Abstract
Purpose
The purpose of this study was to report intermediate-term outcomes following carpal tunnel release using ultrasound guidance and wide-awake local anesthesia no tourniquet, including a subset of patients with preoperative and postoperative magnetic resonance imaging (MRI).
Methods
In this observational study, patients with carpal tunnel syndrome were treated with carpal tunnel release using ultrasound guidance and wide-awake local anesthesia no tourniquet in a procedure room at a single center. Main outcomes were complications; return to activity and work at 2 weeks; Quick Disabilities of the Arm, Shoulder, and Hand and Boston Carpal Tunnel Questionnaire scores through 6 months; and postoperative morphological changes of the transverse carpal ligament, median nerve, and carpal tunnel evaluated using MRI.
Results
No complications were reported among 65 patients (68% women, 96 wrists). By 2 weeks, 97% of patients returned to normal activity and 100% returned to work. Statistically significant improvements in Boston Carpal Tunnel Questionnaire symptom severity scale, Boston Carpal Tunnel Questionnaire functional status scale, and Quick Disabilities of the Arm, Shoulder, and Hand scores occurred by the 2-week follow-up interval and persisted at 6 months (all P < .001). Pre- and postoperative MRI scans were available for 13 patients (17 wrists) at the 3-month mean follow-up. Complete transverse carpal ligament transection was documented in all wrists. Key MRI findings included a 22% increase in carpal tunnel cross-sectional area at the hamate (P < .001), a 52% increase in median nerve cross-sectional area at the hamate (P < .001), an 18% reduction in median nerve signal intensity (P = .002), a 38% reduction in the flattening ratio of the median nerve at the hamate (P < .001), a 33% reduction in the flattening ratio of the median nerve at the pisiform (P < .001), a 20% reduction in the flattening ratio of the carpal tunnel at the hamate (P < .001), and a palmar shift of the median nerve relative to the hamate in all cases.
Conclusions
Carpal tunnel release using ultrasound guidance using wide-awake local anesthesia no tourniquet in a procedure room setting was safe, effective, and resulted in morphological changes that were consistent with carpal tunnel decompression as demonstrated by MRI.
Type of study/level of evidence
Therapeutic IV.
Key words: Carpal tunnel syndrome, Carpal tunnel release, CTR-US, Magnetic resonance imaging, WALANT
Carpal tunnel syndrome (CTS) is the most common entrapment neuropathy1,2 and is associated with high social and economic costs.2 Initial treatment typically includes activity modification, physical therapy, splinting, nonsteroidal anti-inflammatory drugs, or corticosteroid injections.3 For patients with severe or refractory symptoms, surgical release of the transverse carpal ligament (TCL) may be indicated.
Although multiple studies have reported a successful symptomatic relief for patients following traditional open, mini-open, and endoscopic carpal tunnel release (CTR), techniques continue to evolve with a focus on reducing surgical morbidity and accelerating recovery.4, 5, 6, 7 More recently, CTR with ultrasound guidance (CTR-US) has been proposed as a technique with a limited incision size that maintains satisfactory visualization.3,8, 9, 10 CTR-US techniques generally use a minimally invasive approach (incision <5 mm) to transect the TCL while using real-time ultrasound guidance to continuously monitor critical anatomy during the procedure.8, 9, 10
Multiple studies have reported statistically significant and clinically meaningful improvements in patient-reported outcomes following CTR-US.3,8, 9, 10, 11, 12, 13, 14, 15 Despite these encouraging clinical results, the morphological changes within the carpal tunnel following CTR-US have not been fully characterized. Previous research has used magnetic resonance imaging (MRI) to document morphological changes consistent with carpal tunnel decompression (eg, TCL transection, increased carpal tunnel dimensions, and reduced median nerve compression) following open and endoscopic carpal tunnel release (ECTR).16, 17, 18, 19, 20 However, only one study has used MRI to evaluate post–CTR-US changes in the carpal tunnel.21 Although the authors documented complete TCL transection and reduced median nerve compression, they did not report additional important morphological indicators of carpal tunnel decompression such as an increased carpal tunnel cross-sectional area and reduced median nerve edema.16, 17, 18, 19, 20, 21 Consequently, the purpose of this study was to report intermediate-term outcomes following CTR-US and WALANT, including a subset of patients with pre- and post–CTR-US MRI scan including the assessment of the TCL, median nerve, and carpal tunnel dimensions.
Materials and Methods
This study was reviewed and approved by the Wyoming Medical Center institutional review board.
Participants
This was a retrospective observational study of prospectively collected data from patients with CTS treated with CTR-US and WALANT in a procedure room at a single center between July 2019 and October 2021. Eligible patients had moderate to severe CTS confirmed by history, physical examination findings, and electrodiagnostic testing, and they underwent baseline MRI.
Procedures
All procedures were performed by the same physician (who had 13 years of experience in ultrasound-guided procedures) and a single assistant in a procedure room setting using a WALANT technique.22 Through a small incision (4–5 mm) and using ultrasound guidance with augmented reality for ultrasound (McGinley Education Innovations, LLC), the TCL was transected using a commercially available device (UltraGuideCTR, Sonex Health, Inc) designed to facilitate CTR-US by creating space in the carpal tunnel followed by TCL transection using a retrograde knife.
