Abstract
Purpose
Distal radius fractures (DRFs) are among the most common orthopedic injuries, especially in the elderly. A wide variety of approaches have been advocated as successful treatment modalities; yet, there remains variability in practice patterns of DRF in patients with osteoporosis and osteopenia. Using large data set analysis, we sought to determine the risk profile of operative fixation of DRF in patients with low bone mineral density.
Methods
A commercially available health care database, PearlDiver, was queried for all patients who underwent open reduction internal fixation of DRFs between 2010 and 2020. The study population was divided into groups based on the presence or absence of osteopenia or osteoporosis and was further classified by patients who were receiving bisphosphonate therapy. Complication rates were calculated, including rates of malunion, surgical site infection, osteomyelitis, hardware failure, and hardware removal. Five-year future fragility fractures were defined in hip, vertebrae, humerus, and wrist fractures. Chi-square analysis and logistic regression were performed to determine an association between these comorbidities and various postoperative complications.
Results
A total of 152,926 patients underwent open reduction internal fixation of a DRF during the study period. Chi-square analysis of major complications at 3 months showed a statistically significant increase in malunion in patients with osteopenia (P = .05) and patients with osteoporosis (P = .05) who underwent open reduction internal fixation. Logistic regression analysis at 12 months after surgery demonstrated that osteopenia was associated with an increased risk of hardware failure (P < .0001), hardware removal (P < .0001), surgical site infection (P < .0001), and malunion (P = .004). Osteoporosis was associated with a significantly increased risk of hardware failure (P = .01), surgical site infection (P < .0001), and malunion (P < .0001).
Conclusions
We demonstrated, using large data set analysis, that DRF patients with osteopenia and osteoporosis are predicted to be at increased risk of multiple postoperative complications, and thus, bone density should be strongly considered in treatment planning for these patients.
Type of study/level of evidence
Prognostic III.
Key words: Distal radius, Fragility fracture, ORIF, Osteopenia, Osteoporosis
Distal radius fractures (DRFs) are among the most common orthopedic injuries in the western world and are common in the elderly.1, 2, 3, 4 These fractures are one of the most common fragility fractures associated with osteoporosis.5, 6, 7 A wide variety of approaches have been advocated as successful treatment modalities, ranging from surgical (including open reduction internal fixation [ORIF] or closed reduction and percutaneous fixation) to nonsurgical treatment (casting). Regardless of the treatment modality chosen, DRFs in patients with preexisting osteopenia or osteoporosis present a considerable economic and resource utilization burden on the health care system because the incidence of this fracture has been increasing due to the aging of the global population.8, 9, 10, 11
The association between DRFs and various comorbidities, including osteopenia and osteoporosis, has been well described; however, optimal treatment for different fracture patterns of DRFs and in various patient populations is still under investigation.12,13 Because the outcomes for DRF repair are frequently based on objective clinical findings, including pain, strength, and range of motion, these measures may not always correlate with functional recovery in the patient, and as such, complications vary widely in the DRF literature.14 In some smaller studies, osteoporosis has been shown to be associated with early instability, malunion, and late carpal malalignment compared with normal bone mineral density in patients with DRF; however, these studies did not demonstrate large effect sizes, and the study populations were not fully characterized.15,16 Furthermore, patients with low bone mineral density are often treated with bisphosphonate therapy, and surgical outcomes for DRF in this patient population have not been reported.
On the basis of limitations in treatment recommendations and studies analyzing surgical management of DRF in patients with low bone mineral density, we used a large data approach to understand the complex relationship between osteoporosis/osteopenia and postoperative complications after operative fixation of DRF. We retrospectively analyzed these outcomes to provide hand surgeons treating DRF in patients with low bone mineral density an assessment of the risks and benefits of operative management of these fractures, and we further correlated our findings to bisphosphonate treatment in this patient population. Familiarity with complications in this patient population will allow surgeons to make informed decisions on a case-by-case basis.
