Abstract
Background & Aims
Millions of people worldwide are infected chronically with HBV, which results in significant morbidity and mortality. Therapeutic vaccination is a strategy that aims to induce functional cure by restoring cellular immunity to HBV. Previously we have shown the candidate HBV immunotherapeutic vaccine ChAdOx1-HBV, encoding all major HBV antigens and a genetic adjuvant (shark invariant chain), is highly immunogenic in mice.
Methods
Here we report the results of HBV001, a first-in-human, phase I, non-randomised, dose-escalation trial of ChAdOx1-HBV assessed in healthy volunteers and patients with chronic HBV (CHB).
Results
Vaccination with a single dose of ChAdOx1-HBV was safe and well tolerated in both healthy and CHB cohorts. Vaccination induced high magnitude HBV-specific T cell responses against all major HBV antigens (core, polymerase, and surface) in healthy volunteers. Responses were detected but lower in patients with CHB. T cells generated by vaccination were cross-reactive between HBV C and D genotypes.
Conclusions
ChAdOx1-HBV is safe and immunogenic in healthy volunteers and patients with CHB. In further studies, ChAdOx1-HBV will be used in combination with other therapeutic strategies with an aim to overcome the attenuated immunogenicity in patients with CHB.
Impact and implications
Therapeutic vaccine ChAdOx1-HBV, a novel treatment for chronic hepatitis B infection (CHB), has been shown to be immunogenic in preclinical studies. In HBV001, a first-in-human phase I study, we show vaccination with ChAdOx1-HBV is safe and generates high magnitude T cell responses in healthy volunteers and lower levels of responses in patients with CHB. This is an important first step in the development of ChAdOx1-HBV as part of a wider therapeutic strategy to induce hepatitis B functional cure, and is of great interest to patients CHB and clinicians treating the condition.
Clinical Trials Registration
This study is registered at ClinicalTrials.gov (NCT04297917).
Keywords: Hepatitis B, Vaccine, Chimpanzee adenovirus, Shark invariant chain
Graphical abstract
Highlights
-
•
ChAdOx1-HBV is a therapeutic vaccine designed to induce functional cure in patients chronically infected with hepatitis B virus.
-
•
ChAdOx1-HBV is safe in healthy volunteers and patients with chronic HBV.
-
•
ChAdOx1-HBV is immunogenic in healthy volunteers, but immune responses are attenuated in patients with chronic HBV.
Introduction
HBV is estimated to chronically infect over 250 million people worldwide.1 Viral hepatitis-related liver cirrhosis and hepatocellular carcinoma (HCC) contribute to one million global deaths annually.1 Treatment of persons with HBV and HBV elimination as a public health threat by 2030, are targets of the United Nations Agenda for Sustainable Development and the World Health Organization.2,3 Current treatments suppress viral replication, but do not eliminate HBV nor eradicate the risk of developing HCC, and lifelong therapy is therefore often required.4 The development of new treatments that can be given in finite courses is a priority.
HBV-specific T cells, in particular cytotoxic CD8+ T cells, are key players in controlling acute HBV infection.5 However, in chronic disease these cells become functionally impaired and ‘exhausted’, which limits the ability of the immune system to clear the virus.6 One strategy to induce immune control in chronic HBV (CHB) infection is to restore the HBV-specific immune response, analogous to persons that control acute infection.7 Functional HBV cure, defined as long term HBsAg loss, is a major aim of the field as it confers a low risk of developing liver cirrhosis or HCC.8,9 Therapeutic vaccination describes the delivery of an immune activating substance designed to induce new, or boost existing HBV-specific immune responses, as a potential method of restoring HBV-specific immunity.10
Previous attempts at therapeutic vaccination for CHB have not been successful at inducing functional cure.10 A major reason for this is that vaccine candidates have not generated robust CD8+ HBV-specific T cell responses. It is not clear whether this is because the vaccines themselves are not sufficiently immunogenic, or the immunosuppressive environment of CHB and the ‘exhaustion’ of HBV-specific cellular immune responses impedes immunogenicity.7,10
We have designed immunotherapeutic vaccine candidates ChAdOx1-HBV and MVA-HBV for use in heterologous prime/boost strategies and in combination with other therapies such as checkpoint inhibitors, with the aim of inducing functional cure in patients with CHB. Previously we have shown ChAdOx1-HBV and MVA-HBV induce high magnitude HBV-specific T cell responses in mice.11 There are several reasons why these vaccines might be more efficacious than previous HBV therapeutic vaccine candidates. Firstly, chimpanzee adenoviral (ChAd) and modified vaccinia Ankara (MVA) viral vectors used in heterologous vaccination strategies, generate some of the highest magnitude vaccine induced CD8+ T cell responses in humans.12 Secondly, unlike other therapeutic HBV vaccine candidates, the ChAdOx1-HBV immunogen contains near full sequence HBV. Although HBV-specific T cell responses to multiple HBV antigens are necessary to successfully eliminate acute HBV infection,5 protective HBV epitopes are not well defined, and may differ between populations.13 An immunogen containing all the major HBV antigens enables the potential induction of T cell responses towards several HBV epitopes with different major histocompatibility complex (MHC) restrictions. Thirdly, the use of shark invariant chain (sIi) as a genetic adjuvant, which has been used previously in a human malaria vaccine study (A. Flaxman and A. Hill, personal communication) and in our preclinical studies where it enhanced vaccine-induced HBV-specific T cell magnitude.11
HBV001 (NCT04297917), is a first-in-human, phase I, non-randomised, dose-escalation trial of ChAdOx1-HBV in healthy volunteers and patients with CHB.14 The primary outcome was to assess the safety and tolerability of a single dose of ChAdOx1-HBV in both cohorts. Secondary outcomes included the assessment of HBV-specific T cell immunogenicity and quantitative HBsAg after vaccination.