Key procedural steps have been previously published.3,12,15 A preprocedural ultrasound was performed using a high-frequency 17MHz linear transducer (Acuson Freestyle, Siemens USA) to mark the borders of the transverse and longitudinal safe zones as well as the incision site in the distal forearm. The forearm was prepared and draped in the usual sterile fashion. Using a sterile ultrasound cover and sterile gel, relevant anatomical landmarks were again identified, including but not limited to the median nerve, thenar motor branch/recurrent motor branch of the median nerve, palmar cutaneous branch of the median nerve, median and ulnar digital nerves and any communications, ulnar vessels and superficial palmar arch, and distal TCL. The safe zones were re-scanned to ensure acceptable anatomy and sonographic visualization.
A #15 scalpel blade was used to create a 4–5 mm incision at the proximal wrist crease, penetrating the antebrachial fascia. A sterile stainless-steel dilator was then passed into the transverse safe zone using direct ultrasound guidance to further free the synovial tissue from the ligament and facilitate device insertion. Direct ultrasound guidance was then used to pass the cutting device into the carpal tunnel and position it within the transverse and longitudinal safe zones. Following ultrasound confirmation of the appropriate device positioning distal to the TCL and with respect to the surrounding neurovascular structures, balloons were deployed to create space in the carpal tunnel, and the position of the device was re-assessed. Following a satisfactory re-assessment, the cutting knife was activated from its recessed position, and the TCL was transected in a retrograde manner using direct ultrasound guidance and up to three passes. The cutting knife was then placed in its proximal recessed position, the balloons were deflated, and the TCL was probed using the stainless-steel dilator to ensure complete ligament transection and release of the median nerve from the ligament and adjacent synovial tissues. Wounds were closed with sterile adhesive strips; none of the procedures required sutures. Following closure, wounds were dressed in sterile gauze and sterile film, followed by a compression wrap. Patients were instructed to avoid driving and strenuous activity for the remainder of the day and were advised to use nonsteroidal anti-inflammatories or acetaminophen and ice for pain and edema control. Opioids were not prescribed for pain control. Patients were instructed to wear a wrist brace at night for 10 days and were provided with recommended post-CTR stretches and exercises beginning on postoperative day 2 and completed as tolerated. Patients were allowed to return to activities and work as tolerated, starting the day after the procedure. Postprocedure follow-up occurred in the clinic 3 weeks after surgery.
Outcomes
Before surgery, patients provided basic demographic information including age, sex, employment status, and job description (desk-based, repetitive light manual, or heavy manual). Return to activity (normal activities outside of work) and return to work statuses were collected at 2 weeks after surgery. Before and after surgery at 2 weeks, 1 month, 3 months, and 6 months, patients were asked to complete the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) questionnaire and the Boston Carpal Questionnaire symptom severity scale (BCTQ-SSS) and functional status scale (BCTQ-FSS). The QuickDASH is an 11-question survey that assesses upper limb physical symptoms and function of patients on a scale of 1 (asymptomatic/no difficulty) to 5 (extreme/unable to perform). A score is generated from 0 to 100, with higher scores reflecting more symptoms/disability.23 The BCTQ is a commonly used patient-reported outcome measure for CTS.24 The BCTQ-SSS has 11 questions and uses a five-point rating scale, and the BCTQ-FSS has eight items that are rated for a degree of difficulty on a five-point scale. Each scale generates a final score ranging from 1 to 5, with a higher score indicating a greater disability. The BCTQ has undergone extensive testing for validity, reliability, and responsiveness.25 Questionnaires at 1, 3, and 6 months were administered online predominantly or by phone or an email at patient request. Reminder phone calls were placed to patients who did not complete their questionnaires in a timely manner.
Magnetic resonance imaging studies
All patients were offered preoperative and postoperative MRI. Not all patients were able to have MRI scans. Only patients with both pre- and post–CTR-US MRI scans are included in this study. All postoperative MRI measurements were measured blinded to the preoperative MRI data. Following postoperative MRI analyses, the preoperative MRI parameters were measured and comparisons were assessed. All measurements were performed by the primary author and verified by the interventional radiologist who performed all procedures for this study. T1- and T2-weighted axial, coronal, and sagittal high-resolution sequences along with volumetric acquisitions using a Siemens 1.5T and 3.0T scanner were collected. Imaging analysis followed previously published guidelines for measuring morphological changes involved in CTS.16, 17, 18, 19, 20, 21 The TCL status was reported as intact or transected. The TCL was considered to be transected if there was a discontinuity and separation throughout the proximal–distal length of the carpal tunnel on the postoperative MRI. A palmar shift of the median nerve at the level of the hook of hamate was defined as the postoperative change in the distance between the palmar aspect of the carpals and the center of the median nerve. The flattening ratio of the median nerve was measured at the level of the hook of the hamate and the level of the pisiform and was defined as the ratio of the long cross-sectional diameter to the short cross-sectional diameter, with a larger ratio representing a flatter median nerve. The flattening ratio of the carpal tunnel was similarly measured at the level of the hook of the hamate. Median nerve signal intensity was measured to assess changes in median nerve edema. Finally, the cross-sectional areas of the carpal tunnel and median nerve were both outlined digitally and measured at the level of the hamate using Terarecon 3-dimensional software (Terarecon, Inc).