Methods
The commercially available health care database PearlDiver represents 122 million patients across the nation with various insurance types. The database was queried for all patients who underwent ORIF of DRF between 2010 and 2020. The data were extracted using Current Procedural Terminology; International Classification of Diseases (ICD), Ninth Revision; and ICD Tenth Revision codes related to orthopedic procedures (Appendices 1–3, available on the Journal’s website at www.jhsgo.org). Institutional review board approval was not required for the study because all data were deidentified on the basis of Health Insurance Portability Accountability Act compliancy.
Patients who underwent ORIF were divided into groups based on the presence or absence of osteopenia or osteoporosis. To control for bone mass in the osteoporosis/osteopenia group, only men and women older than 50 years and women between the ages of 40 and 50 years with a concurrent diagnosis of premature menopause were included. Analysis of complications at 3, 6, 9, and 12 months after operation included malunion, surgical site infection, osteomyelitis, hardware failure, and hardware removal. Complications were also analyzed in the setting of bisphosphonate therapy. Fragility fractures, defined as hip, vertebrae, humerus, and wrist fractures, were analyzed 5 years after DRF surgery. Patients with osteoporosis/osteopenia were excluded if they did not meet the age criteria or if they were lost to follow-up 1 year after surgery. Patients were excluded from the normal bone mineral density group if they had a diagnosis of osteoporosis or osteopenia at the time of surgery based on ICD Tenth Revision codes. Simple logistic regression and chi-square analysis were performed to determine the association between osteoporosis/osteopenia and these comorbidities and various postoperative complications. All statistical analyses were performed using the R software integrated within the PearlDiver software, and statistical significance was determined at a P value of <.05.
Results
A total of 152,926 patients underwent ORIF of a DRF during the study period. Chi-square analysis of major complications at 3 months demonstrated a marked increase in malunion in patients with osteopenia (odds ratio [OR], 1.16; 95% CI, 0.97–1.39; P = .0526) and patients with osteoporosis (OR, 1.19; 95% CI, 1.01–1.41; P = .0526) who underwent ORIF. This difference was not observed at 6, 9, or 12 months after surgery (Table). Chi-square analysis demonstrated that the risk of fragility fractures in the 5 years after DRF surgery was significantly higher in both osteopenia (OR, 1.58; 95% CI, 1.51–1.66; P < .0001) and osteoporosis (OR, 2.20; 95% CI, 2.11–2.30; P < .0001).
Table.
Demographics of Patients With and Without a Diagnosis of Osteopenia or Osteoporosis Undergoing ORIF of DRF
| Characteristic | (+) Osteopenia | (+) Osteoporosis | Control |
|---|---|---|---|
| Total | 22,659 | 27,531 | 102,736 |
| Women | 21,766 | 26,611 | 78,463 |
| Age (y), mean | 67.56 | 69.38 | 61.6 |
| 40–44 | 141 | 106 | 6,255 |
| 45–49 | 307 | 262 | 8,499 |