Patients and methods
HBV001 study design
HBV001 is a phase I clinical trial registered as NCT04297917.14 Ethical approval was granted by the Berkshire Research Ethics Committee (REC: 19/SC/0419) and all participants gave informed written consent. All study procedures were in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines.15 Participants were sequentially enrolled into four cohorts without randomisation or blinding (Fig. S1). Healthy volunteers were enrolled at the Medicines Evaluation Unit, Manchester, UK and received intramuscular low dose (2.5 × 109 viral particles [vps], cohort 1) or high dose (2.5 × 1010 vps, cohort 2) ChAdOx1-HBV. Patients with CHB on antiviral therapy were enrolled at the Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Oxford, UK and received intramuscular low dose (2.5 × 109 vps, cohort 3) or high dose (2.5 × 1010 vps, cohort 4) ChAdOx1-HBV. The primary outcome was to assess the safety and tolerability of a single dose of ChAdOx1-HBV. Secondary outcomes included the assessment of T cell immunogenicity and changes in quantitative HBsAg levels after vaccination.
Study procedures
At baseline and at the end of study (EOS), HBV serology including HBsAg, anti-HBsAb, and anti-HBcAb were recorded in all participants. In addition, HBV DNA level, quantitative HBsAg level, HBeAg, and anti-HBeAb were measured in patients with CHB. On the day of vaccination and at 14, 28, 56, 84, and 168 days after vaccination, blood was drawn for haematological and biochemical laboratory tests, HBV serology, and to isolate peripheral blood mononuclear cells (PBMCs) for immunological assessment. Solicited adverse events were collected via electronic diary cards for 72 h after vaccination. Unsolicited adverse events were collected until Day 168 after vaccination (EOS). Further information on inclusion and exclusion criteria and study procedures are detailed in the supplementary methods and Tables S1 and S2.
Vaccine design
The HBV immunogen within ChAdOx1-HBV previously described in Chinnakannan et al.,11 includes the entire HBV-core, -polymerase, -pre-S1/pre-S2 and -surface regions of HBV genotype C sequence accession number KJ173426 HBV isolate C2,16 with point mutations in the polymerase region to render it replication deficient (Fig. S2). Truncated sIi and tissue plasminogen activator were included as molecular adjuvants.
Immunological assays
PBMCs for use in immunological assays were isolated from whole blood collected from study participants in sodium heparin vacutainers (Beckton Dickinson, Franklin Lakes, New Jersey, USA) using a Lymphoprep density gradient (Stemcell Technologies, Vancouver, Canada). Cells were counted on a Muse cell analyser (Luminex, Austin, Texas, USA) and used fresh, or cryopreserved in freezing media in 1 ml cryovials in liquid nitrogen. Interferon-gamma (IFNγ) enzyme-linked immunospot (ELISpot, Millipore, Burlington, Massachusetts, USA) assays and intracellular staining (ICS) assays were performed as previously described17 and as further detailed in the Supplementary methods. In brief, PBMCs were incubated with seven pools of HBV peptides (total 396 peptides, 15 amino acids [AAs] long overlapping by 11 AA), corresponding to the entire vaccine immunogen (Tables S3–S5) in ELISpot plates (Millipore, MSIP54510) and IFNγ spot-forming units (SFUs) were counted using an AID ELISpot plate reader. In ICS assays, PBMCs were incubated with pools of HBV peptides (Tables S3–S5) diluted in stimulation cocktail (Table S6) before staining with antibody cocktail for cytokine detection (Table S7). Statistical tests were non-parametric unless otherwise stated and are further detailed in the Supplementary methods. Statistical significance was defined as a p value of <0.05.
Results
Vaccination with ChAdOx1-HBV is safe and well tolerated
Of 32 volunteers screened, 21 were recruited into the study (Fig. S1). Ten healthy volunteers were vaccinated with low dose (2.5 × 109 vps, cohort 1, n = 5) or high dose ChAdOx1-HBV (2.5 × 1010 vps, cohort 2, n = 5). Healthy volunteers had a median age of 38 years (IQR 33–45), 70% of volunteers were male and 10% were of Asian ethnicity (Table S8). Eleven patients with CHB were vaccinated with low dose (2.5 × 109 vps, cohort 3, n = 6) or high dose ChAdOx1-HBV (2.5 × 1010 vps, cohort 4, n = 5). Patients with CHB had a median age of 41 years (IQR 33–47), 55% were male and 55% were of Asian ethnicity; baseline median serum alanine transaminase (ALT) was 27 IU/L, the majority with available baseline data (n = 7/9, 78%) were HBeAg negative and HBeAb positive, HBV DNA was under 40 IU/ml in all patients and the mean HBsAg level was 3.23 log10 IU/ml. Patients were infected with different HBV genotypes, with genotype C (n = 3, 27%) and genotype D (n = 3, 27%) being most common (Table S9).
ChAdOx1-HBV was well tolerated. There were no suspected unexpected serious adverse reactions or related serious adverse events. The most frequently reported adverse events (AEs) were those typically seen in response to intramuscularly administered viral vectored vaccines including warmth at the injection site, fatigue, and myalgia. Overall, 51 local and systemic solicited symptoms were reported within the first 3 days following vaccination (Day 0 through Day 3, Fig. 1); 20 events were reported by six healthy volunteers (n = 2 in cohort 1 and n = 4 in cohort 2), and 31 by six CHB patients (n = 3 cohort 3 and n = 3 in cohort 4). All solicited events were grade 1 (mild, n = 26, 51%) or grade 2 (moderate, n = 25, 49%) in severity and resolved within 3 days, except for four symptoms, which each resolved within 5–7 days. As expected, grade 2 events were reported more frequently after administration of the higher dose of ChAdOx1-HBV. Fifteen unsolicited AEs were reported by 11 participants during the study (Table S10), of which four (27%) were considered related to the vaccination (vomiting, vertigo, transient blurred vision, injection site tenderness). All were mild to moderate in severity and spontaneously resolved within 7 days with no sequelae. No AE led to participant withdrawal from the study.
Fig. 1.
Solicited adverse events within 72 hours of vaccination with ChAdOx1-HBV in healthy volunteers and patients with CHB.
Local and systemic adverse events recorded by self-reported diary cards 72 h after vaccination by healthy volunteers that received (A) low dose (n = 5) or (B) high dose (n = 5) ChAdOx1-HBV, or patients with CHB that received (C) low dose (n = 6) or (D) high dose (n = 5) ChAdOx1-HBV. The percentage of participants by cohort with each solicited symptom is displayed by grade of severity; grade 1 (mild, purple), grade 2 (moderate, green), grade 3 (severe, grey). CHB, chronic hepatitis B virus. (For
interpretation of the references to color in this figure legend, the reader is
referred to the Web version of this article).