Statistical analysis
Based on multiple repeated measurements of BCTQ-SSS, BCQ-FSS, and QuickDASH in individual participants, we used a linear mixed model with Bonferroni correction that incorporates all available data from patients with a pretreatment and at least one posttreatment observation. Data at each follow-up interval were referenced to the pretreatment data and modeled as fixed effects, with a random effect specified at the procedure level. Follow-up data were reported as the mean and 95% confidence interval. Changes in MRI parameters were analyzed with a paired samples t-test for continuous data and a Wilcoxon signed-rank test for paired categorical data. Two-sided P values of less than .05 were considered statistically significant.
Results
A total of 65 patients (96 wrists) were treated consecutively with CTR-US in this study. All procedures were completed successfully using local anesthesia. No procedures were discontinued because of pain or poor visualization. Procedures were typically completed using two to three passes to cut the TCL. No complications occurred intraoperatively or during follow-up. Among the 65 patients (68% women), 34 (52%) had a unilateral procedure, 19 (29%) had simultaneous bilateral procedures, and 12 (18%) had staged bilateral procedures. The CTR-US procedure was performed on the dominant hand in 52 (80%) patients. Among the 41 (63%) employed patients, 20 (49%) reported desk-based duties, 13 (32%) reported repetitive light manual duties, and eight (20%) reported heavy manual duties.
Among the 60 (92%) patients with available time to return to normal activity data, 58 (97%) reported returning to normal activities by 2 weeks. Among the 38 (93%) employed patients with available time to return to work data, 38 (100%) reported returning to work by 2 weeks, with 34 returning to full work duties and four returning to limited duties.
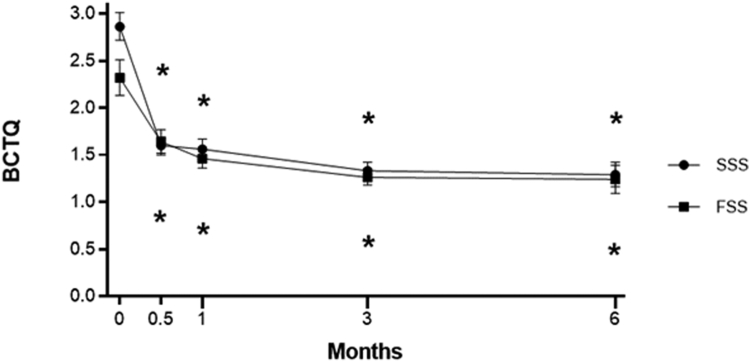
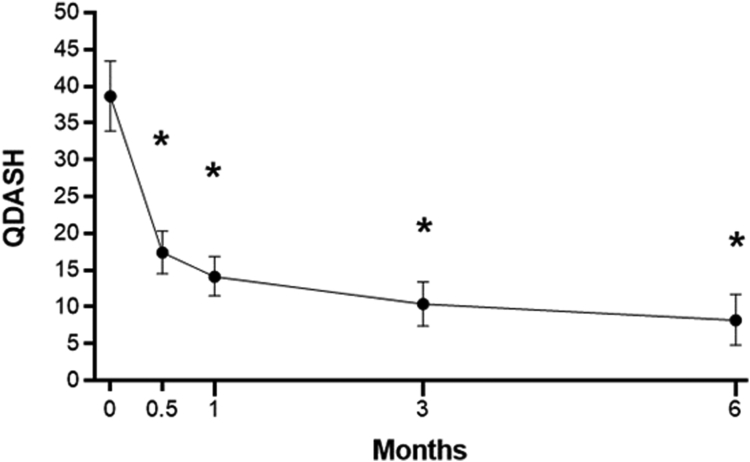
Patient-reported outcome data were available in 65 (100%) patients between 2 weeks and 1 month after surgery and in 39 (60%) patients between 3 and 6 months after surgery. Statistically significant improvements in BCTQ-SSS, BCTQ-FSS, and QuickDASH scores occurred by the 2-week follow-up interval and persisted at 6 months postprocedure (P < .001 for each outcome at each follow-up interval) (Table 1 and Figs. 1 and 2). The improvements exceeded the previously published minimal clinically important difference values of 15 points for QuickDASH,26 1.17 points for BCTQ-SSS,27 and 0.74 points for BCTQ-FSS27 at each follow-up interval, with the exception of BCTQ-FSS at 2 weeks.
Table 1.
Changes in Patient-Reported Outcomes Following CTR-US∗
| Outcome | 2 Wk | 1 Mo | 3 Mo | 6 Mo |
|---|---|---|---|---|
| BCTQ-SSS | −1.26 (−1.46, −1.07) | −1.30 (−1.52, −1.08) | −1.53 (−1.75, −1.31) | −1.58 (−1.83, −1.33) |
| BCTQ-FSS | −0.68 (−0.90, −0.46) | −0.86 (−1.11, −0.61) | −1.06 (−1.32, −0.80) | −1.08 (−1.39, −0.78) |
| QuickDASH | −21.2 (−26.7, −15.8) | −24.5 (−30.8, −18.3) | −28.2 (−35.1, −21.4) | −30.4 (−37.7, −23.1) |
ANOVA, analysis of variance.