| 50–54 | 979 | 799 | 12,329 |
| 55–59 | 2,554 | 2,270 | 16,844 |
| 60–64 | 4,116 | 4,013 | 16,614 |
| 65–69 | 4,394 | 4,900 | 13,883 |
| 70–74 | 4,693 | 6,585 | 16,853 |
| 75–79 | 4,398 | 6,693 | 9,603 |
| 80–84 | 1,087 | 1,903 | 1,856 |
| Region | |||
| Midwest | 6,267 | 6,951 | 27,700 |
| Northeast | 4,271 | 5,411 | 17,670 |
| South | 9,100 | 11,663 | 41,262 |
| West | 2,969 | 3,419 | 15,347 |
| Service Location | |||
| Inpatient | 2,389 | 3,547 | 12,244 |
| Outpatient | 20,160 | 23,854 | 89,950 |
| Unknown/other | 110 | 130 | 542 |
| Plan | |||
| Commercial | 15,116 | 17,051 | 71,417 |
| Medicaid | 537 | 700 | 5,205 |
| Medicare | 6,499 | 9,238 | 23,511 |
| Government | 329 | 347 | 1,621 |
| Unknown/other | 178 | 195 | 982 |
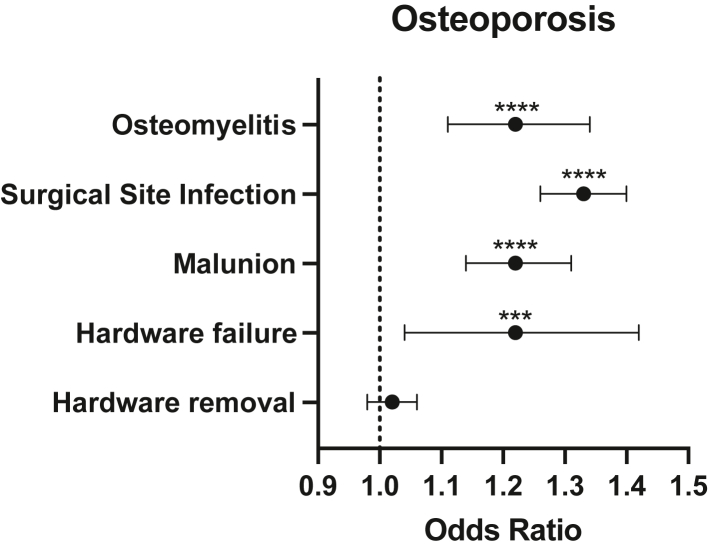
To model risk factors for complications in patients with low bone mineral density undergoing operative fixation of DRF, logistic regression was used. Logistic regression analysis at 12 months after surgery demonstrated that osteopenia was associated with an increased risk of hardware failure (OR, 1.44; 95% CI, 1.22–1.70; P < .0001), hardware removal (OR, 1.11; 95% CI, 1.31–1.16; P < .0001), surgical site infection (OR, 1.28; 95% CI, 1.20–1.36; P < .0001), and malunion (OR, 1.12; 95% CI, 1.03–1.21; P = .04) (Fig. 1). Osteoporosis was not associated with a significantly increased risk of hardware removal (OR, 1.02; 95% CI, 0.98–1.06; P = .21) but was associated with a significantly increased risk of hardware failure (OR, 1.22; 95% CI, 1.04–1.42; P = .01), surgical site infection (OR, 1.33; 95% CI, 1.26–1.40; P < .0001), and malunion (OR, 1.22; 95% CI, 1.14–1.31; P < .0001) (Fig. 2).
Figure 1.
Forest plot demonstrating OR of postoperative complications in patients with osteopenia. ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
Figure 2.
Forest plot demonstrating OR of postoperative complications in patients with osteoporosis. ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
In the logistic regression model, bisphosphonate use was significantly associated with fewer malunion events (OR, 0.93; 95% CI, 0.85–1.02; P < .0001), fewer surgical site infections (OR, 0.82; 95% CI, 0.76–0.88; P < .0001), and fewer instances of hardware removal (OR, 0.89; 95% CI, 0.85–0.93; P < .0001). Bisphosphonates were not significantly associated with hardware failure (OR, 0.87; 95% CI, 0.72–1.06; P = .195) or osteomyelitis (OR, 0.77; 95% CI, 0.63–0.94; P = .221).
Discussion
This study hypothesized that patients with osteopenia and osteoporosis would have an increased risk of postoperative complications after operative fixation of DRFs. In a retrospective analysis, we determined that patients with osteoporosis or osteopenia who underwent ORIF had an increased risk of malunion in the first 3 months after surgery compared with controls with normal bone mineral density (T score, −1 to +1). Furthermore, our logistic regression analysis predicted a significant association (P < .0001) between osteopenia/osteoporosis and several perioperative complications, including surgical site infection, and malunion.
