Abstract
Arsenic contamination in rice poses a significant health risk to rice consumers across the globe. This review examines the impact of water source and type on the speciation and methylation of arsenic in rice. The review highlights that groundwater used for irrigation in arsenic-affected regions can lead to higher total arsenic content in rice grains and lower proportions of methylated arsenic species. The methylation of As in rice is influenced by microbial activity in groundwater, which can methylate arsenic that is taken up by rice plants. Reclaimed water irrigation can also increase the risk of arsenic accumulation in rice crops, although the use of organic amendments and proper water management practices can reduce arsenic accumulation. Different water management regimes, such as continuous flooding irrigation, alternate wetting and drying, aerobic rice cultivation, and subsurface drip irrigation, can affect the speciation and methylation of As in rice. Continuous flooding irrigation reduces methylation of As due to anaerobic conditions, while alternate wetting and drying and aerobic rice cultivation promote methylation by creating aerobic conditions that stimulate the activity of arsenic-methylating microorganisms. Subsurface drip irrigation reduces total arsenic content in rice grains and increases the proportion of less toxic methylated arsenic species. The review also discusses the complex mechanisms of As-methylation and transport in rice, emphasizing the importance of understanding these mechanisms to develop strategies for reducing arsenic uptake in rice plants and mitigating health risks. The review addresses the impact of water source and type on arsenic speciation and methylation in rice and highlights the need for proper water management and treatment measures to ensure the safety of the food supply as well as aiding future research and policies to reduce health risks from rice consumption. The critical information gaps that this review addresses include the specific effects of different water management regimes on As-methylation, the role of microbial communities in groundwater in As-methylation, and the potential risks associated with the use of reclaimed water for irrigation.
Keywords: Rice, As-methylation, As-transporters, Arsenate reductase, Arsenite oxidase
Graphical Abstract

1. Introduction
Arsenic (As) is a group 1 non-threshold carcinogen that poses a significant health risk to humans when consumed through contaminated food, such as rice, being a staple food for a large portion of the world's population(IARC) [1], [2], [3], [4]. Long-term exposure to As can induce oxidative DNA damages, dermatological lesions, keratosis and various forms of internal cancers including lungs, bladder and liver cancers [5], [6], [7].
Arsenic can exist in different oxidation states, but the predominant forms found in the environment are inorganic arsenite (AsIII) and inorganic arsenate (AsV). In recent years, there has been a growing interest in understanding the mechanisms of methylation of As in rice [8], [9], [10], [11], [12]. As-methylation involves the addition of a methyl group (-CH3) to an inorganic arsenic (iAs)-containing molecule, converting it into less toxic organic forms (the organic arsenic (oAs)) such as monomethylarsonous acid (MMA(III)), dimethylarsinous acid (DMA(III)), monomethylarsonic acid (MMA(V)) and dimethylarsinic acid (DMA(V)) [8], [9], [13], [14], [15]. Given that rice is a staple food for over 60% of the global population [16], [17], [18], comprehending the processes that govern the methylation of iAs in rice is crucial for minimizing the health risks associated with rice consumption among consumers across the globe.
Numerous studies have highlighted As contamination in rice as a global issue, with varying levels of iAs detected in rice samples worldwide [2], [8], [9], [13], [14], [19], [20], [21], [22]. However, the extent of contamination depends on soil conditions and cultivation practices [8], [10]. This review aims to consolidate the existing knowledge on As-methylation in rice, to provide a comprehensive overview of the factors influencing As-methylation, to understand the molecular mechanisms underlying As speciation and methylation in rice and to provide the implications of As-methylation in rice to human health. Therefore, this review addresses the critical information gaps that include the specific effects of different water management regimes on As-methylation, the role of microbial communities in groundwater in As-methylation, and the potential risks associated with the use of reclaimed water for irrigation.
To collect the required information, a literature search was carried out on Web of Science, PubMed, and Google Scholar to locate articles pertaining to these topics. The objective of this review is to amalgamate information from diverse fields of study, thereby bridging the gap between different disciplines and offering a comprehensive comprehension of the mechanisms involved As-methylation in rice. Ultimately, this review can contribute to the development of strategies aimed at minimizing the health risks associated with rice consumption.
Despite previous studies on As contamination in rice, several research gaps on role of water management practices, source, and type of irrigation water on speciation and methylation of iAs in rice plants still remain. Currently, there is a lack of comprehensive understanding regarding the comparative influence of groundwater irrigation and reclaimed irrigation water on accumulation and methylation of As in rice [8]. Bridging this knowledge gap is crucial for developing effective strategies to mitigate As contamination.
The role of water management regimes, such as continuous flooding (CF) versus alternate wetting and drying (AWD) in speciation and methylation of As in rice [23] requires further exploration. The current reviews lack a clear understanding of how different water management regimes affect uptake and translocation of methylated As in rice plants. Furthermore, the mechanisms underlying the transfer of As from soil to rice plants remain poorly understood. Investigating the influence of soil properties, such as pH and organic matter content, on As uptake and accumulation in rice is necessary to fill this research gap.
Limited knowledge exists regarding the speciation and methylation of As in rice plants. Understanding and consolidating the factors that influence the conversion of iAs into less toxic forms, such as oAs species, is crucial for reducing potential health implications. Therefore, this study will contribute to a better understanding of As contamination in rice and facilitate the development of effective strategies for its mitigation.
2. Uptake and transport of As in rice plants
2.1. Uptake of As from soil by rice plants
The uptake of As in rice plants is a complex process that involves different mechanisms depending on the specific As species. In soil, the two main forms of As present are arsenate (AsV) and arsenite (AsIII) [24], [25]. Arsenate has similar structure to phosphate, leading to its uptake by the plant through the same transporters involved in phosphate uptake, such as phosphate transporters and high-affinity phosphate transporters [19], [26], [27]. This phenomenon, known as phosphate mimicry, serves as a primary pathway for AsV uptake in rice plants. On the other hand, AsIII is a neutral compound and is taken up by rice plants through aquaporins, which are specialized water channels that allow the passage of small molecules like As.
2.2. Transport of As in a rice plant
Arsenic can be transported to different parts of the rice plant through the xylem and phloem tissues [28], [29], [30] and its accumulation depends on several factors. These factors include the rice genotype, soil type, concentration of As in the soil (level of contamination) and the age and physiological state of the rice plant [26], [31], [32], [33], [34]. The mean As concentration in different parts of rice plants is reported to decrease in the following sequence: roots > leaves > stems > ears > grains [32], [33], [34]. For example, Geng et al. [32] found that roots had the highest concentration of As ranging from 7.330 to 16.4840 mg kg−1, followed by shoots with concentrations ranging from 1.83 to 32.22 mg kg−1, leaves with concentrations ranging from 0.77 to 13.06 mg kg−1, and grains with concentrations ranging from 0.350 to 6.120 mg kg−1. Similarly, Rokonuzzaman et al. [34] reported a similar trends with concentrations as follows: roots (8.260–86.740 mg kg−1), shoots (0.760–17.150 mg kg−1) and leaves 0.250–7.770 mg kg−1).
2.3. Distribution of As in rice grains
Several studies have indicated that the distribution of As in rice grains is not uniform and can vary depending on factors such as rice variety (rice genotype), soil type and environmental conditions [35]. Despite their relatively small weight within the grain, the outermost layers of rice grain (bran and aleurone) have been found to contain the highest concentrations of As [32]. The bran layer, in particular, can contain up to 20 times more As compared to the endosperm, which is the starchy part of the grain commonly consumed [36], [37]. For instance, Pedron et al. [37] observed a 10–20 times higher concentration of As in rice bran compared to the endosperm, while Yao et al. [36] reported a 7.1 times higher As concentration in rice bran compared to polished rice. In terms of variation among rice varieties, a study conducted in Bangladesh found that BR16 accumulated higher levels of As (0.520 mg kg−1) compared to BRRIdhan29 (0.050 mg kg−1) [35]. Additionally, studies have also highlighted that As speciation in rice grains is influenced by factors such as phytotoxicity and cultivars [38], [39]. Understanding these factors is crucial for the development effective strategies to mitigate the health risks associated with As exposure from rice consumption.
2.4. Factors that affect uptake and transport of As
The uptake and transportation of As in rice plants are influenced by various factors, including, the chemical form of As, the presence and availability of nutrient, soil pH, and environmental stressors like drought and flooding [27], [35], [40], [41], [42].
2.4.1. The chemical form of As
The uptake and transportation of As in rice plants are influenced by the various chemical forms of As present in the soil [40], [43], [44], [45], [46]. Specifically, arsenate is the predominant form of As in aerobic soil conditions [47], [48]. It shares similarities with phosphate and is adsorbed by plants through phosphate transporters. Arsenate carries a negative charge at neutral pH, allowing it to be mobile and easily transported in the soil solution. On the other hand, arsenite is the reduced form of As and tends to be dominate in anaerobic or reducing soil conditions. Unlike arsenate, it does not readily adsorb onto soil particles and can freely diffuse across cell membranes. Arsenite uptake in plants occurs through non-specific channels and aquaporins. Organic As compounds, such as MMA and DMA, originate from biological processes involving microbial transformations of iAs. These compounds can be found in soil and water and are also taken up by plants. Organic As (oAs) is less toxic than inorganic forms and generally exhibits lower bioavailability. The primary uptake of As in rice plants occurs through the roots. The transport mechanisms involved vary depending on the specific forms of As. Arsenate is taken up by rice plants through phosphate transporters due to its structural similarity to phosphate. Several studies have identified specific transporters responsible for arsenate uptake in rice, including the phosphate transporters OsPT1, OsPT2, OsPT8, and OsPT11 [14], [26], [49]. Arsenite, on the other hand, enters rice roots via non-specific channels and aquaporins. Studies have shown that members of the NIP (NOD26-like intrinsic protein) subfamily of aquaporins, such as Lsi1 (also known as NIP2;1), play a crucial role in facilitating arsenite uptake in rice roots [11], [40].
After being taken up by the roots, As undergoes transport and translocation to various plant tissues, including the shoots and grains. Chowdhury et al. [50] reported that arsenic accumulation and translocation factors in rice are consistent whether it is grown in pots or in the field. Arsenic initially accumulates in and is primarily translocated into the roots, then progresses to the stem before eventually reaching the leaves and grains, [50]. Chowdhury et al. [50] further reported that a similar pattern of arsenic concentration was observed in Taiwanese rice plants that followed the order: root > straw > grain, irrespective of soil's arsenic concentration.
The specific transport mechanisms involved vary depending on the chemical forms of As. For instance, As is transported from the roots to the shoots through the xylem, the water-conducting tissues of plants. This translocation occurs via the same transporters responsible for arsenate uptake in the roots. Several researches have demonstrated that OsLsi2 (also known as Lsi2 or NIP3;1) - a member of the NIP subfamily of aquaporins – are involved in the translocation of arsenate from the roots to the shoots in rice plants [11], [26], [45]. On the other hand, arsenite can either move upwards or downwards or both within rice plants or get translocated either through the xylem or phloem or both. The translocation of arsenite from the root to the xylem involves various transporters, including OsABCC1 and OsABCC2 [26], [29], [51]. Additionally, arsenite can be sequestered and stored in the vacuoles of different rice plant tissues in roots, which limits its translocation to the shoots [42], [52]. Top of Form For instance, Williams et al. [42] reported a higher transfer factor (TF) of 0.05–3.8 (mean: 0.76) from soil to shoots of rice and a lower TF from soil to grain of 0.01–0.12 (mean: 0.04) demonstrating that larger proportion of As is sequestered and stored in the vacuoles located in the roots.
2.4.2. Nutrient presence and availability
The availability of nutrients in rice paddies significantly impact on the uptake of As by rice plants. For instance, available phosphorus (P) in paddy soils can compete with As uptake and translocation considering that P that is a chemical analogue of As and both are up-taken through the same transporters [35]. This competition ultimately limit As uptake and reduce translocation of As in rice grains. Furthermore, Meharg & Rahman [22] and Mlangeni et al. [16] reported that iron, an essential nutrient for rice plants, can reduce the uptake of As by forming complexes with the As and thereby reducing As bioavailability. Wang et al., [23], [53] also reported that nitrogen soil amendment can increase As uptake of As in rice plants because excessive soil amendment with nitrogen fertilizers increases the soil pH, making As more accessible for uptake to rice plants [54]. Similarly, application of phosphorus fertilizers enhances As availability in the soil and increases As uptake by rice plants, leading to higher levels of As accumulation in the rice grain [54]. The authors suggested that this effect is due to the competition between As and phosphorus for uptake by the plant roots, as well as the increase in the number of As uptake pathways in response to phosphorus fertilization.