Values reported as mean change (95% confidence interval) from pretreatment calculated from linear mixed-model ANOVA with Bonferroni correction.
Figure 1.
Change in BCTQ-SSS and BCTQ-FSS score over 6 months following CTR-US. Plotted data are mean and 95% confidence interval. Asterisk denotes P < .001 for change compared to baseline using a Bonferroni-adjusted linear mixed model.
Figure 2.
Change in QuickDASH score over 6 months following CTR-US. Plotted data are mean and 95% confidence interval. Asterisk denotes P < .001 for change compared to baseline using a Bonferroni-adjusted linear mixed model.
MRI scans were available on 13 patients/17 wrists at a mean of 3 months following surgery (range, 1–7 months). The 3-month clinical outcomes of patients who returned for an MRI were comparable with those who did not (BCTQ-SSS: 1.5 ± 0.4 vs 1.3 ± 0.3; BCTQ-FSS: 1.1 ± 0.2 vs 1.3 ± 0.3; QuickDASH: 7 ± 8 vs 10 ± 10), suggesting that the MRI findings are representative of the entire sample. MRI parameters are summarized in Table 2. Complete transection of the TCL was documented in all 17 wrists. Key postoperative MRI findings including a 22% increase in the cross-sectional area (CSA) of the carpal tunnel at the hamate (P < .001), a 52% increase in the median nerve CSA at the hamate (P < .001), an 18% reduction in the median nerve signal intensity (P = .002), a 38% reduction in the flattening ratio of the median nerve at the hamate (P < .001), a 33% reduction in the flattening ratio of the median nerve at the pisiform (P < .001), a 20% reduction in the flattening ratio of the carpal tunnel at the hamate (P < .001), and a palmar shift of the median nerve relative to the hamate were appreciated in all cases. These observed changes are consistent with the decompression of the carpal tunnel, reduction in median nerve compression in the tunnel (especially distally at the hamate), and reduction in intraneural edema.16, 17, 18, 19, 20, 21 Representative preoperative and postoperative MRI images are provided in Figures 3 and 4.
Table 2.
Changes in MRI Parameters Following CTR-US
| MRI Parameter | Pre∗ | Post∗,† | Change (%) | Change (Absolute) | 95% CI (Absolute) | P Value |
|---|---|---|---|---|---|---|
| Carpal tunnel, hamate level | ||||||
| Width (mm) | 22.0 ± 1.7 | 21.9 ± 2.6 | 0% | −0.1 | −1.1, 0.9 | .82 |
| Height (mm) | 11.3 ± 1.5 | 14.1 ± 2.0 | 24% | 2.7 | 1.9, 3.6 | <.001 |
| CSA (mm2) | 215 ± 39 | 263 ± 44 | 22% | 48 | 34, 62 | <.001 |
| Flattening ratio‡ | 1.96 ± 0.25 | 1.57 ± 0.23 | −20% | −0.39 | −0.55, −0.23 | <.001 |
| Median nerve, hamate level | ||||||
| Long diameter (mm) | 6.73 ± 1.95 | 5.71 ± 2.01 | −15% | −1.02 | −1.77, −0.28 | .01 |
| Short diameter (mm) | 2.25 ± 0.70 | 3.01 ± 0.95 | 34% | 0.76 | 0.35, 1.17 | .001 |
| CSA (mm2)§ | 10.1 ± 6.7 | 15.4 ± 8.4 | 52% | 5.3 | 3.8, 6.8 | <.001 |
| Flattening ratio‡ | 3.17 ± 0.98 | 1.96 ± 0.56 | −38% | −1.21 | −1.68, −0.75 | <.001 |
| Volar distance (mm) | 9.22 ± 1.43 | 11.08 ± 2.10 | 20% | 1.86 | 1.11, 2.61 | <.001 |
| Signal intensity | 912 ± 288 | 749 ± 265 | −18% | −163 | −253, −72 | .002 |
| Median nerve, pisiform level | ||||||
| Short diameter (mm) | 2.84 ± 0.98 | 3.46 ± 1.10 | 22% | 0.62 | 0.18, 1.06 | .008 |
| Long diameter (mm) | 8.10 ± 1.64 | 6.96 ± 1.76 | −14% | −1.13 | −2.1, −0.17 | .02 |
| Flattening ratio‡ | 3.13 ± 1.06 | 2.10 ± 0.54 | −33% | −1.03 | −1.46, −0.6 | <.001 |
| Volar distance (mm) | 11.8 ± 2.7 | 13.3 ± 2.9 | 13% | 1.5 | 0.9, 2.1 | <.001 |
| Median nerve position‖ | ||||||
| Dorsal to line | 6% (1/17) | 0% (0/0) | <.001 | |||
| Crosses line | 94% (16/17) | 6% (1/17) | ||||
| Palmar to line | 0% (0/0) | 94% (16/17) |
CI, confidence interval; CSA, cross-sectional area.