Although this association was not significant (P = .0526) further from surgery, the first 3 months after surgery were associated with an increased risk of malunion in patients with osteopenia and osteoporosis who underwent ORIF. This difference was not observed further from surgery, which may represent delayed bone healing in patients with osteoporosis/osteopenia. Although this is yet to be fully explored in clinical studies, animal models have shown evidence that osteoporosis negatively influences fracture healing and may explain why osteoporotic fractures are delayed in healing at 3 months after surgery.17 Although patients with osteoporosis/osteopenia may show early differences in fracture healing, our data suggest that their ultimate outcome at 12 months is similar to that of patients with normal bone mineral density. Further, osteoporotic bone does not resist the pullout of hardware and fixation devices in the same manner as normal bone and can result in potential loss of reduction or alignment.18 Radiographic alignment is not always predictive of functional outcome, which we were unable to correlate in our data set.19, 20, 21 An interesting area of future investigation would be to understand whether this early difference in radiographic alignment could be correlated with the type of hardware chosen or the severity of the fracture.
With an aging population and osteoporosis-related fracture rates rising, a better understanding of the relationship between low bone mineral density and postoperative outcomes is needed. Distal radius fractures are the most common injury to the upper extremity and are a large source of annual health expenditure because DRFs are often managed surgically, with $170 million of DRF-attributable payments made by Medicare in 2007.8 The increasing rate of operative management of this type of injury is likely secondary to the advent of volar locking plating compared with traditional dorsal plating, in addition to the increase in hand-specific surgical training.22,23
As internal fixation of DRF is more widely used, the financial burden of DRF will continue to grow and may reach $240 million in annual Medicare cost if the internal fixation rate continues to increase and reaches 50%.8 Distal radius fractures were traditionally treated conservatively and cost-effectively with casting, with the mean cost per Medicare beneficiary being $1400 and the mean cost per beneficiary being $3800 for internal fixation.8 Research comparing outcomes of ORIF with those of nonsurgical casting demonstrated better functional results in young patients treated with internal fixation, although these findings may not entirely apply to similar internal fixation procedures in osteoporotic bone.24,25 Yet, the long-term gain measured by quality-adjusted life years only minimally outweighs the shorter-term risks of surgical intervention, and level one evidence to support ORIF in older patients does not exist.25 Medicare ORIF expenditures are nearly triple that of conservative fixation, and accurate appraisal of costs and benefits is important to allocate resources and benefit the correct patient demographic.8
Although osteoporosis has been shown to be associated with increased fracture severity and higher incidence of early instability, measurable outcomes in this patient population have been debated in the literature.15 One group has reported osteoporotic patients to have worse functional outcomes, demonstrated by an average of 15 points higher Disabilities of the Arm, Shoulder, and Hand (questionnaire) scores, despite similar radiographic and range of motion outcomes.26 However, other groups exploring clinical outcomes in patients with osteoporosis have not reported a difference in function or radiologic deformity after DRF.27,28 It is difficult to draw conclusions because these studies were small and the characterization of their study populations was limited; therefore, we sought to analyze these outcomes on a larger scale.
Although our data set did not show any statistically significant differences in the postoperative complications in patients with osteoporosis/osteopenia compared with those in controls, this analysis only accounts for the relationship between bone mineral density and the complication being tested. Therefore, we used simple logistic regression to model the relationship based on the PearlDiver data set to predict the likelihood that patients with osteopenia and osteoporosis would develop postoperative complications. We found that osteopenia was associated with an increased risk of hardware failure, hardware removal, surgical site infection, and malunion. Osteoporosis was not associated with an increased risk of hardware removal but was associated with a significantly (P < .001) increased risk of hardware failure, surgical site infection, and malunion. Because the ICD codes for hardware removal included bridge plate removal, these data indicate that surgeons may be opting to use bridge plates more frequently in patients with osteopenia but not osteoporosis. Further, other groups have shown an association between osteoporosis and surgical site infections in orthopedic procedures other than DRF fixation.29, 30, 31 More research is needed to fully understand this association because other factors, including age, comorbidities, and surgical technique, may influence the risk of surgical site infection. These complications may be attributed to compromised microarchitecture of the bone and increased cortical porosity, as previously described.32 Weak cortices in the osteoporotic bone provide poor screw purchase and may not maintain alignment in the postoperative period.