There were 120 abnormal laboratory tests observed out of the 2,150 tests performed during the study follow-up (5.6%). Severity was defined according to the Division of AIDS table for grading the severity of adult and paediatric AEs.18 The most common laboratory abnormalities across all cohorts were anaemia and neutropaenia (Table S11). The most common abnormalities in healthy volunteers and patients with CHB are shown in Figs S3 and S4, respectively. The majority of the abnormal laboratory tests observed after vaccination were abnormal at baseline (n = 87/120, 73%, Table S11). Of the 33 not present at baseline, 20 remained abnormal at Day 168 (EOS) in four healthy volunteers (neutropaenia and high fibrinogen) and three patients with CHB (high ALT, high aspartate aminotransferase, anaemia, leucopaenia, and lymphopaenia). No abnormal laboratory test was thought to be related to vaccination, as per discretion of the study clinician. The majority of laboratory abnormalities were mild to moderate in severity (grade 1 and 2). There were four grade 3 abnormal laboratory tests reported in one healthy volunteer (raised fibrinogen) and two CHB patients (low neutrophils and low haemoglobin). In patients with CHB, serum ALT remained under 1.5× the upper limit of normal throughout the study and there was no significant difference in ALT between screening and any timepoint tested after vaccination (p = 0.32, Fig. S5).
ChAdOx1-HBV induces high magnitude HBV-specific T cell responses in healthy volunteers
HBV-specific T cell responses induced by vaccination with ChAdOx1-HBV in healthy volunteers assessed in IFNγ ELISpot assays, peaked at Day 14 or Day 28 after vaccination and were significantly higher than baseline responses (Day 0 median 110 [IQR 75–713] IFNγ SFUs per million (106) PBMCs vs. Day 14 median 1,173 [IQR 907–1,553] IFNγ SFUs/106 PBMCs p = 0.0004, or Day 28 median 1,440 [IQR 743–1,798] IFNγ SFUs/106 PBMCs p = 0.0002, Fig. 2A). Responses were detectable at Day 168 (EOS), although they were not significantly different to baseline.
Fig. 2.
Magnitude, breadth, and durability of HBV-specific T cell responses in healthy volunteers after vaccination with ChAdOx1-HBV.
PBMCs from healthy volunteers isolated after low dose (cohort 1, purple) or high dose (cohort 2, green) ChAdOx1-HBV vaccination were incubated with pools of HBV peptides in IFNγ ELISpot assays (IFNγ SFUs/106 PBMCs). (A) Magnitude and durability of total HBV responses compared with baseline. (B) Number of positive peptide pools at Day 0 (baseline) compared with Days 14 and 28 (peak response) and Day 168 (end of study [EOS]). (C) Magnitude and durability of T cell responses to HBV-core (purple), HBV-polymerase (green), HBV-pre-S1/S2 (dark grey) and HBV-surface (light grey) compared with baseline. (D) Magnitude of total HBV-specific T cell responses in individuals vaccinated with low compared with high dose ChAdOx1-HBV. Kruskal-Wallis test with Dunn’s multiple comparisons were used to compare two or more groups with a single control group. A mixed-effects model with Dunnett’s multiple comparisons were used to compare multiple timepoints within two or more groups. Two-way ANOVA with Sidak’s multiple comparisons were used to compare two or more groups at multiple timepoints. Median and IQR shown. ∗p <0.05, ∗∗p <0.01, ∗∗∗∗p <0.0001. ELISpot, enzyme-linked immunospot; IFNγ, interferon-gamma; n.s., not significant; PBMCs, peripheral blood mononuclear cells; SFUs, spot-forming units.
The breadth of T cell responses induced by vaccination (number of positive HBV peptide pools), was significantly higher at Days 14 and 28 after vaccination compared with baseline (Day 0 median 0 [IQR 0–1] positive pools vs. Day 14 median 5 [IQR 4–6] p <0.0001, or Day 28 median 4 [IQR 3–5] p = 0.0038). The breadth of T cell responses was also higher at Day 168 (EOS) as compared with baseline, although this did not reach statistical significance (Fig. 2B).
The magnitude of T cell responses specific to the major HBV antigens (core, polymerase, pre-S1/2 and surface) were increased after vaccination (Fig. 2C). The highest magnitude responses were specific to HBV-polymerase (median 571, range 261–765) and HBV-surface (median 513, range 282–643, Fig. 2C). The magnitude of total HBV-specific T cell responses did not differ between healthy volunteers vaccinated with low dose or high dose ChAdOx1-HBV at any timepoint after vaccination (Fig. 2D).
At baseline two healthy volunteers (00401 and 00404), had high magnitude pre-existing HBV-surface specific T cell responses (658 and 540 IFNγ SFUs/106 PBMCs, Fig. 2C). Further investigation revealed these responses were also present in an unrelated cohort of healthy individuals that had not been exposed to ChAdOx1-HBV, were CD4 specific and targeted regions of HBV-surface containing known epitopes induced by vaccination with recombinant HBsAg vaccine (Supplementary Results, Fig. S6, Table S12).
HBV serology including HBsAg, HBcAb, and HBsAb were evaluated in the healthy volunteers at baseline and Day 168 (EOS, Table S13). At baseline all volunteers had undetectable HBcAb and HBsAg and detectable HBsAb, consistent with previous vaccination with a recombinant HBsAg vaccine. At Day 168 (EOS), HBcAb remained undetectable and HBsAb remained detectable in all volunteers.
HBV-specific T cell responses induced by ChAdOx1-HBV in healthy volunteers are polyfunctional, as assessed by intracellular staining assays
The functionality of HBV-specific T cell responses were investigated in ICS assays (gating strategy Fig. S7). Frozen PBMCs were tested at the timepoint of peak total HBV-specific T cell responses as assessed by ELISpot assay or the closest timepoint if samples at peak were not available (Table S14).