Values reported as mean ± SD or percent (n/N).
MRI performed at mean 3 months (range, 1–7 months) after CTR-US.
Flattening ratio was defined as the ratio of the long cross-sectional diameter to the short-cross-sectional diameter, with the larger ratio representing a flatter median nerve.
Increase in the median nerve CSA is expected at the hamate following successful decompression.
Center of the median nerve was compared to a line drawn from the hook of the hamate to the ridge of the trapezium. All nerves demonstrated a palmar shift at the time of post–CTR-US MRI.
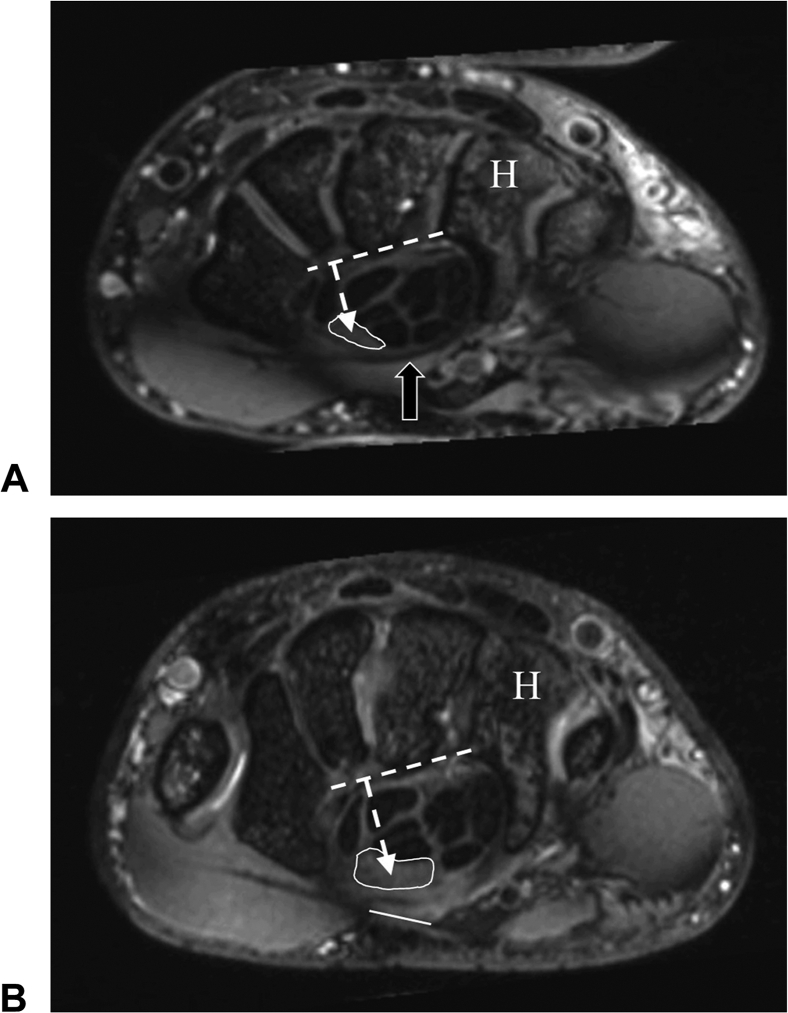
Figure 3.
A Preoperative T2 weighted axial MRI at the hamate (H) level shows the cross-sectional area of median nerve (white outline) with the transverse carpal ligament intact (arrow). B 3-months postoperative T2 weighted axial image at the hamate level shows an increase in the cross-sectional area of median nerve (white outline) and a gap in the transected transverse carpal ligament (white line). Dashed line and arrow in images A and B show a palmar shift of median nerve reported as the difference in postoperative distance measured from the palmer aspect of the carpals (dashed line) and the center of the median nerve (dashed arrow).
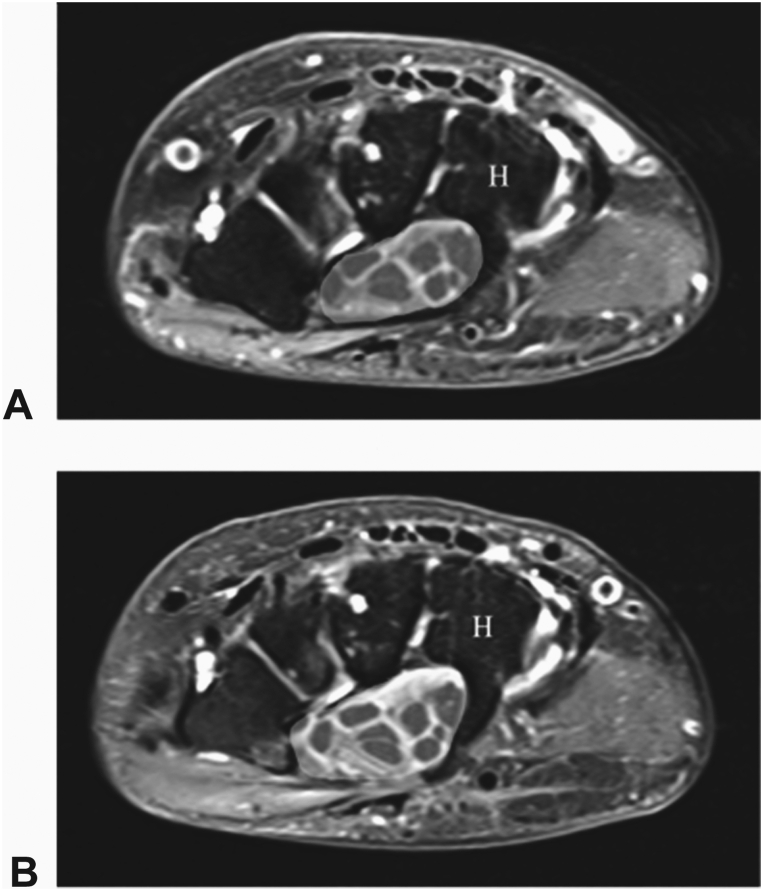
Figure 4.
