Abstract
The present meta-analysis aimed to elucidate the association of Behçet’s disease (BD) with the risk of metabolic syndrome (MetS) and its components. Observational cohort studies were searched from the Embase, Web of Science, Medline, and Cochrane Library databases. The primary outcome was the association of BD with the risk of MetS and its relevant components. Effect estimates with odds ratios (ORs) were pooled using either the random-effects or fixed-effects models, according to heterogeneity. Leave-one-out sensitivity analyses were used to determine the stability of the results. Twenty-three studies, comprising 42,834 patients with BD, were included. Overall, a significant association between BD and the risk of MetS was found (pooled OR 2.26; 95% confidence interval [CI] 1.61–3.17; P < 0.0001). Among the components of MetS, significant associations were found between BD and diabetes mellitus (OR 1.21; 95% CI 1.10–1.33; P < 0.0001), BD and hypertension (OR 1.39; 95% CI 1.13–1.70; P = 0.002), and BD and dyslipidemia (OR 1.21; 95% CI 1.01–1.45; P = 0.04). Our study indicated an association between BD and the risk of MetS and some of its components (diabetes mellitus, hypertension, and dyslipidemia). Physician should consider these associations so that specific treatments are available for patients with comorbidities. Moreover, patients with BD should regularly monitor their blood pressure, fasting plasma glucose, and blood lipid levels.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-023-01044-x.
Keywords: Behçet’s disease, Metabolic syndrome, Meta-analysis, Diabetes mellitus, Hypertension, Dyslipidemia
Introduction
Behçet’s disease (BD) is a multisystemic autoimmune and chronic inflammatory vasculitis, characterized by recurrent painful mouth sores, genital ulcers, uveitis, skin lesions, and other systemic manifestations [1]. It frequently occurs in patients originating in the Middle East, Far East, and Mediterranean, and is also known as Silk Route disease. The reported prevalence of BD in East Asia ranges from 13.5 to 27 per 100,000 persons [2, 3]. BD has significant morbidity and mortality risks, with the main causes of death being ruptured coronary/pulmonary arterial aneurysms, neurological involvement, and thrombosis [4–8]. Although genetic susceptibility, inflammation, and immunological abnormalities have been verified to play decisive roles in BD progression, the pathological mechanism of BD is not completely understood [9]. As research on BD has increased in recent years, it is vital to investigate its comorbidities.
Metabolic syndrome (MetS) affects 14–32% of the world’s population, and its incidence continues to increase [10]. MetS is a set of metabolic abnormalities that includes hypertension, glucose intolerance, abdominal obesity, and atherogenic dyslipidemia, thereby increasing the risk of cardiovascular disease and mortality [11]. The cumulative effects of longstanding inflammation resulting from chronic inflammatory diseases are major contributing factors to MetS. Furthermore, numerous studies have confirmed a link between MetS and inflammatory diseases such as psoriasis and hidradenitis suppurativa [12, 13].
Recent studies have reported that patients with BD are more likely to have MetS than health control (HCs) [14–16]; however, results of studies evaluating the relationship between BD and the risk of MetS and its components remain inconsistent. Therefore, this study was performed to elucidate the relationship of BD and the risk of MetS and its components.
Methods
This study adhered to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) (http://www.prisma-statement.org/) guidelines [17, 18].
Data sources and searches
Two independent authors (TC and XS) searched for studies published before November 31, 2022, using electronic databases (Embase, Web of Science, MEDLINE, and Cochrane Library). The terms used included Behçet syndrome, Behçet disease, Behçet’s syndrome, Silk syndrome, Behçets syndrome, Behçet’s disease, BD, metabolic syndrome, metabolic disorders, hypertension, blood pressure, fasting blood glucose, plasma glucose, dyslipidemia, triglyceride, HDL, waist circumference, obesity, and abdominal obesity. We also performed a manual supplemental search by reviewing the reference lists of relevant articles, systematic reviews, and meta-analyses to avoid potentially missing articles. The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022344815).
Eligibility criteria for selecting studies
The inclusion criteria for studies in the meta-analysis were as follows: (1) prospective or retrospective observational studies; (2) studies involving human participants; (3) studies investigating the association of BD with MetS or its relevant components, and studies describing the prevalence of MetS or its relevant components in BD patients; and (4) studies published in English.
Types of outcome measures
The primary outcome was the association between BD and the risk of MetS and its relevant components.
Data extraction and quality assessment
Two authors (TC and XS) independently engaged in the study selection, data collection, and extraction. In the case of incomplete data, we emailed the authors to obtain supplementary information. The quality of each eligible study was assessed using the Newcastle–Ottawa scale (NOS) by each investigator to evaluate the quality [19]. The NOS awards a maximum of nine points for each study and is based on three major components: selection of the groups, comparability, and exposure; a score of 7–9 indicates high quality (low risk of bias). Any disagreements were resolved by consensus and included a third author (HL).
Statistical analysis
All statistical analyses were performed using Review Manager 5.4 software (The Nordic Cochrane Center, Copenhagen, Denmark) and the Bioconductor programming environment 22 (R, version 4.2.1). Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to assess the prevalence of MetS and its components in the comparison between patients with BD and controls. Statistical heterogeneity between studies was calculated using the I2 test; I2 > 50% indicated that the studies were heterogeneous. If considerable heterogeneity (I2 > 50%) was recognized, the random-effects model was employed (DerSimonian and Laird method); otherwise, a fixed-effects model was used (Mantel–Haenszel method). Begg’s test was conducted to evaluate publication bias [20, 21].