The presence of silicon, another vital nutrient for rice plants, can reduce the uptake and transport of As by rice plants [26], [55], [56]. This is achieved by influencing the chemical forms of As in the soil, resulting in less uptake and accumulation of toxic forms of As in rice plants [26], [49], [57]. Ma et al. [49] reported that silicon enhances the structural integrity of rice plant cell walls, preventing the entry of As into the plant tissues. A study conducted in Bangladesh demonstrated that the application of Si to As-contaminated soil reduced As uptake in rice plants by up to 56% [56], [58], [59]. Similarly, a study in China found that the addition of Si to the soil decreased As accumulation in the roots and shoots of rice plants by up to 42% and 36%, respectively [60]. These findings suggest that soil amendment with Si can be an effective strategy for mitigating As contamination in rice crops.
2.4.3. Soil pH
Soil pH also plays a crucial role in either mobilizing or immobilizing As in porewater. Rahman et al. [35] found that As mobility increased in acidic soils and decreased in alkaline soils due to changes in As speciation and solubility. Similarly, Williams et al. [42] reported that As mobility and bioavailability in soil decreased as soil pH increased. This is because the speciation of As is highly dependent on pH, with As being predominantly present in its more mobile and bioavailable forms at low pH, while at high pH, As is more likely to be adsorbed onto soil particles and immobilised [35], [42].
2.4.4. Environmental stressors
Environmental stressors, such as drought and flooding, can either increase or decrease the uptake and transport of As depending on the specific stressor [42], [60], [61]. Drought stress has the potential to enhance As uptake and accumulation in rice plants by upregulating and promoting the expression of genes involved in As uptake and transport [62]. Conversely, flooding can reduce As uptake and transport in rice plants due to the anaerobic conditions that alter the soil's redox potential, leading to the formation of less mobile As species [27], [35], [40], [41], [42], [63], [64], [65], [66]. To effectively mitigate As contamination in rice crops, it is crucial for agricultural scientists to comprehensively understand the aforementioned factors. This understanding will aid in the development of effective mitigatory strategies aimed at reducing As contamination in rice crops.
2.5. Arsenic speciation and methylation in rice
In rice grains, methylated arsenic species account for 10–90% of the total arsenic, mainly present as DMA, partly as MMA and TMAO [67], [68] which vary with regions, rice genotype and irrigation type. The process of As-methylation is a biotransformation and detoxification process that converts iAs into various methylated forms through enzymatic reactions in living organisms [69], [70]. This methylation process is crucial as it converts highly toxic iAs species into less or non-toxic species as well as more easily excreted forms such as MMA and DMA [69], [70], [71]. As-methylation in rice plants is an important area of research due to the potential health risks associated with consuming rice that has accumulated methylated forms of arsenic. The As-methylation in rice is influenced by various factors, including soil conditions, water source and management, cultivation practices, rice genotypes, microbial interactions, and physicochemical parameters such as soil pH, redox potential, and the presence of iron, sulphur, and phosphate in rice paddies and soil-porewater [69], [70], [71]. Understanding the factors that influence As-methylation in rice plants can help develop strategies to mitigate arsenic contamination and reduce human exposure [69], [71]. Therefore, in this section, we will discuss factors that influence As-methylation in rice plants, including interactions among these factors and their subsequent impact on As dynamics to develop effective strategies for mitigating As contamination in rice.
2.6. Impact of water source and type on As-methylation in rice
2.6.1. Impact of groundwater irrigation on methylation
in certain regions of the world, groundwater (Fig. 1) used for irrigation contains elevated levels of naturally occurring As [31], [67],. This As can be taken up by rice plants through their roots, leading to accumulation and methylation in rice grains [72], [73]. For example, Chowdhury et al. [74], [75] observed that rice cultivated in Bangladesh, where the irrigation water had a high levels of As exhibited significantly higher total As content in the grain compared to rice cultivated non-As contaminated areas. Similarly, several studies have reported that rice samples from As-affected areas of West Bengal, India, had higher total As concentrations but lower proportions of methylated As species compared to rice samples from non-affected areas [76], [77].
Fig. 1.
Factors affecting arsenic (As) methylation indicating main factors and parameters affected.
The methylation of As in rice is influenced by microbial activity (Fig. 1) in groundwater [69], [70], [71], [75], [78], [79]. The presence of microbial communities in groundwater leads to methylation of As, which can then be sequestered by rice plants [69], [70], [71], [78], [80]. The uptake of As by rice plants is impacted by the concentration and speciation of As in the soil and porewater. Implementing appropriate measures such as groundwater monitoring and treatment, improved irrigation techniques, and the utilization of As-resistant rice varieties can help mitigate the risk of As accumulation in rice crops. For instance, studies have shown that high levels of sulphate in groundwater promote As-methylation while high levels of bicarbonate inhibit this process [81]. Additionally, redox conditions in the soil and water can also influence the production of methylated As species [65], [67].
2.6.2. Reclaimed water irrigation
Reclaimed water refers to wastewater that has undergone treatment to remove impurities and is reused for non-potable purposes, such as irrigation. However, numerous studies have indicated that use of reclaimed water for irrigation can increase the risk of As accumulation in rice crops [82], [83]. For instance, a study investigating the impact of reclaimed water irrigation on As accumulation in rice and the effectiveness of water management and organic amendments in reducing As accumulation discovered that using reclaimed water resulted in higher As concentrations in rice grains compared to the use of fresh water for irrigation [84], [85]. Nevertheless, the study also found that a combination of reclaimed water usage and organic amendments reduced As accumulation in rice grains by 18.2–52.7% [84], [85]. The authors recommended that the use of reclaimed water for irrigation should be coupled with appropriate water management practices and organic amendments to minimize As contamination in rice. For instance, research conducted in California's San Joaquin Valley revealed that rice crops irrigated with reclaimed water had higher concentrations of As in their grains compared to rice crops irrigated with fresh water [84], [86]. Similarly, a study conducted in China demonstrated that rice crops irrigated with reclaimed water exhibited higher total As content in their grains compared to crops irrigated with fresh water [33]. While some studies have suggested that the presence of organic matter in reclaimed water can promote the methylation of As in rice, potentially reducing its toxicity, the potential risks associated with the use of reclaimed water for irrigation cannot be overlooked [14], [87]. It is crucial to ensure proper treatment and testing of wastewater before using it for agricultural irrigation, and appropriate measures must be taken to ensure the safety of the food supply.
2.7. Impact of water management regimes on methylation of As in rice
2.7.1. Continuous flooding irrigation (CF)
Paddy rice is traditionally cultivated under continuous flooding irrigation (CF). Under CF, the rice fields are continuously flooded throughout the growing season [88], [89]. Several studies have reported that CF irrigation regime enhances bioaccumulation and decrease methylation of As in rice [90], [91]. CF decrease methylation of As in soil porewater by creating anaerobic conditions that in turn limit the activities of microorganisms responsible for methylation reactions [90], [92], [93], [94]. Methylation of As is an energy-demanding process that requires reducing power, which is supplied by organic matter in the soil [9], [51], [69], [95], [96]. In a flooded soil, the organic matter is rapidly depleted, and the redox potential becomes very low, which limits the activity of As-methylating microorganisms [8], [97], [98]. In addition, the flooded soil can lead to the accumulation of toxic metabolites such as sulphides, which can inhibit the activity of microorganisms [78], [96], [99], [100].
2.8. Alternate wetting and drying (AWD)
Alternate wetting and drying (AWD), which is also referred to as wetting and drying irrigation (WDI) - with slight differences - by other scholars, involves periodically draining the fields to allow the soil to dry out before reflooding [16], [88], [89], [102]. Several studies have reported impacts of AWD on methylation of As in rice plants [16], [26], [34], [68], [88], [89], [99]. For instance, a study conducted in Bangladesh reported that rice cultivated under AWD irrigation had lower iAs and higher oAs concentrations compared to that cultivated under CF [34]. In Mlangeni et al. also reported lower iAs and higher oAs concentrations in rice cultivated under AWD compared to that cultivated under CF in greenhouse experiments in Aberdeen, Scotland [16]. The study reported a 27% lower tAs concentration in rice under AWD (1686 mg kg−1) compared to that under CF (2309 mg kg-1) [16] (Table 1; Table 2). Another study conducted in China investigated the impact of AWD on the accumulation and methylation of As in rice plants [9], [97], [103]. The researchers found that AWD significantly reduced the tAs content in the rice grain compared to continuous flooding [9], [97], [103]. They also found that AWD increased the proportion of dimethylarsinic acid (DMA), which is a less toxic form of As, in the rice grain, suggesting that AWD can enhance the methylation of As in rice plants. A third study conducted in Vietnam compared the impact of CF and AWD on the accumulation and methylation of As in rice plants [97], [103]. The researchers found that AWD significantly reduced the total As content in the rice grain compared to CF. They also found that AWD increased the proportion of DMA in the rice grain, suggesting that AWD can enhance the methylation of As in rice plants [9], [103]. Other studies have also shown that rice grown under AWD has higher levels of DMA and lower levels of MMA compared to rice grown under CF, suggesting that AWD promotes the methylation of iAs to DMA and inhibits the methylation of MMA. The mechanism is that aerobic conditions during the dry period of AWD promote the activity of As-methylating microorganisms in the soil, which preferentially methylate iAs to DMA over MMA. Weber et al. [13] reported lower MMA (0.003 ± 0.004 mg kg−1) (Table 1) in rice bran cultivated under AWD compared to that cultivated under CF (0.01 ± 0.006 mg kg−1). However contrary results have also been reported. For instance, Weber et al. [13] also reported a much lower mean DMA (0.031 ± 0.010 mg kg−1) in rice bran under AWD compared to that under CF (0.149 ± 0.0622 mg kg−1) (Table 1). In contrast, under CF, the anaerobic conditions in the soil promote the methylation of iAs to MMA.
Table 1.
Methylated As (mg kg-ˡ) in rice grains reported by water management regime.
| Analytes | CF | AWD | ARC or LW | References |
|---|---|---|---|---|
| Total arsenic (iAs) | 0.403 | 0.310 | 0.250 | [16] |
| 0.678 | 0.480 | 0.450 | [101] | |
| 1.120 | 0.633 | [13] | ||
| Inorganiic arsenic (iAs) | 0.803 | 0.502 | [13] | |
| 0.185 | 0.150 | 135 | [16] | |
| Arsenites (AsIII) | 0.189 | 0.49 | 0.145 | [101] |
| Arsenenates (AsV) | 0.420 | 0.250 | 0.230 | [101] |
| DMA | 0.149 | 0.010 | [13] | |
| 0.145 | 0.085 | 0.035 | [16] | |
| MMA | 0.01 | 0.003 | [13] |
Note: CF, continuous flooding (also called flooding irrigation (FI)) water management regime; AWD, alternate wetting and drying (also called intermittent wetting and drying) water management regime, ARC, Aerobic rice cultivation (also called low water irrigation).
Table 2.
Impact and mechanism of water management regime on As-methylation indicating effect of the regime and mechanism of iAs conversion to methylated As and references (Ref.).
| Water regime | Effect | Mechanism | Ref. |
|---|---|---|---|
| CF | CF creates anaerobic (anoxic) conditions that cause arsenic-reducing bacteria to thrive | Thriving As-reducing bacteria use AsV as electron acceptor during their metabolic processes which convert AsV to AsIII as follows: AsV + 3e- + 3 H+ → AsIII + H2O Methylation of AsIII to MMA as follows: AsIII + SAM) → MMA + SAH Methylation of MMA to DMA as follows: MMA + SAM → DMA + SAH |
[16] |
| AWD | AWD creates alternate oxic and anoxic conditions | Alternate oxic and anoxic conditions fosters growth of different microbial populations Under oxic conditions, certain bacteria oxidize AsIII to AsV. Under anoxic conditions, other bacteria reduce AsV back to AsIII. Different microbial populations methylate AsV to MMA and DMA. |
[13] |
| ARC | ARC increases oxygen level in root zones | Elevated oxygen levels fosters growth of different microbial populations Different microbial populations transform iAs into oAs species |
[101] |
| SDI | SDI induces variable oxygen availability | Variable oxygen availability influence the activity of different microbial communities. Microorganisms variably impact As transformations. |
[101] |
Note: CF, continuous flooding irrigation; AWD, alternate wetting and drying; ARC, Aerobic rice cultivation; SDI, Subsurface drip irrigation.
