasmic reticulum and development of Ca2+ release mechanisms during meiotic maturation of human oocytes. Biol Reprod. 2010;83(4):578–583. doi: 10.1095/biolreprod.110.085985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211(2):157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 30.Coticchio G, et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21(4):427–454. doi: 10.1093/humupd/dmv011. [DOI] [PubMed] [Google Scholar]
- 31.Mehlmann LM, et al. Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev Biol. 1995;170(2):607–615. doi: 10.1006/dbio.1995.1240. [DOI] [PubMed] [Google Scholar]
- 32.FitzHarris G, Marangos P, Carroll J. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol. 2007;305(1):133–144. doi: 10.1016/j.ydbio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Zhang CH, et al. Maternal diabetes causes abnormal dynamic changes of endoplasmic reticulum during mouse oocyte maturation and early embryo development. Reprod Biol Endocrinol. 2013;11:31. doi: 10.1186/1477-7827-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esposito G, et al. Peptidylarginine deiminase (PAD) 6 is essential for oocyte cytoskeletal sheet formation and female fertility. Mol Cell Endocrinol. 2007;273(1–2):25–31. doi: 10.1016/j.mce.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Kan R, et al. Regulation of mouse oocyte microtubule and organelle dynamics by PADI6 and the cytoplasmic lattices. Dev Biol. 2011;350(2):311–322. doi: 10.1016/j.ydbio.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim B, et al. Potential role for MATER in cytoplasmic lattice formation in murine oocytes. PLoS ONE. 2010;5(9):e12587. doi: 10.1371/journal.pone.0012587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Santis L, et al. Expression and intracytoplasmic distribution of staufen and calreticulin in maturing human oocytes. J Assist Reprod Genet. 2015;32(4):645–652. doi: 10.1007/s10815-015-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehlmann LM, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol. 1996;180(2):489–498. doi: 10.1006/dbio.1996.0322. [DOI] [PubMed] [Google Scholar]
- 39.Stricker SA. Structural reorganizations of the endoplasmic reticulum during egg maturation and fertilization. Semin Cell Dev Biol. 2006;17(2):303–313. doi: 10.1016/j.semcdb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Mao L, et al. Behaviour of cytoplasmic organelles and cytoskeleton during oocyte maturation. Reprod Biomed Online. 2014;28(3):284–299. doi: 10.1016/j.rbmo.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Duan X, et al. Dynamic organelle distribution initiates actin-based spindle migration in mouse oocytes. Nat Commun. 2020;11(1):277. doi: 10.1038/s41467-019-14068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi K, et al. Sequential actin-based pushing forces drive meiosis I chromosome migration and symmetry breaking in oocytes. J Cell Biol. 2013;200(5):567–576. doi: 10.1083/jcb.201211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumollard R, et al. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131(13):3057–3067. doi: 10.1242/dev.01181. [DOI] [PubMed] [Google Scholar]
- 44.Hajnóczky G, et al. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J Physiol. 2000;529(1):69–81. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Udagawa O, Ishihara N. Mitochondrial dynamics and interorganellar communication in the development and dysmorphism of mammalian oocytes. J Biochem. 2020;167(3):257–266. doi: 10.1093/jb/mvz093. [DOI] [PubMed] [Google Scholar]
- 46.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11(5):797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Wakai T, et al. Mitochondrial dynamics controlled by mitofusins define organelle positioning and movement during mouse oocyte maturation. Mol Hum Reprod. 2014;20(11):1090–1100. doi: 10.1093/molehr/gau064. [DOI] [PubMed] [Google Scholar]
- 48.Zhao L, et al. Enriched endoplasmic reticulum-mitochondria interactions result in mitochondrial dysfunction and apoptosis in oocytes from obese mice. J Anim Sci Biotechnol. 2017;8:62. doi: 10.1186/s40104-017-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Istanbul consensus workshop on embryo assessment proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 50.Ebner T, et al. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reprod Biomed Online. 2008;16(1):113–118. doi: 10.1016/S1472-6483(10)60563-9. [DOI] [PubMed] [Google Scholar]
- 51.Nikiforov D, et al. Clusters of smooth endoplasmic reticulum are absent in oocytes from unstimulated women. Reprod Biomed Online. 2021;43(1):26–32. doi: 10.1016/j.rbmo.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Fang T, et al. The impact of oocytes containing smooth endoplasmic reticulum aggregates on assisted reproductive outcomes: a cohort study. BMC Pregnancy Childbirth. 2022;22(1):838. doi: 10.1186/s12884-022-05141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otsuki J, et al. A higher incidence of cleavage failure in oocytes containing smooth endoplasmic reticulum clusters. J Assist Reprod Genet. 2018;35(5):899–905. doi: 10.1007/s10815-018-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dal Canto M, et al. Dysmorphic patterns are associated with cytoskeletal alterations in human oocytes. Hum Reprod. 2017;32(4):750–757. doi: 10.1093/humrep/dex041. [DOI] [PubMed] [Google Scholar]