Results
Description of included studies
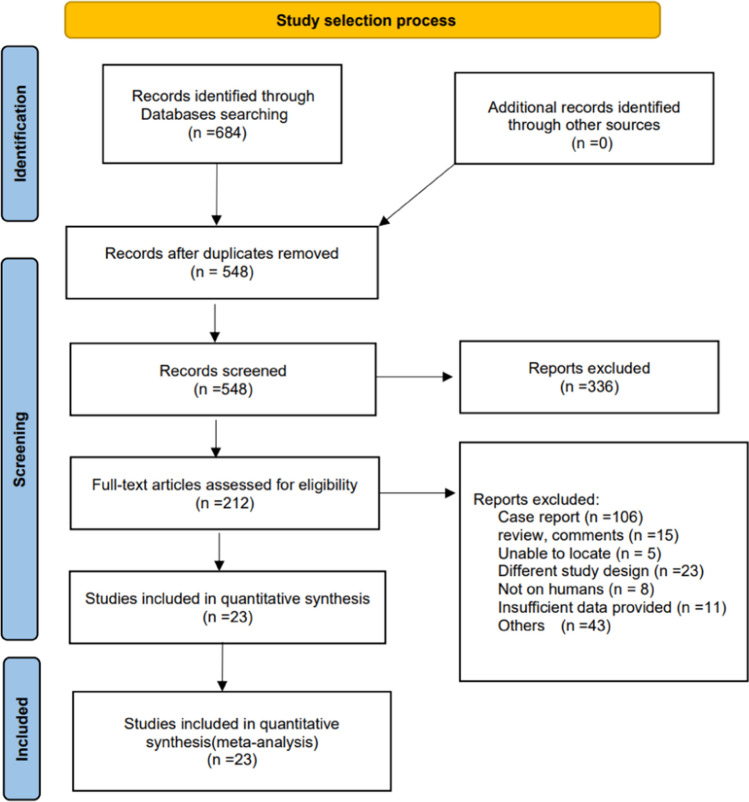
The literature search process is illustrated in Fig. 1. We included 23 studies with 684 records identified through computerized database searches [3, 14, 22–42]; the characteristics of the selected 23 studies are shown in Table 1. The 23 eligible studies, published between 2005 and 2022, comprised 42,834 patients with BD and 26,977 controls. There were five cross-sectional and 18 case–control studies, among which three were from Africa, one from the United States of America, one from Europe, and the remainder from Asia. The quality assessment scores obtained using the NOS for the eligible studies are summarized in Table 2.
Fig. 1.
Selection process for eligible studies included in the systematic review and meta-analysis
Table 1.
Characteristics of the 23 selected studies
| References | Enthnicity | Type of study | Group | Number (n) | Age (mean ± SD, or range, or median [IQR]) (years) | Sex (M/F)(n) | Clinical signs | |||
|---|---|---|---|---|---|---|---|---|---|---|
| MetS (n) | Obesity (n) | BMI (kg/m2) | Waistline (cm) | |||||||
| Erdem et al. [29] | Asian | Case–control | BD | 14 | 23.86 ± 0.89 | / | 21.64 ± 0.82 | |||
| NBD | 15 | 24.13 ± 0.59 | / | 23.00 ± 0.53 | ||||||
| Ugurlu et al. [36] | Asian | Case–control | BD | 225 | 52 ± 8 | 141/84 | 27.4 ± 4.3 | |||
| NBD | 117 | 50 ± 5 | 74/43 | 26.6 ± 4.4 | ||||||
| Kim et al. [24] | Asian | Case–control | BD | 82 | 43.9 ± 11.4 | 26/56 | 5 | 22.9 ± 3.5 | 82.4 ± 9.0 | |
| NBD | 89 | 44.1 ± 9.1 | 40/49 | 8 | 23.6 ± 3.2 | 80.7 ± 9.4 | ||||
| Messedi et al. [33] | African | Case–control | BD | 50 | 48 (41–54) | 35/15 | 88.08 ± 8.6 | |||
| NBD | 50 | 46 (40–54) | 35/15 | 91.10 ± 10.11 | ||||||
| Ulaşoğlu [27] | Asian | Case–control | BD | 115 | 47.6 ± 9.1 | 48/67 | 62 | 28.4 ± 5.0 | 103.8 ± 12.3 | |
| NBD | 65 | 42.7 ± 14.5 | 27/38 | 18 | 23.7 ± 4.9 | 100.6 ± 11.9 | ||||
| Ricart et al. [35] | European | Case–control | BD | 89 | 44 ± 12 | 48/41 | 13 | 25.3 ± 4.3 | ||
| NBD | 89 | 43 ± 10 | 47/42 | 6 | 25.1 ± 3.4 | |||||
| Yalçın et al. [14] | Asian | Case–control | BD | 86 | 39.05 ± 10.1 | 32/54 | 30 | |||
| NBD | 72 | 38.96 ± 11.4 | 23/49 | 14 | ||||||
| Erden et al. [26] | Asian | Case–control | BD | 25 | 33.24 ± 7.18 | 13/12 | 7 | 23.92 ± 1.5 | ||
| NBD | 25 | 34.12 ± 5.74 | 13/12 | 4 | 23.51 ± 1.32 | |||||
| Pandey et al. [42] | America | Cross-sectional | BD | 2540 | 43.9 (0.63) | 642/1898 | ||||
| Gul et al. [22] | Asian | Case–control | BD | 30 | 35.10 ± 7.35 | 15/15 | 5 | 23.59 ± 1.52 | ||
| NBD | 30 | 31.99 ± 6.97 | 15/15 | 3 | 24.32 ± 2.47 | |||||
| Erden et al. [25] | Asian | Case–control | BD | 30 | 36.66 ± 7.9 | 15/15 | 12 | 24.08 ± 1.57 | 85.53 ± 8.37 | |
| NBD | 30 | 37.20 ± 11.3 | 15/15 | 12 | 24.47 ± 1.63 | 87.33 ± 9.58 | ||||