A Preoperative T2 weighted axial MRI at the hamate (H) level shows the cross-sectional area of carpal tunnel (yellow shading). B 4-months postoperative T2 weighted axial image at the hamate level shows an increase in the cross-sectional area of the carpal tunnel (yellow shading).
Discussion
The purpose of this study was to report intermediate-term outcomes following CTR-US using WALANT, including expanded post–CTR-US morphological data and a subset of patients with preoperative and postoperative MRI. Our results demonstrated that CTR-US using WALANT in a procedure room setting was safe, effective, and showed clinical improvements in validated patient-reported outcomes as early as 2 weeks after surgery, which were maintained throughout the 6-month follow-up period. These results of rapid improvement are similar to those reported in previous studies using US-guided techniques and similarly less-invasive CTR techniques such as ECTR.3,8,10,12,15,28,29
Several studies have reported clinical outcomes following CTR-US using QuickDASH and BCTQ,3,8,10,12, 13, 14, 15 but a few have reported return to work and return to normal activity outcomes.3,30,31 These results are important because they demonstrate a major goal of minimally invasive CTR techniques, like CTR-US, of reducing recovery time, thereby lessening the burden of work absenteeism. In the current study, 97% of patients returned to normal activity by 2 weeks and all patients had returned to work. These results with respect to return to work are faster than those reported for open CTR and similar to those reported for ECTR.3,27,29, 30, 31, 32, 33 Although these data are promising, we consider them preliminary because of the relatively small number of patients.
Detailed MRI analysis demonstrated morphological changes consistent with successful carpal tunnel decompression in all cases. A few studies have used ultrasound to evaluate morphological changes following CTR-US,34,35 but only Petrover et al21 has used MRI to quantify these changes. In that study, 129 patients who underwent CTR-US received baseline preoperative and postoperative MRI at 1 month. The results showed a complete transection of the TCL and nerve decompression in all cases. The metrics used to demonstrate nerve decompression were CSA of the median nerve and changes in nerve position, both measured at the level of the hamate.21 Results of our MRI analysis agree with the findings of Petrover et al,11 as both investigations reported a complete transection of the TCL in 100% of the cases, a significant increase in median nerve CSA, and a palmar shift of the median nerve. Our investigation expands this work by documenting increased carpal tunnel dimensions, reduced flattening ratios of the median nerve and carpal tunnel (ie, reduced flattening), and reduced median nerve T2 signal intensities. The results of these expanded metrics are all consistent with postoperative decompression following CTR-US and are consistent with previous data reported for morphological changes of carpal tunnel and its contents using MRI following endoscopic CTR and open CTR.16, 17, 18, 19, 20 Previous work has used signal intensity of the median nerve expressed as the ratio to the hypothenar muscles to avoid variations in signal encountered with surface coils.17,19 For the purpose of this investigation, T2 signal intensity of the median nerve was measured by placing a region of interest over the nerve at the level of the hamate and reporting as a single value. Results showed an 18% decrease in median nerve signal intensity after surgery. Whether this is a clinically significant value remains unclear without other comparable works with set a precedent. However, we hypothesize that this decrease is consistent with some degree of reduction in intraneural edema.17,19
Major strengths of this study are the use of validated patient-reported outcomes (BCTQ and QuickDASH), reporting return to normal activities and work status at 2 weeks, and the inclusion of morphological MRI data measured in blinded fashion for a subset of patients post CTR-US. The novel MRI data presented herein are consistent with carpal tunnel decompression and validate the ability of CTR-US to result in favorable morphological changes similar to those reported following open and ECTR.16, 17, 18, 19, 20 The primary limitations of this study include the retrospective review of prospectively collected data, the relatively small number of patients with 3–6-month clinical follow-up, and the inability to perform pre- and postoperative MRI scans on all patients. Although the clinical results in this study were favorable and commensurate with previously published intermediate-term outcomes for open and ECTR, additional research examining larger numbers of patients with longer follow-up will further define the role of CTR-US in the surgical treatment of patients with CTS.10,29,33 Although only 13 patients (17 wrists) were able or willing to have a postoperative MRI scan, the clinical outcomes of these patients were similar to those in the remainder of the group who did not have postoperative MRI scans, suggesting that the reported MRI results are representative of the entire sample of patients. Furthermore, the number of patients with pre- and postoperative MRI scans in the current study is commensurate with recently published studies using MRI to study morphological changes following CTR.16,20
In conclusion, CTR-US using WALANT in a procedure room setting was safe, effective, and resulted in morphological changes consistent with carpal tunnel decompression as demonstrated by MRI.
