Abstract
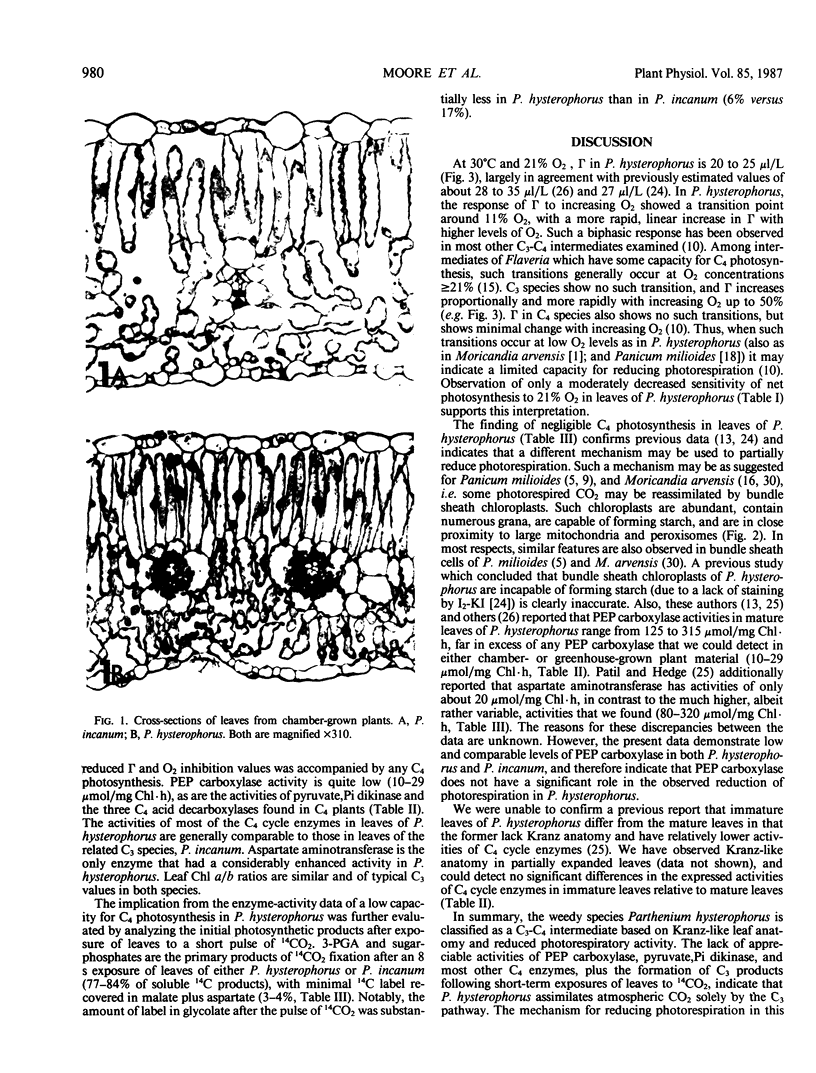
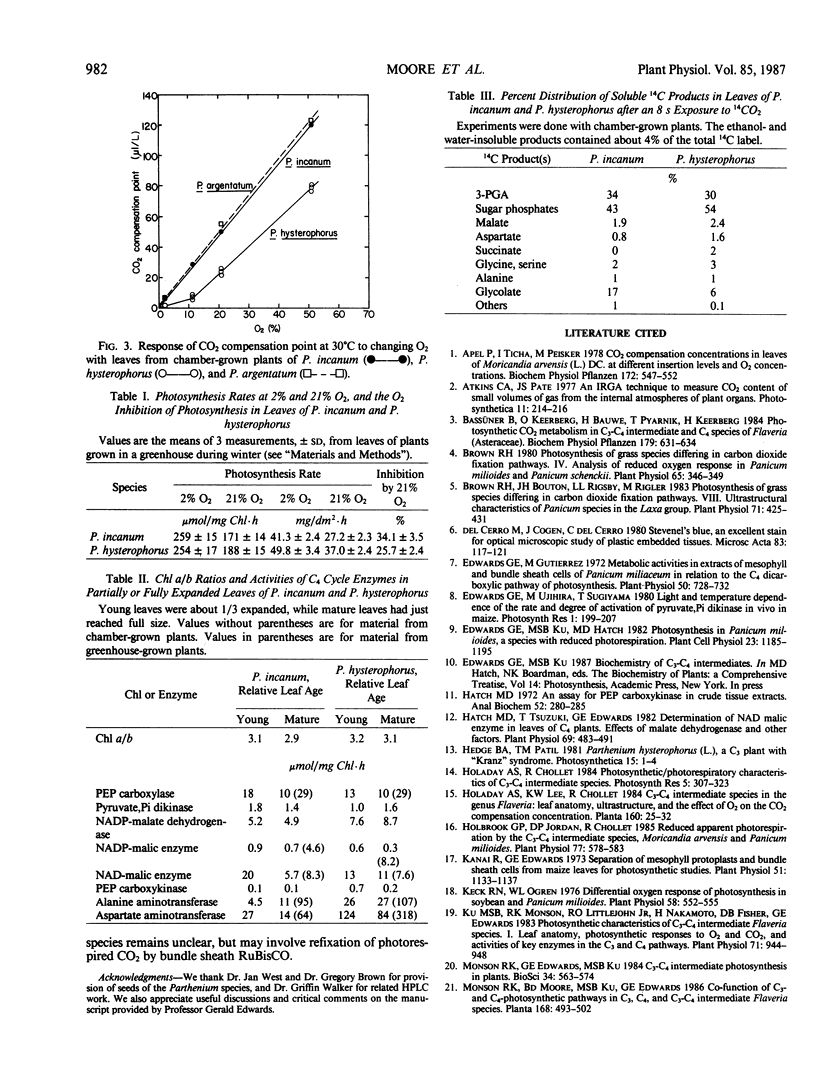
The weedy species Parthenium hysterophorus (Asteraceae) possesses a Kranz-like leaf anatomy. The bundle sheath cells are thick-walled and contain numerous granal chloroplasts, prominent mitochondria, and peroxisomes, all largely arranged in a centripetal position. Both mesophyll and bundle sheath chloroplasts accumulate starch. P. hysterophorus exhibits reduced photorespiration as indicated by a moderately low CO2 compensation concentration (20-25 microliters per liter at 30°C and 21% O2) and by a reduced sensitivity of net photosynthesis to 21% O2. In contrast, the related C3 species P. incanum and P. argentatum (guayule) lack Kranz anatomy, have higher CO2 compensation concentrations (about 55 microliters per liter), and show a greater inhibition of photosynthesis by 21% O2. Furthermore, in P. hysterophorus the CO2 compensation concentration is relatively less sensitive to changes in O2 concentrations and shows a biphasic response to changing O2, with a transition point at about 11% O2. Based on these results, P. hysterophorus is classified as a C3-C4 intermediate. The activities of diagnostic enzymes of C4 photosynthesis in P. hysterophorus were very low, comparable to those observed in the C3 species P. incanum (e.g. phosphoenolpyruvate carboxylase activity of 10-29 micromoles per milligram of chlorophyll per hour). Exposures of leaves of each species to 14CO2 (for 8 seconds) in the light resulted in 3-phosphoglycerate and sugar phosphates being the predominant initial 14C products (77-84%), with ≤4% of the 14C-label in malate plus aspartate. These results indicate that in the C3-C4 intermediate P. hysterophorus, the reduction in leaf photorespiration cannot be attributed to C4 photosynthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. H., Bouton J. H., Rigsby L., Rigler M. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways : VIII. Ultrastructural Characteristics of Panicum Species in the Laxa Group. Plant Physiol. 1983 Feb;71(2):425–431. doi: 10.1104/pp.71.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. H. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways: IV. ANALYSIS OF REDUCED OXYGEN RESPONSE IN PANICUM MILIOIDES AND PANICUM SCHENCKII. Plant Physiol. 1980 Feb;65(2):346–349. doi: 10.1104/pp.65.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Gutierrez M. Metabolic Activities in Extracts of Mesophyll and Bundle Sheath Cells of Panicum miliaceum (L.) in Relation to the C(4) Dicarboxylic Acid Pathway of Photosynthesis. Plant Physiol. 