| El-Gazzar et al. [23] | Asian | Case–control | BD | 38 | 36.2 ± 7.8 | 8/30 | 11 | 26.9 ± 3.9 | ||
| NBD | 38 | 35.4 ± 6.5 | 8/30 | 4 | 25.4 ± 3.1 | |||||
| Yavne et al. [37] | Asian | Case–control | BD | 871 | 49.0 ± 15.5 | 458/413 | ||||
| NBD | 4349 | 49.8 ± 15.4 | 2288/2061 | |||||||
| Koca et al. [31] | Asian | Case–control | BD | 143 | 37.7 ± 10.9 | 61/82 | 18 | 25.3 ± 4.8 | ||
| NBD | 112 | 40.1 ± 13.9 | 48/64 | 23 | 26.3 ± 5.5 | |||||
| Acikgoz et al. [34] | Asian | Case–control | BD | 60 | 44.1 ± 8.3 | 29/31 | 23.0 ± 1.6 | |||
| NBD | 50 | 45.4 ± 7.4 | 27/33 | 22.9 ± 1.5 | ||||||
| Lee et al. [3] | Asian | Cohort study | BD | 19,937 | / | 6502/13435 | ||||
| Gheita et al. [41] | African | Cross-sectional | BD | 1526 | 35.7 ± 9.84 | 1102/424 | 27.57 ± 5.24 | |||
| Chen et al. [39] | Asian | Cohort study | BD | 6508 | 38.1 ± 15.1 | 2837/3671 | ||||
| Lee et al. [40] | Asian | Cohort study | BD | 6178 | / | / | 1609 | |||
| Lin et al. [32] | Asian | Case–control | BD | 1554 | 39.2 ± 12.0 | 653/901 | ||||
| NBD | 3108 | 39.1 ± 12.2 | 1373/1735 | |||||||
| Cebeci Kahraman [28] | Asian | Case–control | BD | 60 | 34.03 ± 8.05 | 44/16 | 24.41 ± 3.06 | |||
| NBD | 45 | 30.87 ± 8.4 | 25/20 | 24.31 ± 2.85 | ||||||
| Jung et al. [30] | Asian | Case–control | BD | 6214 | 46.9 ± 13.2 | 7077/11565 | 85.72 ± 8.4 | |||
| NBD | 18,642 | 46.9 ± 13.2 | 2359/3855 | 82.84 ± 9.8 | ||||||
| ElAdle et al. [38] | African | Case–control | BD | 1028 | 36.8 ± 10.1 | 750/278 | 234 | 28.6 ± 5.8 | 89.7 ± 14.7 | |
| NBD | 51 | 34.3 ± 10.9 | 42/9 | 3 | 28.7 ± 5.4 | 90.5 ± 12.8 | ||||
Table 2.
Quality assessment scores (NOS scale tool) for the eligible studies NOS, Newcastle–Ottawa scale
| Selection | Comparability | Exposure | Scores | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases definition | Cases representativeness | Controls selection | Controls definition | Age matching | Additional matching | Ascentaiment exposure | Same method for cases and controls | Non-response rate | ||
| Erdem et al. [29] | * | * | * | * | * | * | * | * | 8 | |
| Ugurlu et al. [36] | * | * | * | * | * | * | * | 7 | ||
| Kim et al. [24] | * | * | * | * | * | * | * | * | 8 | |
| Messedi et al. [33] | * | * | * | * | * | * | * | * | 8 | |
| Ricart et al. [35] | * | * | * | * | * | * | 6 | |||
| Ulaşoğlu [27] | * | * | * | * | * | * | * | 7 | ||
| Yalçın et al. [14] | * | * | * | * | * | * | * | * | * | 9 |
| Erden et al. [26] | * | * | * | * | * | * | 6 | |||
| Pandey et al. [42] | * | * | * | * | * | * | * | * | 8 | |
| Gul et al. [22] | * | * | * | * | * | * | * | * | 8 | |
| El-Gazzar [23] | * | * | * | * | * | * | * | * | 8 | |
| Erden et al. [25] | * | * | * | * | * | * | * | * | 8 | |
| Koca et al. [31] | * | * | * | * | * | * | * | 7 | ||