Cytokine secretion in response to stimulation with HBV peptide pools HBV-core, -polymerase (designated polymerase regions 1–4 [pol1–4]) and -surface antigens (Tables S3 and S5) were observed, with HBV-polymerase specific CD8+ and CD4+ T cells dominating (Fig. 3A). CD8+ T cells produced predominantly IL-2 (median 0.033% [IQR 0.005–0.113] to pol3 and pol4, followed by IFNγ and tumour necrosis factor-alpha (TNFα). A similar hierarchy of cytokine secretion was observed for CD4+ T cells. The degranulation marker CD107a was expressed by a higher percentage of CD8+ T cells targeting the HBV polymerase region (median 0.115% [IQR 0.0–0.343] to pol1 and pol2, Fig. 3B). The activation marker CD154 was expressed by a higher percentage of CD4+ T cells targeting the HBV pre-S1/S2 and surface regions (median 0.280% [IQR 0.155–0.338], Fig. 3B). Although the majority of HBV-specific CD8+ and CD4+ T cells that were positive for cytokine/surface markers were monofunctional, a proportion were polyfunctional expressing double, triple, or quadruple combinations (Fig. 3C). For example, polyfunctional T cells represented 40% of cytokine producing CD8+ T cells targeting the polymerase region pol3 and pol4 (Fig. 3C).
Fig. 3.
Cytokine production and activation marker expression by T cells after ChAdOx1-HBV vaccination in healthy volunteers.
PBMCs from healthy volunteers isolated at peak timepoint after ChAdOx1-HBV vaccination were incubated with pools of HBV peptides, DMSO (negative control) or PMA/ionomycin (positive control) in intracellular staining assays. (A) Percentage of CD8+ (left) and CD4+ (right) T cells producing IFNγ, TNFα, or IL-2 in response to each peptide pool (HBV-core [white], -polymerase 1 and 2 [pol1 and pol2, grey], -polymerase 3 and 4 [pol3 and pol4, green], -pre-S1/S2, and -surface [purple]). (B) Percentage of CD8+ T cells (left) expressing CD107a and CD4+ T cells (right) expressing CD154 in response to stimulation with each peptide pool. (C) The proportion of IFNγ, TNFα, IL-2, or CD107a/CD154 positive CD8+ T cells (left) and CD4+ T cells (right) producing/expressing single (blue), double (pink), triple (purple), or quadruple (navy) cytokines/activation markers in response to stimulation with each peptide pool. Base of pie chart = median. All results shown after DMSO background subtraction. Median and IQR displayed. IFNγ, interferon-gamma; PBMCs, peripheral blood mononuclear cells; pol1, polymerase 1; pol2, polymerase 2; pol3, polymerase 3; pol4, polymerase 4; PMA, phorbol myristate acetate; pre-S1/S2, pre-surface1 and pre-surface2; TNFα, tumour necrosis factor-alpha.
HBV-specific T cell responses are generated after vaccination with ChAdOx1-HBV in some patients with CHB
HBV-specific T cell responses in patients with CHB were investigated in IFNγ ELISpot assays. At baseline nearly half of the patients (n = 5, 45%) had low but detectable total HBV-specific T cell responses (Day 0 median 47 [IQR 28–163] IFNγ SFUs/106 PBMCs, Fig. 4A). The highest median magnitude of total HBV-specific T cell responses was observed at Day 14 or Day 56 after vaccination, however neither was significantly different from baseline (Day 0 median 47 [IQR 28–163] IFNγ SFUs/106 PBMCs vs. Day 14 median 112 [IQR 35–247] IFNγ SFUs/106 PBMCs p >0.99 or Day 56 median 141 [IQR 24–253] IFNγ SFUs/106 PBMCs p >0.99, Fig. 4A). The breadth of T cell responses induced by vaccination was not significantly different at Day 14 or 56 (peak response) after vaccination, nor at Day 168 (EOS, Fig. 4B). Over half the patients (n = 6, 55%) had a positive ELISpot response at least one timepoint after vaccination (Table S15). At each of Days 14, 28, 56, and 84 after vaccination between two and three patients had positive ELISpot responses, but there were no positive responses at Day 168 (EOS, Table S15). The highest magnitude responses were observed on Day 56 after vaccination towards HBV-polymerase (median 43.3, range 0–252) and HBV-core (median 22, range 0–140). There was no significant difference in the magnitude of T cells that targeted the different HBV antigens, or in the magnitude of the T cell response in those who received low-dose vs. high-dose ChAdOx1-HBV (Fig. S8).
Fig. 4.
Magnitude, breadth, and durability of HBV-specific T cell responses and HBsAg titres in patients with CHB after vaccination with ChAdOx1-HBV.
PBMCs from patients with CHB isolated after low dose (cohort 3, purple) or high dose (cohort 4, green) ChAdOx1-HBV vaccination were incubated with pools of HBV peptides in IFNγ ELISpot assays (IFNγ SFUs/106 PBMCs). (A) Magnitude and durability of total HBV responses after vaccination compared with baseline. (B) Number of positive peptide pools at Day 0 (baseline) compared with Days 14 and 56 (peak response) and Day 168 (EOS). (C) Longitudinal quantitative serum HBsAg levels (log10 IU/ml) after vaccination. The Kruskal-Wallis test with Dunn’s multiple comparisons were used to compare two or more groups with a single control group. Two-way ANOVA with Sidak’s multiple comparisons were used to compare two or more groups at multiple timepoints. Median and IQR shown. CHB, Chronic hepatitis B virus; ELISpot, enzyme-linked immunospot; EOS, end of study; HBsAg, Hepatitis B surface Antigen; IFNγ, interferon-gamma; IU/ml, international units per millilitre; n.s., not significant; PBMCs, peripheral blood mononuclear cells; SFUs, spot-forming units.
Quantitative HBsAg titres did not significantly differ between baseline and any timepoint after vaccination. Between baseline and EOS there was a small non-significant reduction in mean HBsAg levels (mean baseline HBsAg 3.23 log10 IU/ml ± 0.47 vs. mean EOS HBsAg 3.18 log10 IU/ml ± 0.54, mean difference -0.071 log10 IU/ml, p >0.99, Fig. 4C). Univariable and multivariable linear regression did not reveal any significant associations between the change in HBsAg level and other variables (Fig. S9). HBsAb, which was undetectable at baseline (Table S9), remained undetectable at Day 168 (EOS).