The finding showed that the methylation of iAs to oAs was either stimulated by AWD or inhibited by CF and that the total As content in rice grain was higher in plants grown under CF compared to AWD which indicated that AWD inhibited uptake of As. Thus, AWD effectively reduced As accumulation and enhanced methylation of As in rice plants compared to continuous flooding.
2.8.1. Aerobic rice cultivation (ARC)
Aerobic rice cultivation (ARC) involves growing rice in non-flooded soil under aerobic conditions. The impact of ARC on the methylation of As in rice plants has been studied by several researchers. For instance, Chou et al. [101] reported lower tAs and AsIII loading in rice cultivated under ARC compared to that under CF (Table 2). The authors associated the AsIII reduction in ARC to higher redox potential created by aerobic conditions created by non-flooded soil stimulate the growth of microorganisms that promote As-methylation reactions [51], [99], [101] which promotes the methylation of As in soil and water systems [101]. During aerobic conditions, organic matter are oxidized by aerobic microorganism that release carbon dioxide and nitrogen and phosphorus nutrients used by other microorganisms to carry out methylation reactions [41], [89], [101], [104]. A study conducted in China reported that rice grown under ARC had lower levels of iAs and higher levels of oAs compared to rice grown under CF cultivation [105] which also implied that aerobic conditions promotes the methylation of iAs to oAs [105], [106]. Another study conducted in China found that ARC significantly reduced and increased he total As content and percentage of dimethylarsinic acid (DMA), less toxic form of As, in the rice grain compared to CF suggesting that ARC enhances the methylation of As in rice plants. Further studies in conducted in Bangladesh also found that the total As content and proportion of methylated As in the rice grain was higher (>10 folds) and lower (>7 folds), respectively, in plants cultivated under CF compared to ARC [105], [106]. The finding indicated that the total As bioaccumulation and the methylation of As are stimulated and inhibited, respectively by CF conditions [11] confirming that aerobic treatment can effectively reduce the grain bioaccumulation highly toxic As species. These studies have shown that rice grown under ARC has higher levels of DMA and lower levels of MMA compared to rice grown under CF, suggesting that aerobic conditions promote the methylation of iAs to DMA and inhibit the methylation of MMA [41], [89], [106]. The mechanism behind this is that the aerobic conditions in ARC promote the growth of As-methylating microorganisms in the soil, which preferentially methylate iAs to DMA over MMA [41], [89], [106]. In contrast, under CF, the anaerobic conditions in the soil promote the methylation of iAs to MMA [106].
2.8.2. Subsurface drip irrigation (SDI)
A study conducted in California, USA found that rice grown under Subsurface drip irrigation (SDI) had lower levels of iAs and higher levels of oAs compared to rice grown under AWD. This suggests that SDI may promote the methylation of iAs to oAs [34], [84], [90], [93]. SDI is a water management practice that involves the use of drip irrigation lines placed underground, which can improve water use efficiency and reduce water losses due to evaporation and runoff [34], [90]. Studies have shown that SDI can affect the methylation of As in rice plants through various mechanisms [84], [107] (Table 2). Firstly, SDI reduces the amount of water used for irrigation, which can decrease the amount of As available for uptake by the rice plant (Table 2). Besides, reducing available As in the soil porewater SDI improve soil aeration of which both promote the activity of microorganisms that convert iAs to organic methylated As species [108], [109]. Many studies have reported that SDI can reduce the total As (tAs) content in the rice grain compared to continuous flooding. For example, a study conducted in China found that SDI reduced the tAs content in the rice grain by 39% compared to continuous flooding (Table 2). Another study conducted in California, USA, showed that SDI reduced the total As content in the rice grain by 24% compared to flood irrigation. Moreover, these studies have also found that SDI increased the proportion of less toxic methylated As species, particularly dimethylarsinic acid (DMA), in the rice grain (Table 2). A study conducted in California found that SDI increased the proportion of DMA in the rice grain by 68% compared to flood irrigation. Thus, SDI can significantly reduce the total As content in the rice grain and increase the proportion of less toxic methylated As species. The reduction in water used for irrigation and the resulting changes in soil and microbial conditions promote the methylation of As in rice plants.
2.9. Mechanisms of As-methylation
It is reported that the mechanisms of As-methylation in rice are complex that involves several steps and enzymes [99], [107]. The methylation of As primarily occurs in roots of plants [8], [97], [99] and is influenced by a complex interplay of biotic factors involving microbial activity, plant-microbe interactions, and root exudates, [62], [110] as well as abiotic factors including soil pH, redox potential, soil organic matter, and the presence of elements like iron and sulphur [19], [110]. Therefore, understanding these factors is key to managing arsenic contamination in rice cultivation.
The microbes such as bacteria and fungi present in the rhizosphere (the soil zone directly influenced by root secretions) play a significant role in As-methylation [62], [110], [111], [112]. Certain bacteria and fungi can transform iAs such as AsIII into methylated arsenic compounds through metabolic processes. However, dominant methylating microbes of As may vary across distinct soil types or respond to shifts in soil conditions, such as alterations in redox potential [111]. Additionally, it is plausible that diverse rice genotypes could exert varying effects on the rhizosphere, potentially impacting the As-methylating microbes therein that facilitate conversion of iAs into less toxic methylated forms, and subsequently, influencing the As-methylation and accumulation of DMA in rice [111], [112].
Soil pH significantly influences the speciation and methylation of As. In specific pH ranges, like slightly acidic to neutral soils, there's a preference for converting iAs into more harmful forms [112] which can increase the risk of plant uptake of these harmful forms. Conversely, in alkaline soils, As tends to remain in less toxic states. Additionally, since aquaporin channels permit only uncharged molecules, pH can impact the uptake of monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) by altering the balance between protonation and dissociation, thereby affecting substrate availability for membrane transporters. Li et al. [38] demonstrated that raising pH from 4.5 to 6.5 significantly reduced rice's uptake of MMA, and a similar increase from 5.5 to 6.5 substantially decreased DMA uptake as well. These findings suggest that managing soil pH in agricultural settings could be an important strategy for mitigating arsenic uptake by crops. Adjusting pH to slightly acidic to neutral conditions might reduce the uptake of more toxic forms of arsenic, while maintaining alkaline conditions could help minimize the risk of arsenic accumulation in crops.
The redox potential of the soil, which indicates the soil's oxidation-reduction status, affects As speciation [51], [99]. Reduction conditions (low redox potential) can promote the transformation of AsV into the more toxic AsIII (This transformation is concerning because AsIII is generally considered more harmful to living organisms, including plants and humans, than AsV), while oxidation conditions (high redox potential) favour the stability of AsV [99], [101]. This means that in oxidized soils, AsV is less likely to be converted into the more toxic AsIII.
The presence of organic matter in soil can impact As-methylation by binding with As, thereby affecting its availability to microbes as well as rice plants, potentially influencing the As-methylation process [34], [23], [113], [114] considering that organic matter has a greater affinity for arsenic sorption due to the formation of an organo-arsenic complex [114]. Norton et al. [113] highlighted organic matter's role in mobilizing As from paddy fields, as organic matter-consuming microbes reduce oxygen levels, thereby lowering redox potential and facilitating As dissolution from iron oxyhydroxides. Similarly, Syu et al. [115] stressed the importance of considering soil organic matter properties before using organic amendments in As-contaminated soils as they may produce varying effects on As accumulation in rice [115].
The availability of iron (Fe) and sulphur (S) in the paddy soil can impact As-methylation [16], [58], [91], [116], [117], [118]. Iron is known to interact with As and plays an important role in decreasing the absorption of As in rice [114] as well as its speciation [16], [58], [116]. Sulfur also plays a crucial role in reducing accumulation and translocation of As in rice plants by participating in biotic processes that lead to As-methylation [91], [116], [117], [119]. It is reported that application of sulphur induces the formation of Fe plaques on the surfaces of rice roots which reduces the As concentration in soil [119], [120]. It is also reported that sulphur compounds such as sulphates (SO4) may enhance the desorption of AsV from Fe- plaques [119] which increases AsV availability in the soil solution which also can potentially lead to increased arsenic uptake by rice plants through their roots as well as higher substrate pool for methylation processes [119], [120]. This could potentially lead to increased methylation of arsenic within the rice plants, resulting in higher concentrations of organic arsenic species (MMA and DMA) in the plant tissues.
Furthermore, it is also reported that SO4 can inhibit AsV transport into cells in the same way phosphate competes with AsV for transport and metabolism [45], [119], [120], [121] which may also reduce the availability of AsV within the plant. This could lead to a lower concentration of AsV available for conversion into more toxic forms, such as arsenite (AsIII), within the plant tissues. Consequently, the speciation of arsenic within the plant may be shifted towards less toxic forms. In addition, the inhibition of AsV transport into plant cells by SO4 may reduce the availability of AsV for methylation processes within the plant. With inhibited AsV transport, there may be a lower substrate pool for methylation reactions. This could result in reduced production of organic arsenic species (MMA and DMA) within the plant.
The general mechanism of As methylation in rice can be summarized in six steps namely: 1) uptake of As, 2) transport of inorganic As, 3) conversion of arsenite to arsenate, 4) methylation of As, 5) transport of methylation of As, and 6) sequestration of As [11], [30], [96], [122]. Understanding the mechanisms of As methylation is key to developing strategies to reduce As uptake in rice plants, enhance accumulation of methylated As in rice grains compared to highly toxic inorganic As and mitigate the health risks associated with consumption of rice contaminated with As. In this section, we will explore mechanisms of As methylation in rice plants and provide examples and illustrations where applicable.
2.10. Uptake and transport of As by rice plants
2.10.1. Arsenic uptake
The extent of arsenic uptake by the rice plant system is contingent upon the predominant form of arsenic existing within the surrounding environment [123]. However, this assimilation undergoes modulation in response to variations in the redox potential capacity of the said environment [123]. AsV and AsIII can undergo interconversion contingent upon alterations in redox states, consequently instigating the generation of reactive oxygen species (ROS) [27], [123], [124]. Considering that AsIII predominates in waterlogged anaerobic conditions, facilitating its dissolution into the solution and subsequent interaction with proximate soil-water constituents which leads to rice plants uptake As in water logged anoxic conditions mainly as AsIII through the aquaporins in the roots, while AsV, MMA and DMA are less available for uptake compared to AsIII forms [11], [26], [40]. Aquaporins, water channels that allow water and solutes to cross cell membranes, are present in the roots and facilitate the uptake of AsIII [11], [26], [46], [114]. The As uptake process in rice plants is influenced by factors such as presence of iron plaque on the roots [26], [114], [123], soil pH and redox conditions in the soils [125], [126]. Iron plaque, that develop around paddy roots under flooded (waterlogged) conditions exhibit a strong affinity for capturing As(V) within the soil, thereby impeding the upward translocation of arsenic beyond the root zone [16], [50], [110], [123], [127], [128]. These iron complexes effectively adsorb As species from the surrounding rhizosphere, creating an As-rich layer on the root surface, thus reducing the chance of As transportation into the root cell system [72], [129]. The formation of iron plaque is significantly enhanced by a higher degree of Root Oxygen Loss (ROL) and root porosity. Studies suggest that rice cultivars with a greater capacity for oxygen supply to the root zone through ROL mechanisms also exhibit stronger root iron plaque formation, leading to the adsorption of more As species [30], [59], [108]. Higher ROL also promotes the oxidation of AsIII to the more adsorbable AsV form.