1972 Dec;50(6):728–732. doi: 10.1104/pp.50.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. An assat for PEP carboxykinase in crude tissue extracts. Anal Biochem. 1973 Mar;52(1):280–285. doi: 10.1016/0003-2697(73)90350-3. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Tsuzuki M., Edwards G. E. Determination of NAD Malic Enzyme in Leaves of C(4) Plants : EFFECTS OF MALATE DEHYDROGENASE AND OTHER FACTORS. Plant Physiol. 1982 Feb;69(2):483–491. doi: 10.1104/pp.69.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook G. P., Jordan D. B., Chollet R. Reduced Apparent Photorespiration by the C(3)-C(4) Intermediate Species, Moricandia arvensis and Panicum milioides. Plant Physiol. 1985 Mar;77(3):578–583. doi: 10.1104/pp.77.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck R. W. Differential Oxygen Response of Photosynthesis in Soybean and Panicum milioides. Plant Physiol. 1976 Oct;58(4):552–555. doi: 10.1104/pp.58.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M. S., Monson R. K., Littlejohn R. O., Nakamoto H., Fisher D. B., Edwards G. E. Photosynthetic Characteristics of C(3)-C(4) Intermediate Flaveria Species : I. Leaf Anatomy, Photosynthetic Responses to O(2) and CO(2), and Activities of Key Enzymes in the C(3) and C(4) Pathways. Plant Physiol. 1983 Apr;71(4):944–948. doi: 10.1104/pp.71.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpho M. E., Ku M. S., Cheng S. H., Edwards G. E. Photosynthetic Characteristics of C(3)-C(4) Intermediate Flaveria Species : III. Reduction of Photorespiration by a Limited C(4) Pathway of Photosynthesis in Flaveria ramosissima. Plant Physiol. 1984 Aug;75(4):993–996. doi: 10.1104/pp.75.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann P. Separation of phosphate esters and algal extracts by thin-layer electrophoresis and chromatography. J Chromatogr. 1969 Feb 25;39(4):507–509. [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K., Usuda H., Tsuzuki M., Schmitt M., Edwards G. E., Thomas R. J., Evert R. F. Influence of Nitrate and Ammonia on Photosynthetic Characteristics and Leaf Anatomy of Moricandia arvensis. Plant Physiol. 1982 Aug;70(2):616–625. doi: 10.1104/pp.70.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- del Cerro M., Cogen J., del Cerro C. Stevenel's Blue, an excellent stain for optical microscopical study of plastic embedded tissues. Microsc Acta. 1980 May;83(2):117–121. [PubMed] [Google Scholar]