HBV-specific T cell responses are higher after vaccination with ChAdOx1-HBV in healthy volunteers compared with patients with chronic hepatitis B
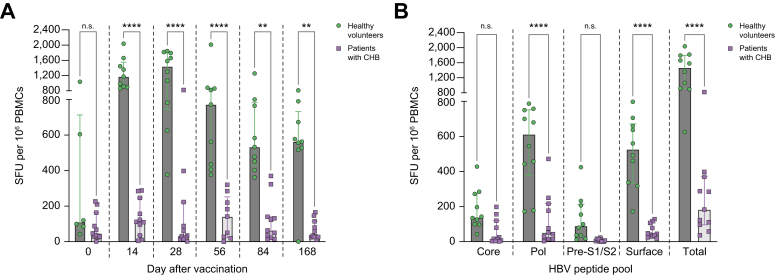
Responses to vaccination were compared between heathy volunteers and patients with CHB. The total magnitude of HBV-specific T cell responses was significantly higher in heathy volunteers compared with patients with CHB at every time point after vaccination (Fig. 5A). At peak response after vaccination, T cell responses were significantly higher in healthy volunteers than in patients with CHB for HBV-polymerase (median 612 [IQR 377–752] IFNγ SFUs/106 PBMCs vs. 52 [IQR 15–216] IFNγ SFUs/106 PBMCs, p <0.0001), HBV-surface (median 527 [IQR 672–333] IFNγ SFUs/106 PBMCs vs. 45 [IQR 30–107] IFNγ SFUs/106 PBMCs, p <0.0001) but not HBV-core or HBV-PreS1/S2 (Fig. 5B).
Fig. 5.
Magnitude, breadth, and durability of HBV-specific T cell responses in patients with Hepatitis B in comparison to healthy volunteers, after vaccination with ChAdOx1-HBV.
PBMCs isolated after ChAdOx1-HBV vaccination were incubated with pools of HBV peptides in IFNγ ELISpot assays (IFNγ SFUs/106 PBMCs). Magnitude of HBV specific T cell responses compared between healthy volunteers (cohorts 1 and 2, green) with patients with HBV (CHB, cohorts 3 and 4, purple). (A) Total HBV responses at each timepoint after vaccination. (B) Peak responses to HBV-core (core), -polymerase (Pol) and -Pre-S1/2 (Pre-S1). Two-way ANOVA with Sidak’s multiple comparisons were used to compare two or more groups at multiple timepoints. Median and IQR shown. ∗∗p <0.01, ∗∗∗∗p <0.0001. CHB, chronic hepatitis B virus; ELISpot, enzyme-linked immunospot; IFNγ, interferon-gamma; ns, not significant; PBMCs, peripheral blood mononuclear cells; SFUs, spot-forming units.
Vaccination with ChAdOx1-HBV induces HBV-specific T cells that cross-react with genotype C and genotype D HBV-peptides
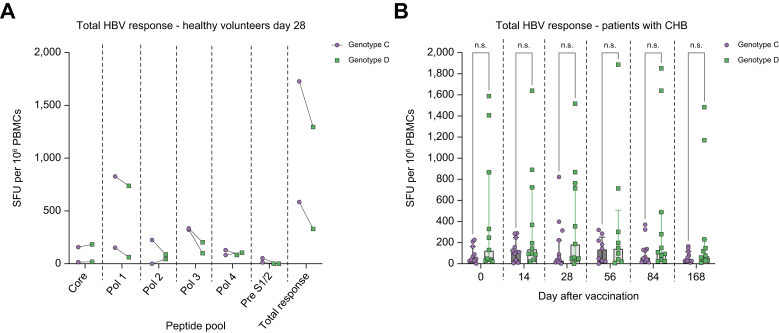
The cross-reactivity of HBV-specific T cells against genotype D, generated with the genotype C ChAdOx1-HBV vaccine were assessed in a subset of volunteers in IFNγ ELISpot assays (healthy volunteers, cohort 2, n = 2) and all patients with CHB (cohorts 3 and 4, n = 11). The two healthy volunteers tested at Day 28 after vaccination (peak response), showed a similar magnitude of HBV-specific T cell responses towards genotype C and genotype D peptide pools (Fig. 6A). In patients with CHB, there was no significant difference between the magnitude of total HBV-specific responses towards genotype C and genotype D peptide pools at baseline or any timepoint after vaccination with ChAdOx1-HBV (Fig. 6B).
Fig. 6.
Magnitude of genotype C compared with genotype D HBV-specific T cell responses after vaccination with ChAdOx1-HBV.
PBMCs isolated after ChAdOx1-HBV vaccination were incubated with pools of HBV genotype C or D peptides in IFNγ ELISpot assays (IFNγ SFUs/106 PBMCs). (A) Magnitude of HBV specific T cell responses in healthy individuals 00407 and 00408 (cohort 2), 28 days after vaccination towards genotype C (purple) compared with genotype D (green) HBV peptide pools (HBV-core, -polymerase [pol 1 – pol 4] and -pre-S1/S2). (B) Magnitude of genotype C (purple) compared with genotype D (green) total HBV specific T cell responses in patients with HBV (CHB, cohorts 3 and 4, n = 11) at all timepoints after vaccination. Two-way ANOVA or mixed effects model with Sidak’s multiple comparisons used for statistical comparison between two groups at multiple timepoints. Median and IQR shown. CHB, chronic hepatitis B virus; ELISpot, enzyme-linked immunospot; IFNγ, interferon-gamma; ns, not significant; PBMCs, peripheral blood mononuclear cells; SFUs, spot-forming units.
ChAdOx1-HBV induces T cell responses specific for the truncated sIi in some healthy volunteers
We have previously shown that sIi significantly enhances HBV-specific T cell responses in preclinical studies.11 We investigated T cell responses generated by vaccination with ChAdOx1-HBV towards sIi and human invariant chain (hIi) using IFNγ ELISpot assays. Unexpectedly, half of healthy volunteers (n = 5, 50%) had detectable sIi-specific T cell responses after vaccination, with magnitudes ranging between 153 and 1072 IFNγ SFUs/106 PBMCs at peak (Fig. 7A). ICS assays showed these were predominantly CD8+ T cells producing IFNγ, TNFα, or IL-2 (Fig. 7B). There were no sIi-specific T cell responses detected in patients with CHB (data not shown). In healthy volunteers there was no significant difference in hIi-specific T cells between baseline and any timepoint after vaccination (Fig. 7C). In addition, at Day 28 after vaccination there was no association between the magnitude of sIi-specific and hIi-specific T cell responses (Fig. 7D), nor sIi-specific and total HBV-specific T cell responses (Fig. 7E).