The factor of soil pH affects the solubility and availability of As in porewater for uptake, with a pH of around 5.5–6.5 being optimal for uptake [12], [130], [131]. For instance, the strong affinity of metal oxides/hydroxides for AsV binding on the surface soil of flooded agricultural land leads to the accumulation of AsV in soils under reduced condition leading to AsV being not readily available for uptake than AsIII under flooded anaerobic conditions, and vice-versa under aerobic conditions [16], [65], [89], [132].
2.10.2. Transport of As
Once AsIII and AsV species are absorbed by the roots, they are transported to the shoots of rice plants through the xylem [11], [46], [112] via the silicic acid and phosphate transporters, respectively [123].
The rate of AsIII translocation in the xylem through a silicon-mediated pathway surpasses that of AsV translocation facilitated by a phosphate-mediated mechanism [123]. This disparity can be attributed to the considerably superior ability of silicon to traverse the xylem sap in contrast to phosphate [123]. Consequently, the transportation efficacy of AsIII species through the xylem system is notably superior to that of AsV [123]. Nevertheless, this preference is subject to the influence of soil acidity, wherein arsenite predominates under acidic conditions [133]. The prevailing redox conditions of the soil also exert an influence on the transport of arsenic. Particularly, arsenite is more readily taken up under anoxic conditions [112], [134].
It has been documented that the loading of arsenic into the rice plant system is contingent upon the prevalent form of arsenic (AsIII or AsV) abundantly present within the surrounding environment. Moreover, these species exhibit interconvertibility contingent upon fluctuations in redox change [27], [123]. Within root cells, arsenic undergoes either conversion into less toxic organic forms or is conveyed to vacuoles in the guise of AsIII or complexed forms such as AsIII-glutathione/phytochelatin complexes [69], [135], [136]. This mechanism is notably efficient within the roots of hypertolerant/non-hyperaccumulator plants, thereby effectively impeding the translocation of As to the aerial parts of the plants. On the other hand, AsV reduction to AsIII has been observed to transpire efficiently in hyperaccumulator plants [137], [138], [139].
Previous investigations have consistently identified AsIII as the predominant species of arsenic transported via the xylem sap from roots to shoots, irrespective of whether the plant is supplied with AsV or AsIII plants [123], [124]. The remarkably efficient translocation of As in As hyperaccumulators could potentially be attributed to several factors, including the proficient reduction of AsV to AsIII within the roots, heightened efflux of AsIII from cortical cells to the xylem, limited complexation of AsIII with thiol compounds and subsequent sequestration within root vacuoles, alongside minimal efflux of AsIII from roots to the external milieu [110], [124], [135].
2.11. Mechanisms of methylation of iAs
2.11.1. Conversion of arsenite to arsenate
Arsenite (AsIII) is more toxic to plants and animals than arsenate (AsV) [74], [114], [140]. The first step of As-methylation process involves the conversion of AsV to AsIII, which is helped by the presence of arsenate reductase (AR) enzymes situated in the cytoplasm of root cells [20], [51], [141]. Thus, the more toxic As species, AsIII is converted to less toxic species, AsV. This process which is crucial for the subsequent methylation reactions [18], [142]. The conversion of AsIII to AsV is mediated by AsIII-oxidase (AO) and glutathione S-transferase (GST) enzymes [52], [143], [144]. AsIII is mechanistically converted to AsV through oxidation with molecular oxygen (O2) or reactive oxygen species (ROS) and through enzymatic oxidation by AsIII-oxidase (AO) [18]. It is reported in plants such as rice, the enzymatic pathway could be the main mechanism of AsIII oxidation[18], [132].
The study conducted by Majumdar et al. [123] and Mitra et al. [26] sheds light on the conversion of AsIII to AsV and the mechanisms underlying this transformation. The researchers reported that AsIII can be converted to AsV through various pathways, including oxidation with molecular oxygen (O2), reactive oxygen species (ROS), and enzymatic oxidation by AsIII-oxidase (AO) [26], [123]. This conversion process is particularly relevant in the context of environmental and health concerns related to arsenic contamination in rice plants.
One of the mechanisms identified by Majumdar et al. [123], [139]. and Mitra et al. [26] is the oxidation of AsIII through interactions with molecular oxygen (O2) or reactive oxygen species (ROS) [123], [139]. This chemical transformation involves the transfer of electrons from AsIII to oxygen molecules or ROS, leading to the conversion of AsIII to AsV [26], [123], [145], [146]. This pathway often occurs in oxidative environments and contributes to the detoxification of AsIII.
Another significant mechanism is the enzymatic oxidation of AsIII by AsIII-oxidase (AO) [23], [114], [135] This enzymatic pathway is particularly relevant in plants, such as rice. AsIII-oxidase is an enzyme that catalyzes the oxidation of AsIII to AsV by utilizing molecular oxygen [26], [123], [145], [146]. This enzyme plays a crucial role in detoxifying AsIII within plants and microbial systems [123], [146] suggesting that enzymatic pathway through AsIII-oxidase could be the primary mechanism responsible for the conversion of AsIII to AsV, especially in rice plants.
AsIII-oxidase, enzymes found in some bacteria, fungi, and algae resident in the roots of rice plants, catalyse the conversion of AsIII to AsV through the addition of oxygen (O2) or hydrogen peroxide (H2O2) [147]. The reaction mechanism of arsenite oxidation by AsIII-oxidase is believed to involve the transfer of electrons from AsIII to a cofactor such as a quinone which in turn reduces O2 to H2O2 [109], [132]. According to Rawlings [109], the produced H2O2 reacts with AsIII to produce AsV as following:
| AsIII + H2O2 → AsV + 2 H+ + H2O | (1) |
Some studies have also reported that peroxidases are involved in the oxidation of AsIII to AsV in rice plants by catalysing the reduction of H2O2to H2O which is followed by oxidation of AsIII to AsV as in Eq. 1 [18]. and Superoxide dismutase (SOD) is also reported to be involved in the oxidation of AsIII to AsV by catalysing the conversion of O2 to H2O2, which can then be used by AsIII-oxidase to oxidise AsIII [18].
2.11.2. Mechanism of As-methylation
Methylation of As primarily occurs in the roots of rice plants and serves as an important detoxification mechanism involving a series of enzymes and pathways. The first step involves the conversion of arsenate (AsV) to arsenite (AsIII), which is helped by the presence of arsenate reductase (AR) enzymes in the cytoplasm of root cells [141], [148]. Subsequently, the process involves the methylation of As, specifically the addition of a methyl group, resulting in the formation of monomethylarsonic acid (MMA). This methylation step is mediated by arsenite methyltransferase (AS3MT) enzymes and relies on S-adenosylmethionine (SAM) as the methyl donor, leading to the production of MMAIII and MMAV facilitated by the presence of MMA(V) reductase enzymes in the cytoplasm of root cells [51], [141]. Further progression is as follows:
2.11.2.1. Conversion of AsV to AsIII
Inorganic arsenate (AsV) present in the soil is taken up by rice plant roots. Within the plant, AsV is reduced to inorganic arsenite (AsIII), the more reactive and toxic form of arsenic. This reduction step is facilitated by arsenate reductase enzymes [30], [65].
2.11.2.2. Methylation of AsIII by ASMT
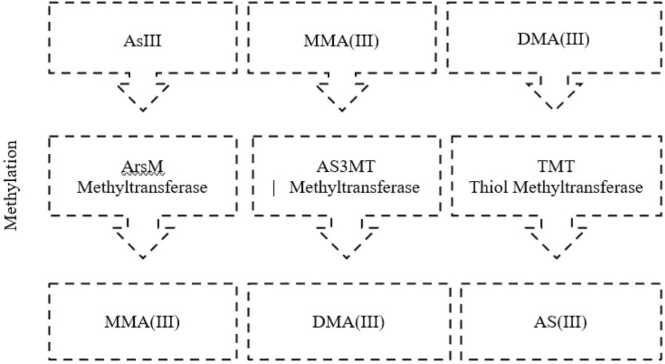
The methylated forms of arsenic are produced through the enzymatic activity of arsenate methyltransferase (ASMT) in rice plants [149] (Fig. 2). ASMT transfers a methyl group from folate to AsIII, leading to the formation of monomethylarsonic acid (MMA(V)) [149].
Fig. 2.
Mechanisms of methylation of As showing three main pathways of methylation of As.
2.11.2.3. Methylation of MMA(V) to DMA(V)
In some rice plants, MMA(V) can be further methylated to form dimethylarsinic acid (DMA(V)) [11] (Fig. 2). It is reported that the exact mechanism and enzymes involved in this step are not yet fully understood and can vary between different rice cultivars [11].
2.11.3. Pathways of As-methylation
Several studies have reported that As-methylation follows three distinct pathways: ArsM pathway [97], glutathione (GSH)-dependent pathway [30], [71], and the folate-dependent pathway which are discussed below.
2.11.3.1. ArsM pathway
The ArsM pathway serves as the primary route for As-methylation in microorganisms [8], [150]. This pathway involves the enzymatic activity of ArsM methyltransferase, which directly methylates AsIII (Fig. 2). Consequently, ArsM transfers a methyl group from S-adenosyl methionine (SAM) to AsIII, leading to the formation of monomethylarsonous acid (MMAIII) [8], [150]. MMAIII can then undergo further methylated to produce dimethylarsinous acid (DMAIII) [8], [150] (Fig. 2). Both MMAIII and DMAIII exhibit lower toxicity compared to iAs and can be efficiently excreted in humans. For instance, arsenic-methylating bacteria, such as Escherichia coli, possess ArsM genes that encode the ArsM methyltransferase enzyme. The ArsM enzyme catalyses the methylation of AsIII using SAM as the methyl donor (Fig. 2). This mechanism helps the bacteria to survive in arsenic-rich environments. For instance, arsenic-methylating bacteria, such as Escherichia coli, have been found to possess ArsM genes that encode the ArsM methyltransferase enzyme [8], [150]. This ArsM enzyme catalyses the methylation of AsIII using SAM as the methyl donor [8], [150]. This mechanism provides a significant advantage for these bacteria, allowing them to thrive and survive in arsenic-rich environments [8], [150].
2.11.3.2. Glutathione (GSH)-dependent Pathway
The GSH-dependent pathway is the primary As-methylation mechanism in mammals, including humans [26], [27], [51], [151]. It involves the sequential methylation of AsIII by the action of two enzymes: arsenite methyltransferase (AS3MT) and thiol methyltransferase (TMT) (Fig. 2) [26], [151]. AS3MT catalyses the transfer of a methyl group from SAM to AsIII, resulting in the formation of MMA(III). MMA(III) is then further methylated by TMT using GSH as the methyl donor, producing DMA(V) [51], [151]. DMA(V) can be reduced to DMA(III) inside the cells. In root cells, As is either converted to less toxic organic forms or is transported to vacuoles as AsIII or as AsIII-glutathione/phytochelatin complexes [30], [151].
2.11.3.3. Folate-dependent pathway
The folate-dependent pathway is an alternative pathway for As-methylation, mainly found in plants and fungi [151], [152]. It involves the methylation of AsV rather than AsIII. This pathway requires the presence of folate as a cofactor and involves the enzymatic activity of arsenate methyltransferase (ASMT). In rice For example, folate-dependent pathway is also used for As-methylation. ASMT enzymes in rice methylate AsV using folate as a methyl donor [151]. The methylated forms of arsenic, such as monomethylarsonic acid (MMA(V)) and dimethylarsinic acid (DMA(V)), can accumulate in rice grains, potentially posing a risk to human health.
2.12. Transport of methylated As
Once methylated, As can be transported from the roots to the shoots of plants through the xylem. This transportation process is facilitated by various transporters, including silicon transporters and aquaporins [11], [40], [46], [78], [122], [153], [154]. The transport of methylated As to the cell wall is facilitated by transporters, such as the NIP-type aquaporins [11], [26], [40], which are integral membrane proteins that regulate the transport of water and small solutes across the cell membrane [11], [40]. These transporters have been shown to help the uptake of DMA into the root cells of rice plants, which is then transported to the shoots and sequestered in the cell walls.
Silicon transporters, such as Lsi1, have been observed to aid in the uptake and transport of As in rice plants. Lsi1 is a nodulin 26-like intrinsic protein (NIP) aquaporin responsible for transporting silicon and other small molecules across the plasma membrane, such as urea and glycerol [11], [40]. Some studies have proposed that Lsi1 may also facilitate the transport of As, particularly DMA, in rice plants. Aquaporins, a group of membrane proteins, are also involved in the transportation of water and small solutes across cell membranes. However, the mechanisms involved in the transport of methylated As in plants are complex and not yet fully understood [155].