| Yavne et al. [37] | * | * | * | * | * | * | * | * | * | 9 |
| Acikgoz et al. [34] | * | * | * | * | * | * | * | * | 8 | |
| Lee et al. [3] | * | * | * | * | * | * | * | * | 8 | |
| Gheita et al. [41] | * | * | * | * | * | * | * | * | 8 | |
| Chen et al. [39] | * | * | * | * | * | * | * | * | * | 9 |
| Lin et al. [32] | * | * | * | * | * | * | * | * | * | 9 |
| Lee et al. [40] | * | * | * | * | * | * | * | * | 8 | |
| Cebeci Kahraman [28] | * | * | * | * | * | * | * | 7 | ||
| Jung et al. [30] | * | * | * | * | * | * | * | * | * | 9 |
| ElAdle et al. [38] | * | * | * | * | * | * | * | * | * | 9 |
Behcet’s disease and metabolic syndrome
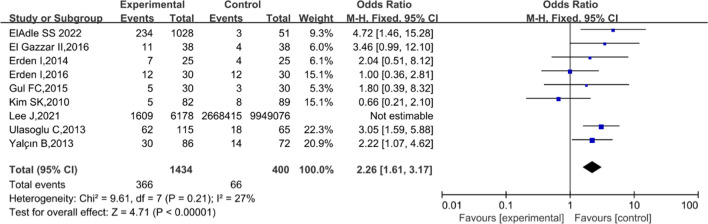
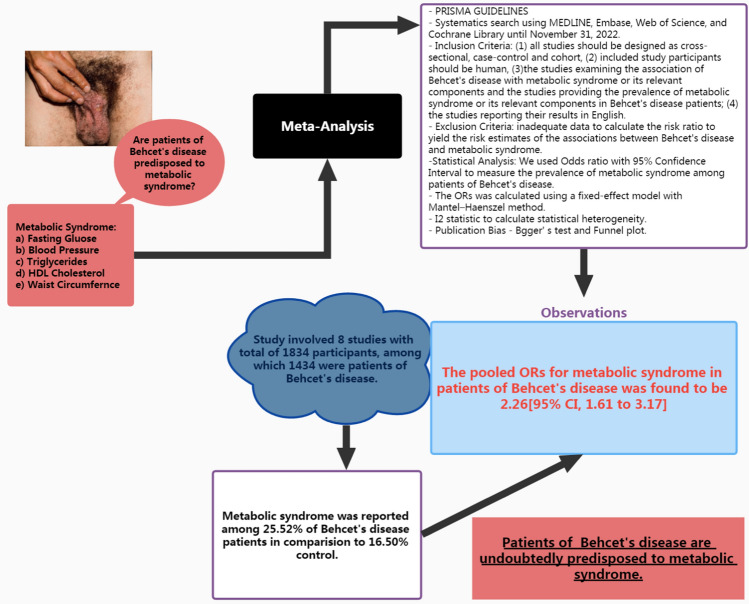
The prevalence of MetS in patients with BD was reported in eight studies [14, 22–27]. Among the included studies, MetS was reported in 25.52% of patients with BD (366/1434), whereas only 16.50% were found to be affected by MetS in the non-BD group (66/400). The pooled analysis showed that BD was significantly associated with MetS (OR 2.26; 95% CI 1.61–3.17; P < 0.0001; Fig. 2). Moreover, the fixed-effects model was used because eligible trials demonstrated low heterogeneity (I2 = 27%; P = 0.21). Additionally, we did not examine publication bias based on the symmetry of the funnel plot and the Begg’s test (P = 0.4579).
Fig. 2.
Association between Behçet’s disease and metabolic syndrome. Forest plot for the association between Behçet’s disease and metabolic syndrome
Behcet’s disease and diabetes mellitus
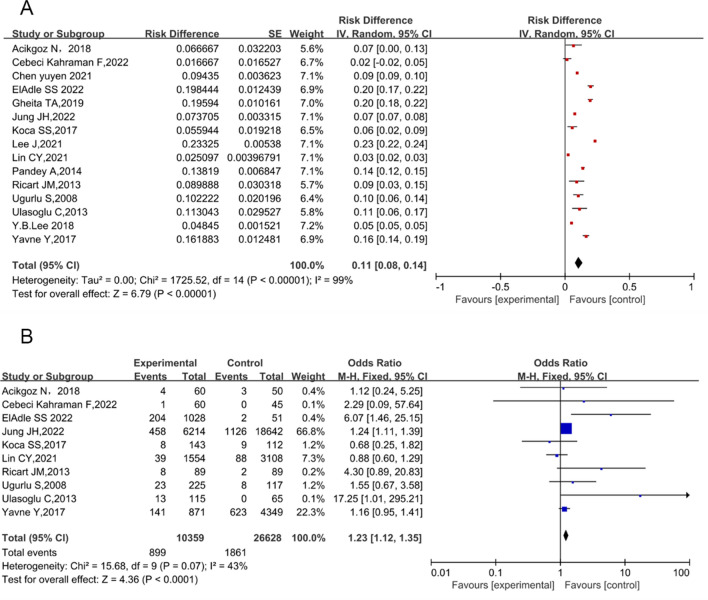
Fifteen studies reported the prevalence of diabetes mellitus in patients with BD, including 10 case–control studies, three cohort studies, and two cross-sectional studies. The pooled prevalence of diabetes mellitus in patients with BD was 11% (95% CI 8% to 14%; P < 0.0001), and the heterogeneity of these studies was evident (I2 = 99%; P = 0.000; Fig. 3A). Excluding five studies that did not report the number of patients with diabetes mellitus among non-BD participants, pooled analysis of the other 10 case–control studies [27, 28, 30–32, 34–38] showed that diabetes mellitus was detected in 7.45% of patients with BD, and 7.00% of controls. The association between BD and diabetes mellitus was considered significant (OR 1.23; 95% CI 1.12–1.35; P < 0.0001; Fig. 3B), with low heterogeneity (I2 = 43%; P = 0.07). The prevalence of diabetes mellitus in patients with BD using the Begg’s test (P = 0.3252) revealed no publication bias. Moreover, seven studies that reported fasting blood glucose levels in patients with BD were included, and no significant difference in fasting blood glucose was observed (mean difference [MD], 1.00; 95% CI −3.12–5.11; P = 0.64; Supplementary Fig. 1) [22–24, 26, 28, 34, 35].
Fig. 3.