Acknowledgments
The authors thank Larry Miller, PhD, PStat for statistical analysis and critical review. The funding source provided financial support for statistical analysis, but was not involved in study design; in the interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Declaration of interests: J.C.M. reports consultancy with Sonex Health, Inc unrelated to the current manuscript and is Founder and CEO of McGinley Education Innovations, LLC, which owns, produces, and sells the McGinley Augmented Reality for Ultrasound System. No benefits in any form have been received or will be received by the other authors related directly to this article.
References
- 1.Atroshi I., Englund M., Turkiewicz A., Tägil M., Petersson I.F. Incidence of physician-diagnosed carpal tunnel syndrome in the general population. Arch Intern Med. 2011;171(10):943–944. doi: 10.1001/archinternmed.2011.203. [DOI] [PubMed] [Google Scholar]
- 2.Palmer D.H., Hanrahan L.P. Social and economic costs of carpal tunnel surgery. Instr Course Lect. 1995;44:167–172. [PubMed] [Google Scholar]
- 3.Fowler J.R., Chung K.C., Miller L.E. Multicenter pragmatic study of carpal tunnel release with ultrasound guidance. Expert Rev Med Devices. 2022;19(3):273–280. doi: 10.1080/17434440.2022.2048816. [DOI] [PubMed] [Google Scholar]
- 4.Badger S.A., O’Donnell M.E., Sherigar J.M., Connolly P., Spence R.A. Open carpal tunnel release—still a safe and effective operation. Ulster Med J. 2008;77(1):22–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Okutsu I., Ninomiya S., Takatori Y., Ugawa Y. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5(1):11–18. doi: 10.1016/0749-8063(89)90084-4. [DOI] [PubMed] [Google Scholar]
- 6.Aslani H.R., Alizadeh K., Eajazi A., et al. Comparison of carpal tunnel release with three different techniques. Clin Neurol Neurosurg. 2012;114(7):965–968. doi: 10.1016/j.clineuro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Benson L.S., Bare A.A., Nagle D.J., et al. Complications of endoscopic and open carpal tunnel release. Arthroscopy. 2006;22(9):919–924.e9242. doi: 10.1016/j.arthro.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Capa-Grasa A., Rojo-Manaute J.M., Rodríguez F.C., Martín J.V. Ultra minimally invasive sonographically guided carpal tunnel release: an external pilot study. Orthop Traumatol Surg Res. 2014;100(3):287–292. doi: 10.1016/j.otsr.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Nakamichi K., Tachibana S., Yamamoto S., Ida M. Percutaneous carpal tunnel release compared with mini-open release using ultrasonographic guidance for both techniques. J Hand Surg Am. 2010;35(3):437–445. doi: 10.1016/j.jhsa.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Rojo-Manaute J.M., Capa-Grasa A., Chana-Rodríguez F., et al. Ultra-minimally invasive ultrasound-guided carpal tunnel release: a randomized clinical trial. J Ultrasound Med. 2016;35(6):1149–1157. doi: 10.7863/ultra.15.07001. [DOI] [PubMed] [Google Scholar]
- 11.Petrover D., Hakime A., Silvera J., Richette P., Nizard R. Ultrasound-guided surgery for carpal tunnel syndrome: a new interventional procedure. Semin Intervent Radiol. 2018;35(4):248–254. doi: 10.1055/s-0038-1673360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leiby B.M., Beckman J.P., Joseph A.E. Long-term clinical results of carpal tunnel release using ultrasound guidance. Hand (N Y) 2022;17(6):1074–1081. doi: 10.1177/1558944720988080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph A.E., Leiby B.M., Beckman J.P. Clinical results of ultrasound-guided carpal tunnel release performed by a primary care sports medicine physician. J Ultrasound Med. 2020;39(3):441–452. doi: 10.1002/jum.15120. [DOI] [PubMed] [Google Scholar]
- 14.Kamel S.I., Freid B., Pomeranz C., Halpern E.J., Nazarian L.N. Minimally invasive ultrasound-guided carpal tunnel release improves long-term clinical outcomes in carpal tunnel syndrome. AJR Am J Roentgenol. 2021;217(2):460–468. doi: 10.2214/AJR.20.24383. [DOI] [PubMed] [Google Scholar]
- 15.Bergum R.A., Ciota M.R. Office-based carpal tunnel release using ultrasound guidance in a community setting: long-term results. Cureus. 2022;14(7) doi: 10.7759/cureus.27169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters B.R., Martin A.M., Memauri B.F., et al. Morphologic analysis of the carpal tunnel and median nerve following open and endoscopic carpal tunnel release. Hand (N Y) 2021;16(3):310–315. doi: 10.1177/1558944719861711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng A.W.H., Griffith J.F., Tsai C.S.C., et al. MRI of the carpal tunnel 3 and 12 months after endoscopic carpal tunnel release. AJR Am J Roentgenol. 2021;216(2):464–470. doi: 10.2214/AJR.20.23066. [DOI] [PubMed] [Google Scholar]