2.13. Sequestration of As
Methylated As, once in the shoots, can be sequestered in plant vacuoles and cell walls. This sequestration is facilitated by transporters such as MATE and ABC transporters [11], [26], [45], [49]. ABCC-type transporters, belonging to the MRP family, play a role in transporting methylated As from the cytoplasm to the vacuoles, reducing its toxicity [30], [135]. Another sequestration mechanism involves the binding of methylated As compounds to the cell wall components, like pectin and lignin, through electrostatic and covalent interactions [30], [135], [156]. This binding reduces the mobility and bioavailability of As, preventing its uptake and translocation within the plant. The sequestration of methylated As through these mechanisms, involving various transporters, is vital for minimizing toxicity and safeguarding human health by preventing its accumulation in edible plant parts. The above-mentioned mechanism of As methylation in rice is based on several studies such as those conducted by [18], [23], [61], [65], [90], [157], [158]. These studies have supplied important insights into the enzymes involved in As methylation and the transporters that help the movement of As within the plant.
3. Conclusions and recommendations
This review emphasizes that the choice of water source for irrigation has a significant influence on arsenic levels in rice. Groundwater used for irrigation in arsenic-affected regions contributes to higher total arsenic content in rice grains, while reclaimed water irrigation can also elevate arsenic accumulation in rice crops, necessitating proper monitoring and treatment. It is also noted that different water management practices have varying effects on As-methylation in rice. Continuous flooding (CF) irrigation leads to reduced methylation due to anaerobic conditions, whereas techniques like alternate wetting and drying (AWD), aerobic rice cultivation (ARC) and subsurface drip irrigation (SDI) promote As-methylation by creating aerobic conditions favourable for methylation-related microorganisms. SDI stands out as a promising method to mitigate arsenic-related risks as it reduces the total arsenic content in rice grains and increases the proportion of less toxic methylated arsenic species, thereby potentially enhancing the safety of rice consumption. Furthermore, As-methylation involves complex biotic and abiotic processes in rice roots, requiring multiple enzymes. Understanding these mechanisms is crucial for developing interventions to reduce arsenic contamination in rice and protect human health. Multiple methylation pathways in rice plants allow detoxification of inorganic arsenic to less toxic forms.
It is worth highlighting that several elements, including iron, silicon, and phosphate ions, perform pivotal functions in the transportation and methylation processes of As within rice plants. Consequently, comprehending the functions of these elements in facilitating As transport and methylation in rice plants offers valuable insights into potential approaches for alleviating the adverse effects of arsenic contamination in rice grains. This underscores the importance of implementing sound soil and nutrient management practices, which may encompass the deliberate use of tailored soil amendments (fertilizers). These amendments are designed to curtail As uptake by rice plants and, in turn, enhance the safety of rice production. To achieve this goal, further research is warranted to identify and evaluate specific formulations of these elements for their effectiveness in practical applications.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Angstone Thembachako Mlangeni reports financial support was provided by Commonwealth Scholarship Commission.
Acknowledgements
This research received no external funding.
Handling Editor: Prof. L.H. Lash
Data Availability
No data was used for the research described in the article.
References
- 1.Rafiq M.T., Aziz R., Yang X., Xiao W., Rafiq M.K., Ali B., Li T. Cadmium phytoavailability to rice (Oryza sativa L.) grown in representative chinese soils. a model to improve soil environmental quality guidelines for food safety. Ecotoxicol. Environ. Saf. 2014;103:101–107. doi: 10.1016/j.ecoenv.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Rahman M.A., Hasegawa H. High levels of inorganic arsenic in rice in areas where arsenic-contaminated water is used for irrigation and cooking. Sci. Total Environ. 2011;409:4645–4655. doi: 10.1016/j.scitotenv.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 3.International agency for research on cancer (IARC) IARC monographs: cadmium and cadmium compounds in plastics. Eur. Chem. Agency. 2012;100C:13. doi: 10.1002/14356007.a04. [DOI] [Google Scholar]
- 4.International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 84 : Some Drinking-Water Disinfectants and Contaminants Including Arsenic.; International Agency for Cancer Research (IARC): Lyon, 2004; Vol. 84;. [PMC free article] [PubMed]
- 5.Hojsak I., Braegger C., Bronsky J., Campoy C., Colomb V., Decsi T., Domellöf M., Fewtrell M., Mis N.F., Mihatsch W., et al. Arsenic in rice: a cause for concern. J. Pediatr. Gastroenterol. Nutr. 2015;60:142–145. doi: 10.1097/MPG.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 6.Rintala E., Ekholm P., Koivisto P., Peltonen K., Venäläinen E. The intake of inorganic arsenic from long grain rice and rice-based baby food in finland – low safety margin warrants follow up. Food Chem. 2014;150:199–205. doi: 10.1016/j.foodchem.2013.10.155. [DOI] [PubMed] [Google Scholar]
- 7.Nriagu, J.O.; Bhattacharya, P.; Mukherjee, A.B.; Bundschuh, J. Chapter 1 Arsenic in Soil and Groundwater: An Overview. In Trace Metals and other Contaminants in the Environment; Bhattacharya, P., Mukherjee, A.B., Bundschuh, J., Zevenhoven, R., Loeppert, R.H., Eds.; 2007; Vol. 9, pp. 3–60.
- 8.Reid, M.C.; Maillard, J.; Bagnoud, A.; Falquet, L.; Vo, P.Le; Bernier-latmani, R. Arsenic Methylation Dynamics in a Rice Paddy Soil Anaerobic Enrichment Culture. 2017, 10.1021/acs.est.7b02970. [DOI] [PubMed]
- 9.Zavala Y.J., Gerads R., Gürleyük H., Duxbury J.M. Arsenic in rice: II. Arsenic speciation in USA grain and implications for human health. Environ. Sci. Technol. 2008;42:3861–3866. doi: 10.1021/es702748q. [DOI] [PubMed] [Google Scholar]
- 10.Arao T. a, Kawasaki A., Baba K., Matsumoto S., Maejima Y. Arsenic contamination in soils and crops in japan and various countermeasures. Pedologist. 2011:202–213. [Google Scholar]
- 11.Li R.-Y., Ago Y., Mitani N., Feldmann J., P.Mcgrath S., Ma J.F., Zhao F.-J. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009;150:2071–2080. doi: 10.1104/pp.109.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q., Yang Z., Zhang Q., Liu X., Zhuo X., Wu T., Wang L., Wei X., Ji J. Ecological risk assessment of cd and other heavy metals in soil-rice system in the karst areas with high geochemical background of Guangxi, China. Sci. China Earth Sci. 2021;64:1126–1139. doi: 10.1007/s11430-020-9763-0. [DOI] [Google Scholar]
- 13.Weber A.M., Baxter B.A., McClung A., Lamb M.M., Becker-Dreps S., Vilchez S., Koita O., Wieringa F., Ryan E.P. Arsenic speciation in rice bran: agronomic practices, postharvest fermentation, and human health risk assessment across the lifespan. Environ. Pollut. 2021;290 doi: 10.1016/j.envpol.2021.117962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abedi T., Mojiri A. Arsenic uptake and accumulation mechanisms in rice species. Plants. 2020;9:1–17. doi: 10.3390/plants9020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abedin M.J., Meharg A.A. Uptake kinetics of arsenic species in rice plants. Plant Physiol. 2002;128:1120–1128. doi: 10.1104/pp.010733.0.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mlangeni A.T., Perez M., Raab A., Krupp E.M., Norton G.J., Feldmann J. Simultaneous stimulation of arsenic methylation and inhibition of cadmium bioaccumulation in rice grain using zero valent iron and alternate wetting and drying water management. Sci. Total Environ. 2020;711 doi: 10.1016/j.scitotenv.2019.134696. [DOI] [PubMed] [Google Scholar]
- 17.Fageria N.K. Yield physiology of rice. J. Plant Nutr. 2017;30:843–879. doi: 10.1080/15226510701374831. [DOI] [Google Scholar]
- 18.Meharg A.A., Zhao F.J. In: Arsenic & Rice. Meharg A.A., Zhao F.J., editors. Springer Science+Business Media B.V; 2012. Chapter 2: arsenic in rice grain; pp. 11–30. ISBN 9789400729476. [Google Scholar]
- 19.Meharg A.A., Hartley-Whitaker J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant speciest species. New Phytol. 2002;154:29–43. [Google Scholar]
- 20.Meharg A.A., Zhao F.-J. In: first ed. Meharg A.A., Zhao F.-J., editors. Vol. 60. Springer Science+Business Media; New York: 2012. (Arsenic & Rice). ISBN ISBN 978-94-007-2946-9. [Google Scholar]
- 21.Islam M.S., Niazi N.K., Akter S., Rahman M.M., Bhattacharya P., Arsenic Cadmium, and lead in soil and rice samples from paddy fields of bangladesh: levels, sources, and health risks. Environ. Sci. Pollut. Res. 2021;28:32403–32413. [Google Scholar]
- 22.Meharg A.A., Rahman M. Arsenic contamination of bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ. Sci. Technol. 2003;37:229–234. doi: 10.1021/es0259842. [DOI] [PubMed] [Google Scholar]
- 23.Wang M., Tang Z., Chen X., Wang X., Zhou W., Tang Z., Zhang J., Zhao F. Water management impacts the soil microbial communities and total arsenic and methylated arsenicals in rice grains *. Environ. Pollut. 2019;247:736–744. doi: 10.1016/j.envpol.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Hung D.Q., Nekrassova O., Compton R.G. Analytical methods for inorganic arsenic in water. A Rev. 2004;64:269–277. doi: 10.1016/j.talanta.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Juskelis R., Li W., Nelson J., Cappozzo J.C. Arsenic speciation in rice cereals for infants. J. Agric. Food Chem. 2013;61:10670–10676. doi: 10.1021/jf401873z. [DOI] [PubMed] [Google Scholar]
- 26.Mitra A., Chatterjee S., Moogouei R., Gupta D.K. Arsenic accumulation in rice and probable mitigation approaches: a review. Agronomy. 2017;7:1–22. doi: 10.3390/agronomy7040067. [DOI] [Google Scholar]
- 27.Tripathi R.D., Srivastava S., Mishra S., Singh N., Tuli R., Gupta D.K., Maathuis F.J.M. Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol. 2007;25 doi: 10.1016/j.tibtech.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Ye W., Zhang J., Fan T., Lu H., Chen H., Li X. Arsenic speciation in the phloem exudates of rice and its role in arsenic accumulation in rice grains. Ecotoxicol. Environ. Saf. 2017;143:87–91. doi: 10.1016/j.ecoenv.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Punshon T., Jackson B.P., Meharg A.A., Warczack T., Scheckel K., Lou M. Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Sci. Total Environ. 2017;581–582:209–220. doi: 10.1016/j.scitotenv.2016.12.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suriyagoda L.D.B., Dittert K., Lambers H. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric. Ecosyst. Environ. 2018;253:23–37. doi: 10.1016/j.agee.2017.10.017. [DOI] [Google Scholar]