Forest plots for the association between Behçet’s disease and diabetes mellitus. Observational studies of the association between Behçet’s disease and diabetes mellitus (A). Case–control studies of the association between Behçet’s disease and diabetes mellitus (B)
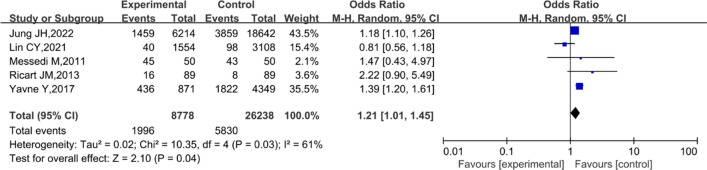
Behcet’s disease and dyslipidemia
As illustrated in Fig. 4, the prevalence of dyslipidemia was significantly associated with BD (OR 1.21; 95% CI 1.01–1.45; P = 0.04; Fig. 4), with a substantial heterogeneity (I2 = 61%; P = 0.03) [30, 32, 33, 35, 37]. In addition, the sensitivity analysis showed that the overall statistical significance of the meta-analysis did not change after removal of any individual study, indicating that the results were stable and credible. Moreover, a non-significant publication bias was reported based on the results of the Begg’s test (P = 1.0000).
Fig. 4.
Forest plot for the association between Behçet’s disease and dyslipidemia
Twelve studies recorded triglyceride levels [22–29, 31, 34, 35, 38], and nine studies recorded high-density lipoprotein (HDL) levels [22–28, 34, 38] in patients with BD. The meta-analysis illustrated that elevated triglyceride levels were not significantly related to BD (MD, 9.36; 95% CI −1.96–20.68; P = 0.11; Supplementary Fig. 2). A lower HDL level was observed in patients with BD in most studies; however, the difference was not markedly significant (MD, −2.28; 95% CI −5.38–0.81; P = 0.15; Supplementary Fig. 3).
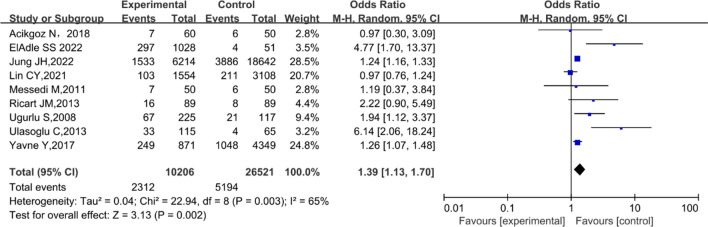
Behcet’s disease and hypertension
The results of the nine trials including 35,648 patients, also demonstrated an increased OR for hypertension in association with BD [27, 30, 32–38]. The meta-analysis revealed a significant association between BD and hypertension (pooled OR 1.39; 95% CI 1.13–1.70; P = 0.002), with considerable heterogeneity (I2 = 65%; P = 0.003; Fig. 5). The funnel plot of studies assessing the association between BD and hypertension indicated no publication bias, which was supported by the results of the Begg’s test (P = 0.4042). Of these studies, two recorded systolic and diastolic blood pressure (DBP) levels in patients with BD [23, 24]. The pooled analysis showed no significant difference in systolic blood pressure (MD, 5.90; 95% CI −1.43–13.24; P = 0.11); however, an evident difference in DBP between patients with BD and HCs (MD, 3.73; 95% CI 1.20–6.27; P = 0.004) was observed (Supplementary Fig. 4).
Fig. 5.
Forest plot for the association between Behçet’s disease and hypertension
Behcet’s disease and obesity
The pooled analysis of ORs of two individual studies showed no significant association of BD with obesity (OR 1.09; 95% CI 0.26–4.49; P = 0.90; Supplementary Fig. 5A) [31, 35]. Thirteen case–control studies that included body mass index [22–29, 31, 34–36, 38], and six studies that included the waistline [24, 25, 27, 30, 33, 38] indicated no significant correlation between BD and body mass index (MD, 0.18; 95% CI −0.48–0.84; P = 0.59; Supplementary Fig. 5B), or between BD and waistline (MD, 0.77; 95% CI −1.33–2.88; P = 0.47; Supplementary Fig. 5C).
Behcet’s disease and insulin resistance
Results for the analysis of the relationship between BD and insulin resistance are shown in Supplementary Fig. 6 [22, 26, 27, 29]. There was no obvious association between BD and insulin resistance (OR 2.12; 95% CI 0.75–6.03; P = 0.16), with substantial heterogeneity (I2 = 57%; P = 0.08; Supplementary Fig. 6). We conducted sensitivity analyses for BD and insulin resistance; the results did not show any significant alteration in the pooled OR when any individual study was sequentially omitted, demonstrating its stability and credibility. No evidence of potential publication bias was shown using Begg’s test (P = 0.4969). A subgroup analysis performed primarily based on different diagnostic tools of included trials revealed that patient with Behçet disease was associated with elevated incidence of insulin resistance when the diagnostic criteria for insulin resistance was hyperinsulinaemic-euglycaemic glucose clamp technique (OR 14.00; 95% CI 1.43–137.32; P = 0.02). But no obvious association between BD and insulin resistance was observed when the diagnostic criteria was the homeostasis model assessment of insulin resistance (HOMA-IR) formal score (OR 1.59; 95% CI 0.59–4.33; P = 0.36; Supplementary Fig. 7).
The influences of additional factors
Besides potential immunologic interactions with metabolic processes, other factors including disease activity and treatment can also affect the risk of diabetes mellitus, arterial hypertension and dyslipidemia. In order to exclude the effect of drugs on the study, we excluded patients receiving drugs which may lead to metabolic complications such as steroids and DMARDs. The analyses showed similar results, and BD is significantly correlated with arterial hypertension (OR 1.26; 95% CI 1.07–1.48; P = 0.02; Supplementary Fig. 8A) and dyslipidemia (OR 1.14; 95% CI 1.05–1.24; P = 0.002; Supplementary Fig. 8B). Instead, the result indicated that there is no significant association between BD and diabetes mellitus (OR 1.20; 95% CI 0.94–1.53; P = 0.14; Supplementary Fig. 8C).