- 55.Sfontouris IA, et al. Complex chromosomal aberrations in a fetus originating from oocytes with smooth endoplasmic reticulum (SER) aggregates. Syst Biol Reprod Med. 2018;64(4):283–290. doi: 10.1080/19396368.2018.1466375. [DOI] [PubMed] [Google Scholar]
- 56.Akarsu C, et al. Smooth endoplasmic reticulum aggregations in all retrieved oocytes causing recurrent multiple anomalies: case report. Fertil Steril. 2009;92(4):1496.e1–1496.e3. doi: 10.1016/j.fertnstert.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 57.Otsuki J, et al. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19(7):1591–1597. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 58.Gurunath S, et al. Live birth rates in in vitro fertilization cycles with oocytes containing smooth endoplasmic reticulum aggregates and normal oocytes. J Hum Reprod Sci. 2019;12(2):156–163. doi: 10.4103/jhrs.JHRS_92_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J, et al. Oocytes with smooth endoplasmic reticulum aggregates are not associated with impaired reproductive outcomes: a matched retrospective cohort study. Front Endocrinol (Lausanne) 2021;12:688967. doi: 10.3389/fendo.2021.688967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreux L, et al. Is it time to reconsider how to manage oocytes affected by smooth endoplasmic reticulum aggregates? Hum Reprod. 2019;34(4):591–600. doi: 10.1093/humrep/dez010. [DOI] [PubMed] [Google Scholar]
- 61.Stigliani S, et al. Presence of aggregates of smooth endoplasmic reticulum in MII oocytes affects oocyte competence: molecular-based evidence. Mol Hum Reprod. 2018;24(6):310–317. doi: 10.1093/molehr/gay018. [DOI] [PubMed] [Google Scholar]
- 62.Zhang T, et al. Mitochondrial dysfunction and endoplasmic reticulum stress involved in oocyte aging: an analysis using single-cell RNA-sequencing of mouse oocytes. J Ovarian Res. 2019;12(1):53. doi: 10.1186/s13048-019-0529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barbe A, et al. Mechanisms of adiponectin action in fertility: an overview from gametogenesis to gestation in humans and animal models in normal and pathological conditions. Int J Mol Sci, 2019; 20(7). [DOI] [PMC free article] [PubMed]
- 64.Zhao H, et al. Single-cell transcriptomics of human oocytes: environment-driven metabolic competition and compensatory mechanisms during oocyte maturation. Antioxid Redox Signal. 2019;30(4):542–559. doi: 10.1089/ars.2017.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang F, et al. Effects of mitochondria-associated Ca(2+) transporters suppression on oocyte activation. Cell Biochem Funct. 2021;39(2):248–257. doi: 10.1002/cbf.3571. [DOI] [PubMed] [Google Scholar]
- 66.Shoshan-Barmatz V, et al. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med. 2010;31(3):227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Yuan J, et al. MYBL2 guides autophagy suppressor VDAC2 in the developing ovary to inhibit autophagy through a complex of VDAC2-BECN1-BCL2L1 in mammals. Autophagy. 2015;11(7):1081–1098. doi: 10.1080/15548627.2015.1040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang D, et al. The inositol 1,4,5-trisphosphate receptor (Itpr) gene family in Xenopus: identification of type 2 and type 3 inositol 1,4,5-trisphosphate receptor subtypes. Biochem J. 2007;404(3):383–391. doi: 10.1042/BJ20070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grabmayr H, Romanin C, and Fahrner M. STIM Proteins: an ever-expanding family. Int J Mol Sci, 2020; 22(1). [DOI] [PMC free article] [PubMed]
- 70.Zhou Y, et al. The STIM-Orai coupling interface and gating of the Orai1 channel. Cell Calcium. 2017;63:8–13. doi: 10.1016/j.ceca.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Penna A, et al. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008;456(7218):116–120. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Green KN, et al. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J Cell Biol. 2008;181(7):1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernhardt ML, et al. Store-operated Ca(2+) entry is not required for fertilization-induced Ca(2+) signaling in mouse eggs. Cell Calcium. 2017;65:63–72. doi: 10.1016/j.ceca.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burton KA, McKnight GS. PKA, germ cells, and fertility. Physiology (Bethesda) 2007;22:40–46. doi: 10.1152/physiol.00034.2006. [DOI] [PubMed] [Google Scholar]
- 75.Bornslaeger EA, Wilde MW, Schultz RM. Regulation of mouse oocyte maturation: involvement of cyclic AMP phosphodiesterase and calmodulin. Dev Biol. 1984;105(2):488–499. doi: 10.1016/0012-1606(84)90306-3. [DOI] [PubMed] [Google Scholar]
- 76.Nutt LK, et al. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell. 2005;123(1):89–103. doi: 10.1016/j.cell.2005.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Labrecque R, Sirard MA. The study of mammalian oocyte competence by transcriptome analysis: progress and challenges. Mol Hum Reprod. 2014;20(2):103–116. doi: 10.1093/molehr/gat082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and material in this article are available.