Fig. 7.
Magnitude of T cell responses specific to invariant chain after vaccination with ChAdOx1-HBV.
PBMCs isolated from healthy volunteers (cohort 1 purple, cohort 2 green) after vaccination were incubated with peptides corresponding to the truncated sIi and hIi in IFNγ ELISpot assays or intracellular staining assays. (A) Magnitude and durability of sIi T cell responses after vaccination (IFNγ SFUs/106 PBMCs). (B) Percentage of CD8+ (purple symbols) and CD4+ (green symbols) T cells producing IFNγ, TNFα, or IL-2 in response to sIi. (C) Magnitude and durability of hIi specific T cell responses after vaccination. Comparison between (D) sIi- and hIi-specific T cell response magnitude and (E) sIi-specific and total HBV-specific T cell responses after vaccination. Kruskal-Wallis test with Dunn’s multiple comparisons were used to compare two or more groups with a single control group. Linear regression was performed to compare two continuous variables. Median shown. ELISpot, enzyme-linked immunospot; hIi, human invariant chain; IFNγ, interferon-gamma; PBMCs, peripheral blood mononuclear cells; SFUs, spot-forming units; sIi, shark invariant chain; TNFα, tumour necrosis factor-alpha.
Discussion
We have developed a novel viral vectored vaccine, ChAdOx1-HBV, encoding all major HBV antigens alongside a genetic adjuvant sIi, and assessed this in a first-in-human, phase I, dose-escalation study, in both healthy volunteers and in patients with CHB. We show ChAdOx1-HBV is safe and well tolerated, with an AE profile typical for those previously observed with viral vectored vaccines.[19], [20], [21], [22], [23] In addition, we saw no evidence of ‘flares’ in liver enzymes24 in vaccinated patients with CHB.
In healthy volunteers, we show ChAdOx1-HBV induces high magnitude, polyfunctional, and durable HBV-specific T cell responses towards all major HBV antigens. This is important because HBV T cell responses with multiple specificities are required for clearance of acute HBV.5 In addition, ChAdOx1-HBV generated higher magnitude responses than previously reported for another HBV therapeutic vaccine candidate (GS-4774) trialled in healthy volunteers.25 HBV-surface-specific T cell responses detected in selected healthy volunteers at baseline were also detected in an unrelated cohort of healthy volunteers with no exposure to ChAdOx1-HBV. These responses were likely present as a result of previous vaccination with recombinant HBsAg prophylactic vaccine, supported by their CD4 predominance, specificity to regions of the HBV-surface antigen containing known MHC class II restricted epitopes induced by recombinant HBsAg vaccination,26,27 and all healthy volunteers having HBV serology consistent with previous HBsAg vaccination.
We show that in some patients with CHB, T cell responses specific to HBV are de-novo generated or boosted after vaccination with ChAdOx1-HBV. Although most previous trials of HBV therapeutic vaccine candidates have found that HBV-specific T cell responses are not reliably induced or boosted after vaccination (reviewed in Cargill et al.10), our results are consistent with a subset of studies which have found that between 22% and 67% of participants have detectable ex-vivo T cell responses after vaccination.[28], [29], [30] There is scope to further enhance the magnitude and breadth of ChAdOx1-HBV primed HBV-specific T cell responses in CHB patients using heterologous viral vectors encoding HBV antigens (e.g. MVA) as we have previously demonstrated in preclinical studies,11 and has been demonstrated in human vaccines for HCV and malaria.12,31
The magnitude of total HBV T cell responses was significantly lower in patients with CHB than in healthy volunteers after ChAdOx1-HBV and were limited to HBV-core and -polymerase. As ChAdOx1-HBV was highly immunogenic in healthy volunteers, the attenuated T cell responses observed in patients with CHB is likely attributable to ‘exhausted’ cellular immune responses, characteristic of CHB infection.6,7 The relative inability of ChAdOx1-HBV vaccination to enhance HBV-surface specific T cells is consistent with previous data showing they are most dysfunctional and difficult to restore.30,[32], [33], [34] Similar findings have been observed in HCV vaccine trials, which showed the magnitude and functionality of T cell responses to vaccination in patients with chronic HCV were significantly attenuated compared with healthy volunteers.35,36
The ChAdOx1-HBV vaccine includes sIi as genetic adjuvant,37 which has an adjuvant effect irrespective of HLA type.17,37 We previously showed sIi significantly enhanced HBV specific T cell responses induced by ChAdOx1-HBV in preclinical studies.11 In some HBV001 participants, high magnitude T cell responses against sIi were generated. This appears to be limited to HLA-A∗02 restricted individuals, as responses are induced in humanised HLA-A∗02 HHD mice but no other mouse strains after vaccination with ChAdOx1-HBV (data not shown) and HLA-A∗02 restricted sIi-specific T cells have been previously identified in response to an sIi containing malaria vaccine (A. Flaxman and A. Hill, pers. commun.). The magnitude of sIi T cell responses and total HBV-specific T cell responses were not related, suggesting presence of sIi specific T cell responses does not impair the generation of HBV-specific T cell responses. Despite low homology between sIi and hIi,37 there is a theoretical risk of T cell autoreactivity. Reassuringly, we found minimal T cell responses to hIi and no association between the magnitude of sIi and hIi responses in HBV001, consistent with a trial of HCV-vaccine containing full length hIi adjuvant in humans, which did not detect autoreactive immune responses.17
Our study has several limitations. Firstly, the sample size was small, although this is typical for first-in-human studies. Secondly, although we demonstrated broad cross-reactivity of HBV-specific T cells generated by ChAdOx1-HBV towards HBV genotype C and D peptides, study participants were recruited in the UK and are unlikely to be fully representative of patients with CHB from endemic areas where dominant HLA haplotypes differ and genotype C or D infection predominates.38 Thirdly, HBsAb titres were not precisely quantified in healthy volunteers, so we were unable to determine whether vaccination with ChAdOx1-HBV boosted levels of pre-existing HBsAb. Finally, we assessed a single vaccine modality, and although we show robust T cell responses in healthy volunteers, responses in CHB patients were reduced. This suggests that future therapeutic vaccine studies with ChAdOx1-HBV will need to combine with additional strategies to be efficacious.7
Several of these limitations will be addressed in a phase II clinical trial HBV-002 (NCT0477890439), which is currently recruiting patients with HBV in genotype C endemic areas to combinations of ChAdOx1-HBV, MVA-HBV, and nivolumab to assess safety, immunogenicity, and efficacy. This approach is supported by animal models and a small human pilot study, which demonstrated the combination of therapeutic HBV vaccine with checkpoint inhibitor nivolumab can induce HBV functional cure.40,41
This is a first-in-human study to show vaccination with ChAdOx1-HBV is safe and well tolerated in healthy volunteers and patients with CHB, generating high-magnitude T-cell responses against all major HBV antigens in healthy volunteers, and lower levels of responses in patients with CHB. T cells generated by vaccination were cross-reactive between HBV genotypes. This is an important first step in the development of ChAdOx1-HBV as part of a broader therapeutic strategy aiming to induce HBV functional cure, in which ChAdOx1-HBV is combined with therapeutic vaccine MVA-HBV and checkpoint inhibition. Further studies in larger cohorts of patients where HBV is endemic are necessary to evaluate the efficacy of this approach.