Discussion
This meta-analysis quantitatively investigated the relationship between BD and the risk of MetS and its components. Twenty-three observational studies were included to investigate the prevalence of MS and its components in patients with BD [14, 22–37]. In total, 9,686 patients with BD and 26,926 controls were enrolled in our analysis. The cumulative assessment of this meta-analysis indicates that MetS has emerged as an important associative factor in patients with BD (Fig. 6). Individual risk factors for diabetes mellitus, hypertension, and dyslipidemia are linked to comorbidities in patients with BD.
Fig. 6.
Outline to assess methodology and key observations of Behçet’s disease and its association with metabolic syndrome
Consistent with previous reports, our study demonstrated a prominent association between BD and MetS. In the present study, the risk of MetS in patients with BD was 126% higher than in the healthy control group (OR 2.26; 95% CI 1.61–3.17; P < 0.0001). Furthermore, there was no significant heterogeneity or publication bias in the analysis. Further, the results of sensitivity analysis were stable and credible. Our findings revealed that BD is significantly associated with insulin resistance, diabetes mellitus and hypertension. Moreover, based on all the included studies, the association of BD with fasting blood glucose (a diabetes-associated parameter) and systolic blood pressure (a hypertension-associated parameter) was not statistically significant. However, there was an evident difference in DBP levels between patients with BD and non-BD controls; therefore, the DBP probability is a potential marker for predicting the incidence of BD-related hypertension. Still, the number of included studies reporting DBP levels was limited, and more studies are needed to confirm this result. In addition, when we excluded the effect of medications, the relationship between BD and diabetes was not statistically significant. Therefore, physicians should pay attention to the distinction between complications and drug effects in order to facilitate further treatment of patients.
Additionally, although substantial heterogeneity was observed across the included studies, a significant association between dyslipidemia and BD was reported in our study, and decreased HDL levels in patients with BD were observed in the pooled analysis. A previous study reported that low HDL levels were closely related to the dysfunction and inflammation of vascular in patients with BD; however, no significant association between BD and HDL level have been reported in our study. Further observational studies are urgently needed to analyze the relationship between HDL levels and BD.
MetS is a clinical condition characterized by a series of metabolic risk factors [11], and is an important risk factor for cardiovascular diseases [11]. BD can be described as a multifactorial disease that may also affect the cardiovascular system [42]. The association of BD with MetS, diabetes mellitus, hypertension, and dyslipidemia potentially arise from a similar etiopathogenesis between BD and these metabolic disorders. Inflammation plays a vital role in BD, and elevated circulatory proinflammatory cytokines—including interleukin-1, interleukin-6, interleukin-8, and tumor necrosis factor-alpha (TNF-α)—can be found in patients with BD [9, 43]. These inflammatory markers also cause the downregulation of insulin activity, which leads to insulin resistance, endothelial dysfunction, and the development of MetS [44]. TNF-α has also been associated with the pathogenesis of insulin resistance and diabetes mellitus [45, 46]. Regarding immune disorders, the dysfunction of regulatory T cells also regulates the development of both BD and hypertension [43, 47]. Moreover, BD and MetS negatively impact patients’ quality of life and psychological health [48, 49]. The inflammatory process of BD and MetS may explain the psychological damage. Moreover, the level of inflammation in vivo can also be enhanced by emotional stress, and psychological factors play an essential role in the development of BD and MetS.
Limitation
Given the limited number of studies assessing the prevalence of MetS or its different components in patients with BD and diverse clinical characteristics, no data were available to perform subgroup analyses based on the type, severity, or activity of BD. In addition, the inclusion of patients in this study was limited to few regions and included various study designs.
Conclusion
In summary, our systematic review and meta-analysis suggests that patients with BD (25.52%) are more predisposed to MetS than the general population (16.50%). An association between BD and the risk of MetS components—including diabetes mellitus, dyslipidemia, and hypertension—was also identified. Based on these findings, we recommend that physician consider these associations so that specific attention and treatment are available for patients with particular comorbidities. We also advise that patients with BD regularly monitor their blood pressure, triglyceride, fasting plasma glucose, and HDL cholesterol levels, as well as waist circumference.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed extensively to this work. TC and XS contributed equally to the study design and analysis. The search strategies were conducted by HL. The study selection, assessment of validity, and data extraction were performed by YC and LL. The manuscript was drafted by JZ with contributions from all authors.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No.: n82073462).
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Declarations
Conflict of Interest
The authors report no potential conflict of interest.
Ethical approval
All analyses were based on previous published studies. Thus, no ethical approval and patient consent are required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tingqiao Chen and Xinyi Shao contributed equally to this work.