Our study presents a useful model to identify high-risk populations undergoing DRF fixation that can potentially affect treatment options, patient selection, and perioperative counseling. Statistical modeling of our data set predicts that surgical management of patients with low bone mineral density carries the risk of poor bone healing and infection. The decision to proceed with operative fixation of DRF should be carried out on a case-by-case basis, and the risks and benefits should be weighed in a patient-specific manner.
Beyond the management of DRF, the occurrence of DRF can be an important opportunity to diagnose and treat osteoporosis to prevent future fragility fractures. In accordance with the literature, we found that DRFs are associated with increased risk of subsequent fragility fractures, including hip, vertebrae, humerus, and distal radius, and are thus an important indicator of underlying bone mineral disease.33,34 We encourage surgeons to consider bone mineral density testing and vitamin D/calcium levels and to maintain endocrinopathy, hematologic disorders, and medication side-effects on their differential diagnosis for secondary causes of osteoporosis.18
Furthermore, our data support the protective effect of bisphosphonates in operative outcomes in DRF. In addition to fewer postoperative complications, bisphosphonate use protects these patients from future fragility fractures, as previously shown.35, 36, 37 Although bisphosphonates have not been shown to have a marked effect on fracture healing time, the secondary effects of increasing bone mineral density, reducing bone synthesis, and reducing resorption markers may result in a lower bone turnover state, thus improving surgical outcomes.38 Some authors have suggested that DRF is an important point of intervention in osteoporosis treatment, and previous analysis has shown that initiation of bisphosphonate therapy at the time of DRF significantly (P < .05) reduces the overall burden of hip fracture.35 Our data indicate that bisphosphonate treatment prior to DRF not only would prevent fragility fractures but also may improve surgical outcomes if a fragility fracture were to occur.
The limitations of our study include those inherent to large-volume databases, such as reliance on ICD Ninth Revision and Current Procedural Terminology coding and potential confounding variables not recorded in the data set. Additionally, our study is retrospective and was limited to patients who underwent surgical repair of their DRF. This indicates that our patient population had more clinically and radiographically severe fractures, necessitating surgical intervention. The nature and severity of these fractures may predispose patients to postoperative complications. Although these are major complications, they may not necessarily correlate with functional outcomes that we were unable to analyze in our study.
Footnotes
K.R.T. and M.H.F. contributed equally to this work.
Declaration of interests: No benefits in any form have been received or will be received related directly to this article.
Supplementary Data
References
- 1.Nellans K.W., Kowalski E., Chung K.C. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113–125. doi: 10.1016/j.hcl.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung K.C., Spilson S.V. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908–915. doi: 10.1053/jhsu.2001.26322. [DOI] [PubMed] [Google Scholar]
- 3.Karl J.W., Olson P.R., Rosenwasser M.P. The epidemiology of upper extremity fractures in the United States, 2009. J Orthop Trauma. 2015;29(8):e242–e244. doi: 10.1097/BOT.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 4.Baron J.A., Karagas M., Barrett J., et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612–618. doi: 10.1097/00001648-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Court-Brown C.M., Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691–697. doi: 10.1016/j.injury.2006.04.130. [DOI] [PubMed] [Google Scholar]