Financial support
The trial was funded by Vaccitech Limited, without other outside support. TC was funded by a Wellcome Trust Training Fellowship for Clinicians (211042/Z/18/Z) and is a National Institute for Health and Care Research (NIHR) Academic Clinical Lecturer. SC was funded by the Medical Research Council UK, Oxford NIHR Biomedical Research Centre and Vaccitech. PK is funded by the Wellcome Trust (222426/Z/21/Z) and is an NIHR Senior Investigator. EB is funded by the Medical Research Council UK (MR/K010239/1), the Oxford NIHR Biomedical Research Centre and is an NIHR Senior Investigator.
Authors’ contributions
Conceptualisation: TE, EB. Data curation TC, PC, AB, BK, LH, RM. Formal analysis: TC, KR. Funding acquisition: PK, TE, EB. Investigation TC, PC, AB, LH, EA, SC. Methodology TC, PC, BK, SC, SS, LB, HS, TE, EB. Project administration BK, RM, LB. Resources PK, TE, EB. Supervision KR, HS, PK, TE, EB. Visualisation TC. Writing original draft TC. Writing review and editing: all authors.
Data availability statement
Data will be made available on reasonable request. All data are available in the main text or the Supplementary materials.
Conflicts of interest
SC and EB are named inventors, and TC is a contributor on a patent application describing the ChAdOx1-HBV vaccine reported in this manuscript (International Application No. PCT/GB2018/050948). BK, RM, SS, LB, KR, HS, and TE are employed by Vaccitech. The other authors declare that they have no competing interests.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We would like to acknowledge the staff of the Medicines Evaluation Unit (Manchester, UK), as well as the volunteers and patients who participated. Pedro Folegatti acted as Medical Monitor, and trial support for data management, monitoring, statistics, and safety reporting were undertaken by S-cubed (Abingdon, UK). We would like to thank the Viral Vector Core Facility at the Jenner Institute (Oxford, UK) for their help with vaccine production.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100885.
Supplementary data
The following are the supplementary data to this article:
References
- 1.World Health Organization . April 2017. Global hepatitis report.https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ Available from: [Google Scholar]
- 2.United Nations . 2015. Transforming our world: the 2030 agenda for sustainable development.https://sdgs.un.org/2030agenda Available from: [Google Scholar]
- 3.World Health Organization . 2016. Global health sector strategy on viral hepatitis 2016–2021.https://www.who.int/publications/i/item/WHO-HIV-2016.06 Available from: [Google Scholar]
- 4.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Thimme R., Wieland S., Steiger C., Ghrayeb J., Reimann K., Purcell R.H., et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisicaro P., Barili V., Rossi M., Montali I., Vecchi A., Acerbi G., et al. Pathogenetic mechanisms of T cell dysfunction in chronic HBV infection and related therapeutic approaches. Front Immunol. 2020;11:849. doi: 10.3389/fimmu.2020.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maini M.K., Pallett L.J. Defective T-cell immunity in hepatitis B virus infection: why therapeutic vaccination needs a helping hand. Lancet Gastroenterol Hepatol. 2018;3:192–202. doi: 10.1016/S2468-1253(18)30007-4. [DOI] [PubMed] [Google Scholar]
- 8.Lok A.S., Zoulim F., Dusheiko G., Ghany M.G. Hepatitis B cure: from discovery to regulatory approval. J Hepatol. 2017;67:847–861. doi: 10.1016/j.jhep.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Cornberg M., Lok A.S.F., Terrault N.A., Zoulim F., EASL-AASLD HBV Treatment Endpoints Conference Faculty Guidance for design and endpoints of clinical trials in chronic hepatitis B – Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. J Hepatol. 2020;72:539–557. doi: 10.1016/j.jhep.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Cargill T., Barnes E. Therapeutic vaccination for treatment of chronic hepatitis B. Clin Exp Immunol. 2021;205:106–118. doi: 10.1111/cei.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinnakannan S.K., Cargill T.N., Donnison T.A., Ansari M.A., Sebastian S., Lee L.N., et al. The design and development of a multi-HBV antigen encoded in chimpanzee adenoviral and modified vaccinia Ankara viral vectors; a novel therapeutic vaccine strategy against HBV. Vaccines (Basel) 2020;8:184. doi: 10.3390/vaccines8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capone S., Brown A., Hartnell F., Del Sorbo M., Traboni C., Vassilev V., et al. Optimising T cell (re)boosting strategies for adenoviral and modified vaccinia Ankara vaccine regimens in humans. NPJ Vaccin. 2020;5:94. doi: 10.1038/s41541-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y., Ding Y., Shen C. A systematic review of T cell epitopes defined from the proteome of hepatitis B virus. Vaccines. 2022;10:257. doi: 10.3390/vaccines10020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaccitech Limited . 2020. NCT04297917 A phase 1 monotherapy study to evaluate the safety, tolerability & immunogenicity of vaccination with candidate chimpanzee adenovirus-vectored HepB virus vaccine ChAdOx1 HBV in healthy participants & participants with chronic HepB infection.https://clinicaltrials.gov/ct2/show/NCT04297917 Available from: [Google Scholar]
- 15.Good Clinical Practice Network. ICH harmonised guideline integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice ICH E6(R2) ICH Consensus Guideline. Available from: https://ichgcp.net/.