- 31.Ma R., Shen J., Wu J., Tang Z., Shen Q., Zhao F. Impact of agronomic practices on arsenic accumulation and speciation in rice grain. Environ. Pollut. 2014;194:217–223. doi: 10.1016/j.envpol.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Geng A., Wang X., Wu L., Wang F., Chen Y., Yang H., Zhang Z., Zhao X. Arsenic accumulation and speciation in rice grown in arsanilic acid-elevated paddy soil. Ecotoxicol. Environ. Saf. 2017;137:172–178. doi: 10.1016/j.ecoenv.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Liu R., Altschul E.B., Hedin R.S., Nakles D.V., Dzombak D.A. Sequestration enhancement of metals in soils by addition of iron oxides recovered from coal mine drainage sites. Soil Sediment Contam. 2014;23:374–388. doi: 10.1080/15320383.2014.831027. [DOI] [Google Scholar]
- 34.Rokonuzzaman M., Li W.C., Man Y.B., Tsang Y.F., Ye Z. Arsenic accumulation in rice: sources, human health impact and probable mitigation approaches. Rice Sci. 2022;29:309–327. doi: 10.1016/j.rsci.2022.02.002. [DOI] [Google Scholar]
- 35.Rahman M.A., Hasegawa H., Rahman M.M., Rahman M.A., Miah M.A.M. Accumulation of arsenic in tissues of rice plant (Oryza sativa L.) and its distribution in fractions of rice grain. Chemosphere. 2007;69:942–948. doi: 10.1016/j.chemosphere.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 36.Yao B.M., Chen P., Sun G.X. Distribution of elements and their correlation in bran, polished rice, and whole grain. Food Sci. Nutr. 2020;8:982–992. doi: 10.1002/fsn3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedron T., Segura F.R., Paniz F.P., de Moura Souza F., dos Santos M.C., de Magalhães Júnior A.M., Batista B.L. Mitigation of arsenic in rice grains by polishing and washing: evidencing the benefit and the cost. J. Cereal Sci. 2019;87:52–58. doi: 10.1016/j.jcs.2019.03.003. [DOI] [Google Scholar]
- 38.Syu C.H., Huang C.C., Jiang P.Y., Lee C.H., Lee D.Y. Arsenic accumulation and speciation in rice grains influenced by arsenic phytotoxicity and rice genotypes grown in arsenic-elevated paddy soils. J. Hazard. Mater. 2015;286:179–186. doi: 10.1016/j.jhazmat.2014.12.052. [DOI] [PubMed] [Google Scholar]
- 39.Smedley P.L., Kinniburgh D.G. In: In Essentials of Medical Geology. Selinus O., Alloway B., Centeno J.A., Finkelman R.B., Fuge R., Lindh U., Smedley P., editors. Springer; 2013. Arsenic in groundwater and the environment; pp. 279–310. [Google Scholar]
- 40.Ma J.F., Yamaji N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015;20:435–442. doi: 10.1016/j.tplants.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Meharg A.A., Norton G., Deacon C., Williams P., Adomako E.E., Price A., Zhu Y., Li G., Zhao F., Mcgrath S., et al. Variation in rice cadmium related to human exposure. Environ. Sci. Technol. 2013;47:5613–5618. doi: 10.1021/es400521h. [DOI] [PubMed] [Google Scholar]
- 42.Williams P.N., Villada A., Figuerola J., Green A.J. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 2007;41:6854–6859. doi: 10.1021/es070627i. [DOI] [PubMed] [Google Scholar]
- 43.NaziaTahir, Ullah A., Tahir A., Rashid H.U., Rehman T. ur, Danish S., Hussain B., Akca H. Strategies for reducing Cd concentration in paddy soil for rice safety. J. Clean. Prod. 2021;316 doi: 10.1016/j.jclepro.2021.128116. [DOI] [Google Scholar]
- 44.Hua, B.; Yan, W.; Wang, J.; Deng, B.; Yang, J. Arsenic Accumulation in Rice Grains: Effects of Cultivars and Water Management Practices 1. 2011, 28, 591–596, 10.1089/ees.2010.0481. [DOI]
- 45.Dixit G., Singh A.P., Kumar A., Mishra S., Dwivedi S., Kumar S., Trivedi P.K., Pandey V., Tripathi R.D. Reduced arsenic accumulation in rice (Oryza Sativa L.) shoot involves sulfur mediated improved thiol metabolism, antioxidant system and altered arsenic transporters. Plant Physiol. Biochem. 2016;99:86–96. doi: 10.1016/j.plaphy.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Meharg A.A., Jardine L. Arsenite transport into paddy rice (Oryza sativa) roots. New Phytol. 2003;157:39–44. doi: 10.1046/j.1469-8137.2003.00655.x. [DOI] [PubMed] [Google Scholar]
- 47.Wolthers M., Charlet L., van Der Weijden C.H., van der Linde P.R., Rickard D. Arsenic mobility in the ambient sulfidic environment: sorption of arsenic(V) and arsenic(III) onto disordered mackinawite. Geochim. Cosmochim. Acta. 2005;69:3483–3492. doi: 10.1016/j.gca.2005.03.003. [DOI] [Google Scholar]
- 48.Wolthers M., Butler I.B., Rickard D. Influence of arsenic on iron sulfide transformations. Chem. Geol. 2007;236:217–227. doi: 10.1016/j.chemgeo.2006.09.010. [DOI] [Google Scholar]
- 49.Ma J.F., Yamaji N., Mitani N., Xu X., Su Y., Mcgrath S.P., Zhao F. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. 2008:9931–9935. doi: 10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chowdhury N.R., Das R., Joardar M., Ghosh S., Bhowmick S., Roychowdhury T. Arsenic accumulation in paddy plants at different phases of pre-monsoon cultivation. Chemosphere. 2018;210:987–997. doi: 10.1016/j.chemosphere.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y., Han Y., Cao Y., Zhu Y., Rathinasabapathi B. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front. Plant Sci. 2017;8:1–11. doi: 10.3389/fpls.2017.00268. (|) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majumder S., Banik P. Geographical variation of arsenic distribution in paddy soil, rice and rice- based products: a meta-analytic approach and implications to human health. J. Environ. Manag. 2019;233:184–199. doi: 10.1016/j.jenvman.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z., Zhang W., Beebout S.S., Zhang H., Liu L., Yang J., Zhang J. Grain yield, water and nitrogen use efficiencies of rice as influenced by irrigation regimes and their interaction with nitrogen rates. F. Crop. Res. 2016;193:54–69. doi: 10.1016/j.fcr.2016.03.006. [DOI] [Google Scholar]
- 54.Zheng Y., Chen T.B., Lei M., Huang Z.C., Wu H.T., Chen H., Fan K.K. Soil environmental quality standards for heavy metals in China: policy and progress. J. Environ. Manag. 2020;254 doi: 10.1016/j.jenvman.2019.109774. [DOI] [Google Scholar]
- 55.Wu C., Zou Q., Xue S., Pan W., Huang L., Hartley W., Mo J., Wong M. The effect of silicon on iron plaque formation and arsenic accumulation in rice genotypes with different radial oxygen loss ( ROL) Environ. Pollut. 2016;212:27–33. doi: 10.1016/j.envpol.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Tripathi P., Tripathi R.D., Singh R.P., Dwivedi S., Goutam D., Shri M., Trivedi P.K., Chakrabarty D. Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol. Eng. 2013;52:96–103. doi: 10.1016/j.ecoleng.2012.12.057. [DOI] [Google Scholar]
- 57.Wu C., Huang L., Xue S., Pan W., Zou Q., Hartley W., Wong M. Oxic and anoxic conditions affect arsenic ( As) accumulation and arsenite transporter expression in rice. Chemosphere. 2017;168:969–975. doi: 10.1016/j.chemosphere.2016.10.114. [DOI] [PubMed] [Google Scholar]
- 58.LI G., ZHENG M., TANG J., SHIM H., CAI C. Effect of silicon on arsenic concentration and speciation in different rice tissues. Pedosphere. 2018;28:511–520. doi: 10.1016/S1002-0160(17)60409-0. [DOI] [Google Scholar]
- 59.Wu C., Zou Q., Xue S., Mo J., Pan W., Lou L., Wong M.H. Effects of silicon (Si) on arsenic (As) accumulation and speciation in rice (Oryza sativa L.) genotypes with different radial oxygen loss (ROL) Chemosphere. 2015;138:447–453. doi: 10.1016/j.chemosphere.2015.06.081. [DOI] [PubMed] [Google Scholar]
- 60.Liang Y., Nikolic M., Bélanger R., Gong H., Song A. In: Liang Y., Nikolic M., Bélanger R., Gong H., Song A., editors. Vol. 22. Springer: New York; 2015. (Silicon in Agriculture From Theory to Practice). (first.) ISBN 9789401799775. [Google Scholar]
- 61.Li B., Zhou S., Wei D., Long J., Peng L., Tie B. Mitigating arsenic accumulation in rice ( Oryza sativa L.) from typical arsenic contaminated paddy soil of southern china using nanostructured α -MnO 2: pot experiment and Fi eld application. Sci. Total Environ. 2019;650:546–556. doi: 10.1016/j.scitotenv.2018.08.436. [DOI] [PubMed] [Google Scholar]
- 62.Meharg C., Meharg A.A. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ. Exp. Bot. 2015;120:8–17. doi: 10.1016/j.envexpbot.2015.07.001. [DOI] [Google Scholar]
- 63.Zulqarnain Haider, Mehboob A., Razaq A., Khalid U. bin, Rasool N., Mehmood K. Effect of drought stress on some grain quality traits in rice ( Oryza sativa L.) Acad. J. Agric. Res. 2014;2:128–133. [Google Scholar]
- 64.Oliveira L.M.De, Suchismita D., Silva E.B., Gao P., Vardanyan L. Interactive effects of chromate and arsenate on their uptake and speciation in pteris ensiformis. Plant Soil. 2017 [Google Scholar]
- 65.Zhao F.J., Ma J.F., Meharg A.A., McGrath S.P. Arsenic uptake and metabolism in rice. N. Phytol. 2009;181:779–794. doi: 10.1111/j.1469-8137.2008.02716.x. [DOI] [PubMed] [Google Scholar]
- 66.Chang C.Y., Yu H.Y., Chen J.J., Li F.B., Zhang H.H., Liu C.P. Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the pearl river delta, South China. Environ. Monit. Assess. 2014;186:1547–1560. doi: 10.1007/s10661-013-3472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meharg A.A., Williams P.N., Adomako E., Lawgali Y., Deacon L., Villada A., Cambell R.C.J., Sun G., Zhu Y.-G., Feldmann J., et al. Geographical variation in total and inorganic arsenic content of polished ( White) rice. Environ. Sci. Technol. 2009;43:1612–1617. doi: 10.1021/es802612a. [DOI] [PubMed] [Google Scholar]
- 68.Arao T., Kawasaki A., Baba K., Matsumoto S. Effects of arsenic compound amendment on arsenic speciation in rice grain. Environ. Sci. Technol. 2011;45:1291–1297. doi: 10.1021/es1033316. [DOI] [PubMed] [Google Scholar]
- 69.Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181–182:211–217. doi: 10.1016/S0300-483X(02)00285-8. [DOI] [PubMed] [Google Scholar]
- 70.Francesconi K., Kuehnelt D. Determination of arsenic species a critical review. Analyst. 2004;129:373–395. doi: 10.1039/b401321m. [DOI] [PubMed] [Google Scholar]
- 71.Styblo M., Del Razo L.M., LeCluyse E.L., Hamilton G.A., Wang C., Cullen W.R., Thomas D.J. Metabolism of arsenic in primary cultures of human and rat hepatocytes. Chem. Res. Toxicol. 1999;12:560–565. doi: 10.1021/tx990050l. [DOI] [PubMed] [Google Scholar]
- 72.Williams P.N., Islam M.R., Adomako E.E., Raab A., Hossain S.A., Zhu Y.G., Feldmann J., Meharg A.A. Increase in rice grain arsenic for regions of bangladesh irrigating paddies with elevated arsenic in groundwaters. Environ. Sci. Technol. 2006;40:4903–4908. doi: 10.1021/es060222i. [DOI] [PubMed] [Google Scholar]
- 73.Signes-pastor A.J., Carey M., Meharg A.A. Inorganic arsenic removal in rice bran by percolating cooking water. Food Chem. 2017;234:76–80. doi: 10.1016/j.foodchem.2017.04.140. [DOI] [PubMed] [Google Scholar]
- 74.Das D., Chatterjee A., Mandai B.K., Chowdhury T.R., Samanta G., Chakraborti D. Arsenic in ground water in six districts of West Bengal, India: the biggest arsenic calamity in the world: part I. Arsenic species in drinking water and urine of the affected people. Analyst. 1995;120:643–650. doi: 10.1039/AN9952000643. [DOI] [PubMed] [Google Scholar]