- 6.Hung L.K., Wu H.T., Leung P.C., Qin L. Low BMD is a risk factor for low-energy Colles’ fractures in women before and after menopause. Clin Orthop Relat Res. 2005;435:219–225. doi: 10.1097/01.blo.0000155345.04782.14. [DOI] [PubMed] [Google Scholar]
- 7.Kanterewicz E., Yañez A., Pérez-Pons A., Codony I., Del Rio L., Díez-Pérez A. Association between Colles’ fracture and low bone mass: age-based differences in postmenopausal women. Osteoporos Int. 2002;13(10):824–828. doi: 10.1007/s001980200114. [DOI] [PubMed] [Google Scholar]
- 8.Shauver M.J., Yin H., Banerjee M., Chung K.C. Current and future national costs to medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36(8):1282–1287. doi: 10.1016/j.jhsa.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Huetteman H.E., Zhong L., Chung K.C. Cost of surgical treatment for distal radius fractures and the implications of episode-based bundled payments. J Hand Surg Am. 2018;43(8):720–730. doi: 10.1016/j.jhsa.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Shauver M.J., Clapham P.J., Chung K.C. An economic analysis of outcomes and complications of treating distal radius fractures in the elderly. J Hand Surg Am. 2011;36(12):1912–1918.e3. doi: 10.1016/j.jhsa.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 11.Farner S., Malkani A., Lau E., Day J., Ochoa J., Ong K. Outcomes and cost of care for patients with distal radius fractures. Orthopedics. 2014;37(10):e866–e878. doi: 10.3928/01477447-20140924-52. [DOI] [PubMed] [Google Scholar]
- 12.Gehrmann S.V., Windolf J., Kaufmann R.A. Distal radius fracture management in elderly patients: a literature review. J Hand Surg Am. 2008;33(3):421–429. doi: 10.1016/j.jhsa.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Schneppendahl J., Windolf J., Kaufmann R.A. Distal radius fractures: current concepts. J Hand Surg Am. 2012;37(8):1718–1725. doi: 10.1016/j.jhsa.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Goldhahn J., Angst F., Simmen B.R. What counts: outcome assessment after distal radius fractures in aged patients. J Orthop Trauma. 2008;22(suppl 8):S126–S130. doi: 10.1097/BOT.0b013e31817614a1. [DOI] [PubMed] [Google Scholar]
- 15.Clayton R.A., Gaston M.S., Ralston S.H., Court-Brown C.M., McQueen M.M. Association between decreased bone mineral density and severity of distal radial fractures. J Bone Joint Surg Am. 2009;91(3):613–619. doi: 10.2106/JBJS.H.00486. [DOI] [PubMed] [Google Scholar]
- 16.Xu W., Ni C., Yu R., Gu G., Wang Z., Zheng G. Risk factors for distal radius fracture in postmenopausal women. Orthopade. 2017;46(5):447–450. doi: 10.1007/s00132-017-3403-9. [DOI] [PubMed] [Google Scholar]
- 17.Gorter E.A., Reinders C.R., Krijnen P., Appelman-Dijkstra N.M., Schipper I.B. The effect of osteoporosis and its treatment on fracture healing a systematic review of animal and clinical studies. Bone Rep. 2021;15 doi: 10.1016/j.bonr.2021.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostergaard P.J., Hall M.J., Rozental T.D. Considerations in the treatment of osteoporotic distal radius fractures in elderly patients. Curr Rev Musculoskelet Med. 2019;12(1):50–56. doi: 10.1007/s12178-019-09531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ring D., Jupiter J.B. Treatment of osteoporotic distal radius fractures. Osteoporos Int. 2005;16(suppl 2):S80–S84. doi: 10.1007/s00198-004-1808-x. [DOI] [PubMed] [Google Scholar]
- 20.Synn A.J., Makhni E.C., Makhni M.C., Rozental T.D., Day C.S. Distal radius fractures in older patients: is anatomic reduction necessary? Clin Orthop Relat Res. 2009;467(6):1612–1620. doi: 10.1007/s11999-008-0660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung K.C., Cho H.E., Kim Y., Kim H.M., Shauver M.J., WRIST Group Assessment of anatomic restoration of distal radius fractures among older adults: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3(1) doi: 10.1001/jamanetworkopen.2019.19433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung K.C., Watt A.J., Kotsis S.V., Margaliot Z., Haase S.C., Kim H.M. Treatment of unstable distal radial fractures with the volar locking plating system. J Bone Joint Surg Am. 2006;88(12):2687–2694. doi: 10.2106/JBJS.E.01298. [DOI] [PubMed] [Google Scholar]