- 16.GenBank . 2015. Hepatitis B virus isolate C2_CLP(3), complete genome. GenBank: KJ173426.1.https://www.ncbi.nlm.nih.gov/nuccore/KJ173426 Available from: [Google Scholar]
- 17.Esposito I., Cicconi P., D’Alise A.M., Brown A., Eposito M., Swadling L., et al. MHC class II invariant chain-adjuvanted viral vectored vaccines enhances T cell responses in humans. Sci Transl Med. 2020;12:eaaz7715. doi: 10.1126/scitranslmed.aaz7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS . 2017. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, corrected version 2.1.https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf Available from: [Google Scholar]
- 19.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsey A.R., Sobieszczyk M.E., Hirsch I., Sproule S., Robb M.L., Corey L., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asano M., Okada H., Itoh Y., Hirata H., Ishikawa K., Yoshida E., et al. Immunogenicity and safety of AZD1222 (ChAdOx1 nCoV-19) against SARS-CoV-2 in Japan: a double-blind, randomized controlled phase 1/2 trial. Int J Infect Dis. 2022;114:165–174. doi: 10.1016/j.ijid.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghany M.G., Feld J.J., Chang K.M., Chan H.L.Y., Lok A.S.F., Visvanathan K., et al. Serum alanine aminotransferase flares in chronic hepatitis B infection: the good and the bad. Lancet Gastroenterol Hepatol. 2020;5:406–417. doi: 10.1016/S2468-1253(19)30344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaggar A., Coeshott C., Apelian D., Rodell T., Armstrong B.R., Shen G., et al. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine. 2014;32:4925–4931. doi: 10.1016/j.vaccine.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Werner J.M., Abdalla A., Gara N., Ghany M.G., Rehermann B. The hepatitis B vaccine protects re-exposed health care workers, but does not provide sterilizing immunity. Gastroenterology. 2013;145:1026–1034. doi: 10.1053/j.gastro.2013.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua C.G., Mehrotra A., Mazzulli T., Wong D.K., Feld J.J., Janssen L.A., et al. Optimized ex vivo stimulation identifies multi-functional HBV-specific T cells in a majority of chronic hepatitis B patients. Sci Rep. 2020;10 doi: 10.1038/s41598-020-68226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon S.K., Seo Y.B., Im S.J., Bae S.H., Song M.J., You C.R., et al. Safety and immunogenicity of therapeutic DNA vaccine with antiviral drug in chronic HBV patients and its immunogenicity in mice. Liver Int. 2015;35:805–815. doi: 10.1111/liv.12530. [DOI] [PubMed] [Google Scholar]
- 29.Lok A.S., Pan C.Q., Han S.H.B., Trinh H.N., Fessel W.J., Rodell T., et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol. 2016;65(3):509–516. doi: 10.1016/j.jhep.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Boni C., Janssen H.L.A., Rossi M., Yoon S.K., Vecchi A., Barili V., et al. Combined GS-4774 and tenofovir therapy can improve HBV-specific T-cell responses in patients with chronic hepatitis. Gastroenterology. 2019;157:227–241.e7. doi: 10.1053/j.gastro.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 31.Ewer K.J., O’Hara G.A., Duncan C.J.A., Collins K.A., Sheehy S.H., Reyes-Sandoval A., et al. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun. 2013;4:2836. doi: 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuch A., Salimi Alizei E., Heim K., Wieland D., Kiraithe M.M., Kemming J., et al. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8+ T cells in chronically HBV-infected patients with low viral load. Gut. 2019;68:905–915. doi: 10.1136/gutjnl-2018-316641. [DOI] [PubMed] [Google Scholar]
- 33.Hoogeveen R.C., Robidoux M.P., Schwarz T., Heydmann L., Cheney J.A., Kvistad D., et al. Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection. Gut. 2019;68:893–904. doi: 10.1136/gutjnl-2018-316644. [DOI] [PubMed] [Google Scholar]
- 34.Le Bert N., Gill U.S., Hong M., Kunasegaran K., Tan D.Z.N., Ahmad R., et al. Effects of hepatitis B surface antigen on virus-specific and global T cells in patients with chronic hepatitis B virus infection. Gastroenterology. 2020;159:652–664. doi: 10.1053/j.gastro.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Kelly C., Swadling L., Capone S., Brown A., Richardson R., Halliday J., et al. Chronic hepatitis C viral infection subverts vaccine-induced T-cell immunity in humans. Hepatology. 2016;63:1455–1470. doi: 10.1002/hep.28294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swadling L., Halliday J., Kelly C., Brown A., Capone S., Ansari M.A., et al. Highly-immunogenic virally-vectored T-cell vaccines cannot overcome subversion of the T-cell response by HCV during chronic infection. Vaccines. 2016;4:27. doi: 10.3390/vaccines4030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halbroth B.R., Sebastian S., Poyntz H.C., Bergu M., Cottingham M.G., Hill A.V.S., et al. Development of a molecular adjuvant to enhance antigen-specific CD8+ T cell responses. Sci Rep. 2018;8 doi: 10.1038/s41598-018-33375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velkov S., Ott J.J., Protzer U., Michler T. The global hepatitis B virus genotype distribution approximated from available genotyping data. Genes. 2018;9:495. doi: 10.3390/genes9100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaccitech Limited . 2021. A Phase 1b/2a, open-label study to evaluate the safety, tolerability and immunogenicity of VTP-300 with or without nivolumab in participants with chronic hepatitis B infection. NCT04778904.https://clinicaltrials.gov/ct2/show/NCT04778904 Available from: [Google Scholar]
- 40.Gane E., Verdon D.J., Brooks A.E., Gaggar A., Nguyen A.H., Subramanian G.M., et al. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: a pilot study. J Hepatol. 2019;71:900–907. doi: 10.1016/j.jhep.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 41.Liu J., Zhang E., Ma Z., Wu W., Kosinska A., Zhang X., et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on reasonable request. All data are available in the main text or the Supplementary materials.