- 75.Chowdhury U.K., Biswas B.K., Chowdhury T.R., Samanta G., Mandal B.K., Basu G.C., Chanda C.R., Lodh D., Saha K.C., Mukherjee S.K., et al. Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ. Health Perspect. 2000;108:393–397. doi: 10.1289/ehp.00108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Islam S., Mahmudur M., Islam M.R., Naidu R. Geographical variation and age-related dietary exposure to arsenic in rice from Bangladesh. Sci. Total Environ. J. 2017;602:122–131. doi: 10.1016/j.scitotenv.2017.05.184. [DOI] [PubMed] [Google Scholar]
- 77.Sandhi A., Yu C., Rahman M., Amin N. Arsenic in the water and agricultural crop production system: Bangladesh perspectives. Environ. Sci. Pollut. Res. 2022:51354–51366. doi: 10.1007/s11356-022-20880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattacharya P., Welch A.H., Stollenwerk K.G., McLaughlin M.J., Bundschuh J., Panaullah G. Arsenic in the environment: biology and chemistry. Sci. Total Environ. 2007;379:109–120. doi: 10.1016/j.scitotenv.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 79.Chakraborty M., Mukherjee A., Ahmed K.M. A review of groundwater arsenic in the Bengal Basin, Bangladesh and India: from source to sink. Curr. Pollut. Rep. 2015;1:220–247. doi: 10.1007/s40726-015-0022-0. [DOI] [Google Scholar]
- 80.Yang Y., Zhang H., Yuan H., Duan G., Jin D., Zhao F., Zhu Y. Microbe mediated arsenic release from iron minerals and arsenic methylation in rhizosphere controls arsenic fate in soil-rice system. Environ. Pollut. 2018;236:598–608. doi: 10.1016/j.envpol.2018.01.099. [DOI] [PubMed] [Google Scholar]
- 81.Song R., Zhao C.-Y., Liu J., Zhang J., Du Y.-X., Li J.-Z., Sun H.-Z., Zhao H.-B., Zhao Q.-Z. Effect of sulphate nutrition on arsenic translocation and photosynthesis of rice seedlings. Acta Physiol. Plant. 2013;35:3237–3243. doi: 10.1007/s11738-013-1358-y. [DOI] [Google Scholar]
- 82.Martínez-Cortijo J., Ruiz-Canales A. Effect of heavy metals on rice irrigated fields with waste water in High PH mediterranean soils: the particular case of the valencia area in Spain. Agric. Water Manag. 2018;210:108–123. doi: 10.1016/j.agwat.2018.07.037. [DOI] [Google Scholar]
- 83.Sandil S., Óvári M., Dobosy P., Vetési V., Endrédi A., Takács A., Füzy A., Záray G. Effect of arsenic-contaminated irrigation water on growth and elemental composition of tomato and cabbage cultivated in three different soils, and related health risk assessment. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111098. [DOI] [PubMed] [Google Scholar]
- 84.Abi-Saab M.T., Jomaa I., El Hage R., Skaf S., Fahed S., Rizk Z., Massaad R., Romanos D., Khairallah Y., Azzi V., et al. Are fresh water and reclaimed water safe for vegetable irrigation? Empirical evidence from Lebanon. Water. 2022;14 doi: 10.3390/w14091437. [DOI] [Google Scholar]
- 85.Yanala S.R., Pagilla K.R. Use of biochar to produce reclaimed water for irrigation use. Chemosphere. 2020;251 doi: 10.1016/j.chemosphere.2020.126403. [DOI] [PubMed] [Google Scholar]
- 86.Ding C., Zhang T., Wang X., Zhou F., Yang Y., Yin Y. Effects of soil type and genotype on lead concentration in rootstalk vegetables and the selection of cultivars for food safety. J. Environ. Manag. 2013;122:8–14. doi: 10.1016/j.jenvman.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 87.Zeng F., Ali S., Zhang H., Ouyang Y., Qiu B., Wu F., Zhang G. The influence of PH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011;159:84–91. doi: 10.1016/j.envpol.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 88.Norton G.J., Shafaei M., Travis A.J., Deacon C.M., Danku J., Pond D., Cochrane N., Lockhart K., Salt D., Zhang H., et al. Impact of alternate wetting and drying on rice physiology, grain production, and grain quality. F. Crop. Res. 2017;205:1–13. doi: 10.1016/j.fcr.2017.01.016. [DOI] [Google Scholar]
- 89.Price A.H., Norton G.J., Salt D.E., Ebenhoeh O., Meharg A.A., Meharg C., Islam M.R., Sarma R.N., Dasgupta T., Ismail A.M., et al. Alternate wetting and drying irrigation for rice in Bangladesh: is it sustainable and has plant breeding something to offer? Food Energy Secur. 2013;2:120–129. doi: 10.1002/fes3.29. [DOI] [Google Scholar]
- 90.Li C., Carrijo D.R., Nakayama Y., Linquist B.A., Green P.G., Parikh S.J. Impact of alternate wetting and drying irrigation on arsenic uptake and speciation in flooded rice systems. Agric. Ecosyst. Environ. 2019;272:188–198. doi: 10.1016/j.agee.2018.11.009. [DOI] [Google Scholar]
- 91.Honma T., Ohba H., Kaneko A., Nakamura K., Makino T., Katou H. Effects of soil amendments on arsenic and cadmium uptake by rice plants (Oryza sativa L. Cv. Koshihikari) under different water management practices. Soil Sci. Plant Nutr. 2016;62:349–356. doi: 10.1080/00380768.2016.1196569. [DOI] [Google Scholar]
- 92.Harine I.J., Islam M.R., Hossain M., Afroz H., Jahan R., Siddique A.B., Uddin S., Hossain M.A., Alamri S., Siddiqui M.H., et al. Arsenic accumulation in rice grain as influenced by water management: human health risk assessment. Agronomy. 2021;11:1–13. doi: 10.3390/agronomy11091741. [DOI] [Google Scholar]
- 93.Linquist B.A., Anders M.M., Adviento-Borbe M.A.A., Chaney R.L., Nalley L.L., da Rosa E.F.F., van Kessel C. Reducing greenhouse gas emissions, water use, and grain arsenic levels in rice systems. Glob. Chang. Biol. 2015;21:407–417. doi: 10.1111/gcb.12701. [DOI] [PubMed] [Google Scholar]
- 94.Wan Y., Camara A.Y., Yu Y., Wang Q., Guo T., Zhu L., Li H. Cadmium dynamics in soil pore water and uptake by rice: influences of soil-applied selenite with different water managements. Environ. Pollut. 2018;240:523–533. doi: 10.1016/j.envpol.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 95.De Francisco P., Martín-González A., Rodriguez-Martín D., Díaz S. Interactions with arsenic: mechanisms of toxicity and cellular resistance in eukaryotic microorganisms. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph182212226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Di X., Beesley L., Zhang Z., Zhi S., Jia Y., Ding Y. Microbial arsenic methylation in soil and uptake and metabolism of methylated arsenic in plants: a review. Int. J. Environ. Res. Public Health. 2019;16:1–13. doi: 10.3390/ijerph16245012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zeng X.C., Yang Y., Shi W., Peng Z., Chen X., Zhu X., Wang Y. Microbially mediated methylation of arsenic in the arsenic-rich soils and sediments of jianghan plain. Front. Microbiol. 2018;9:1–13. doi: 10.3389/fmicb.2018.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin Z., Wang X., Wu X., Liu D., Yin Y., Zhang Y., Xiao S., Xing B. Nitrate reduced arsenic redox transformation and transfer in Fl ooded paddy soil-rice system. Environ. Pollut. 2018;243:1015–1025. doi: 10.1016/j.envpol.2018.09.054. [DOI] [PubMed] [Google Scholar]
- 99.Chen J., Rosen B.P. The arsenic methylation cycle: how microbial communities adapted methylarsenicals for use as weapons in the continuing war for dominance. Front. Environ. Sci. 2020;8:1–14. doi: 10.3389/fenvs.2020.00043. [DOI] [Google Scholar]
- 100.Roberta F., Maria J., Souza D.O., Silva E., Paula D., Martins C., Carolina A., Paulelli C., Barbosa F., Lemos B. Arsenic speciation in Brazilian rice grains organically and traditionally cultivated: is there any difference in arsenic content ? FRIN. 2016;89:169–176. doi: 10.1016/j.foodres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 101.Chou M.L., Jean J.S., Sun G.X., Yang C.M., Hseu Z.Y., Kuo S.F., Tseng H.Y., Yang Y.J. Irrigation practices on rice crop production in arsenic-rich paddy soil. Crop Sci. 2016;56:422–431. doi: 10.2135/cropsci2015.04.0233. [DOI] [Google Scholar]
- 102.Zhang J., Li H., Zhou Y., Dou L., Cai L. Bioavailability and soil-to-crop transfer of heavy metals in farmland soils: a case study in the pearl River Delta, South China. Environ. Pollut. 2018;235:710–719. doi: 10.1016/j.envpol.2017.12.106. [DOI] [PubMed] [Google Scholar]
- 103.Xu Y., Ge J., Tian S., Li S., Nguy-robertson A.L., Zhan M., Cao C. Effects of water-saving irrigation practices and drought resistant rice variety on greenhouse gas emissions from a no-till paddy in the central lowlands of China. Sci. Total Environ. 2015;505:1043–1052. doi: 10.1016/j.scitotenv.2014.10.073. [DOI] [PubMed] [Google Scholar]
- 104.Halder D., Biswas A., Zdenka Š., Chatterjee D., Nriagu J., Jacks G., Bhattacharya P. Arsenic species in raw and cooked rice: implications for human health in rural Bengal. Sci. Total Environ. 2014;498:200–208. doi: 10.1016/j.scitotenv.2014.07.075. [DOI] [PubMed] [Google Scholar]
- 105.Fang Y., Sun X., Yang W., Ma N., Xin Z., Fu J., Liu X., Liu M., Mariga A.M., Zhu X., et al. Concentrations and health risks of lead, cadmium, arsenic, and mercury in rice and edible mushrooms in China. Food Chem. 2014;147:147–151. doi: 10.1016/j.foodchem.2013.09.116. [DOI] [PubMed] [Google Scholar]
- 106.Hu P., Ouyang Y., Wu L., Shen L., Luo Y., Christie P. Effects of water management on arsenic and cadmium speciation and accumulation in an upland rice cultivar. J. Environ. Sci. (China) 2015;27:225–231. doi: 10.1016/j.jes.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 107.Okeke P.N., Orisakwe O.E., Oyelola O.T. Influence of climate change on heavy metal bioavailability and human health implications in rice ecosystems. JEnvironmental Sci. Pollut. Res. 2018;25:2181–2182. doi: 10.1007/s11356-017-0763-8. [DOI] [Google Scholar]
- 108.Wu C., Ye Z., Shu W., Zhu Y., Wong M. Arsenic accumulation and speciation in rice are affected by root aeration and variation of genotypes. J. Exp. Bot. 2011;62:2889–2898. doi: 10.1093/jxb/erq462. [DOI] [PubMed] [Google Scholar]
- 109.Rawlings D.E. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Fact. 2005;4(1):15. doi: 10.1186/1475-2859-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Upadhyay M.K., Majumdar A., Kumar J.S. Arsenic in rice agro-ecosystem: solutions for safe and sustainable rice production. Front. Sustain. Food Syst. 2020;4 doi: 10.3389/fsufs.2020.00053. [DOI] [Google Scholar]
- 111.Zhao F.J., Zhu Y.G., Meharg A.A. Methylated arsenic species in rice: geographical variation, origin, and uptake mechanisms. Environ. Sci. Technol. 2013;47:3957–3966. doi: 10.1021/es304295n. [DOI] [PubMed] [Google Scholar]
- 112.Zhao F.J., McGrath S.P. Biofortification and phytoremediation. Curr. Opin. Plant Biol. 2009;12:373–380. doi: 10.1016/j.pbi.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 113.Norton G.J., Adomako E.E., Deacon C.M., Carey A.M., Price A.H., Meharg A.A. Effect of organic matter amendment, arsenic amendment and water management regime on rice grain arsenic species. Environ. Pollut. 2013;177:38–47. doi: 10.1016/j.envpol.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 114.Mitra A. In: Arsen. Contam. Environ. Issues Solut. Chatterjee S., Gupta D.K., editors. 2017. Arsenic contamination in the environment: the issues and solutions; pp. 1–218. [DOI] [Google Scholar]