- 18.Momose T., Uchiyama S., Kobayashi S., Nakagawa H., Kato H. Structural changes of the carpal tunnel, median nerve and flexor tendons in MRI before and after endoscopic carpal tunnel release. Hand Surg. 2014;19(2):193–198. doi: 10.1142/S0218810414500191. [DOI] [PubMed] [Google Scholar]
- 19.Cudlip S.A., Howe F.A., Clifton A., Schwartz M.S., Bell B.A. Magnetic resonance neurography studies of the median nerve before and after carpal tunnel decompression. J Neurosurg. 2002;96(6):1046–1051. doi: 10.3171/jns.2002.96.6.1046. [DOI] [PubMed] [Google Scholar]
- 20.Grandizio L.C., Rocha D.F.B., Beck J.D., et al. Median nerve and carpal tunnel morphology before and after endoscopic carpal tunnel release: a 6-year follow-up study. Hand (N Y) 2023;18(suppl 1):56S–61S. doi: 10.1177/15589447211058819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrover D., Silvera J., De Baere T., Vigan M., Hakimé A. Percutaneous ultrasound-guided carpal tunnel release: study upon clinical efficacy and safety. Cardiovasc Intervent Radiol. 2017;40(4):568–575. doi: 10.1007/s00270-016-1545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalonde D. Minimally invasive anesthesia in wide awake hand surgery. Hand Clin. 2014;30(1):1–6. doi: 10.1016/j.hcl.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Beaton D.E., Wright J.G., Katz J.N. Upper Extremity Collaborative Group. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87(5):1038–1046. doi: 10.2106/JBJS.D.02060. [DOI] [PubMed] [Google Scholar]
- 24.Sambandam S.N., Priyanka P., Gul A., Ilango B. Critical analysis of outcome measures used in the assessment of carpal tunnel syndrome. Int Orthop. 2008;32(4):497–504. doi: 10.1007/s00264-007-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leite J.C., Jerosch-Herold C., Song F. A systematic review of the psychometric properties of the Boston Carpal Tunnel Questionnaire. BMC Musculoskelet Disord. 2006;7:78. doi: 10.1186/1471-2474-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franchignoni F., Vercelli S., Giordano A., et al. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH) J Orthop Sports Phys Ther. 2014;44(1):30–39. doi: 10.2519/jospt.2014.4893. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.K., Jeon S.H. Minimal clinically important differences in the Carpal Tunnel Questionnaire after carpal tunnel release. J Hand Surg Eur Vol. 2013;38(1):75–79. doi: 10.1177/1753193412442137. [DOI] [PubMed] [Google Scholar]
- 28.Vasiliadis H.S., Xenakis T.A., Mitsionis G., Paschos N., Georgoulis A. Endoscopic versus open carpal tunnel release. Arthroscopy. 2010;26(1):26–33. doi: 10.1016/j.arthro.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Trumble T.E., Diao E., Abrams R.A., Gilbert-Anderson M.M. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84(7):1107–1115. doi: 10.2106/00004623-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 30.de la Fuente J., Aramendi J.F., Ibañez J.M., et al. Minimally invasive ultrasound-guided vs open release for carpal tunnel syndrome in working population: a randomized controlled trial. J Clin Ultrasound. 2021;49(7):693–703. doi: 10.1002/jcu.23019. [DOI] [PubMed] [Google Scholar]
- 31.Miller L.E., Chung K.C. Determinants of return to activity and work after carpal tunnel release: a systematic review and meta-analysis. Expert Rev Med Devices. 2023;20(5):417–425. doi: 10.1080/17434440.2023.2195549. [DOI] [PubMed] [Google Scholar]
- 32.Helm R.H., Vaziri S. Evaluation of carpal tunnel release using the Knifelight instrument. J Hand Surg Br. 2003;28(3):251–254. doi: 10.1016/s0266-7681(02)00395-9. [DOI] [PubMed] [Google Scholar]
- 33.Atroshi I., Larsson G.U., Ornstein E., et al. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: randomised controlled trial. BMJ. 2006;332(7556):1473. doi: 10.1136/bmj.38863.632789.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chappell C.D., Beckman J.P., Baird B.C., Takke A.V. Ultrasound (US) changes in the median nerve cross-sectional area after microinvasive us-guided carpal tunnel release. J Ultrasound Med. 2020;39(4):693–702. doi: 10.1002/jum.15146. [DOI] [PubMed] [Google Scholar]
- 35.Latzka E.W., Henning P.T., Pourcho A.M. Sonographic changes after ultrasound-guided release of the transverse carpal ligament: a case report. PM R. 2018;10(10):1125–1129. doi: 10.1016/j.pmrj.2018.02.018. [DOI] [PubMed] [Google Scholar]