References
- 1.Mendes D, Correia M, Barbedo M, et al. Behçet’s disease—a contemporary review. J Autoimmun. 2009;32:178–188. doi: 10.1016/j.jaut.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Kim JB, Kim S, Lee T, et al. Colchicine-induced rhabdomyolysis caused by interaction with clarithromycin in a patient with Behcet disease. J Clin Rheumatol. 2013;19:108–109. doi: 10.1097/RHU.0b013e31828639e0. [DOI] [PubMed] [Google Scholar]
- 3.Lee YB, Lee SY, Choi JY, et al. Incidence, prevalence, and mortality of Adamantiades-Behçet’s disease in Korea: a nationwide, population-based study (2006–2015) J Eur Acad Dermatol Venereol. 2018;32:999–1003. doi: 10.1111/jdv.14601. [DOI] [PubMed] [Google Scholar]
- 4.Alpsoy E, Donmez L, Onder M, et al. Clinical features and natural course of Behçet’s disease in 661 cases: a multicentre study. Br J Dermatol. 2007;157:901–906. doi: 10.1111/j.1365-2133.2007.08116.x. [DOI] [PubMed] [Google Scholar]
- 5.Farouk H. Behçet’s disease, echocardiographers, and cardiac surgeons: together is better. Echocardiography. 2014;31:783–787. doi: 10.1111/echo.12524. [DOI] [PubMed] [Google Scholar]
- 6.Fei Y, Li X, Lin S, et al. Major vascular involvement in Behçet’s disease: a retrospective study of 796 patients. Clin Rheumatol. 2013;32:845–852. doi: 10.1007/s10067-013-2205-7. [DOI] [PubMed] [Google Scholar]
- 7.Misra DP, Shenoy SN. Cardiac involvement in primary systemic vasculitis and potential drug therapies to reduce cardiovascular risk. Rheumatol Int. 2017;37:151–167. doi: 10.1007/s00296-016-3435-1. [DOI] [PubMed] [Google Scholar]
- 8.Kural-Seyahi E, Fresko I, Seyahi N, et al. The long-term mortality and morbidity of Behçet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore) 2003;82:60–76. doi: 10.1097/00005792-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 9.de Chambrun PM, Wechsler B, Geri G, Cacoub P, Saadoun D. New insights into the pathogenesis of Behçet’s disease. Autoimmun Rev. 2012;11:687–698. doi: 10.1016/j.autrev.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Kalan Farmanfarma K, Kaykhaei MA, Adineh HA, Mohammadi M, Dabiri S, Ansari-Moghaddam A. Prevalence of metabolic syndrome in Iran: a meta-analysis of 69 studies. Diabetes Metab Syndr. 2019;13:792–799. doi: 10.1016/j.dsx.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 11.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 12.Choudhary S, Pradhan D, Pandey A, et al. The association of metabolic syndrome and psoriasis: a systematic review and meta-analysis of observational study. Endocr Metab Immune Disord Drug Targets. 2020;20:703–717. doi: 10.2174/1871530319666191008170409. [DOI] [PubMed] [Google Scholar]
- 13.Phan K, Charlton O, Smith SD. Hidradenitis suppurativa and metabolic syndrome—systematic review and adjusted meta-analysis. Int J Dermatol. 2019;58:1112–1117. doi: 10.1111/ijd.14500. [DOI] [PubMed] [Google Scholar]
- 14.Yalçın B, Gür G, Artüz F, Allı N. Prevalence of metabolic syndrome in Behçet disease: a case-control study in Turkey. Am J Clin Dermatol. 2013;14:421–425. doi: 10.1007/s40257-013-0034-8. [DOI] [PubMed] [Google Scholar]
- 15.Oguz A, Dogan EG, Uzunlulu M, Oguz FM. Insulin resistance and adiponectin levels in Behçet’s syndrome. Clin Exp Rheumatol. 2007;25(Suppl 45):S118–S119. [PubMed] [Google Scholar]
- 16.Seremet S, Gurel MS. Miscellaneous skin disease and the metabolic syndrome. Clin Dermatol. 2018;36:94–100. doi: 10.1016/j.clindermatol.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Anal Obs Stud Epidemiol (Moose) Group JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive—trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009;38:287–298. doi: 10.1093/ije/dyn188. [DOI] [PubMed] [Google Scholar]
- 21.Langhorne P. Bias in meta-analysis detected by a simple, graphical test. Prospectively identified trials could be used for comparison with meta-analyses. BMJ. 1998;316:471. [PMC free article] [PubMed] [Google Scholar]
- 22.Gul FC, Cicek D, Kaman D, Demir B, Nazik H. Changes of serum lipocalin-2 and retinol binding protein-4 levels in patients with psoriasis and Behçet’s disease. Eur J Dermatol. 2015;25:195–197. doi: 10.1684/ejd.2014.2490. [DOI] [PubMed] [Google Scholar]
- 23.El-Gazzar I, El-Dakrony A-H, Sayed S, et al. Clinical significance of metabolic syndrome and carotid intima-media thickness in Behҫet’s disease patients: relation to disease activity. Egypt Rheumatol. 2017;39:171–174. doi: 10.1016/j.ejr.2016.11.001. [DOI] [Google Scholar]
- 24.Kim SK, Choe JY, Park SH, Lee SW, Lee GH, Chung WT. Increased insulin resistance and serum resistin in Korean patients with Behçet’s disease. Arch Med Res. 2010;41:269–274. doi: 10.1016/j.arcmed.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Erden I, Uçak H, Demir B, et al. Serum ghrelin levels in patients with Behcet’s disease. Postepy Dermatol Alergol. 2016;33:450–456. doi: 10.5114/ada.2016.63884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erden I, Demir B, Uçak H, Cicek D, Dertlioğlu SB, Aydin S. Serum salusin-α and salusin-β levels in patients with Behcet’s disease. Eur J Dermatol. 2014;24:577–582. doi: 10.1684/ejd.2014.2397. [DOI] [PubMed] [Google Scholar]