- 115.Syu C.H., Wu P.R., Lee C.H., Juang K.W., Lee D.Y. Arsenic phytotoxicity and accumulation in rice seedlings grown in arsenic-contaminated soils as influenced by the characteristics of organic matter amendments and soils. J. Plant Nutr. Soil Sci. 2019;182:60–71. doi: 10.1002/jpln.201800337. [DOI] [Google Scholar]
- 116.Naggar Y.Al, Naiem E., Mona M., Giesy J.P., Seif A. Metals in agricultural soils and plants in Egypt. Toxicol. Environ. Chem. 2014;96:730–742. doi: 10.1080/02772248.2014.984496. [DOI] [Google Scholar]
- 117.Zhang Q., Zhang L., Liu T., Liu B., Huang D., Zhu Q., Xu C. The influence of liming on cadmium accumulation in rice grains via iron-reducing bacteria. Sci. Total Environ. 2018;645:109–118. doi: 10.1016/j.scitotenv.2018.06.316. [DOI] [PubMed] [Google Scholar]
- 118.Sun M., Liu G., Wu Q. Speciation of organic and inorganic selenium in selenium-enriched rice by graphite furnace atomic absorption spectrometry after cloud point extraction. Food Chem. 2013;141:66–71. doi: 10.1016/j.foodchem.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 119.Finnegan P.M., Chen W. Arsenic toxicity: the effects on plant metabolism. Front. Physiol. 2012;3:1–18. doi: 10.3389/fphys.2012.00182. (JUN) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu Z.Y., Zhu Y.G., Li M., Zhang L.G., Cao Z.H., Smith F.A. Sulfur (S)-induced enhancement of iron plaque formation in the rhizosphere reduces arsenic accumulation in rice (Oryza sativa L.) seedlings. Environ. Pollut. 2007;147:387–393. doi: 10.1016/j.envpol.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 121.Dixit G., Singh A.P., Kumar A., Singh P.K., Kumar S., Dwivedi S., Trivedi P.K., Pandey V., Norton G.J., Dhankher O.P., et al. Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J. Hazard. Mater. 2015;298:241–251. doi: 10.1016/j.jhazmat.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 122.Tripathi R.D., Tripathi P., Dwivedi S., Kumar A., Mishra A., Chauhan P.S., Norton G.J., Nautiyal C.S. Roles for root iron plaque in sequestration and uptake of heavy metals and metalloids in aquatic and wetland plants. Metallomics. 2014;6:1789–1800. doi: 10.1039/c4mt00111g. [DOI] [PubMed] [Google Scholar]
- 123.Majumdar A., Bose S. In: Mechanisms of Arsenic Toxicity and Tolerance in Plants. Hasanuzzaman M., Nahar K., Fujita M., editors. Springer; Singapore: 2018. A glimpse on uptake kinetics and molecular responses of arsenic tolerance in rice plants; pp. 299–315. ISBN 9789811312922. [Google Scholar]
- 124.Majumdar A., Upadhyay M.K., Giri B., Srivastava S., Srivastava A.K., Jaiswal M.K., Bose S. Arsenic dynamics and flux assessment under drying-wetting irrigation and enhanced microbial diversity in paddy soils: a four year study in bengal delta plain. J. Hazard. Mater. 2020 doi: 10.1016/j.jhazmat.2020.124443. [DOI] [PubMed] [Google Scholar]
- 125.Flora S.J.S. In: Handbook of Arsenic Toxicology. Flora S.J.S., editor. Elsevier Inc; Amsterdam: 2015. Arsenic: chemistry, occurrence, and exposure; pp. 1–49. ISBN 9780124199552. [Google Scholar]
- 126.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Syu C., Lee C., Jiang P., Chen M., Lee D. Comparison of as sequestration in iron plaque and uptake by different genotypes of rice plants grown in as-contaminated paddy soils. Plant Soil. 2014:411–422. doi: 10.1007/s11104-013-1893-8. [DOI] [Google Scholar]
- 128.Mandal B.K., Suzuki K.T. Arsenic round the world: a review. Talanta. 2002;58:201–235. doi: 10.1016/S0039-9140(02)00268-0. [DOI] [PubMed] [Google Scholar]
- 129.Liu W.J., Zhu Y.G., Smith F.A., Smith S.E. Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? J. Exp. Bot. 2004;55:1707–1713. doi: 10.1093/jxb/erh205. [DOI] [PubMed] [Google Scholar]
- 130.Mlangeni A.T., Lancaster S.T., Raab A., Krupp E.M., Norton G.J., Feldmann J. Higher zero valent iron soil amendments dosages markedly inhibit accumulation of as in faya and kilombero cultivars compared to Cd. Sci. Total Environ. 2021;794 doi: 10.1016/j.scitotenv.2021.148735. [DOI] [PubMed] [Google Scholar]
- 131.Guo B., Hong C., Tong W., Xu M., Huang C., Yin H., Lin Y., Fu Q. Health risk assessment of heavy metal pollution in a soil-rice system: a case study in the Jin-Qu Basin of China. Sci. Rep. 2020;10(1):11. doi: 10.1038/s41598-020-68295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu Y.G., Pilon-Smits E.A.H., Zhao F.J., Williams P.N., Meharg A.A. Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009;14:436–442. doi: 10.1016/j.tplants.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 133.Smith F.W., Ealing P.M., Hawkesford M.J., Clarkson D.T. Plant members of a family of sulfate transporters reveal functional subtypes. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9373–9377. doi: 10.1073/pnas.92.20.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yamaguchi, N.; Ohkura, T.; Takahashi, Y.; Maejima, Y.; Arao, T. Arsenic Distribution and Speciation near Rice Roots In Fl Uenced by Iron Plaques and Redox Conditions of the Soil Matrix. 2014. [DOI] [PubMed]
- 135.Souri Z., Karimi N., Sandalio L.M. Arsenic hyperaccumulation strategies: an overview. Front. Cell Dev. Biol. 2017;5:1–8. doi: 10.3389/fcell.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tripathi R.D., Singh R., Tripathi P., Dwivedi S., Chauhan R., Adhikari B., Trivedi P.K. Arsenic accumulation and tolerance in rootless macrophyte najas indica are mediated through antioxidants, amino acids and phytochelatins. Aquat. Toxicol. 2014;157:70–80. doi: 10.1016/j.aquatox.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 137.Dinh N.T., Vu D.T., Mulligan D., Nguyen A.V. Accumulation and distribution of zinc in the leaves and roots of the hyperaccumulator noccaea caerulescens. Environ. Exp. Bot. 2015;110:85–95. doi: 10.1016/j.envexpbot.2014.10.001. [DOI] [Google Scholar]
- 138.Raskin I., Ensley B.D. (Burt D. phytoremediation of toxic metals: using plants to clean the environment. J. Plant Biotechnol. 1999;1:304. doi: 10.1016/S0958-1669(97)80106-1. [DOI] [Google Scholar]
- 139.Majumdar A., Upadhyay M.K., Kumar J.S., Sheena, Barla A., Srivastava S., Jaiswal M.K., Bose S. Ultra-structure alteration via enhanced silicon uptake in arsenic stressed rice cultivars under intermittent irrigation practices in bengal delta basin. Ecotoxicol. Environ. Saf. 2019;180:770–779. doi: 10.1016/j.ecoenv.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 140.Ma L., Wang L., Jia Y., Yang Z. Arsenic speciation in locally grown rice grains from hunan province, china: spatial distribution and potential health risk. Sci. Total Environ. 2016;557–558:438–444. doi: 10.1016/j.scitotenv.2016.03.051. [DOI] [PubMed] [Google Scholar]
- 141.Nurchi V.M., Djordjevic A.B., Crisponi G., Alexander J., Bjørklund G., Aaseth J. Arsenic toxicity: molecular targets and therapeutic agents. Biomolecules. 2020;10:1–15. doi: 10.3390/biom10020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun G.X., Williams P.N., Carey A.M., Zhu Y.G., Deacon C., Raab A., Feldmann J., Islam R.M., Meharg A.A. Inorganic arsenic in rice bran and its products are an order of magnitude higher than in bulk grain. Environ. Sci. Technol. 2008;42:7542–7546. doi: 10.1021/es801238p. [DOI] [PubMed] [Google Scholar]
- 143.Dubey A.K., Kumar N., Sahu N., Kumar P., Chakrabarty D., Behera S.K., Mallick S. Response of two rice cultivars differing in their sensitivity towards arsenic, differs in their expression of glutaredoxin and glutathione s transferase genes and antioxidant usage. Ecotoxicol. Environ. Saf. 2016;124:393–405. doi: 10.1016/j.ecoenv.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 144.Li X., Qiao J., Li S., Häggblom M.M., Li F., Hu M. Bacterial communities and functional genes stimulated during anaerobic arsenite oxidation and nitrate reduction in a paddy soil. Environ. Sci. Technol. 2020;54:2172–2181. doi: 10.1021/acs.est.9b04308. [DOI] [PubMed] [Google Scholar]
- 145.Honma T., Ohba H., Kaneko-Kadokura A., Makino T., Nakamura K., Katou H. Optimal Soil Eh, PH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ. Sci. Technol. 2016;50:4178–4185. doi: 10.1021/acs.est.5b05424. [DOI] [PubMed] [Google Scholar]
- 146.ZHENG R., SUN G., ZHU Y. Effects of microbial processes on the fate of arsenic in paddy soil. Chin. Sci. Bull. 2013;58:186–193. doi: 10.1007/s11434-012-5489-0. [DOI] [Google Scholar]
- 147.Oremland R.S., Stolz J.F. The ecology of arsenic. Science. 2003;300(80-.):939–944. doi: 10.1126/science.1081903. [DOI] [PubMed] [Google Scholar]
- 148.Bacaicoa E., García-Mina J.M. Iron efficiency in different cucumber cultivars: the importance of optimizing the use of foliar iron. J. Am. Soc. Hortic. Sci. 2009;134:405–416. doi: 10.21273/jashs.134.4.405. [DOI] [Google Scholar]
- 149.Khan K.M., Chakraborty R., Bundschuh J., Bhattacharya P., Parvez F. Health effects of arsenic exposure in latin america: an overview of the past eight years of research. Sci. Total Environ. 2020;710 doi: 10.1016/j.scitotenv.2019.136071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pereira H.S., Melo L.C., Faria L.C. de, Ferreira E.P. de B., Mercante F.M., Wendland A., Souza T.L.P.O. de. Common bean elite lines cultivated under nitrogen fertilization and inoculation with rhizobium tropici. Ciência Rural. 2015;45:2168–2173. doi: 10.1590/0103-8478cr20141135. [DOI] [Google Scholar]
- 151.Drobna Z., Styblo M., Thomas D.J., Carolina N., Hill C., Hill C., Carolina N. An overview of arsenic metabolism and toxicity. Curr. Protoc. Toxicol. 2009:1–6. doi: 10.1002/0471140856.tx0431s42. ISBN 0471140856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shankar S., Shanker U., Shikha Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J. 2014;2014 doi: 10.1155/2014/304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bienert G.P., Schjoerring J.K., Jahn T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta - Biomembr. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 154.Rashdan N.A., Pattillo C.B. Hydrogen peroxide in the ER: a tale of triage. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xue J., Bakker M.R., Milin S., Graham D. Enhancement in soil fertility, early plant growth and nutrition and mycorrhizal colonization by vermicompost application varies with native and exotic tree species. J. Soils Sediment. 2022;22:1662–1676. doi: 10.1007/s11368-022-03180-5. [DOI] [Google Scholar]
- 156.Rahimi G., Kolahchi Z., Charkhabi A. Uptake and translocation of some heavy metals by rice crop (Oryza sativa) in paddy soils. Agriculture. 2017;63:163–175. doi: 10.1515/agri-2017-0016. [DOI] [Google Scholar]
- 157.Zhu J., Johnson T.M., Finkelman R.B., Zheng B., Sýkorová I., Pe J. The occurrence and origin of selenium minerals in Se-rich stone coals, spoils and their adjacent soils in Yutangba, China. Chem. Geol. 2012;331:27–38. doi: 10.1016/j.chemgeo.2012.08.023. [DOI] [Google Scholar]
- 158.Carrijo D.R., Li C., Parikh S.J., Linquist B.A. Irrigation management for arsenic mitigation in rice grain: timing and severity of a single soil drying. Sci. Total Environ. 2019;649:300–307. doi: 10.1016/j.scitotenv.2018.08.216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.