- 23.Chung K.C., Petruska E.A. Treatment of unstable distal radial fractures with the volar locking plating system. Surgical technique. J Bone Joint Surg Am. 2007;89(suppl 2 Pt 2):256–266. doi: 10.2106/JBJS.G.00283. [DOI] [PubMed] [Google Scholar]
- 24.Rozental T.D., Blazar P.E., Franko O.I., Chacko A.T., Earp B.E., Day C.S. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837–1846. doi: 10.2106/JBJS.H.01478. [DOI] [PubMed] [Google Scholar]
- 25.Koenig K.M., Davis G.C., Grove M.R., Tosteson A.N., Koval K.J. Is early internal fixation preferred to cast treatment for well-reduced unstable distal radial fractures? J Bone Joint Surg Am. 2009;91(9):2086–2093. doi: 10.2106/JBJS.H.01111. [DOI] [PubMed] [Google Scholar]
- 26.FitzPatrick S.K., Casemyr N.E., Zurakowski D., Day C.S., Rozental T.D. The effect of osteoporosis on outcomes of operatively treated distal radius fractures. J Hand Surg Am. 2012;37(10):2027–2034. doi: 10.1016/j.jhsa.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Boymans T.A., van Helden S., Kessels A., Ten Broeke R., Brink P.R. Bone mineral density is not correlated with one-year functional outcome in distal radial fractures: a preliminary study. Eur J Trauma Emerg Surg. 2009;35(3):281–286. doi: 10.1007/s00068-008-8120-y. [DOI] [PubMed] [Google Scholar]
- 28.Semel J., Gray J.M., Ahn H.J., Nasr H., Chen J.J. Predictors of outcome following hip fracture rehabilitation. PM R. 2010;2(9):799–805. doi: 10.1016/j.pmrj.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Lai Q., Song Q., Guo R., et al. Risk factors for acute surgical site infections after lumbar surgery: a retrospective study. J Orthop Surg Res. 2017;12(1):116. doi: 10.1186/s13018-017-0612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruffilli A., Manzetti M., Cerasoli T., et al. Osteopenia and sarcopenia as potential risk factors for surgical site infection after posterior lumbar fusion: a retrospective study. Microorganisms. 2022;10(10):1905. doi: 10.3390/microorganisms10101905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babu J.M., Kalagara S., Durand W., Antoci V., Deren M.E., Cohen E. Sarcopenia as a risk factor for prosthetic infection after total hip or knee arthroplasty. J Arthroplasty. 2019;34(1):116–122. doi: 10.1016/j.arth.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 32.Shah G.M., Gong H.S., Chae Y.J., Kim Y.S., Kim J., Baek G.H. Evaluation and management of osteoporosis and sarcopenia in patients with distal radius fractures. Clin Orthop Surg. 2020;12(1):9–21. doi: 10.4055/cios.2020.12.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordvall H., Glanberg-Persson G., Lysholm J. Are distal radius fractures due to fragility or to falls? A consecutive case-control study of bone mineral density, tendency to fall, risk factors for osteoporosis, and health-related quality of life. Acta Orthop. 2007;78(2):271–277. doi: 10.1080/17453670710013799. [DOI] [PubMed] [Google Scholar]
- 34.Cuddihy M.T., Gabriel S.E., Crowson C.S., O’Fallon W.M., Melton L.J., III Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int. 1999;9(6):469–475. doi: 10.1007/s001980050172. [DOI] [PubMed] [Google Scholar]
- 35.Bhat S.B., Ilyas A.M. Economic Analysis of bisphosphonate use after distal radius fracture for prevention of hip fracture. Arch Bone Jt Surg. 2017;5(6):380–383. [PMC free article] [PubMed] [Google Scholar]
- 36.Hoff M., Skovlund E., Meyer H.E., et al. Does treatment with bisphosphonates protect against fractures in real life? The HUNT study, Norway. Osteoporos Int. 2021;32(7):1395–1404. doi: 10.1007/s00198-021-05845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imam B., Aziz K., Khan M., Zubair T., Iqbal A. Role of bisphosphonates in postmenopausal women with osteoporosis to prevent future fractures: a literature review. Cureus. 2019;11(8):e5328. doi: 10.7759/cureus.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y., Liu X., Gu Y., et al. The effect of bisphosphonates on fracture healing time and changes in bone mass density: a meta-analysis. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.688269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.