- 27.Ulaşoğlu C. Frequency of metabolic syndrome and nonalcoholic fatty liver in Behçet’s disease 2013
- 28.Cebeci Kahraman F, Kayataş K, Savaş Erdoğan S, Onsun N. Do nonobese patients with Behçet’s disease have insulin resistance? J Cosmet Dermatol. 2022;21:1688–1694. doi: 10.1111/jocd.14276. [DOI] [PubMed] [Google Scholar]
- 29.Erdem H, Dinc A, Pay S, Simsek I, Turan M. Peripheral insulin resistance in patients with Behçet’s disease. J Eur Acad Dermatol Venereol. 2006;20:391–395. doi: 10.1111/j.1468-3083.2006.01457.x. [DOI] [PubMed] [Google Scholar]
- 30.Jung JH, Han KD, Lee YB, Park YG. Behçet’s disease is associated with multiple sclerosis and rheumatoid arthritis: A Korean population-based study. Dermatology. 2022;238:86–91. doi: 10.1159/000514634. [DOI] [PubMed] [Google Scholar]
- 31.Koca SS, Kara M, Özgen M, et al. Low prevalence of obesity in Behçet’s disease is associated with high obestatin level. Eur J Rheumatol. 2017;4:113–117. doi: 10.5152/eurjrheum.2017.160095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CY, Chen HA, Wu CH, Su YJ, Hsu TC, Hsu CY. Is Behçet’s syndrome associated with an increased risk of ischemic heart disease? A real-world evidence in Taiwan. Arthritis Res Ther. 2021;23:161. doi: 10.1186/s13075-021-02543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messedi M, Frigui M, Ben Mahfoudh K, et al. Intima-media thickness of carotid artery in patients with Behçet’s disease. Arch Med Res. 2011;42:398–404. doi: 10.1016/j.arcmed.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Acikgoz N, Kurtoğlu E, Yagmur J, Kapicioglu Y, Cansel M, Ermis N. Elevated monocyte to high-density lipoprotein cholesterol ratio and endothelial dysfunction in Behçet disease. Angiology. 2018;69:65–70. doi: 10.1177/0003319717704748. [DOI] [PubMed] [Google Scholar]
- 35.Ricart JM, España F, Navarro S, Todolí J, De la Fuente JM, Vayá A. Mean platelet volume does not seem to relate to thrombosis or posterior uveitis in Behçet’s disease. Clin Hemorheol Microcirc. 2013;54:51–57. doi: 10.3233/CH-2012-1564. [DOI] [PubMed] [Google Scholar]
- 36.Ugurlu S, Seyahi E, Yazici H. Prevalence of angina, myocardial infarction and intermittent claudication assessed by Rose Questionnaire among patients with Behcet’s syndrome. Rheumatology (Oxford) 2008;47:472–475. doi: 10.1093/rheumatology/kem385. [DOI] [PubMed] [Google Scholar]
- 37.Yavne Y, Tiosano S, Watad A, Comaneshter D, Cohen AD, Amital H. Investigating the link between ischemic heart disease and Behcet’s disease: a cross-sectional analysis. Int J Cardiol. 2017;241:41–45. doi: 10.1016/j.ijcard.2017.02.135. [DOI] [PubMed] [Google Scholar]
- 38.Eladle SS, Latif EA, Abdel-Fattah YH, et al. Metabolic syndrome in Behçets disease patients: keep an eye on the eye. Curr Rheumatol Rev. 2022 doi: 10.2174/1573397118666220610123242. [DOI] [PubMed] [Google Scholar]
- 39.Chen YY, Lai YJ, Yen YF, Chen HH, Chou P. Uveitis as a potential predictor of acute myocardial infarction in patients with Behcet’s disease: a population-based cohort study. BMJ Open. 2021;11:e042201. 10.1136/bmjopen-2020-042201 [DOI] [PMC free article] [PubMed]
- 40.Lee J, Yoo S, Han KD, et al. Association between metabolic syndrome and Behçet’s disease: a nationwide population-based study. J Dermatol. 2021;48:1062–1066. doi: 10.1111/1346-8138.15809. [DOI] [PubMed] [Google Scholar]
- 41.Gheita TA, El-Latif EA, El-Gazzar II, et al. Behçet’s disease in Egypt: a multicenter nationwide study on 1526 adult patients and review of the literature. Clin Rheumatol. 2019;38:2565–2575. doi: 10.1007/s10067-019-04570-w. [DOI] [PubMed] [Google Scholar]
- 42.Pandey A, Garg J, Krishnamoorthy P, et al. Predictors of coronary artery disease in patients with Behçet’s disease. Cardiology. 2014;129:203–206. doi: 10.1159/000365139. [DOI] [PubMed] [Google Scholar]
- 43.Tong B, Liu X, Xiao J, Su G. Immunopathogenesis of Behcet’s disease. Front Immunol. 2019;10:665. doi: 10.3389/fimmu.2019.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11:215–225. doi: 10.1177/1753944717711379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38:183–191. doi: 10.1016/j.diabet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Agita A, Alsagaff MT. Inflammation, immunity, and hypertension. Acta Med Indones. 2017;49:158–165. [PubMed] [Google Scholar]
- 48.El Hasbani G, Uthman I, Jawad S, Jawad ASM. The psychological impact of Behçet’s disease. Musculoskelet Care. 2022;20:742–757. doi: 10.1002/msc.1632. [DOI] [PubMed] [Google Scholar]
- 49.Rivera M, Porras-Segovia A, Rovira P, Molina E, Gutiérrez B, Cervilla J. Associations of major depressive disorder with chronic physical conditions, obesity and medication use: results from the PISMA-ep study. Eur Psychiatry. 2019;60:20–27. doi: 10.1016/j.eurpsy.2019.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.